Multi-Component Synthesis of New Fluorinated-Pyrrolo[3,4-b]pyridin-5-ones Containing the 4-Amino-7-chloroquinoline Moiety and In Vitro–In Silico Studies Against Human SARS-CoV-2
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.1.1. Synthesis of Precursors
2.1.2. Optimization of Reaction Conditions
2.1.3. Synthesis of the Assayed Polyheterocycles
2.2. In Vitro Studies
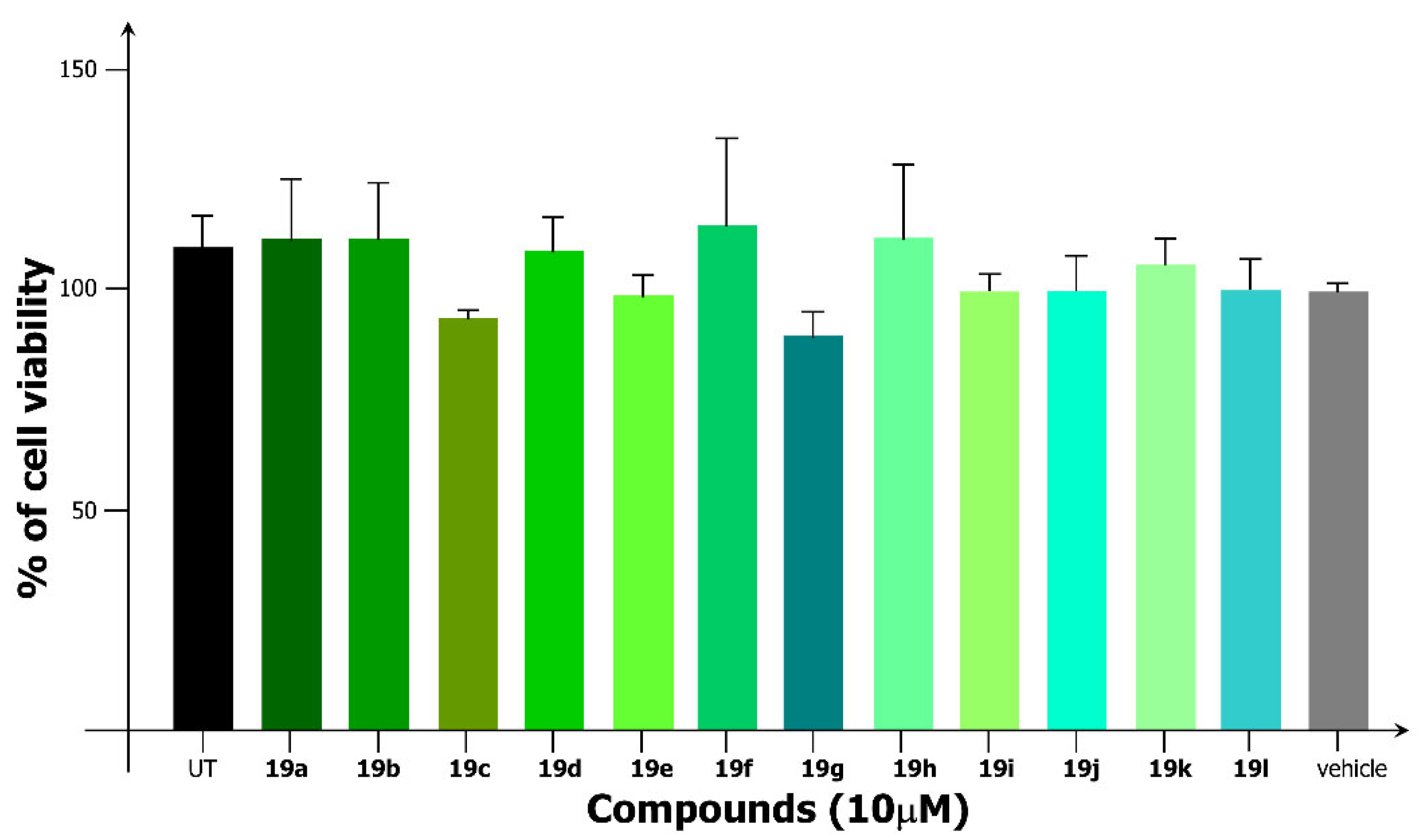
2.2.1. Cytotoxicity Assay
2.2.2. Evaluation of Antiviral Activity
2.3. In Silico Studies: (Docking and Molecular Dynamics)
2.3.1. Introduction
2.3.2. ADMETox
2.3.3. Cavity Search and Binding Site Validation
2.3.4. Docking Studies
2.3.5. Dynamics of the Complexes with 19d
2.3.6. Dynamics of the Complexes with 19i
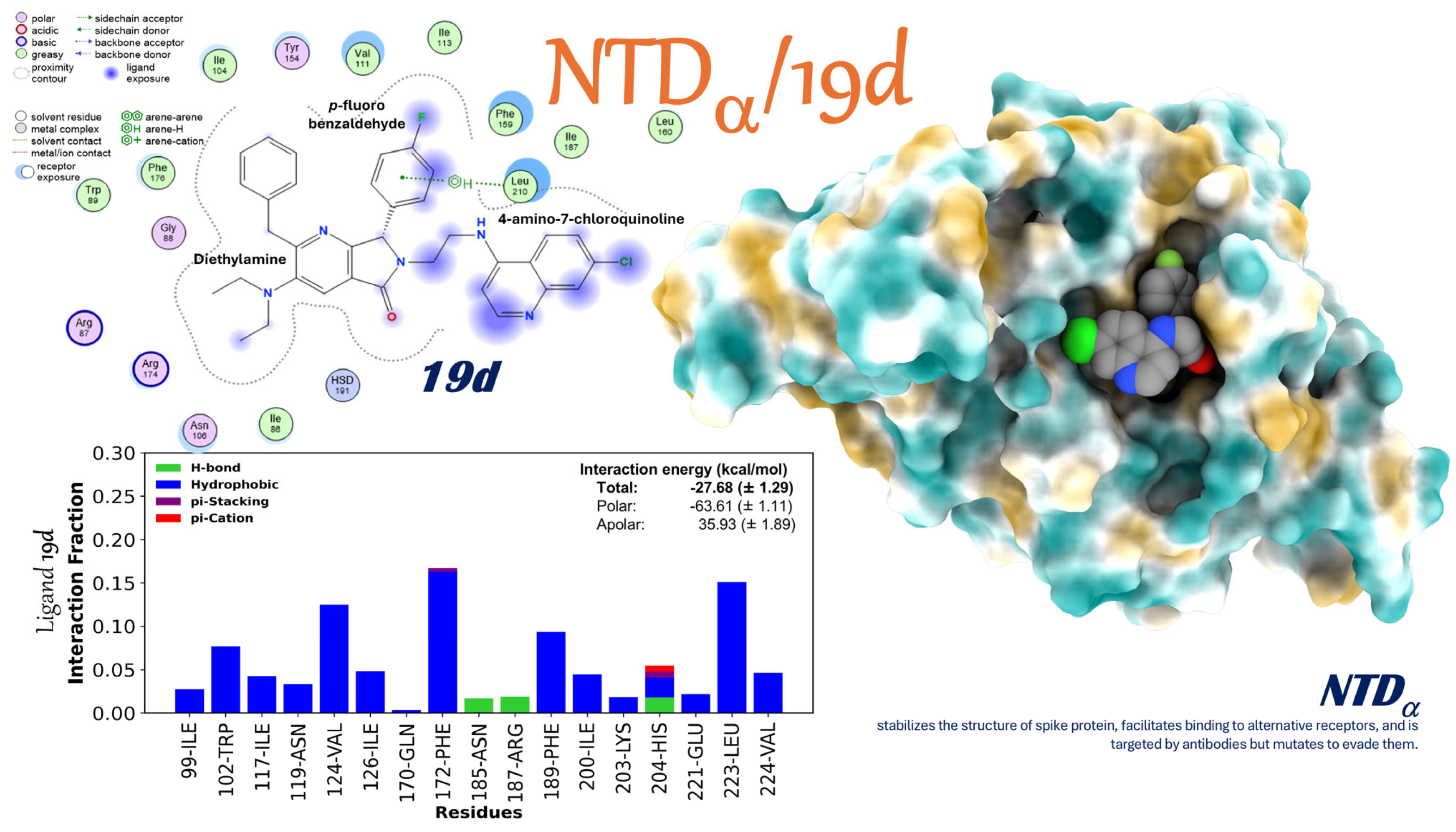
