Minimal-Access Coronary Revascularization: Past, Present, and Future
Abstract
1. Introduction
2. Materials and Methods
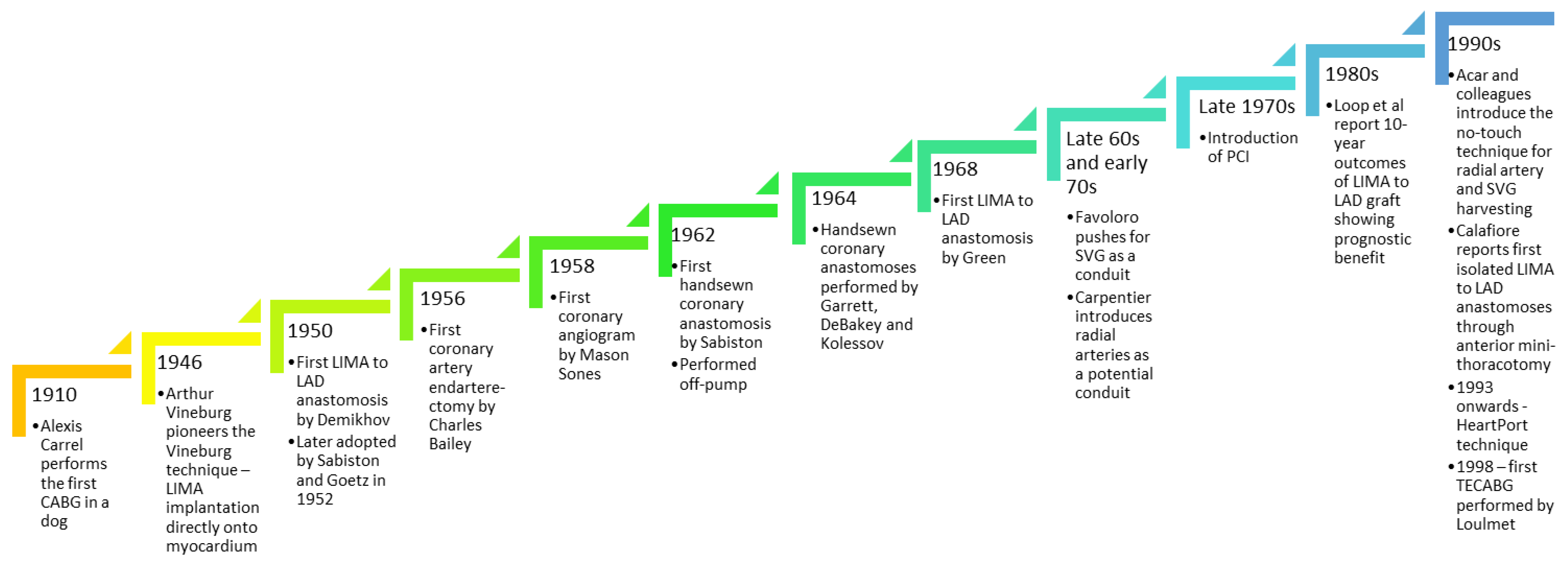
3. History of Coronary Artery Bypass Grafting
4. Minimal-Access Coronary Revascularisation—International Guidelines Perspective
5. Patient Selection and Rationale for Minimal-Access Coronary Intervention
6. Contraindications to Minimal-Access Coronary Revascularisation
7. Techniques of Minimal-Access Coronary Artery Revascularisation
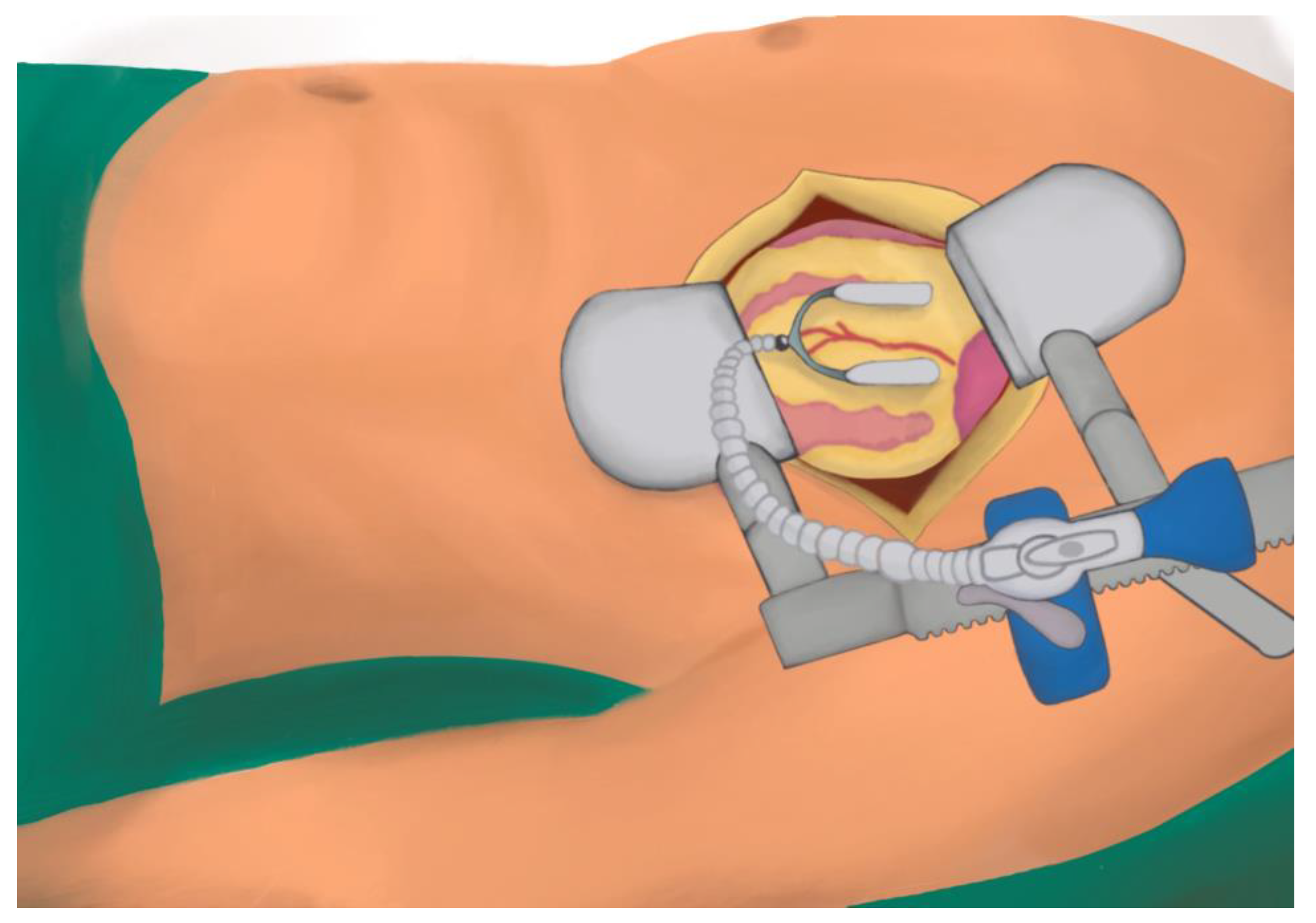
7.1. MIDCABG
7.1.1. Description
7.1.2. Positioning and Monitoring
7.1.3. Operative Steps
7.1.4. Evidence
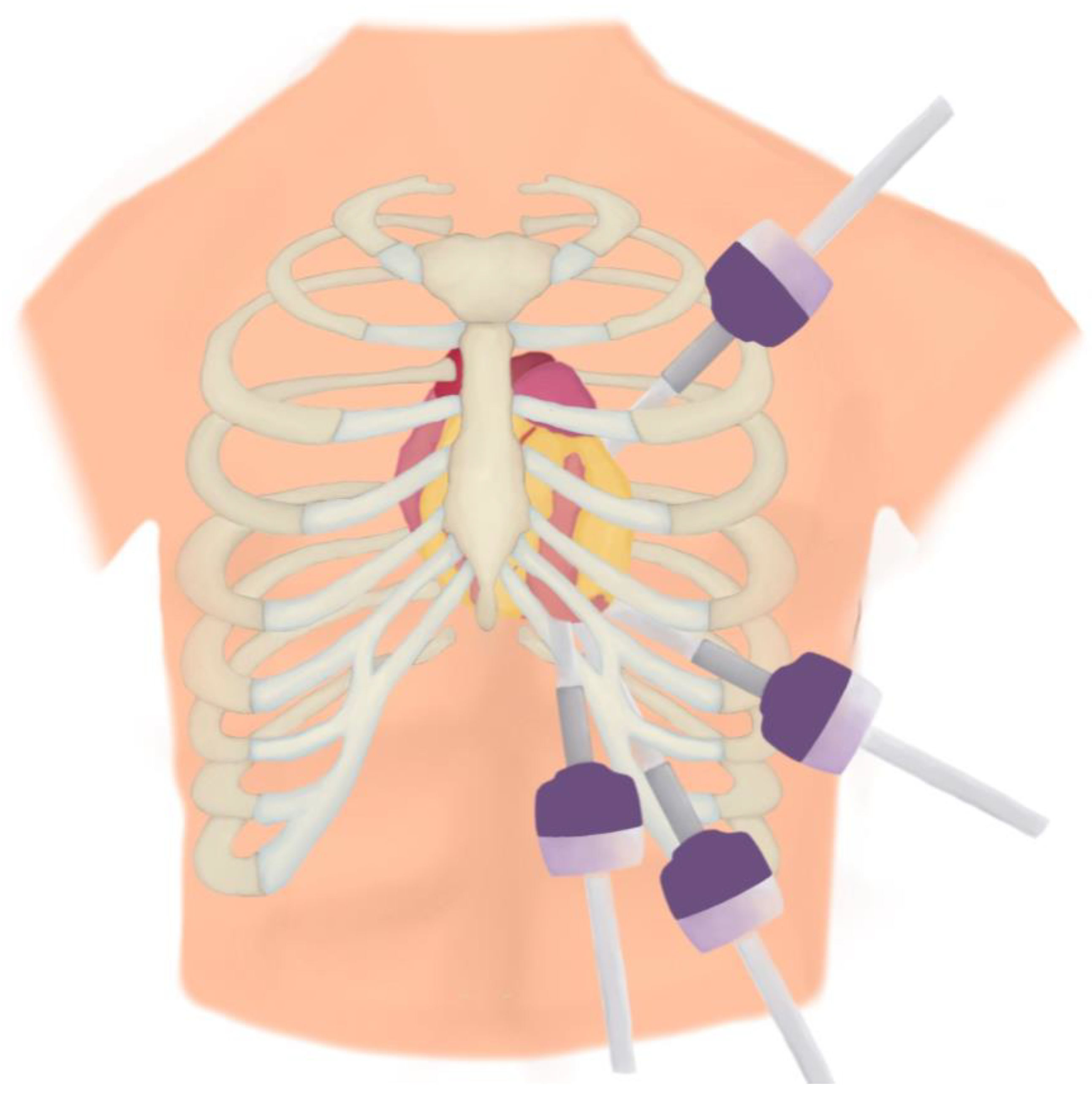
7.2. TECABG/RACABG
7.2.1. Description
7.2.2. Positioning and Monitoring
7.2.3. Operative Steps
7.2.4. Evidence
7.3. HCR
7.3.1. Definition
7.3.2. Evidence
8. Nomenclatures
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AH-TECABG | Totally Endoscopic Arrested Heart Coronary Artery Bypass Grafting |
| CABG | Coronary Artery Bypass Grafting |
| CPB | Cardiopulmonary bypass |
| HCR | Hybrid Coronary Revascularisation |
| LAD | Left Anterior Descending Artery |
| LIMA | Left Internal Mammary Artery |
| MICS CABG | Minimally Invasive Coronary Artery Bypass Grafting |
| MIDCABG | Minimally invasive Coronary Artery Bypass Grafting |
| PA CABG | Port-Access Coronary Artery Bypass Grafting |
| RACABG | Robotic-Assisted Coronary Artery Bypass Grafting |
| RATS | Robot-Assisted Thoracoscopic Surgery |
| RCT | Randomised Control Trial |
| RIMA | right Internal Mammary Artery |
| TECABG | Totally Endoscopic Coronary Artery Bypass Grafting |
| VATS | Video-Assisted Thoracoscopic Surgery |
References
- Carrel, A., VIII. On the Experimental Surgery of the Thoracic Aorta and Heart. Ann. Surg. 1910, 52, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Melly, L.; Torregrossa, G.; Lee, T.; Jansens, J.-L.; Puskas, J.D. Fifty years of coronary artery bypass grafting. J. Thorac. Dis. 2018, 10, 1960–1967. [Google Scholar] [PubMed]
- Diodato, M.; Chedrawy, E.G. Coronary artery bypass graft surgery: The past, present, and future of myocardial revascularisation. Surg. Res. Pract. 2014, 2014, 726158. [Google Scholar] [PubMed]
- Vineberg, A.; Miller, G. Internal mammary coronary anastomosis in the surgical treatment of coronary artery insufficiency. Can. Med. Assoc. J. 1951, 64, 204–210. [Google Scholar]
- Thomas, J.L. The Vineberg legacy: Internal mammary artery implantation from inception to obsolescence. Tex. Heart Inst. J. 1999, 26, 107–113. [Google Scholar]
- Rozsival, V. Outcome of Vineberg’s operation after 31 years. Heart 2006, 92, 1070. [Google Scholar] [CrossRef][Green Version]
- Sabiston, D.C., Jr. The William F. Rienhoff, Jr. lecture. The coronary circulation. Johns Hopkins Med. J. 1974, 134, 314–329. [Google Scholar]
- Goetz, R.H.; Rohman, M.; Haller, J.D.; Dee, R.; Rosenak, S.S. Internal mammary-coronary artery anastomosis. A nonsuture method employing tantalum rings. J. Thorac. Cardiovasc. Surg. 1961, 41, 378–386. [Google Scholar]
- Bailey, C.P.; May, A.; Lemmon, W.M. Survival after coronary endarterectomy in man. J. Am. Med. Assoc. 1957, 164, 641–646. [Google Scholar] [CrossRef]
