Interventions to Address Cardiovascular Risk in Obese Patients: Many Hands Make Light Work
Abstract
1. Introduction: Relationship between Obesity and Cardiovascular Disease
2. Methods
3. Lifestyle Modifications: Diet and Physical Activity
3.1. Diet
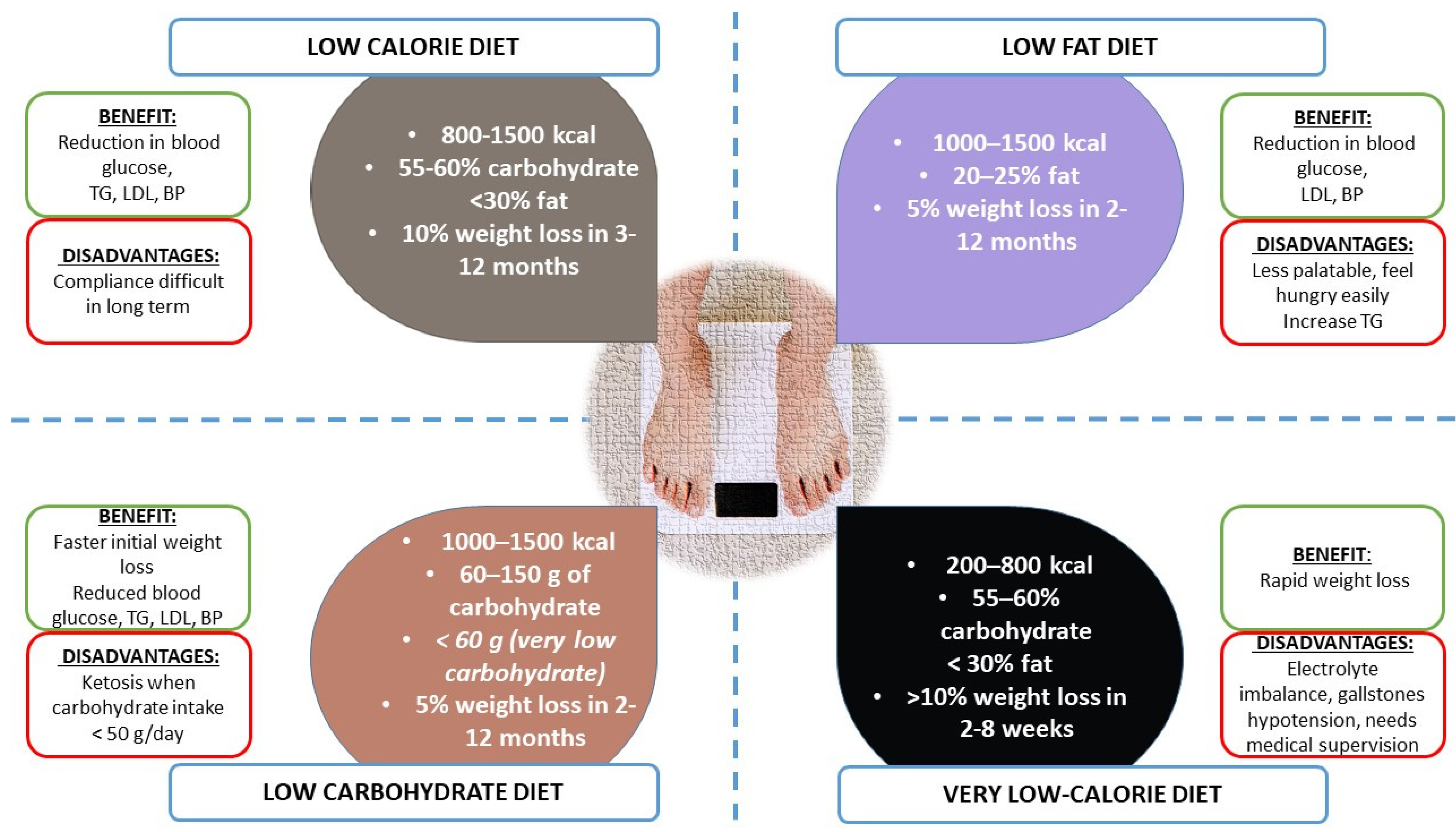
- Low-calorie diet (LCD)
- Low-fat diet
- Low-carbohydrate diet (e.g., Mediterranean Diet)
3.2. Physical Activity
3.2.1. Type of Exercise
Aerobic Exercise
Resistance Exercise
Combined Aerobic and Resistance Exercise
3.2.2. Exercise Duration and Frequency
3.2.3. Dose–Response Relationship between Exercise and Health Outcomes
3.2.4. Exercise Adherence and Strategies for Maintaining Regular Exercise
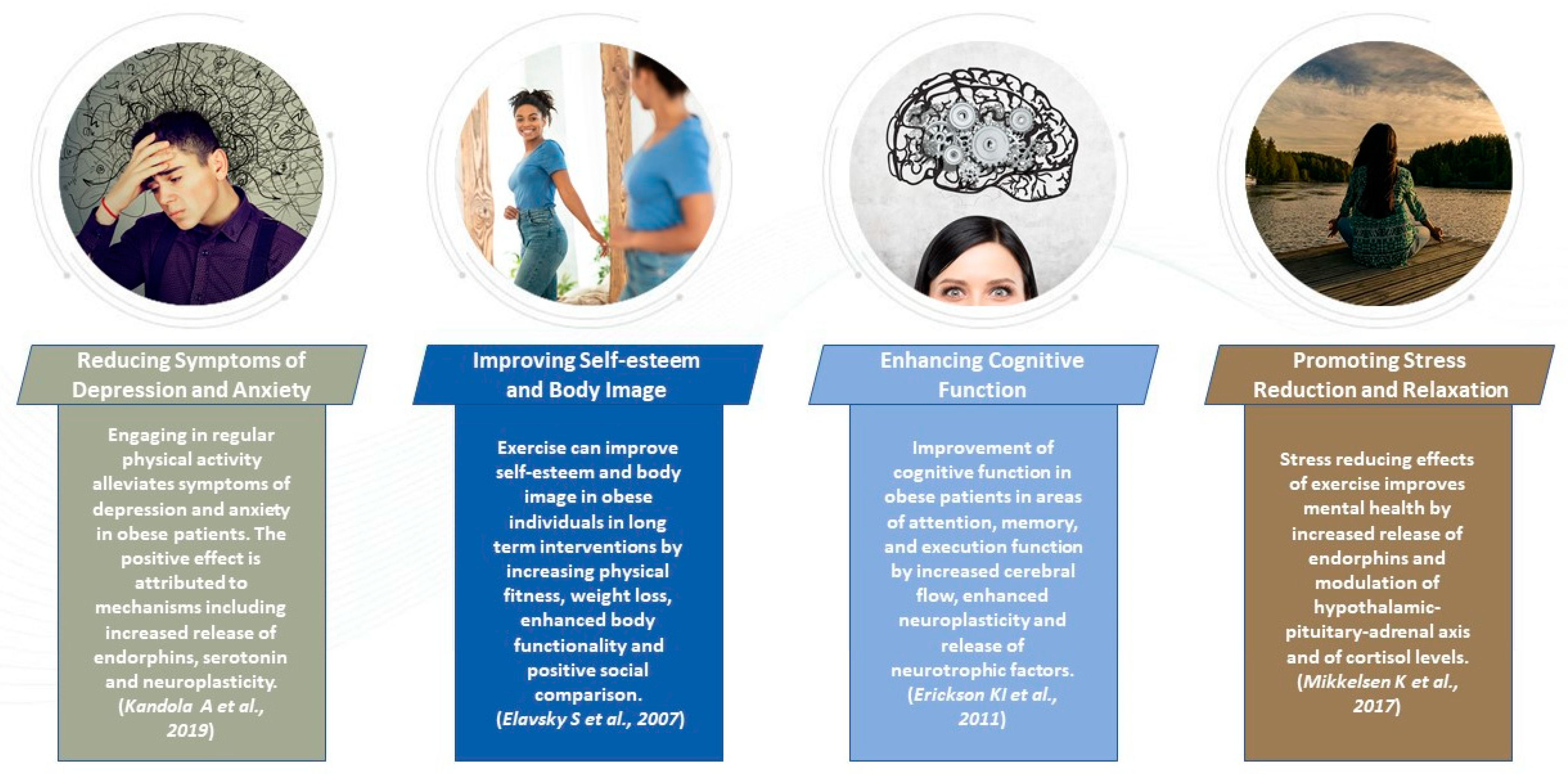
3.2.5. Exercise and Mental Health in Obese Patients
3.2.6. Exercise and Appetite Regulation in Obese Patients
Impact on Hunger and Satiety Hormones
Exercise-Induced Changes in Energy Expenditure
Role of Exercise Intensity and Duration
4. Contribution of Diet and Physical Activity in Improving Inflammatory Conditions in Obese Patients
5. Pharmacological Treatments for Weight Reduction
5.1. Appetite Suppression
5.1.1. Phentermine
5.1.2. Sibutramine (Withdrawn from the Market)
5.1.3. Lorcaserin (Withdrawn from the Market)
5.1.4. Bupropion–Naltrexone
5.2. Fat Absorption Inhibition—Orilstat
5.3. Increased Energy Expenditure
5.3.1. Thermogenic Supplements
5.3.2. Thyroid Hormone Analogs
5.3.3. Modulation of Brown Adipose Tissue (BAT) Activity
5.4. Combination Therapies and Future Directions
5.4.1. Phentermine–Topiramate
5.4.2. GLP-1 Receptor Agonists
5.4.3. Liraglutide
5.4.4. Semaglutide
5.5. Future Directions
6. Surgical Treatments
7. How to Select the Most Appropriate Intervention
8. Artificial Intelligence in Obesity Management
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Jimenez, F.; Almahmeed, W.; Bays, H.; Cuevas, A.; Di Angelantonio, E.; le Roux, C.W.; Sattar, N.; Sun, M.C.; Wittert, G.; Pinto, F.J.; et al. Obesity and cardiovascular disease: Mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur. J. Prev. Cardiol. 2022, 29, 2218–2237. [Google Scholar] [CrossRef]
- Congdon, P.; Amugsi, D. Editorial: The obesity epidemic: Causes, context, prevention. Front. Public Health 2022, 10, 1030180. [Google Scholar] [CrossRef] [PubMed]
- Goryakin, Y.; Lobstein, T.; James, W.P.; Suhrcke, M. The impact of economic, political and social globalization on overweight and obesity in the 56 low and middle income countries. Soc. Sci. Med. 2015, 133, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- d’Errico, M.; Pavlova, M.; Spandonaro, F. The economic burden of obesity in Italy: A cost-of-illness study. Eur. J. Health Econ. 2022, 23, 177–192. [Google Scholar] [CrossRef]
- McLaren, L. Socioeconomic status and obesity. Epidemiol. Rev. 2007, 29, 29–48. [Google Scholar] [CrossRef]
- Larson, N.I.; Story, M.T.; Nelson, M.C. Neighborhood environments: Disparities in access to healthy foods in the U.S. Am. J. Prev. Med. 2009, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Story, M.; Kaphingst, K.M.; Robinson-O’Brien, R.; Glanz, K. Creating healthy food and eating environments: Policy and environmental approaches. Annu. Rev. Public Health 2008, 29, 253–272. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Morland, K.; Diez Roux, A.V.; Wing, S. Supermarkets, other food stores, and obesity: The atherosclerosis risk in communities study. Am. J. Prev. Med. 2006, 30, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Duffey, K.; Gordon-Larsen, P. Environmental influences on food choice, physical activity and energy balance. Physiol. Behav. 2005, 86, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Giskes, K.; van Lenthe, F.; Avendano-Pabon, M.; Brug, J. A systematic review of environmental factors and obesogenic dietary intakes among adults: Are we getting closer to understanding obesogenic environments? Obes. Rev. 2011, 12, e95–e106. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Jankowiak, N.; Nederkoorn, C.; Raynor, H.A.; French, S.A.; Finkelstein, E. Experimental research on the relation between food price changes and food-purchasing patterns: A targeted review. Am. J. Clin. Nutr. 2012, 95, 789–809. [Google Scholar] [CrossRef]
- Sallis, J.F.; Glanz, K. Physical activity and food environments: Solutions to the obesity epidemic. Milbank Q. 2009, 87, 123–154. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.H.; Vierboom, Y.C.; Stokes, A. The role of obesity in exceptionally slow US mortality improvement. Proc. Natl. Acad. Sci. USA 2018, 115, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Visco, V.; Pascale, A.V.; Virtuoso, N.; Mongiello, F.; Cinque, F.; Gioia, R.; Finelli, R.; Mazzeo, P.; Manzi, M.V.; Morisco, C.; et al. Serum Uric Acid and Left Ventricular Mass in Essential Hypertension. Front. Cardiovasc. Med. 2020, 7, 570000. [Google Scholar] [CrossRef]