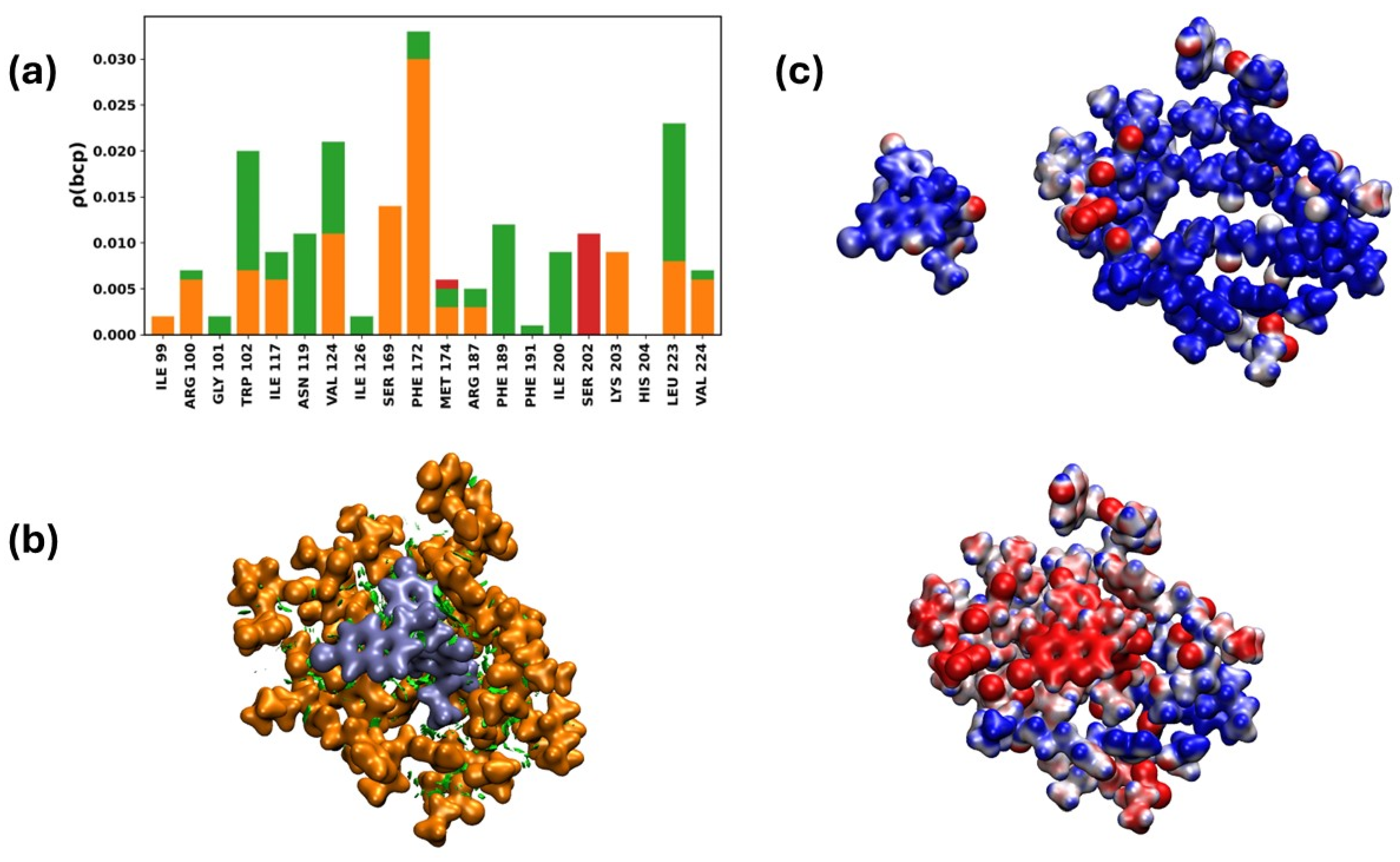
2.3.7. Interaction of the NTDα/19d Complex
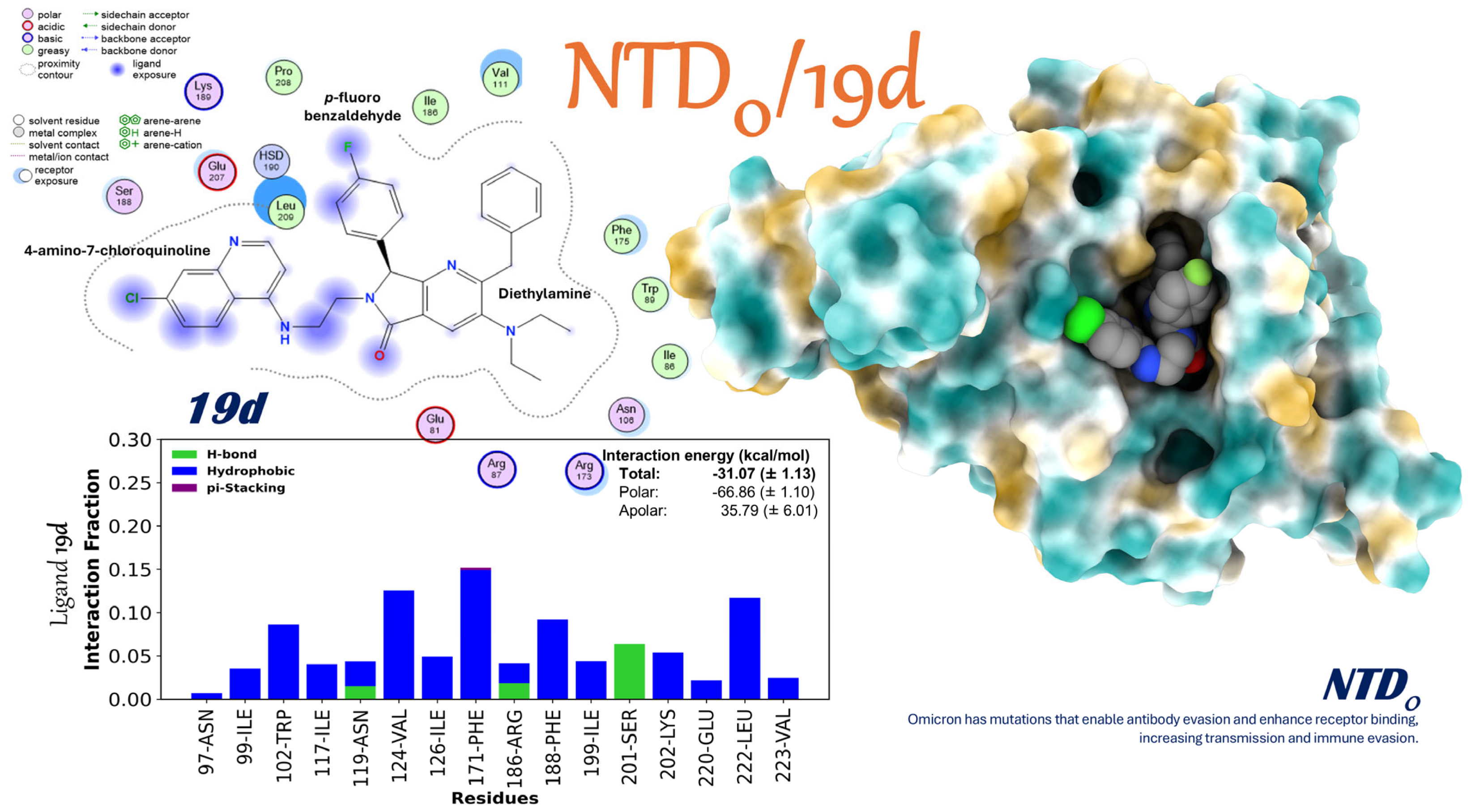
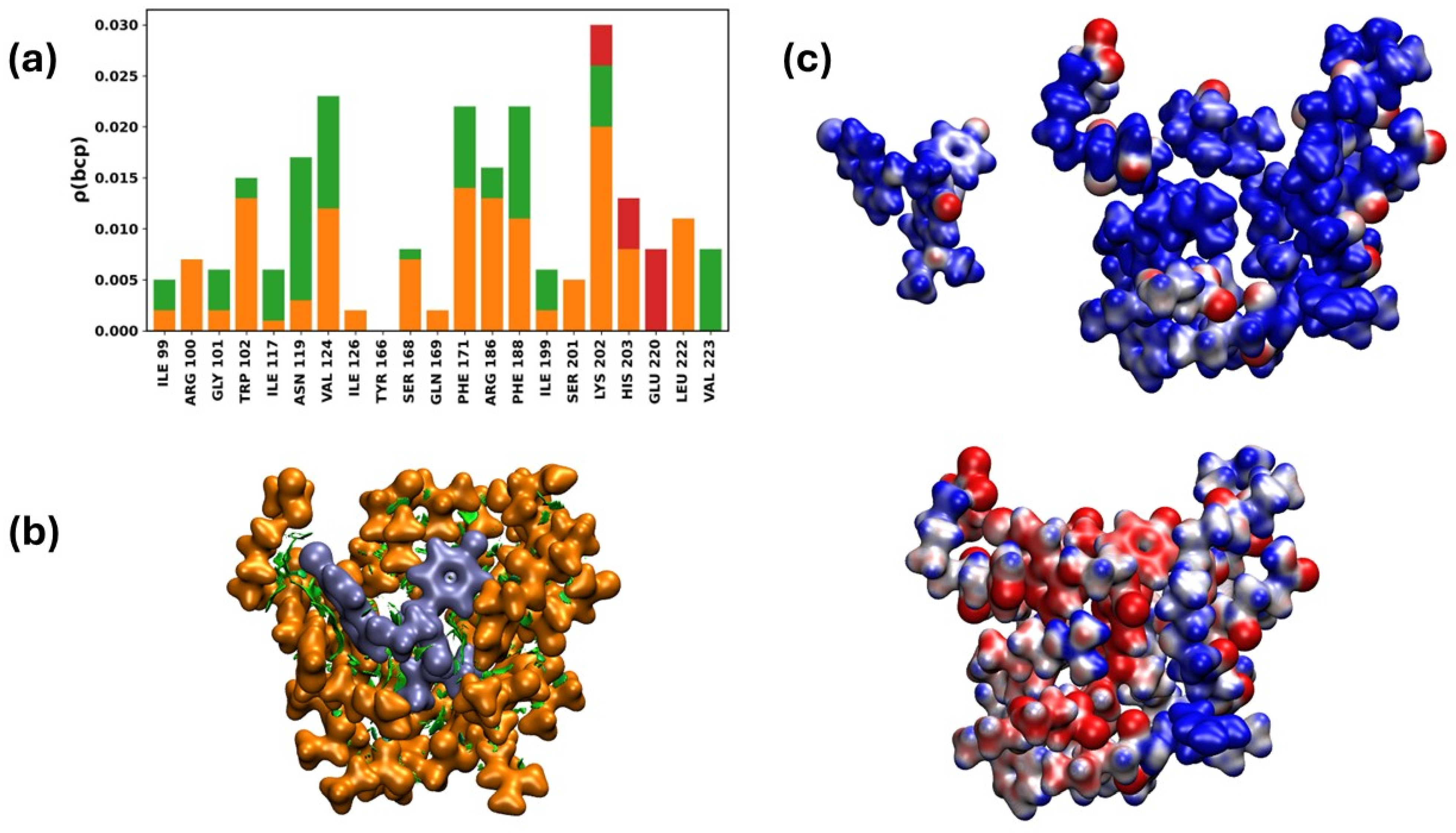
2.3.8. Interaction of the NTDo/19d Complex
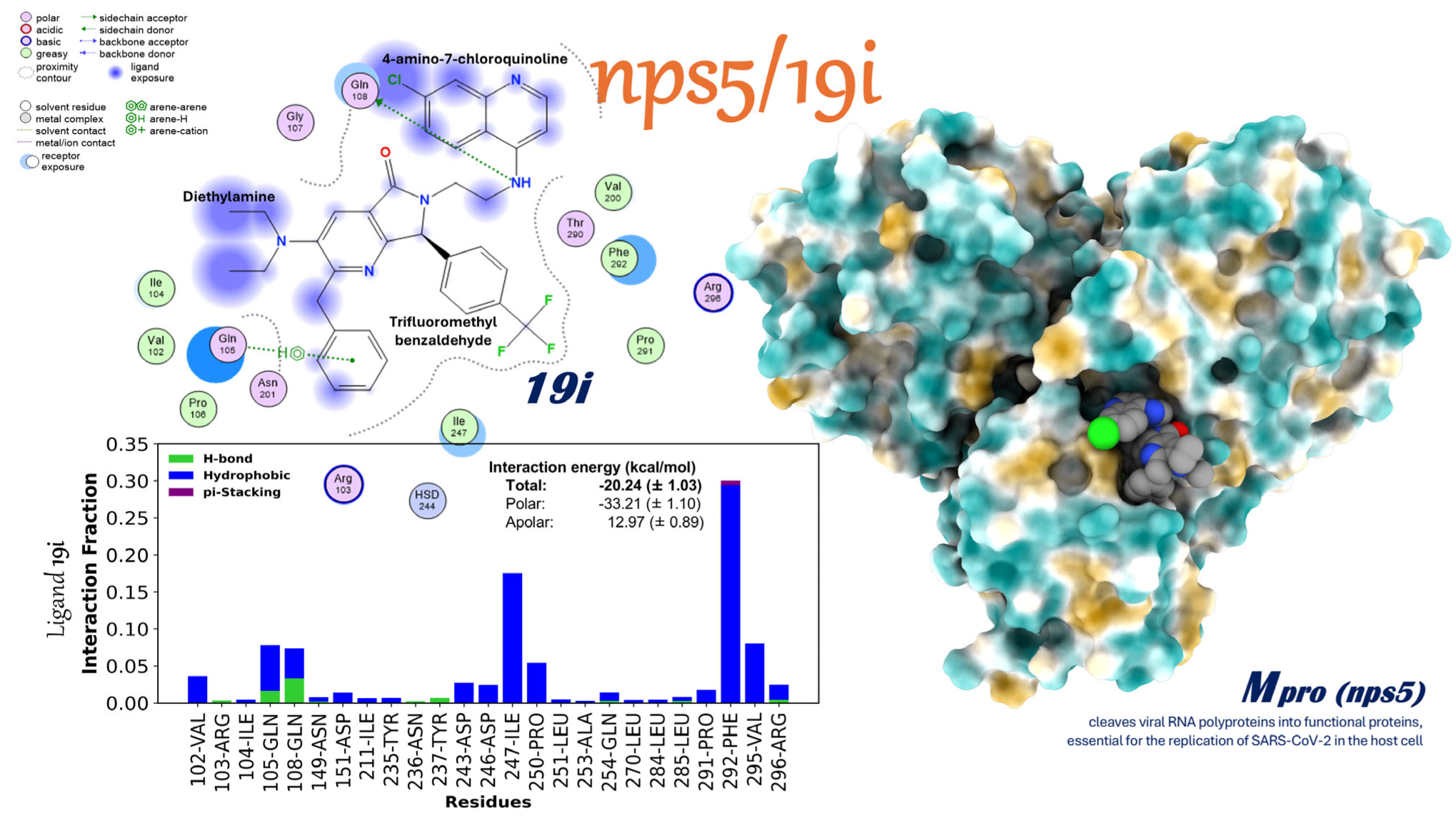
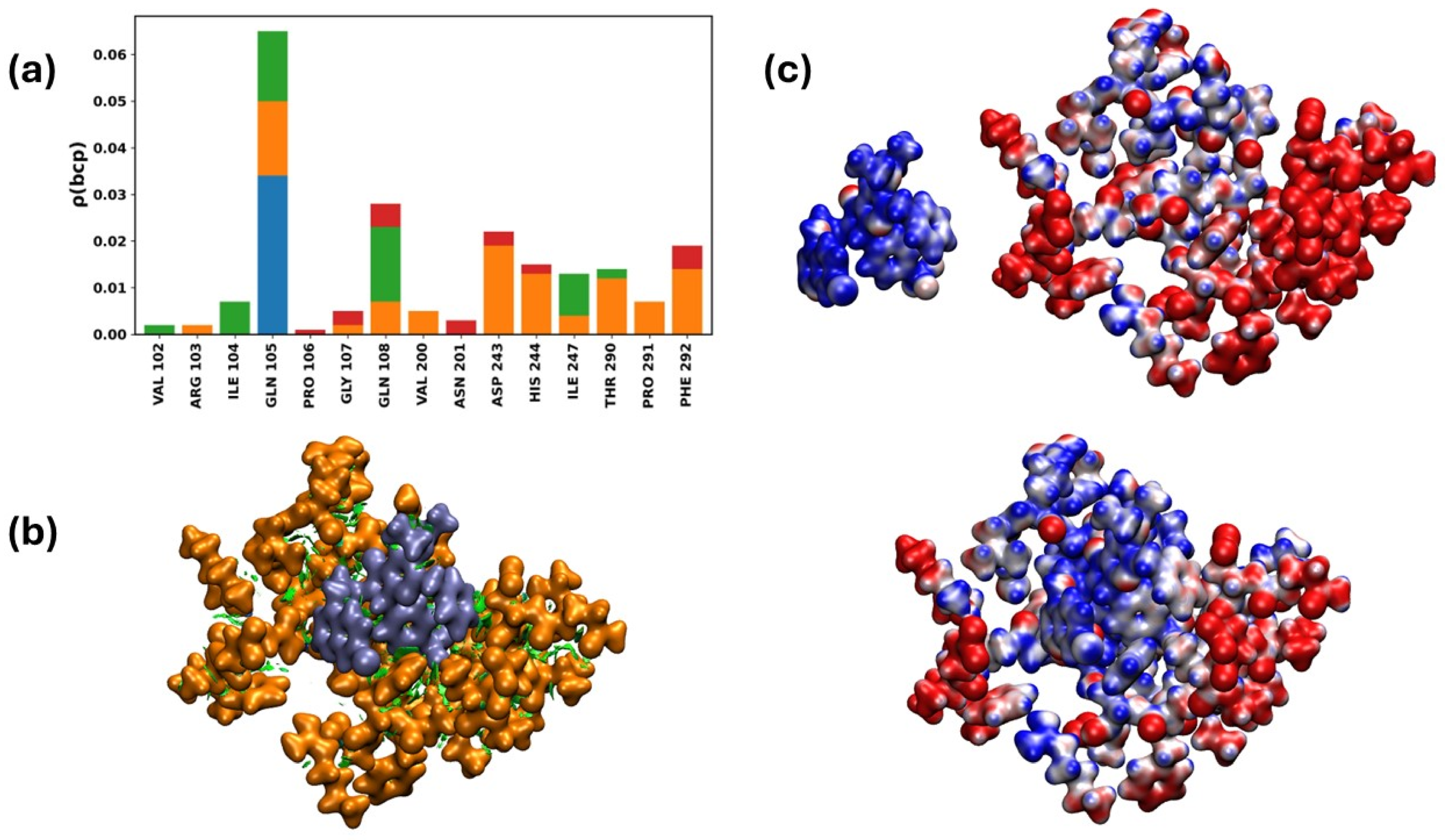
2.3.9. Interactions of the Mpro/19i Complex
2.4. In Silico Studies (Molecular Structure)
Quantum Chemistry Analysis of Non-Covalent Interactions
- Changing exposed and changeable domains: 19d shows a lot of adaptive flexibility when binding to the NTD’s polar regions in both the Alpha and Omicron versions. The strength of this binding suggests that there may be an allosteric or structural disruption that could make it harder for the virus to enter and avoid the immune response of the host.