- Sones, F.M., Jr.; Shirey, E.K. Cine coronary arteriography. Mod. Concepts Cardiovasc. Dis. 1962, 31, 735–738. [Google Scholar]
- Garrett, H.E.; Dennis, E.W.; DeBakey, M.E. Aortocoronary bypass with saphenous vein graft. Seven-year follow-up. JAMA 1973, 223, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Kolessov, V.I. Mammary artery-coronary artery anastomosis as method of treatment for angina pectoris. J. Thorac. Cardiovasc. Surg. 1967, 54, 535–544. [Google Scholar] [PubMed]
- Green, G.E.; Stertzer, S.H.; Reppert, E.H. Coronary arterial bypass grafts. Ann. Thorac. Surg. 1968, 5, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, R.G. Saphenous vein autograft replacement of severe segmental coronary artery occlusion: Operative technique. Ann. Thorac. Surg. 1968, 5, 334–339. [Google Scholar] [CrossRef]
- Marti, M.C.; Bouchardy, B.; Cox, J.N. Aorto-coronary by-pass with autogenous saphenous vein grafts: Histopathological aspects. Virchows Arch. A Pathol. Pathol. Anat. 1971, 352, 255–266. [Google Scholar] [CrossRef]
- Cuminetti, G.; Gelsomino, S.; Curello, S.; Lorusso, R.; Maessen, J.G.; Hoorntje, J.C.A. Contemporary use of arterial and venous conduits in coronary artery bypass grafting: Anatomical, functional and clinical aspects. Neth. Heart J. 2017, 25, 4–13. [Google Scholar] [CrossRef]
- Carpentier, A.; Guermonprez, J.L.; Deloche, A.; Frechette, C.; DuBost, C. The aorta-to-coronary radial artery bypass graft. A technique avoiding pathological changes in grafts. Ann. Thorac. Surg. 1973, 16, 111–121. [Google Scholar] [CrossRef]
- Acar, C.; Jebara, V.A.; Portoghese, M.; Beyssen, B.; Pagny, J.Y.; Grare, P.; Chachques, J.C.; Fabiani, J.N.; Deloche, A.; Guermonprez, J.L. Revival of the radial artery for coronary artery bypass grafting. Ann. Thorac. Surg. 1992, 54, 652–659, discussion 659–660. [Google Scholar] [CrossRef]
- Acar, C.; Ramsheyi, A.; Pagny, J.Y.; Jebara, V.; Barrier, P.; Fabiani, J.N.; Deloche, A.; Guermonprez, J.L.; Carpentier, A. The radial artery for coronary artery bypass grafting: Clinical and angiographic results at five years. J. Thorac. Cardiovasc. Surg. 1998, 116, 981–989. [Google Scholar] [CrossRef]
- Loop, F.D.; Lytle, B.W.; Cosgrove, D.M.; Stewart, R.W.; Goormastic, M.; Williams, G.W.; Golding, L.A.; Gill, C.C.; Taylor, P.C.; Sheldon, W.C.; et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 1986, 314, 1–6. [Google Scholar] [CrossRef]
- Gruntzig, A. Transluminal dilatation of coronary-artery stenosis. Lancet 1978, 1, 263. [Google Scholar] [CrossRef]
- Calafiore, A.M.; Giammarco, G.D.; Teodori, G.; Bosco, G.; D’Annunzio, E.; Barsotti, A.; Maddestra, N.; Paloscia, L.; Vitolla, G.; Sciarra, A.; et al. Left anterior descending coronary artery grafting via left anterior small thoracotomy without cardiopulmonary bypass. Ann. Thorac. Surg. 1996, 61, 1658–1663, discussion 1664–1665. [Google Scholar]
- Fann, J.I.; Burdon, T.A.; Pompili, M.F. Minimally invasive cardiac surgery using the HEARTPORT technique. Asia Pac. Heart J. 1999, 8, 19–26. [Google Scholar] [CrossRef]
- Loulmet, D.; Carpentier, A.; d’Attellis, N.; Berrebi, A.; Cardon, C.; Ponzio, O.; Aupecle, B.; Relland, J.Y. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J. Thorac. Cardiovasc. Surg. 1999, 118, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Colet, J.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar]