- Sorriento, D.; Rusciano, M.R.; Visco, V.; Fiordelisi, A.; Cerasuolo, F.A.; Poggio, P.; Ciccarelli, M.; Iaccarino, G. The Metabolic Role of GRK2 in Insulin Resistance and Associated Conditions. Cells 2021, 10, 167. [Google Scholar] [CrossRef]
- Di Pietro, P.; Lizio, R.; Izzo, C.; Visco, V.; Damato, A.; Venturini, E.; De Lucia, M.; Galasso, G.; Migliarino, S.; Rasile, B.; et al. A Novel Combination of High-Load Omega-3 Lysine Complex (AvailOm((R))) and Anthocyanins Exerts Beneficial Cardiovascular Effects. Antioxidants 2022, 11, 896. [Google Scholar] [CrossRef]
- Visco, V.; Izzo, C.; Mancusi, C.; Rispoli, A.; Tedeschi, M.; Virtuoso, N.; Giano, A.; Gioia, R.; Melfi, A.; Serio, B.; et al. Artificial Intelligence in Hypertension Management: An Ace up Your Sleeve. J. Cardiovasc. Dev. Dis. 2023, 10, 74. [Google Scholar] [CrossRef]
- Grover, S.A.; Kaouache, M.; Rempel, P.; Joseph, L.; Dawes, M.; Lau, D.C.; Lowensteyn, I. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: A modelling study. Lancet Diabetes Endocrinol. 2015, 3, 114–122. [Google Scholar] [CrossRef]
- Peto, R.; Whitlock, G.; Jha, P. Effects of obesity and smoking on U.S. life expectancy. N. Engl. J. Med. 2010, 362, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, P.; Izzo, C.; Abate, A.C.; Iesu, P.; Rusciano, M.R.; Venturini, E.; Visco, V.; Sommella, E.; Ciccarelli, M.; Carrizzo, A.; et al. The Dark Side of Sphingolipids: Searching for Potential Cardiovascular Biomarkers. Biomolecules 2023, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Lakka, H.M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Pingitore, A.; Conte, E.; Halasz, G.; Ambrosetti, M.; Peruzzi, M.; Cavarretta, E. Obesity and Cardiovascular Risk: Systematic Intervention Is the Key for Prevention. Healthcare 2023, 11, 902. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Despres, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef]
- Fruhbeck, G.; Busetto, L.; Dicker, D.; Yumuk, V.; Goossens, G.H.; Hebebrand, J.; Halford, J.G.C.; Farpour-Lambert, N.J.; Blaak, E.E.; Woodward, E.; et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes. Facts 2019, 12, 131–136. [Google Scholar] [CrossRef]
- Fall, T.; Mendelson, M.; Speliotes, E.K. Recent Advances in Human Genetics and Epigenetics of Adiposity: Pathway to Precision Medicine? Gastroenterology 2017, 152, 1695–1706. [Google Scholar] [CrossRef]
- Di Pietro, P.; Carrizzo, A.; Sommella, E.; Oliveti, M.; Iacoviello, L.; Di Castelnuovo, A.; Acernese, F.; Damato, A.; De Lucia, M.; Merciai, F.; et al. Targeting the ASMase/S1P pathway protects from sortilin-evoked vascular damage in hypertension. J. Clin. Investig. 2022, 132, e146343. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, M.; Giallauria, F.; Carrizzo, A.; Visco, V.; Silverio, A.; Cesaro, A.; Calabro, P.; De Luca, N.; Mancusi, C.; Masarone, D.; et al. Artificial intelligence in cardiovascular prevention: New ways will open new doors. J. Cardiovasc. Med. 2023, 24, e106–e115. [Google Scholar] [CrossRef] [PubMed]
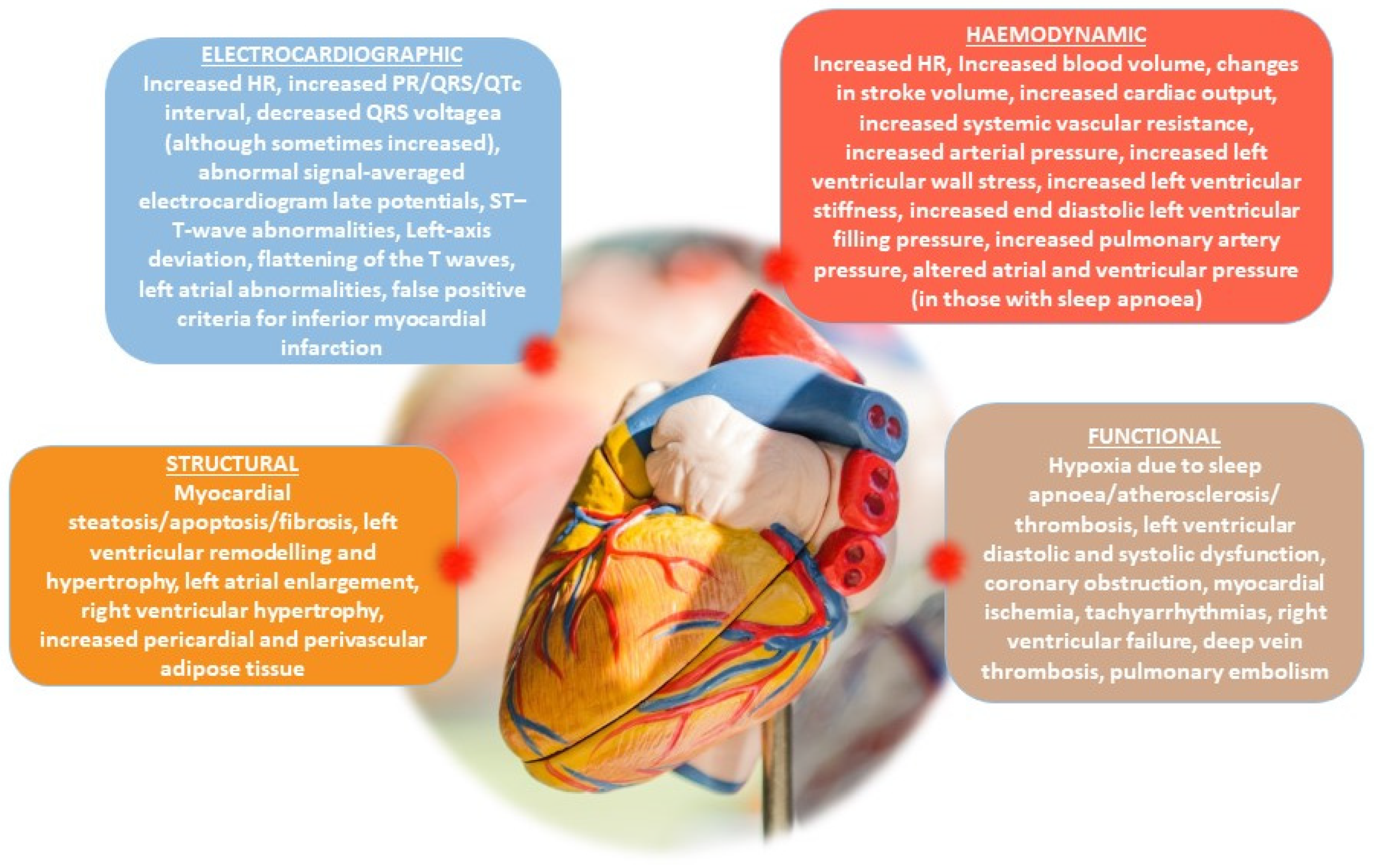
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Heart Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R. The complex relationship between weight and sleep apnoea. Thorax 2015, 70, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Jacob, S. Cardiovascular outcome trials in obesity: A review. Obes. Rev. 2021, 22, e13112. [Google Scholar] [CrossRef]
- Bays, H.E. Adiposopathy is “sick fat” a cardiovascular disease? J. Am. Coll. Cardiol. 2011, 57, 2461–2473. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Bays, H.E.; Taub, P.R.; Epstein, E.; Michos, E.D.; Ferraro, R.A.; Bailey, A.L.; Kelli, H.M.; Ferdinand, K.C.; Echols, M.R.; Weintraub, H.; et al. Ten things to know about ten cardiovascular disease risk factors. Am. J. Prev. Cardiol. 2021, 5, 100149. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Almeida, A.L.; Teixido-Tura, G.; Choi, E.Y.; Opdahl, A.; Fernandes, V.R.; Wu, C.O.; Bluemke, D.A.; Lima, J.A. Metabolic syndrome, strain, and reduced myocardial function: Multi-ethnic study of atherosclerosis. Arq. Bras. Cardiol. 2014, 102, 327–335. [Google Scholar] [CrossRef]
- Aurigemma, G.P.; Silver, K.H.; Priest, M.A.; Gaasch, W.H. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J. Am. Coll. Cardiol. 1995, 26, 195–202. [Google Scholar] [CrossRef]
- Wong, C.Y.; O’Moore-Sullivan, T.; Leano, R.; Byrne, N.; Beller, E.; Marwick, T.H. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004, 110, 3081–3087. [Google Scholar] [CrossRef]
- Avelar, E.; Cloward, T.V.; Walker, J.M.; Farney, R.J.; Strong, M.; Pendleton, R.C.; Segerson, N.; Adams, T.D.; Gress, R.E.; Hunt, S.C.; et al. Left ventricular hypertrophy in severe obesity: Interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension 2007, 49, 34–39. [Google Scholar] [CrossRef]