- Stopping viral replication in targets that have been around for a long time: 19i interacts with the active site of Mpro, an important and highly conserved enzyme, through a network of contacts mainly made up of hydrophobic forces and stable hydrogen bonds. This method, which is similar to competitive inhibition, stops the viral proteolysis and may still work even if changes are made to other parts of the genome.
3. Materials and Methods
3.1. Chemistry (Software, Instrumentation and Chemicals)
3.2. General Procedure for the Synthesis of the Fluorinated 4-Amino-7-chloroquinoline-5-aminoxazole 14a and fluorinated 4-amino-7-chloroquinoline-pyrrolo[3,4-b]pyridin-5-ones 19a–l
3.2.1. N1-((4-Benzyl-5-morpholinooxazol-2-yl)(4-fluorophenyl)methyl)-N2-(7-chloroquinolin-4-yl)ethane-1,2-diamine 14a
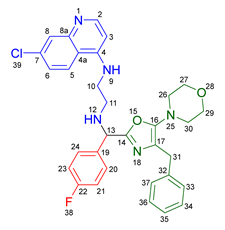
3.2.2. 2-Benzyl-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-7-(4-fluorophenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19a
3.2.3. 2-Benzyl-6-(3-((7-chloroquinolin-4-yl)amino)propyl)-7-(4-fluorophenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19b
3.2.4. 2-Benzyl-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-7-(4-fluorophenyl)-3-(piperidin-1-yl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19c
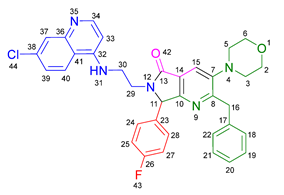
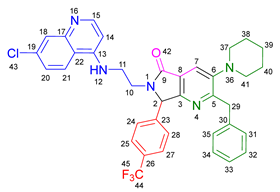
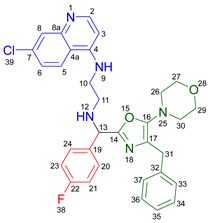
3.2.5. 2-Benzyl-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-3-(diethylamino)-7-(4-fluorophenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19d
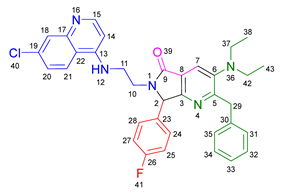
3.2.6. 2-Benzyl-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-7-(2-fluorophenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19e
3.2.7. 2-Benzyl-6-(3-((7-chloroquinolin-4-yl)amino)propyl)-7-(2-fluorophenyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19f

3.2.8. 2-Benzyl-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-3-morpholino-7-(4-(trifluoromethyl)phenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19g
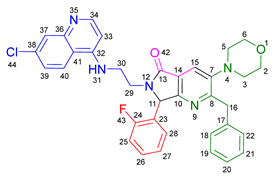
3.2.9. 2-Benzyl-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-3-(piperidin-1-yl)-7-(4-(trifluoromethyl)phenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19h
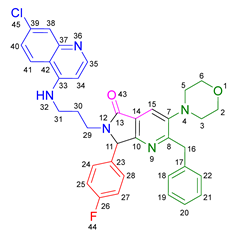
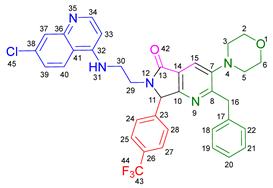
3.2.10. 2-Benzyl-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-3-(diethylamino)-7-(4-(trifluoromethyl)phenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19i
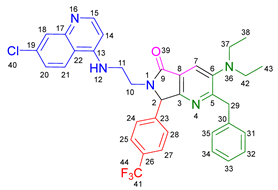
3.2.11. 2-Benzyl-7-(3,5-bis(trifluoromethyl)phenyl)-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19j
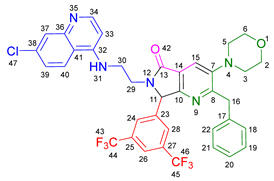
3.2.12. 2-Benzyl-7-(3,5-bis(trifluoromethyl)phenyl)-6-(2-((7-chloroquinolin-4-yl)amino)ethyl)-3-(piperidin-1-yl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19k
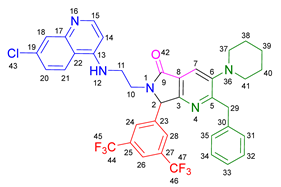
3.2.13. 2-Benzyl-6-(3-((7-chloroquinolin-4-yl)amino)propyl)-3-morpholino-7-(perfluorophenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one 19l

3.3. In Vitro Studies
3.3.1. Cell Line and Virus
3.3.2. Cell Viability Assay
3.3.3. Time Addition Assay
3.4. In Silico Studies (Docking and Molecular Dynamics)
3.4.1. ADME and Tox Properties
3.4.2. Target Identification and Active Pocket Assessment
3.4.3. Homology Modeling and Docking Simulations
3.4.4. Preparation of the System for Docking
3.4.5. Docking Protocol
3.4.6. Molecular Dynamics Simulations: System Construction
3.4.7. Calculations of Binding Free Energy: MM/GBSA Methodology
3.5. In Silico Studies (Molecular Structure)
Non-Covalent Interactions by Using the Electron Density
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whiter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Park, S.B. Privileged Structures: Efficient Chemical “Navigators” toward Unexplored Biologically Relevant Chemical Spaces. J. Am. Chem. Soc. 2014, 136, 14629–14638. [Google Scholar] [CrossRef]
- Patchett, A.A.; Nargund, R.P. Chapter 26. Privileged structures-An update. Annu. Rep. Med. Chem. 2000, 35, 289–298. [Google Scholar]
- Schneider, P.; Schneider, G. Privileged Structures Revisited. Angew. Chem. Int. Ed. 2017, 56, 7971–7974. [Google Scholar] [CrossRef]
- Barreiro, E.J. Privileged Scaffolds in Medicinal Chemistry: An introduction. In Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; Bräse, S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 1–15. [Google Scholar]
- Upadhyay, S.P.; Thapa, P.; Sharma, R.; Sharma, M. 1-Isoindolinone scaffold-based natural products with a promising diverse bioactivity. Fitoterapia 2020, 146, 104722. [Google Scholar] [CrossRef]
- Sanwer, S.; Nasreen, A.; Swain, B.; Peddapaka, J.; Alvala, R.; Arifuddin, M. Synthesis and antimicrobial evaluation of novel 8-hydroxyquinoline containing benzimidazole hybrids. Discov. Chem. 2025, 2, 17. [Google Scholar] [CrossRef]
- Bazine, I.; Cheraiet, Z.; Bensegueni, R.; Bensouici, C.; Boukhari, A. Synthesis, antioxidant and anticholinesterase activities of novel quinoline-aminophosphonate derivatives. J. Heterocycl. Chem. 2020, 57, 2139–2149. [Google Scholar] [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a Privileged Scaffold in Cancer Drug Discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Zhao, J.; Cheng, N.; Wang, Y.; Fu, Y.; Zheng, Y.; Wang, J.; Zhu, M.; Cen, S.; et al. Synthesis and antiviral activity of a series of novel quinoline derivatives as anti-RSV or anti-IAV agents. Eur. J. Med. Chem. 2021, 214, 113208. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Tung, C.-W.; Wu, C.-H.; Tzeng, C.-C.; Chen, Y.-H.; Hwang, T.-L.; Chen, Y.-L. Discovery of Indeno [1,2-c]quinoline Derivatives as Potent Dual Antituberculosis and Anti-Inflammatory Agents. Molecules 2017, 22, 1001. [Google Scholar] [CrossRef]