- Gasior, M.; Zembala, M.O.; Tajstra, M.; Filipiak, K.; Gierlotka, M.; Hrapkowicz, T.; Hawranek, M.; Polonski, L.; Zembala, M.; POL-MIDES (HYBRID) Investigators. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc. Interv. 2014, 7, 1277–1283. [Google Scholar] [PubMed]
- Tajstra, M.; Hrapkowicz, T.; Hawranek, M.; Filipiak, K.; Gierlotka, M.; Zembala, M.; Gasior, M.; Zembala, M.O. Hybrid coronary revascularization in selected patients with Multivessel disease. JACC Cardiovasc. Interv. 2018, 11, 847–852. [Google Scholar] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Gohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI guideline for Coronary artery revascularization: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [PubMed]
- Guan, Z.; Zhang, Z.; Gu, K.; Wang, H.; Lin, J.; Zhou, W.; Wan, F. Minimally invasive CABG or hybrid coronary revascularization for Multivessel Coronary Diseases: Which is best? A systematic review and metaanalysis. Heart Surg. Forum 2019, 22, E493–E502. [Google Scholar]
- Misfeld, M.; Brereton, R.J.; Sweetman, E.A.; Doig, G.S. Neurologic complications after off-pump coronary artery bypass grafting with and without aortic manipulation: Meta-analysis of 11,398 cases from 8 studies. J. Thorac. Cardiovasc. Surg. 2011, 142, e11–e17. [Google Scholar]
- Czerny, M.; Baumer, H.; Kilo, J.; Lassnigg, A.; Hamwi, A.; Vukovich, T.; Wolner, E.; Grimm, M. Inflammatory response and myocardial injury following coronary artery bypass grafting with or without cardiopulmonary bypass. Eur. J. Cardiothorac. Surg. 2000, 17, 737–742. [Google Scholar] [PubMed]
- Shroyer, A.L.; Hattler, B.; Wagner, T.H.; Collins, J.F.; Baltz, J.H.; Quin, J.A.; Hossein Almassi, G.; Kozora, E.; Bakaeen, F.; Cleveland, J.C., Jr.; et al. Five-year outcomes after on-pump and off-pump coronary-artery bypass. N. Engl. J. Med. 2017, 377, 623–632. [Google Scholar] [PubMed]
- Diegeler, A.; Walther, T.; Metz, S.; Falk, V.; Krakor, R.; Autschbach, R.; Mohr, F.W. Comparison of MIDCAB versus conventional CABG surgery regarding pain and quality of life. Heart Surg. Forum 1999, 2, 290–295. [Google Scholar] [PubMed]
- Garg, S.; Raja, S.G. Minimally invasive direct coronary artery bypass (MIDCAB) grafting. AME Med. J. 2020, 5, 19. [Google Scholar]
- McGinn, J.T.; Usman, S.; Lapierre, H.; Pothula, V.R.; Mesana, T.G.; Ruel, M. Minimally invasive coronary artery bypass grafting. Circulation 2009, 120 (Suppl. 1), S78–S84. [Google Scholar] [PubMed]
- Subramanian, V.A. Midcab approach for Single Vessel Coronary Artery Bypass Graft. Oper. Tech. Card. Thorac. Surg. 1998, 3, 2–15. [Google Scholar] [CrossRef]
- Ruel, M. Nonsternotomy multivessel coronary artery bypass grafting: A key development in cardiac surgery. J. Thorac. Cardiovasc. Surg. Tech. 2021, 10, 162–167. [Google Scholar]
- Bonatti, J.; Schachner, T.; Bonaros, N.; Oehlinger, A.; Ruetzler, E.; Friedrich, G.; Feuchner, G.; Laufer, G. How to improve performance of robotic totally endoscopic coronary artery bypass grafting. Am. J. Surg. 2008, 195, 711–716. [Google Scholar]
- Ruel, M.; Shariff, M.A.; Lapierre, H.; Goyal, N.; Dennie, C.; Sadel, S.M.; Sohmer, B.; McGinn, J.T., Jr. Results of the minimally invasive coronary artery bypass grafting angiographic patency study. J. Thorac. Cardiovasc. Surg. 2014, 147, 203–209. [Google Scholar]