- Thakker, G.D.; Frangogiannis, N.G.; Bujak, M.; Zymek, P.; Gaubatz, J.W.; Reddy, A.K.; Taffet, G.; Michael, L.H.; Entman, M.L.; Ballantyne, C.M. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2504–H2514. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, S.; Yamashita, N.; Otsu, K.; Kuzuya, T.; Hori, M. Cholesterol feeding exacerbates myocardial injury in Zucker diabetic fatty rats. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H256–H262. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, M.S.; Schaffer, S.W. Myocardial ischemic-reperfusion injury in a rat model of metabolic syndrome. Obesity 2008, 16, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Moberly, S.P.; Mather, K.J.; Berwick, Z.C.; Owen, M.K.; Goodwill, A.G.; Casalini, E.D.; Hutchins, G.D.; Green, M.A.; Ng, Y.; Considine, R.V.; et al. Impaired cardiometabolic responses to glucagon-like peptide 1 in obesity and type 2 diabetes mellitus. Basic. Res. Cardiol. 2013, 108, 365. [Google Scholar] [CrossRef]
- Sassoon, D.J.; Goodwill, A.G.; Noblet, J.N.; Conteh, A.M.; Herring, B.P.; McClintick, J.N.; Tune, J.D.; Mather, K.J. Obesity alters molecular and functional cardiac responses to ischemia/reperfusion and glucagon-like peptide-1 receptor agonism. Basic. Res. Cardiol. 2016, 111, 43. [Google Scholar] [CrossRef]
- Dincer, U.D.; Araiza, A.; Knudson, J.D.; Shao, C.H.; Bidasee, K.R.; Tune, J.D. Dysfunction of cardiac ryanodine receptors in the metabolic syndrome. J. Mol. Cell Cardiol. 2006, 41, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; Franssen, C.; Lourenco, A.; Falcao-Pires, I.; Fontoura, D.; Leite, S.; Plettig, L.; Lopez, B.; Ottenheijm, C.A.; Becher, P.M.; et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ. Heart Fail. 2013, 6, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- McDowell, K.; Petrie, M.C.; Raihan, N.A.; Logue, J. Effects of intentional weight loss in patients with obesity and heart failure: A systematic review. Obes. Rev. 2018, 19, 1189–1204. [Google Scholar] [CrossRef]
- Knudson, J.D.; Dincer, U.D.; Bratz, I.N.; Sturek, M.; Dick, G.M.; Tune, J.D. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation 2007, 14, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Berwick, Z.C.; Dick, G.M.; Tune, J.D. Heart of the matter: Coronary dysfunction in metabolic syndrome. J. Mol. Cell Cardiol. 2012, 52, 848–856. [Google Scholar] [CrossRef]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Gaber, M.; Hainer, J.; Klein, J.; Dorbala, S.; Blankstein, R.; Di Carli, M.F. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012, 126, 1858–1868. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Salas-Salvado, J.; Rubio, M.A.; Barbany, M.; Moreno, B. SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med. Clin. 2007, 128, 184–196. [Google Scholar] [CrossRef]
- Perez, E.A.; Gonzalez, M.P.; Martinez-Espinosa, R.M.; Vila, M.D.M.; Reig Garcia-Galbis, M. Practical Guidance for Interventions in Adults with Metabolic Syndrome: Diet and Exercise vs. Changes in Body Composition. Int. J. Environ. Res. Public Health 2019, 16, 3481. [Google Scholar] [CrossRef]
- Purcell, K.; Sumithran, P.; Prendergast, L.A.; Bouniu, C.J.; Delbridge, E.; Proietto, J. The effect of rate of weight loss on long-term weight management: A randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Carrizzo, A.; Lizio, R.; Di Pietro, P.; Ciccarelli, M.; Damato, A.; Venturini, E.; Iannece, P.; Sommella, E.; Campiglia, P.; Ockermann, P.; et al. Healthberry 865((R)) and Its Related, Specific, Single Anthocyanins Exert a Direct Vascular Action, Modulating Both Endothelial Function and Oxidative Stress. Antioxidants 2021, 10, 1191. [Google Scholar] [CrossRef]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- San-Cristobal, R.; Navas-Carretero, S.; Martinez-Gonzalez, M.A.; Ordovas, J.M.; Martinez, J.A. Contribution of macronutrients to obesity: Implications for precision nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef]
- Larsen, T.M.; Dalskov, S.M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesova, M.; Pihlsgard, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Fock, K.M.; Khoo, J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 59–63. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Santulli, G.; Pascale, V.; Finelli, R.; Visco, V.; Giannotti, R.; Massari, A.; Morisco, C.; Ciccarelli, M.; Illario, M.; Iaccarino, G.; et al. We are What We Eat: Impact of Food from Short Supply Chain on Metabolic Syndrome. J. Clin. Med. 2019, 8, 2061. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hebert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention with Vegetarian Diet). Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Haghighatdoost, F.; Rahimlou, M.; Rodrigues, A.P.S.; Gaskarei, M.K.; Okhovat, P.; de Oliveira, C.; Silveira, E.A.; Sarrafzadegan, N. The Effect of Ketogenic Diet on Shared Risk Factors of Cardiovascular Disease and Cancer. Nutrients 2022, 14, 3499. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef]