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC Adv. 2020, 10, 20784–20793. [Google Scholar] [CrossRef]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Flores-Reyes, J.C.; Islas-Jácome, A.; González-Zamora, E. The Ugi three-component reaction and its variants. Org. Chem. Front. 2021, 8, 5460–5515. [Google Scholar] [CrossRef]
- Sun, X.; Janvier, P.; Zhao, G.; Bienaymé, H.; Zhu, J. A Novel Multicomponent Synthesis of Polysubstituted 5-Aminooxazole and its New Scaffold-Generating Reaction to Pyrrolo[3,4-b]pyridine. Org. Lett. 2001, 3, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Janvier, P.; Sun, X.; Bienaymé, H.; Zhu, J. Ammonium Chloride-Promoted Four-Component Synthesis of Pyrrolo[3,4-b]pyridin-5-one. J. Am. Chem. Soc. 2002, 124, 2560–2567. [Google Scholar] [CrossRef]
- Savela, R.; Méndez-Gálvez, C. Isoindolinone Synthesis via One-Pot Type Transition Metal Catalyzed C-C Bond Forming Reactions. Chem. Eur. J. 2021, 27, 5344–5378. [Google Scholar] [CrossRef]
- Blanco-Carapia, R.E.; Islas-Jácome, P.; Gutiérrez-Carrillo, A.; García-Sánchez, M.A.; González-Zamora, E.; Islas-Jácome, A. One-pot synthesis of phenyl- and biphenyl-linked bis-pyrrolo[3,4-b]pyridin-5-ones via a pseudo-repetitive Ugi-Zhu-5CR coupled to a double cascade process (aza-Diels–Alder/N-acylation/decarboxylation/dehydration). Tetrahedron Lett. 2024, 151, 155322. [Google Scholar] [CrossRef]
- Guevara-Pulido, J.; Jiménez, R.A.; Morantes, S.J.; Jaramillo, D.N.; Acosta-Guzmán, P. Design, Synthesis, and Development of 4-[(7-Chloroquinoline-4-yl)amino]phenol as a Potential SARS-CoV-2 Mpro Inhibitor. ChemistrySelect 2022, 7, e202200125. [Google Scholar] [CrossRef]
- Henary, E.; Casa, S.; Dost, T.L.; Sloop, J.C.; Henary, M. The Role of Small Molecules Containing Fluorine Atoms in Medicine and Imaging Applications. Pharmaceuticals 2024, 17, 281. [Google Scholar] [CrossRef]
- Jaiswal, G.; Kumar, V. In-silico design of a potential inhibitor of SARS-CoV-2 S protein. PLoS ONE 2020, 15, e0240004. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Shaikh, M.; tul-Wahab, A.; ur-Rahman, A. In silico identification of potential inhibitors of key SARS-CoV-2 3CL hydrolase (Mpro) via molecular docking, MMGBSA predictive binding energy calculations, and molecular dynamics simulation. PLoS ONE 2020, 15, e0235030. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, A.K.; Kushwaha, P.P.; Prajapati, K.S.; Shuaib, M.; Senapati, S.; Kumar, S. Identification of potential natural inhibitors of SARS-CoV2 main protease by molecular docking and simulation studies. J. Biomol. Struct. Dyn. 2021, 39, 4334–4345. [Google Scholar] [CrossRef]
- Pandey, P.; Rane, J.S.; Chatterjee, A.; Kumar, A.; Khan, R.; Prakash, A.; Ray, S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: An in silico study for drug development. J. Biomol. Struct. Dyn. 2021, 39, 6306–6316. [Google Scholar] [CrossRef]
- Niazi, S.K.; Mariam, Z. Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis. Pharmaceuticals 2023, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Han, D.; Tang, W.; Sun, L. Recent advances in application of computer-aided drug design in anti-COVID-19 Virials Drug Discovery. Biomed. Pharmacother. 2024, 173, 116423. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bai, Y.; Guo, Y.; Wang, M.; Ji, X.; Wang, Y. Drug Discovery for SARS-CoV-2 Utilizing Computer-Aided Drug Design Approaches. COVID 2025, 5, 32. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Menon, S. Insilico insights to mutational and evolutionary aspects of sars-COV2. Int. J. Multidiscip. Res. Dev. 2021, 8, 167–172. [Google Scholar]
- Castiglione, F.; Deb, D.; Srivastava, A.P.; Lio, P.; Liso, A. From infection to immunity: Understanding the response to SARS-CoV2 through in-silico modeling. Front. Immunol. 2021, 12, 646972. [Google Scholar] [CrossRef]
- Fayol, A.; Housseman, C.; Sun, X.; Janvier, P.; Bienaymé, H.; Zhu, J. Synthesis of α-Isocyano-α-alkyl(aryl)acetamides and their Use in the Multicomponent Synthesis of 5-Aminooxazole, Pyrrolo[3,4-b]pyridin-5-one and 4,5,6,7-Tetrahydrofuro [2,3-c]pyridine. Synthesis 2005, 1, 161–165. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Mendoza, E.J.; Manguiat, K.; Wood, H.; Drebot, M. Two Detailed Plaque Assay Protocols for the Quantification of Infectious SARS-CoV-2. Curr. Protoc. Microbiol. 2020, 57, cpmc105. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; Van Ranst, M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004, 323, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Rebeaud, M.E.; Zores, F. SARS-CoV-2 and the Use of Chloroquine as an Antiviral Treatment. Front. Med. 2020, 7, 184. [Google Scholar] [CrossRef]
- Rolain, J.M.; Colson, P.; Raoult, D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents 2007, 30, 297–308. [Google Scholar] [CrossRef]
- Randolph, V.B.; Winkler, G.; Stollar, V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 1990, 174, 450–458. [Google Scholar] [CrossRef]
- Li, H.; Komori, A.; Li, M.; Chen, X.; Yang, A.W.H.; Sun, X.; Liu, Y.; Hung, A.; Zhao, X.; Zhou, L. Multi-ligand molecular docking, simulation, free energy calculations and wavelet analysis of the synergistic effects between natural compounds baicalein and cubebin for the inhibition of the main protease of SARS-CoV-2. J. Mol. Liq. 2023, 374, 121253. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef]
- Cevallos, A.M.; Herrera, J.; López-Villaseñor, I.; Hernández, R. Differential Effects of Two Widely Used Solvents, DMSO and Ethanol, on the Growth and Recovery of Trypanosoma cruzi Epimastigotes in Culture. Korean J. Parasitol. 2017, 55, 81–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morales-Salazar, I.; Castañón-Alonso, S.L.; Canseco-González, D.; Díaz-Cervantes, E.; González-Zamora, E.; Islas-Jácome, A. Synthesis of new bis-furanyl-pyrrolo[3,4-b]pyridin-5-ones via the Ugi-Zhu reaction and docking studies on the main protease (Mpro) from SARS-CoV-2. Chem. Proc. 2022, 4, 84. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Schyman, P.; Liu, R.; Desai, V.; Wallqvist, A. vNN web server for ADMET predictions. Front. Pharmacol. 2017, 8, 889. [Google Scholar] [CrossRef]