- Bonatti, J.; Schachner, T.; Bernecker, O.; Chevtchik, O.; Bonaros, N.; Ott, H.; Friedrich, G.; Weidiner, F.; Laufer, G. Robotic totally endoscopic coronary artery bypass: Program development and learning curve issues. J. Thorac. Cardiovasc. Surg. 2004, 127, 504–510. [Google Scholar]
- Van Praet, K.M.; Kofler, M.; Nazari Shafti, T.Z.; El Al, A.A.; van Kampen, A.; Amabile, A.; Torregrossa, G.; Kempfert, J.; Falk, V.; Balkhy, H.H.; et al. Minimally invasive coronary revascularisation surgery: A focused review of the available literature. Interv. Cardiol. Rev. 2021, 16, e08. [Google Scholar]
- Ruel, M. Commentary: Sternotomy for every cardiac surgery patient ain’t the future, so let’s get going. J. Thorac. Cardiovasc. Surg. 2023, 165, 129–131. [Google Scholar] [PubMed]
- Greenspu, H.G.; Adourian, U.A.; Fonger, J.D.; Fan, J.S. Minimally invasive direct coronary artery bypass (MIDCAB): Surgical techniques and anesthetic considerations. J. Cardiothorac. Vasc. Anes. 1996, 10, 507–509. [Google Scholar]
- Patel, A.J.; Yates, M.T.; Soppa, G.K. What is the optimal revascularization technique for isolated disease of the left anterior descending artery: Minimally invasive direct coronary artery bypass or percutaneous coronary intervention? Interact. Cardiovasc. Thorac. Surg. 2014, 19, 144–148. [Google Scholar] [CrossRef]
- Raja, S.G.; Benedetto, U.; Alkizwini, E.; Gupta, S.; Amrani, M.; Harefield Cardiac Outcomes Research Group. Propensity Score Adjusted Comparison of MIDCAB Versus Full Sternotomy Left Anterior Descending Artery Revascularization. Innovations 2015, 10, 174–178. [Google Scholar] [CrossRef]
- Raja, S.G.; Garg, S.; Rochon, M.; Daley, S.; De Robertis, F.; Bahrami, T. Short-term clinical outcomes and long-term survival of minimally invasive direct coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2018, 7, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Repossini, A.; Di Bacco, L.; Nicoli, F.; Passaretti, B.; Stara, A.; Jonida, B.; Muneretto, C. Minimally invasive coronary artery bypass: Twenty-year experience. J. Thorac. Cardiovasc. Surg. 2019, 158, 127–138.e1. [Google Scholar] [CrossRef]
- Manuel, L.; Fong, L.S.; Betts, K.; Bassin, L.; Wolfenden, H. LIMA to LAD grafting returns patient survival to age-matched population: 20-year outcomes of MIDCAB surgery. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac243. [Google Scholar] [CrossRef]
- Benetti, F.J.; Ballester, C.; Sani, G.; Doonstra, P.; Grandjean, J. Video assisted coronary bypass surgery. J. Card. Surg. 1995, 10, 620–625. [Google Scholar] [CrossRef]
- Bonatti, J.; Wehman, B.; de Biasi, A.R.; Jeudy, J.; Griffith, B.; Lehr, E.J. Totally endoscopic quadruple coronary artery bypass grafting is feasible using robotic technology. Ann. Thorac. Surg. 2012, 93, e111–e112. [Google Scholar] [CrossRef]
- Balkhy, H.H. Robotic totally endoscopic coronary artery bypass grafting: It’s now or never! JTCVS Tech. 2021, 10, 153–157. [Google Scholar] [CrossRef]
- Lee, J.D.; Srivastava, M.; Bonatti, J. History and current status of robotic totally endoscopic coronary artery bypass. Circ. J. 2012, 76, 2058–2065. [Google Scholar] [CrossRef][Green Version]