- Lorenzo, P.M.; Sajoux, I.; Izquierdo, A.G.; Gomez-Arbelaez, D.; Zulet, M.A.; Abete, I.; Castro, A.I.; Baltar, J.; Portillo, M.P.; Tinahones, F.J.; et al. Immunomodulatory effect of a very-low-calorie ketogenic diet compared with bariatric surgery and a low-calorie diet in patients with excessive body weight. Clin. Nutr. 2022, 41, 1566–1577. [Google Scholar] [CrossRef]
- Teixeira, G.P.; Guimaraes, K.C.; Soares, A.; Marqueze, E.C.; Moreno, C.R.C.; Mota, M.C.; Crispim, C.A. Role of chronotype in dietary intake, meal timing, and obesity: A systematic review. Nutr. Rev. 2022, 81, 75–90. [Google Scholar] [CrossRef]
- Ekiz Erim, S.; Sert, H. The relationship between chronotype and obesity: A systematic review. Chronobiol. Int. 2023, 40, 529–541. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfor, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity—An update. Biomed. Pharmacother. 2021, 140, 111789. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Swift, D.L.; Nevels, T.R.; Solar, C.A.; Brophy, P.M.; McGee, J.E.; Brewer, S.B.; Clark, A.; Houmard, J.A.; Lutes, L.D. The Effect of Aerobic Training and Increasing Nonexercise Physical Activity on Cardiometabolic Risk Factors. Med. Sci. Sport. Exerc. 2021, 53, 2152–2163. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.A.; Tran, Z.V. Aerobic exercise and resting blood pressure: A meta-analytic review of randomized, controlled trials. Prev. Cardiol. 2001, 4, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Ermolao, A.; van Baak, M.A.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraca, E.V.; Encantado, J.; Dicker, D.; Farpour-Lambert, N.; et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: Focus on blood pressure, insulin resistance, and intrahepatic fat-A systematic review and meta-analysis. Obes. Rev. 2021, 22 (Suppl. 4), e13269. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Bredin, S.S.D.; Guan, Y.; Dickinson, K.; Kim, D.D.; Chua, Z.; Kaufman, K.; Warburton, D.E.R. Cardiovascular Health Benefits of Exercise Training in Persons Living with Type 1 Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 253. [Google Scholar] [CrossRef]
- Strasser, B.; Schobersberger, W. Evidence for resistance training as a treatment therapy in obesity. J. Obes. 2011, 2011, 482564. [Google Scholar] [CrossRef] [PubMed]
- Westcott, W.L. Resistance training is medicine: Effects of strength training on health. Curr. Sport. Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sport. Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Garcia-Hermoso, A.; Ramirez-Velez, R.; Lubans, D.R.; Izquierdo, M. Effects of physical education interventions on cognition and academic performance outcomes in children and adolescents: A systematic review and meta-analysis. Br. J. Sport. Med. 2021, 55, 1224–1232. [Google Scholar] [CrossRef]
- Batrakoulis, A. Psychophysiological Adaptations to Pilates Training in Overweight and Obese Individuals: A Topical Review. Diseases 2022, 10, 71. [Google Scholar] [CrossRef]
- Clark, J.E. Diet, exercise or diet with exercise: Comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18–65 years old) who are overfat, or obese; systematic review and meta-analysis. J. Diabetes Metab. Disord. 2015, 14, 31. [Google Scholar] [CrossRef]
- Donini, L.M.; Dernini, S.; Lairon, D.; Serra-Majem, L.; Amiot, M.J.; Del Balzo, V.; Giusti, A.M.; Burlingame, B.; Belahsen, R.; Maiani, G.; et al. A Consensus Proposal for Nutritional Indicators to Assess the Sustainability of a Healthy Diet: The Mediterranean Diet as a Case Study. Front. Nutr. 2016, 3, 37. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Matsuo, T.; So, R.; Takahashi, M. Estimating cardiorespiratory fitness from heart rates both during and after stepping exercise: A validated simple and safe procedure for step tests at worksites. Eur. J. Appl. Physiol. 2020, 120, 2445–2454. [Google Scholar] [CrossRef]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef]
- Maillard, F.; Pereira, B.; Boisseau, N. Effect of High-Intensity Interval Training on Total, Abdominal and Visceral Fat Mass: A Meta-Analysis. Sport. Med. 2018, 48, 269–288. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports, M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sport. Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Rogers, R.J.; Davis, K.K.; Collins, K.A. Role of Physical Activity and Exercise in Treating Patients with Overweight and Obesity. Clin. Chem. 2018, 64, 99–107. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Herrmann, S.D.; Lambourne, K.; Szabo, A.N.; Honas, J.J.; Washburn, R.A. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS ONE 2014, 9, e83498. [Google Scholar] [CrossRef]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A.; et al. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sport. Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Carraca, E.V.; Markland, D.; Silva, M.N.; Ryan, R.M. Exercise, physical activity, and self-determination theory: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Visco, V.; Ferruzzi, G.J.; Nicastro, F.; Virtuoso, N.; Carrizzo, A.; Galasso, G.; Vecchione, C.; Ciccarelli, M. Artificial Intelligence as a Business Partner in Cardiovascular Precision Medicine: An Emerging Approach for Disease Detection and Treatment Optimization. Curr. Med. Chem. 2021, 28, 6569–6590. [Google Scholar] [CrossRef] [PubMed]