- Braga, R.C.; Alves, V.M.; Muratov, E.N.; Strickland, J.; Kleinstreuer, N.; Tropsha, A.; Andrade, C.H. Pred-skin: A fast and reliable web application to assess skin sensitization effect of chemicals. J. Chem. Inf. Model. 2017, 57, 1013–1017. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- de Freitas, R.F.; Schapira, M. A systematic analysis of atomic protein–ligand interactions in the PDB. Med. Chem. Comm. 2017, 8, 1970–1981. [Google Scholar] [CrossRef]
- D’Oliveira, A.; Dai, X.; Mottaghinia, S.; Olson, S.; Geissler, E.P.; Etienne, L.; Zhang, Y.; Mugridge, J.S. Recognition and cleavage of human tRNA methyltransferase TRMT1 by the SARS-CoV-2 main protease. bioRxiv 2024, 529306. [Google Scholar]
- Calleja, D.J.; Kuchel, N.; Lu, B.G.C.; Birkinshaw, R.W.; Klemm, T.; Doerflinger, M.; Cooney, J.P.; Mackiewicz, L.; Au, A.E.; Yap, Y.Q.; et al. Insights into drug repurposing, as well as specificity and compound properties of piperidine-based SARS-CoV-2 PLpro inhibitors. Front. Chem. 2022, 10, 861209. [Google Scholar] [CrossRef]
- Shannon, A.; Fattorini, V.; Sama, B.; Selisko, B.; Feracci, M.; Falcou, C.; Gauffre, P.; El Kazzi, P.; Delpal, A.; Decroly, E.; et al. A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase. Nat. Commun. 2022, 13, 621. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Y.; Ge, J.; Zheng, L.; Gao, Y.; Wang, T.; Jia, Z.; Wang, H.; Huang, Y.; Li, M.; et al. Architecture of a SARS-CoV-2 mini replication and transcription complex. Nat. Commun. 2020, 11, 5874. [Google Scholar] [CrossRef]
- Xia, S.; Wen, Z.; Wang, L.; Lan, Q.; Jiao, F.; Tai, L.; Wang, Q.; Sun, F.; Jiang, S.; Lu, L.; et al. Structure-based evidence for the enhanced transmissibility of the dominant SARS-CoV-2 B. 1.1. 7 variant (Alpha). Cell Discov. 2021, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, H.; Nishimura, M.; Tjan, L.H.; Sutandhio, S.; Marini, M.I.; Effendi, G.B.; Shigematsu, H.; Kato, K.; Hasegawa, N.; Aoki, K.; et al. Identification and analysis of monoclonal antibodies with neutralizing activity against diverse SARS-coV-2 variants. J. Virol. 2023, 97, e00286-23. [Google Scholar] [CrossRef]
- Santana, C.A.; Silveira, S.A.; Moraes, J.P.A.; Izidoro, S.C.; de Melo-Minardi, R.C.; Ribeiro, A.J.M.; Tyzack, J.D.; Borkakoti, N.; Thornton, J.M. GRaSP: A graph-based residue neighborhood strategy to predict binding sites. Bioinformatics 2020, 36, i726–i734. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.A.; Izidoro, S.C.; de Melo-Minardi, R.C.; Tyzack, J.D.; Ribeiro, A.J.M.; Pires, D.E.V.; Thornton, J.M.; Silveira, S.A. GRaSP-web: A machine learning strategy to predict binding sites based on residue neighborhood graphs. Nucleic Acids Res. 2022, 50, W392–W397. [Google Scholar] [CrossRef]
- Jakubec, D.; Skoda, P.; Krivak, R.; Novotny, M.; Hoksza, D. PrankWeb 3: Accelerated ligand-binding site predictions for experimental and modelled protein structures. Nucleic Acids Res. 2022, 50, W593–W597. [Google Scholar] [CrossRef]
- Eguida, M.; Rognan, D. Estimating the similarity between protein pockets. Int. J. Mol. Sci. 2022, 23, 12462. [Google Scholar] [CrossRef]
- Behera, S.; Hahn, D.F.; Wilson, C.J.; Marsili, S.; Tresadern, G.; Gapsys, V.; de Groot, B.L. Quantification of the Impact of Structure Quality on Predicted Binding Free Energy Accuracy. J. Chem. Inf. Model. 2025, 65, 6927–6938. [Google Scholar] [CrossRef]
- Vakser, I.A. Challenges in protein docking. Curr. Opin. Struct. Biol. 2020, 64, 160–165. [Google Scholar] [CrossRef]
- Robin, X.; Waterhouse, A.M.; Bienert, S.; Studer, G.; Alexander, L.T.; Tauriello, G.; Schwede, T.; Pereira, J. The SWISS-model repository of 3D protein structures and models. In Open Access Databases and Datasets for Drug Discovery; Diana, A., Przewosny, M., Zoete, V., Eds.; Wiley-VCH: Weinheim, Germany, 2023; pp. 175–199. [Google Scholar]
- Chen, V.B.; Arendall, W.B., III; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods Enzym. 1997, 277, 396–404. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Malone, B.F.; Perry, J.K.; Olinares, P.D.B.; Lee, H.W.; Chen, J.; Appleby, T.C.; Feng, J.Y.; Bilello, J.P.; Ng, H.; Sotiris, J.; et al. Structural basis for substrate selection by the SARS-CoV-2 replicase. Nature 2023, 614, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Ozvoldik, K.; Stockner, T.; Krieger, E. YASARA model–interactive molecular modeling from two dimensions to virtual realities. J. Chem. Inf. Model. 2023, 63, 6177–6182. [Google Scholar] [CrossRef]
- López-López, D.; Razo-Hernández, R.S.; Millán-Pacheco, C.; Leyva-Peralta, M.A.; Peña-Morán, O.A.; Sánchez-Carranza, J.N.; Rodríguez-López, V. Ligand-Based Drug Design of Genipin Derivatives with Cytotoxic Activity against HeLa Cell Line: A Structural and Theoretical Study. Pharmaceuticals 2023, 16, 1647. [Google Scholar] [CrossRef]
- Morales-Salazar, I.; Garduño-Albino, C.E.; Montes-Enríquez, F.P.; Nava-Tapia, D.A.; Navarro-Tito, N.; Herrera-Zúñiga, L.D.; González-Zamora, E.; Islas-Jácome, A. Synthesis of Pyrrolo[3,4-b]pyridin-5-ones via Ugi-Zhu Reaction and In Vitro-In Silico Studies against Breast Carcinoma. Pharmaceuticals 2023, 16, 1562. [Google Scholar] [CrossRef]
- Millán-Pacheco, C.; Ríos-Soto, L.; Corral-Rodríguez, N.; Sierra-Campos, E.; Valdez-Solana, M.; Téllez-Valencia, A.; Avitia-Domínguez, C. Discovery of Potential Noncovalent Inhibitors of Dehydroquinate Dehydratase from Methicillin-Resistant Staphylococcus aureus through Computational-Driven Drug Design. Pharmaceuticals 2023, 16, 1148. [Google Scholar] [CrossRef]
- Stevenson, G.A.; Kirshner, D.; Bennion, B.J.; Yang, Y.; Zhang, X.; Zemla, A.; Torres, M.W.; Epstein, A.; Jones, D.; Kim, H.; et al. Clustering protein binding pockets and identifying potential drug interactions: A novel ligand-based featurization method. J. Chem. Inf. Model. 2023, 63, 6655–6666. [Google Scholar] [CrossRef]