- Yilmaz, A.; Robic, B.; Starinieri, P.; Polus, F.; Stinkens, R.; Stessel, B. A new viewpoint on endoscopic CABG: Technique description and clinical experience. J. Cardiol. 2020, 75, 614–620. [Google Scholar] [PubMed]
- Cao, C.; Indraratna, P.; Doyle, M.; Tian, D.H.; Liou, K.; Munkholm-Larsen, S.; Uys, C.; Virk, S. A systematic review on robotic coronary artery bypass graft surgery. Ann. Cardiothorac. Surg. 2016, 5, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.; Stastny, L.; Reinstadler, S.; Dumfarth, J.; Kilo, J.; Friedrich, G.; Schachner, T.; Grimm, M.; Bonatti, J.; Bonaros, N. Robotic Versus Conventional Coronary Artery Bypass Grafting. Direct Comparison of Long-Term Clinical Outcome. Innovations 2017, 12, 239–246. [Google Scholar] [PubMed]
- Leonard, J.R.; Rahouma, M.; Abouarab, A.A.; Schwann, A.N.; Scuderi, G.; Lau, C.; Guy, T.S.; Demetres, M.; Puskas, J.D.; Taggart, D.P.; et al. Totally endoscopic coronary artery bypass surgery: A meta-analysis of the current evidence. Int. J. Cardiol. 2018, 261, 42–46. [Google Scholar]
- Göbölös, L.; Ramahi, J.; Obeso, A.; Bartel, T.; Hogan, M.; Traina, M.; Edris, A.; Hasasn, F.; El Banna, M.; Tuzcu, E.M.; et al. Robotic Totally Endoscopic Coronary Artery Bypass Grafting: Systematic Review of Clinical Outcomes from the Past two Decades. Innovations 2019, 14, 5–16. [Google Scholar] [CrossRef]
- Hammal, F.; Nagase, F.; Menon, D.; Ali, I.; Nagendran, J.; Stafinski, T. Robot-assisted coronary artery bypass surgery: A systematic review and meta-analysis of comparative studies. Can. J. Surg. 2020, 63, E491. [Google Scholar]
- Holm, N.R.; Mäkikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.; Trovik, T.; Kellerth, T.; Kalinaukas, G.; Mogensen, L.J.H.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: Updated 5-year outcomes from the randomised, non-inferiority Noble trial. Lancet 2020, 395, 191–199. [Google Scholar]
- Kappetein, A.P.; Head, S.J. CABG, stents, or hybrid procedures for left main disease? EuroIntervention 2015, 11 (Suppl. V), V111–V114. [Google Scholar]
- Katz, M.R.; Van Praet, F.; de Canniere, D.; Murphy, D.; Siwek, L.; Seshadri-Kreaden, U.; Friedricj, G.; Bonatti, J. Integrated coronary revascularization: Percutaneous coronary intervention plus robotic totally endoscopic coronary artery bypass. Circulation 2006, 114, I473–I476. [Google Scholar] [CrossRef]
- Deppe, A.-C.; Liakopoulos, O.J.; Kuhn, E.W.; Slottosch, I.; Scherner, M.; Choi, Y.-H.; Rahmanian, P.B.; Wahlers, T. Minimally invasive direct coronary bypass grafting versus percutaneous coronary intervention for single-vessel disease: A meta-analysis of 2885 patients. Eur. J. Cardiothorac. Surg. 2014, 47, 397–406. [Google Scholar] [PubMed]
- Kiaii, B.; Teefy, P. Hybrid coronary artery revascularization: A review and current evidence. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2019, 14, 394–404. [Google Scholar]
- Saha, T.; Naqvi, S.Y.; Goldberg, S. Hybrid revascularization: A Review. Cardiology 2018, 140, 35–44. [Google Scholar] [PubMed]
- Harskamp, R.E.; Zheng, Z.; Alexander, J.H.; Williams, J.B.; Xian, Y.; Halkos, M.E.; Brennan, J.M.; de Winter, R.J.; Smith, P.K.; Lopes, R.D. Status quo of hybrid coronary revascularization for multi-vessel coronary artery disease. Ann. Thorac. Surg. 2013, 96, 2268–2277. [Google Scholar]