- Esch, T.; Stefano, G.B. Endogenous reward mechanisms and their importance in stress reduction, exercise and the brain. Arch. Med. Sci. 2010, 6, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yoneshiro, T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr. Opin. Lipidol. 2013, 24, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, S.; Piacentino, D.; Sani, G.; Aromatario, M. Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Martins, C.; Kulseng, B.; King, N.A.; Holst, J.J.; Blundell, J.E. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J. Clin. Endocrinol. Metab. 2010, 95, 1609–1616. [Google Scholar] [CrossRef]
- Broom, D.R.; Batterham, R.L.; King, J.A.; Stensel, D.J. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R29–R35. [Google Scholar] [CrossRef]
- Blundell, S.W.; Shepherd, R.B.; Dean, C.M.; Adams, R.D.; Cahill, B.M. Functional strength training in cerebral palsy: A pilot study of a group circuit training class for children aged 4–8 years. Clin. Rehabil. 2003, 17, 48–57. [Google Scholar] [CrossRef]
- Deighton, K.; Barry, R.; Connon, C.E.; Stensel, D.J. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1147–1156. [Google Scholar] [CrossRef]
- King, J.A.; Wasse, L.K.; Ewens, J.; Crystallis, K.; Emmanuel, J.; Batterham, R.L.; Stensel, D.J. Differential acylated ghrelin, peptide YY3-36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J. Clin. Endocrinol. Metab. 2011, 96, 1114–1121. [Google Scholar] [CrossRef]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity, F. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Ryan, L.; Grunwald, G.K.; Storgaard, M.; Saris, W.; Melanson, E.; Hill, J.O. The role of dietary fat in body fatness: Evidence from a preliminary meta-analysis of ad libitum low-fat dietary intervention studies. Br. J. Nutr. 2000, 83 (Suppl. 1), S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Majzoub, J.A.; Al-Zahrani, A.; Dallal, G.E.; Blanco, I.; Roberts, S.B. High glycemic index foods, overeating, and obesity. Pediatrics 1999, 103, E26. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Mills, K.T.; Yao, L.; Demanelis, K.; Eloustaz, M.; Yancy, W.S., Jr.; Kelly, T.N.; He, J.; Bazzano, L.A. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: A meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol. 2012, 176 (Suppl. 7), S44–S54. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Clark, K.; Coleman, E.; Donnelly, J.E.; Foreyt, J.; Melanson, E.; Volek, J.; Volpe, S.L.; American College of Sports, M. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sport. Exerc. 2001, 33, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jonsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Izzo, C.; Forte, M.; Sommella, E.; Di Pietro, P.; Venturini, E.; Ciccarelli, M.; Galasso, G.; Rubattu, S.; Campiglia, P.; et al. A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 8706. [Google Scholar] [CrossRef]
- Carrizzo, A.; Moltedo, O.; Damato, A.; Martinello, K.; Di Pietro, P.; Oliveti, M.; Acernese, F.; Giugliano, G.; Izzo, R.; Sommella, E.; et al. New Nutraceutical Combination Reduces Blood Pressure and Improves Exercise Capacity in Hypertensive Patients Via a Nitric Oxide-Dependent Mechanism. J. Am. Heart Assoc. 2020, 9, e014923. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA 2003, 289, 1799–1804. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; You, T.; Pahor, M. Behavioural treatments for chronic systemic inflammation: Effects of dietary weight loss and exercise training. CMAJ 2005, 172, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Busceti, C.L.; Marchitti, S.; Bianchi, F.; Di Pietro, P.; Riozzi, B.; Stanzione, R.; Cannella, M.; Battaglia, G.; Bruno, V.; Volpe, M.; et al. Dickkopf-3 Upregulates VEGF in Cultured Human Endothelial Cells by Activating Activin Receptor-Like Kinase 1 (ALK1) Pathway. Front. Pharmacol. 2017, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sport. 2006, 16 (Suppl. 1), 3–63. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjostrom, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef]
- Davidson, M.H.; Hauptman, J.; DiGirolamo, M.; Foreyt, J.P.; Halsted, C.H.; Heber, D.; Heimburger, D.C.; Lucas, C.P.; Robbins, D.C.; Chung, J.; et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: A randomized controlled trial. JAMA 1999, 281, 235–242. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- van Can, J.; Sloth, B.; Jensen, C.B.; Flint, A.; Blaak, E.E.; Saris, W.H. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 784–793. [Google Scholar] [CrossRef]