- Ganyukov, V.; Kochergin, N.; Shilov, A.; Tarasov, R.; Skupien, J.; Szot, W.; Kokov, A.; Popov, V.; Kozyrin, K.; Barbarash, O.; et al. Randomized clinical trial of surgical vs. Percutaneous vs. hybrid revascularization in multivessel coronary artery disease: Residual myocardial ischemia and clinical outcomes at one year—Hybrid coronary revascularization versus stenting or surgery (HREVS). J. Interv. Cardiol. 2020, 2020, 5458064. [Google Scholar] [PubMed]
- Esteves, V.; Oliveira, M.A.; Feitosa, F.S.; Mariani, J.; Campos, C.M.; Hajjar, L.A.; Lisboa, L.A.; Jatene, F.B.; Filho, R.K.; Lemos Neto, P.A. Late clinical outcomes of myocardial hybrid revascularization versus coronary artery bypass grafting for complex triple-vessel disease: Long-term follow-up of the randomized merging clinical trial. Catheter. Cardiovasc. Interv. 2020, 97, 259–264. [Google Scholar] [PubMed]
- Nagraj, S.; Tzoumas, A.; Kakargias, F.; Giannopoulos, S.; Ntoumaziou, A.; Kokkinidis, D.G.; Villela, M.A.; Latib, A. Hybrid coronary revascularization (HCR) versus coronary artery bypass grafting (CABG) in multivessel coronary artery disease (MVCAD): A meta-analysis of 14 studies comprising 4226 patients. Catheter. Cardiovasc. Interv. 2022, 100, 1182–1194. [Google Scholar] [PubMed]
- Dixon, L.K.; Akberali, U.; Di Tommaso, E.; George, S.J.; Johnson, T.W.; Bruno, V.D. Hybrid coronary revascularization versus coronary artery bypass grafting for Multivessel coronary artery disease: A systematic review and meta-analysis. Int. J. Cardiol. 2022, 359, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, H.; Chan, V.; Sohmer, B. Minimally invasive coronary artery bypass grafting via a small thoracotomy versus off-pump: A case-matched study. Eur. J. Cardiothorac. Surg. 2011, 40, 804–810. [Google Scholar]
- Ziankou, A.; Ostrovsky, Y. Early and midterm results of notouch aorta multivessel small thoracotomy coronary artery bypass grafting: A propensity score-matched study. Innovations 2015, 10, 258–267. [Google Scholar] [PubMed]
- Rodriguez, M.L.; Lapierre, H.R.; Sohmer, B.F.; Ruel, M.A. Repeat revascularization after minimally invasive coronary artery bypass grafting: Is it a problem? Innovations 2017, 12, 269–274. [Google Scholar] [PubMed]
- Nambiar, P.; Kumar, S.; Mittal, C.M.; Sarkar, I.C. Outcomes of bilateral internal thoracic arteries in minimally invasive coronary artery bypass grafting with analogy to the SYNTAX trial. Innovations 2019, 14, 227–235. [Google Scholar]
- Bonaros, N.; Schachner, T.; Lehr, E.; Kofler, M.; Wiedemann, D.; Hong, P.; Wehman, B.; Zimrin, D.; Vesely, M.K.; Friedrich, G.; et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: Predictors of success and safety. Ann. Thorac. Surg. 2013, 95, 803–812. [Google Scholar] [PubMed]
- Weidinger, F.; Schachner, T.; Bonaros, N.; Hofauer, B.; Lehr, E.J.; Vesely, M.; Zimrin, D.; Bonatti, J. Predictors and consequences of postoperative atrial fibrillation following robotic totally endoscopic coronary bypass surgery. Eur. J. Cardiothorac. Surg. 2014, 45, 318–322. [Google Scholar] [PubMed]
- Kitahara, H.; McCrorey, M.; Patel, B.; Nisivaco, S.; Balkhy, H.H. Does robotic beating heart connector totally endoscopic coronary artery bypass bridge the gender gap in coronary bypass surgery? Innovations 2018, 13, 35–39. [Google Scholar] [PubMed]