- Astrup, A.; Rossner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E.; Group, N.N.S. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K.E.; Prcela, L.; Wadden, T.; Buse, J.B.; Bakris, G.; Perez, A.; Smith, S.R. Effect of Naltrexone-Bupropion on Major Adverse Cardiovascular Events in Overweight and Obese Patients with Cardiovascular Risk Factors: A Randomized Clinical Trial. JAMA 2016, 315, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Aronne, L.; Rubino, D.; Still, C.; Wyatt, H.; Burns, C.; Kim, D.; Dunayevich, E.; Group, C.-I.S. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity 2013, 21, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L.; Fujioka, K.; Plodkowski, R.A.; Mudaliar, S.; Guttadauria, M.; Erickson, J.; Kim, D.D.; Dunayevich, E.; Group, C.-I.S. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010, 376, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Gadde, K.M.; Garvey, W.T.; Peterson, C.A.; Schwiers, M.L.; Najarian, T.; Tam, P.Y.; Troupin, B.; Day, W.W. Controlled-release phentermine/topiramate in severely obese adults: A randomized controlled trial (EQUIP). Obesity 2012, 20, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Ryan, D.H.; Bohannon, N.J.; Kushner, R.F.; Rueger, M.; Dvorak, R.V.; Troupin, B. Weight-loss therapy in type 2 diabetes: Effects of phentermine and topiramate extended release. Diabetes Care 2014, 37, 3309–3316. [Google Scholar] [CrossRef]
- Gadde, K.M.; Allison, D.B.; Ryan, D.H.; Peterson, C.A.; Troupin, B.; Schwiers, M.L.; Day, W.W. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 1341–1352. [Google Scholar] [CrossRef]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults with Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef]
- Davies, M.; Faerch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Petrie, J.R.; Sesti, G.; Mannucci, E.; Courreges, J.P.; Lindegaard, M.L.; Jensen, C.B.; Atkin, S.L.; Study, I. A Phase 2, Randomized, Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog, Semaglutide, Compared with Placebo and Open-Label Liraglutide in Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Song, W.K.; Park, J.Y. Association of CYP2B6, CYP3A5, and CYP2C19 genetic polymorphisms with sibutramine pharmacokinetics in healthy Korean subjects. Clin. Pharmacol. Ther. 2009, 86, 511–518. [Google Scholar] [CrossRef]
- James, W.P.; Caterson, I.D.; Coutinho, W.; Finer, N.; Van Gaal, L.F.; Maggioni, A.P.; Torp-Pedersen, C.; Sharma, A.M.; Shepherd, G.M.; Rode, R.A.; et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N. Engl. J. Med. 2010, 363, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.S.; Majumdar, S.R. Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. Lancet 2007, 369, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Weissman, N.J.; Anderson, C.M.; Sanchez, M.; Chuang, E.; Stubbe, S.; Bays, H.; Shanahan, W.R.; Behavioral, M.; Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010, 363, 245–256. [Google Scholar] [CrossRef]
- Thomsen, W.J.; Grottick, A.J.; Menzaghi, F.; Reyes-Saldana, H.; Espitia, S.; Yuskin, D.; Whelan, K.; Martin, M.; Morgan, M.; Chen, W.; et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: In vitro and in vivo pharmacological characterization. J. Pharmacol. Exp. Ther. 2008, 325, 577–587. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Yanovski, J.A. Long-term drug treatment for obesity: A systematic and clinical review. JAMA 2014, 311, 74–86. [Google Scholar] [CrossRef]
- Hendricks, E.J.; Rothman, R.B.; Greenway, F.L. How physician obesity specialists use drugs to treat obesity. Obesity 2009, 17, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundar, B.; Perez, C.I.; Luna, A.; Solorio, J.; Moreno, M.G.; Elias, D.; Simon, S.A.; Gutierrez, R. D1 and D2 antagonists reverse the effects of appetite suppressants on weight loss, food intake, locomotion, and rebalance spiking inhibition in the rat NAc shell. J. Neurophysiol. 2015, 114, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.Y.; Kang, J.H.; Park, Y.W.; Park, S.W. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes. Metab. 2010, 12, 876–882. [Google Scholar] [CrossRef]
- Aronne, L.J.; Wadden, T.A.; Peterson, C.; Winslow, D.; Odeh, S.; Gadde, K.M. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity 2013, 21, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Astrup, A.; Engeli, S.; Narkiewicz, K.; Day, W.W.; Finer, N. Cardiovascular effects of phentermine and topiramate: A new drug combination for the treatment of obesity. J. Hypertens. 2014, 32, 1178–1188. [Google Scholar] [CrossRef]
- Bray, G.A.; Ryan, D.H. Update on obesity pharmacotherapy. Ann. N. Y. Acad. Sci. 2014, 1311, 1–13. [Google Scholar] [CrossRef]