- Repossini, A.; Tespili, M.; Saino, A.; Kotelnikov, I.; Moggi, A.; Di Bacco, L.; Muneretto, C. Hybrid revascularization in multivessel coronary artery disease. Eur. J. Cardiothorac. Surg. 2013, 44, 288–294. [Google Scholar]
- Halkos, M.E.; Walker, P.F.; Vassiliades, T.A.; Douglas, J.S.; Devireddy, C.; Guyton, R.A.; Finn, A.V.; Tanveer Rab, S.; Puskas, J.D.; Liberman, H.A. Clinical and angiographic results after hybrid coronary revascularization. Ann. Thorac. Surg. 2014, 97, 484–490. [Google Scholar]
- Puskas, J.D.; Halkos, M.E.; DeRose, J.J.; Bagiella, E.; Miller, M.A.; Overbey, J.; Bonatti, J.; Srinivas, V.S.; Vesely, M.; Sutter, F.; et al. Hybrid coronary revascularization for the treatment of multivessel coronary artery disease: A multicenter observational study. J. Am. Coll. Cardiol. 2016, 68, 356–365. [Google Scholar]
- Bonatti, J.; Schachner, T.; Bonaros, N.; Öhlinger, A.; Danzmayr, M.; Jonetzko, P.; Friedrich, G.; Kolbitsch, C.; Mair, P.; Laufer, G. Technical challenges in totally endoscopic robotic coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2006, 131, 146–153. [Google Scholar]
- Groh, M.A.; Sutherland, S.E.; Burton, H.G.; Johnson, A.M.; Ely, S.W. Port-access coronary artery bypass grafting: Technique and comparative results. Ann. Thorac. Surg. 1999, 68, 1506–1508. [Google Scholar] [PubMed]
- Guo, M.H.; Wells, G.A.; Glineur, D.; Fortier, J.; Davierwala, P.M.; Kikuchi, K.; Lemma, M.G.; Mishra, Y.K.; McGinn, J.; Ramchandani, M.; et al. Minimally invasive coronary surgery compared to sternotomy coronary artery bypass grafting: The MIST trial. Contemp. Clin. Trials 2019, 78, 140–145. [Google Scholar] [PubMed]
| MIDCABG | MICS CABG | TECABG/RACABG | HCR | |
|---|---|---|---|---|
| Contra- indications | Absolute: Emergency surgery with haemodynamic compromise Severe pectus excavatum Severe pulmonary disease In TECABG/RACABG, the presence of severe left pleural scarring Relative: Left subclavian artery stenosis Haemodialysis arteriovenous fistula on the patient’s left side Re-do surgery Morbid obesity Severe LV dysfunction Need for right coronary artery graft with no posterior descending or left ventricular branch target Need for circumflex coronary artery graft with no adequate marginal branch target and absence of femoral pulses bilaterally | |||
| Advantages | Avoids the use of CPB | Allows complete revascularization in the presence of three-vessel or diffuse coronary artery disease Allows complete harvest of the LIMA, whether skeletonised or not Allows access to all coronary arteries and their territories Allows proximal anastomoses to be routinely performed | Transthoracic assistance may not be necessary for RACABG if a fourth robotic arm is available Minimal surgical trauma Allows multivessel revascularization Smaller incisions Less pain because no retractor is required for LIMA harvest | Avoids the use of CPB Still obtains the prognostic benefit of LIMA to LAD graft but complete revascularisation of other territories as well through PCIs |