- Peri, G.; Introna, M.; Corradi, D.; Iacuitti, G.; Signorini, S.; Avanzini, F.; Pizzetti, F.; Maggioni, A.P.; Moccetti, T.; Metra, M.; et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 2000, 102, 636–641. [Google Scholar] [CrossRef]
- Fidler, M.C.; Sanchez, M.; Raether, B.; Weissman, N.J.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M.; Group, B.C.T. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: The BLOSSOM trial. J. Clin. Endocrinol. Metab. 2011, 96, 3067–3077. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Smith, S.R.; Weissman, N.J.; Fidler, M.C.; Sanchez, M.; Zhang, J.; Raether, B.; Anderson, C.M.; Shanahan, W.R. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: The BLOOM-DM study. Obesity 2012, 20, 1426–1436. [Google Scholar] [CrossRef]
- Weissman, N.J.; Sanchez, M.; Koch, G.G.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M. Echocardiographic assessment of cardiac valvular regurgitation with lorcaserin from analysis of 3 phase 3 clinical trials. Circ. Cardiovasc. Imaging 2013, 6, 560–567. [Google Scholar] [CrossRef]
- Rucker, D.; Padwal, R.; Li, S.K.; Curioni, C.; Lau, D.C. Long term pharmacotherapy for obesity and overweight: Updated meta-analysis. BMJ 2007, 335, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Glinni, D.; Moreno, M.; Cioffi, F.; Silvestri, E.; Goglia, F. Thyroid hormone analogues and derivatives: Actions in fatty liver. World J. Hepatol. 2014, 6, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Zhou, Y.; Li, D.; Rossulek, M.; Dong, J.; Somayaji, V.; Weng, Y.; Clark, R.; Lanba, A.; Owen, B.M.; et al. A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab. 2016, 23, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Franquet Elia, E.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Clemmensen, C.; Muller, T.D.; Woods, S.C.; Berthoud, H.R.; Seeley, R.J.; Tschop, M.H. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017, 168, 758–774. [Google Scholar] [CrossRef]
- Karmali, S.; Johnson Stoklossa, C.; Sharma, A.; Stadnyk, J.; Christiansen, S.; Cottreau, D.; Birch, D.W. Bariatric surgery: A primer. Can. Fam. Physician 2010, 56, 873–879. [Google Scholar]
- Billeter, A.T.; Fischer, L.; Wekerle, A.L.; Senft, J.; Muller-Stich, B. Malabsorption as a Therapeutic Approach in Bariatric Surgery. Viszeralmedizin 2014, 30, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Benraouane, F.; Litwin, S.E. Reductions in cardiovascular risk after bariatric surgery. Curr. Opin. Cardiol. 2011, 26, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Czupryniak, L.; Strzelczyk, J.; Pawlowski, M.; Loba, J. Circadian blood pressure variation in morbidly obese hypertensive patients undergoing gastric bypass surgery. Am. J. Hypertens. 2005, 18, 446–451. [Google Scholar] [CrossRef]
- Schiavon, C.A.; Bersch-Ferreira, A.C.; Santucci, E.V.; Oliveira, J.D.; Torreglosa, C.R.; Bueno, P.T.; Frayha, J.C.; Santos, R.N.; Damiani, L.P.; Noujaim, P.M.; et al. Effects of Bariatric Surgery in Obese Patients with Hypertension: The GATEWAY Randomized Trial (Gastric Bypass to Treat Obese Patients with Steady Hypertension). Circulation 2018, 137, 1132–1142. [Google Scholar] [CrossRef]
- Salminen, P.; Helmio, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavisto, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients with Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA 2018, 319, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Ikramuddin, S.; Korner, J.; Lee, W.J.; Connett, J.E.; Inabnet, W.B.; Billington, C.J.; Thomas, A.J.; Leslie, D.B.; Chong, K.; Jeffery, R.W.; et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The Diabetes Surgery Study randomized clinical trial. JAMA 2013, 309, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Stoll, C.R.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef]
- Dietz, W.H.; Baur, L.A.; Hall, K.; Puhl, R.M.; Taveras, E.M.; Uauy, R.; Kopelman, P. Management of obesity: Improvement of health-care training and systems for prevention and care. Lancet 2015, 385, 2521–2533. [Google Scholar] [CrossRef]
- Caixas, A.; Villaro, M.; Arraiza, C.; Montalva, J.C.; Lecube, A.; Fernandez-Garcia, J.M.; Corio, R.; Bellido, D.; Llisterri, J.L.; Tinahones, F.J. SEEDO-SEMERGEN consensus document on continuous care of obesity between Primary Care and Specialist Hospital Units 2019. Med. Clin. 2020, 155, 267.e1–267.e11. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. Reviewers of the AACEOCPG: American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22 (Suppl. 3), 1–203. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.M.; Kushner, R.F. A proposed clinical staging system for obesity. Int. J. Obes. 2009, 33, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Fitch, A.; Cuda, S.; Gonsahn-Bollie, S.; Rickey, E.; Hablutzel, J.; Coy, R.; Censani, M. Artificial intelligence and obesity management: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023. Obes. Pillars 2023, 6, 100065. [Google Scholar] [CrossRef]
- An, R.; Shen, J.; Xiao, Y. Applications of Artificial Intelligence to Obesity Research: Scoping Review of Methodologies. J. Med. Internet Res. 2022, 24, e40589. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Mallio, C.A. Artificial intelligence and abdominal adipose tissue analysis: A literature review. Quant. Imaging Med. Surg. 2021, 11, 4461–4474. [Google Scholar] [CrossRef]
- Wang, B.; Torriani, M. Artificial Intelligence in the Evaluation of Body Composition. Semin. Musculoskelet. Radiol. 2020, 24, 30–37. [Google Scholar] [CrossRef]
- Han, K.; Cao, P.; Wang, Y.; Xie, F.; Ma, J.; Yu, M.; Wang, J.; Xu, Y.; Zhang, Y.; Wan, J. A Review of Approaches for Predicting Drug-Drug Interactions Based on Machine Learning. Front. Pharmacol. 2021, 12, 814858. [Google Scholar] [CrossRef]
- Fitch, A.K.; Bays, H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes. Pillars 2022, 1, 100004. [Google Scholar] [CrossRef]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Ferreras, A.; Sumalla-Cano, S.; Martínez-Licort, R.; Elío, I.; Tutusaus, K.; Prola, T.; Vidal-Mazón, J.L.; Sahelices, B.; de la Torre Díez, I. Systematic Review of Machine Learning applied to the Prediction of Obesity and Overweight. J. Med. Syst. 2023, 47, 8. [Google Scholar] [CrossRef] [PubMed]
| Drug Name | Drug Class | Mechanism of Action | Indications | Common Side Effects |
|---|---|---|---|---|
| Orlistat (Xenical) [129,130,131] | Lipase inhibitor | Inhibits gastric and pancreatic lipases, reducing fat absorption in the gut | Obesity weight management | Gastrointestinal issues, oily stools, flatulence |
| Liraglutide (Saxenda) [132,133,134] | GLP-1 receptor agonist | Mimics the action of incretin hormones, increasing satiety and reducing appetite | Obesity weight management type 2 diabetes | Nausea, vomiting, diarrhea, constipation |
| Naltrexone–Bupropion (Contrave) [135,136,137] | Opioid antagonist and dopamine/norepinephrine reuptake inhibitor | Reduces appetite by blocking opioid receptors and increasing dopamine/norepinephrine levels | Obesity weight management | Nausea, constipation, headache, dizziness, dry mouth |
| Phentermine-Topiramate (Qsymia) [138,139,140] | Sympathomimetic amine and anticonvulsant | Increases satiety and reduces appetite through increased norepinephrine release and GABA receptor modulation | Obesity weight management | Dizziness, dry mouth, constipation, insomnia, palpitations |
| Semaglutide (Wegovy) [141,142,143,144] | GLP-1 receptor agonist | Mimics the action of incretin hormones, increasing satiety and reducing appetite | Obesity weight management type 2 diabetes | Nausea, vomiting, diarrhea, constipation, abdominal pain |
| Sibutramine (Meridia) [145,146,147] | Serotonin-norepinephrine reuptake inhibitor | Increases satiety and reduces appetite by enhancing serotonin and norepinephrine release | Obesity weight management | Dry mouth, constipation, insomnia, increased heart rate |
| Lorcaserin (Belviq) [148,149] | Serotonin 2C receptor agonist | Activates serotonin 2C receptors in the brain to promote satiety and reduce appetite | Obesity weight management | Headache, dizziness, fatigue, nausea, dry mouth, constipation |
| Thermogenic Supplements | Dietary supplements | Increase metabolism and promote fat burning by increasing thermogenesis | Weight management fat loss | Varies depending on ingredients: insomnia, increased heart rate, anxiety, gastrointestinal discomfort |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visco, V.; Izzo, C.; Bonadies, D.; Di Feo, F.; Caliendo, G.; Loria, F.; Mancusi, C.; Chivasso, P.; Di Pietro, P.; Virtuoso, N.; et al. Interventions to Address Cardiovascular Risk in Obese Patients: Many Hands Make Light Work. J. Cardiovasc. Dev. Dis. 2023, 10, 327. https://doi.org/10.3390/jcdd10080327
Visco V, Izzo C, Bonadies D, Di Feo F, Caliendo G, Loria F, Mancusi C, Chivasso P, Di Pietro P, Virtuoso N, et al. Interventions to Address Cardiovascular Risk in Obese Patients: Many Hands Make Light Work. Journal of Cardiovascular Development and Disease. 2023; 10(8):327. https://doi.org/10.3390/jcdd10080327
Chicago/Turabian StyleVisco, Valeria, Carmine Izzo, Davide Bonadies, Federica Di Feo, Giuseppe Caliendo, Francesco Loria, Costantino Mancusi, Pierpaolo Chivasso, Paola Di Pietro, Nicola Virtuoso, and et al. 2023. "Interventions to Address Cardiovascular Risk in Obese Patients: Many Hands Make Light Work" Journal of Cardiovascular Development and Disease 10, no. 8: 327. https://doi.org/10.3390/jcdd10080327
APA StyleVisco, V., Izzo, C., Bonadies, D., Di Feo, F., Caliendo, G., Loria, F., Mancusi, C., Chivasso, P., Di Pietro, P., Virtuoso, N., Carrizzo, A., Vecchione, C., & Ciccarelli, M. (2023). Interventions to Address Cardiovascular Risk in Obese Patients: Many Hands Make Light Work. Journal of Cardiovascular Development and Disease, 10(8), 327. https://doi.org/10.3390/jcdd10080327