| Dis- advantages | Restricted to single LIMA to LAD graft Cannot access all coronary artery territories Still requires a thoracotomy, which can be painful Does not lend itself to intramyocardial targets | Difficult to harvest RIMA Reasonable patency rate at 6 months | A long learning curve with higher initial rates of LIMA to LAD anastomosis failure, LIMA injuries, and longer bypass times Access depends on the port position | LIMA to LAD anastomosis failure more common than with standard CABG The use of antithrombotic medications and contrast are required for PCIs very soon before or after a major cardiac procedure More than one major intervention within days of each other |
| Authors | Surgical Technique | Patients | Retrospective vs. Prospective | Survival | Follow-Up/Months | Sternotomy Conversion | Peri-Op Stroke | LOS | Number of Grafts | Complete Revascularisation | LIMA–LAD Patency | Repeat Revascularisation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McGinn et al. (2009) [35] | MIDCABG | 450 | Retrospective | 98.7% | 1 | 3.8% | 0.4% | 5.9 +/− 3.4 | 2.1 +/− 0.7 | 95% | - | 2.7% |
| Lapierre et al. (2011) [70] | MIDCABG | 150 | Retrospective | 100.0% | 3 | 6.7% | 0.0% | 5.0 | 1.8 +/− 0.7 | 100% | - | 3.3% |
| Zianku et al. (2015) [71] | MIDCABG | 151 | Retrospective | 99.3% | 40.3 | 2.7% | 0.0% | 4.5 | 2.9 +/− 0.5 | 100% | 100.0% | - |
| Rodriguez et al. (2017) [72] | MIDCABG | 306 | Retrospective | 100.0% | 33.6 | 3.3% | 0.0% | 5.8 +/− 5.5 | 1.8 +/− 0.7 | 93% | - | 6.9% |
| Nambiar et al. (2019) [73] | MIDCABG | 940 | Retrospective | 99.1% | 2.9 | 0.6% | 0.2% | 3.1 +/− 1.2 | 3.2 | 97.90% | 99.80% | 1.1% |
| Bonaros et al. (2013) [74] | TECABG | 500 | Retrospective | 99.0% | 120 | 10.0% | 9.0% | 6.0 | - | - | 90–95% | - |
| Weldinger et al. (2014) [75] | TECABG | 384 | Retrospective | 99.2% | 60 | 14.0% | 1.8% | 7.0 | - | - | - | - |
| Kitahara et al. (2018) [76] | TECABG | 263 | Retrospective | 98.5% | 1 | 3.0% | 0.0% | 3.5 +/− 2.9 | - | - | - | - |
| Repossini et al. (2013) [77] | HCR with MIDCABG | 166 | Retrospective | 95.8% | 54 | 2.4% | N/A | 6.5 | - | Functionally complete 100%, anatomically incomplete 16.9% | 100% before PCI | 7.2% |
| Halkos et al. (2014) [78] | HCR with RA-MIDCABG | 300 | Retrospective | 98.7% | 1 | 2.0% | 1.0% | 5.0 | - | - | 97.60% | 4.3% |
| Puskas et al. (2016) [79] | HCR (variable) | 200 | Prospective | 98.5% | 1.5 years | 0.5% | 0.0% | - | - | 75.20% | - | 7.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purmessur, R.; Wijesena, T.; Ali, J. Minimal-Access Coronary Revascularization: Past, Present, and Future. J. Cardiovasc. Dev. Dis. 2023, 10, 326. https://doi.org/10.3390/jcdd10080326
Purmessur R, Wijesena T, Ali J. Minimal-Access Coronary Revascularization: Past, Present, and Future. Journal of Cardiovascular Development and Disease. 2023; 10(8):326. https://doi.org/10.3390/jcdd10080326
Chicago/Turabian StylePurmessur, Rushmi, Tharushi Wijesena, and Jason Ali. 2023. "Minimal-Access Coronary Revascularization: Past, Present, and Future" Journal of Cardiovascular Development and Disease 10, no. 8: 326. https://doi.org/10.3390/jcdd10080326
APA StylePurmessur, R., Wijesena, T., & Ali, J. (2023). Minimal-Access Coronary Revascularization: Past, Present, and Future. Journal of Cardiovascular Development and Disease, 10(8), 326. https://doi.org/10.3390/jcdd10080326