- Chen, T.; Shu, X.; Zhou, H.; Beckford, F.A.; Misir, M. Algorithm selection for protein-ligand docking: Strategies and analysis on ACE. Sci. Rep. 2023, 13, 8219. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35, W522–W525. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D., Jr. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Jo, S.; Brooks, C.L., III; Lee, H.S.; Im, W. CHARMM-GUI ligand reader and modeler for CHARMM force field generation of small molecules. J. Comput. Chem. 2017, 38, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Balusek, C.; Hwang, H.; Lau, C.H.; Lundquist, K.; Hazel, A.; Pavlova, A.; Lynch, D.L.; Reggio, P.H.; Wang, Y.; Gumbart, J.C. Accelerating membrane simulations with hydrogen mass repartitioning. J. Chem. Theory Comput. 2019, 15, 4673–4686. [Google Scholar] [CrossRef]
- Naughton, F.B.; Alibay, I.; Barnoud, J.; Barreto-Ojeda, E.; Beckstein, O.; Bouysset, C.; Cohen, O.; Gowers, R.J.; MacDermott-Opeskin, H.; Matta, M.; et al. MDAnalysis 2.0 and beyond: Fast and interoperable, community driven simulation analysis. Biophys. J. 2022, 121, 272a–273a. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef]
- Forouzesh, N.; Mishra, N. An effective MM/GBSA protocol for absolute binding free energy calculations: A case study on SARS-CoV-2 spike protein and the human ACE2 receptor. Molecules 2021, 26, 2383. [Google Scholar] [CrossRef]
- Dasmahapatra, U.; Kumar, C.K.; Das, S.; Subramanian, P.T.; Murali, P.; Isaac, A.E.; Ramanathan, K.; Balamurali, M.M.; Chanda, K. In-silico molecular modelling, MM/GBSA binding free energy and molecular dynamics simulation study of novel pyrido fused imidazo [4,5-c] quinolines as potential anti-tumor agents. Front. Chem. 2022, 10, 991369. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Miller, B.R., III; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Makeneni, S.; Thieker, D.F.; Woods, R.J. Applying pose clustering and MD simulations to eliminate false positives in molecular docking. J. Chem. Inf. Model. 2018, 58, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Knapp, B.; Ospina, L.; Deane, C.M. Avoiding false positive conclusions in molecular simulation: The importance of replicas. J. Chem. Theory Comput. 2018, 14, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Daura, X.; Conchillo-Solé, O. On quality thresholds for the clustering of molecular structures. J. Chem. Inf. Model. 2022, 62, 5738–5745. [Google Scholar] [CrossRef]
- Hunkler, S.; Diederichs, K.; Kukharenko, O.; Peter, C. Fast conformational clustering of extensive molecular dynamics simulation data. J. Chem. Phys. 2023, 158, 144109. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Atanda, H.; Balogun, T.A.; Alshehri, M.M.; Olivos-Ramirez, G.; Vilca-Quispe, J.; Chenet-Zuta, M.; Cárdenas-Cárdenas, R.; Delgado, W.H.; Ropón-Palacios, G.; Umar, H.I. In silico study revealed the inhibitory activity of selected phytomolecules of C. rotundus against VacA implicated in gastric ulcer. J. Biomol. Struct. Dyn. 2023, 41, 10713–10724. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Grabowski, S.J. What is the covalency of hydrogen bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V. Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Vargas, R.; Garza, J.; Martínez, A. Exploring Intermolecular and Intramolecular Interactions: A Review beyond Hydrogen Bonds. J. Mex. Chem. Soc. 2024, 68, 970–980. [Google Scholar] [CrossRef]
- Vargas, R.; Garza, J.; Martínez, A.; Ibarra, I.A. Computational tools to study non-covalent interactions and confinement effects in chemical systems. Chem. Commun. 2024, 60, 3008–3018. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Padilla-Bernal, G.; Vargas, R.; Martínez, A. Salt bridge: Key interaction between antipsychotics and receptors. Theor. Chem. Acc. 2023, 142, 65. [Google Scholar] [CrossRef]
- Martínez, A.; García-Gutiérrez, P.; Zubillaga, R.A.; Garza, J.; Vargas, R. Main interactions of dopamine and risperidone with the dopamine D2 receptor. Phys. Chem. Chem. Phys. 2021, 23, 14224–14230. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, P.; Zubillaga, R.A.; Ibarra, I.A.; Martínez, A.; Vargas, R.; Garza, J. Non-conventional interactions of N3 inhibitor with the main protease of SARS-CoV and SARS-CoV-2. Comput. Struct. Biotechnol. J. 2021, 19, 4669–4675. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 5, 799–805. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Ufimtsev, I.S.; Martínez, T.J. Quantum chemistry on graphical processing units. 1. Strategies for two-electron integral evaluation. J. Chem. Theory Comput. 2008, 4, 222–231. [Google Scholar] [CrossRef]
- Ufimtsev, I.S.; Martínez, T.J. Quantum chemistry on graphical processing units. 2. Direct self-consistent-field implementation. J. Chem. Theory Comput. 2009, 5, 1004–1015. [Google Scholar] [CrossRef]
- Ufimtsev, I.S.; Martínez, T.J. Quantum chemistry on graphical processing units. 3. Analytical energy gradients, geometry optimization, and first principles molecular dynamics. J. Chem. Theory Comput. 2009, 5, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.C.; Hernández-Esparza, R.; Vázquez-Mayagoitia, Á.; Vargas, R.; Garza, J. Implementation of the molecular electrostatic potential over graphics processing units. J. Chem. Inf. Model. 2019, 59, 3120–3127. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Esparza, R.; Mejía-Chica, S.-M.; Zapata-Escobar, A.D.; Guevara-García, A.; Martínez-Melchor, A.; Hernández-Pérez, J.-M.; Vargas, R.; Garza, J. Grid-based algorithm to search critical points, in the electron density, accelerated by graphics processing units. J. Comput. Chem. 2014, 35, 2272–2278. [Google Scholar] [CrossRef]
- Hernández-Esparza, R.; Vázquez-Mayagoitia, Á.; Soriano-Agueda, L.A.; Vargas, R.; Garza, J. GPUs as boosters to analyze scalar and vector fields in quantum chemistry. Int. J. Quantum Chem. 2019, 119, e25671. [Google Scholar] [CrossRef]
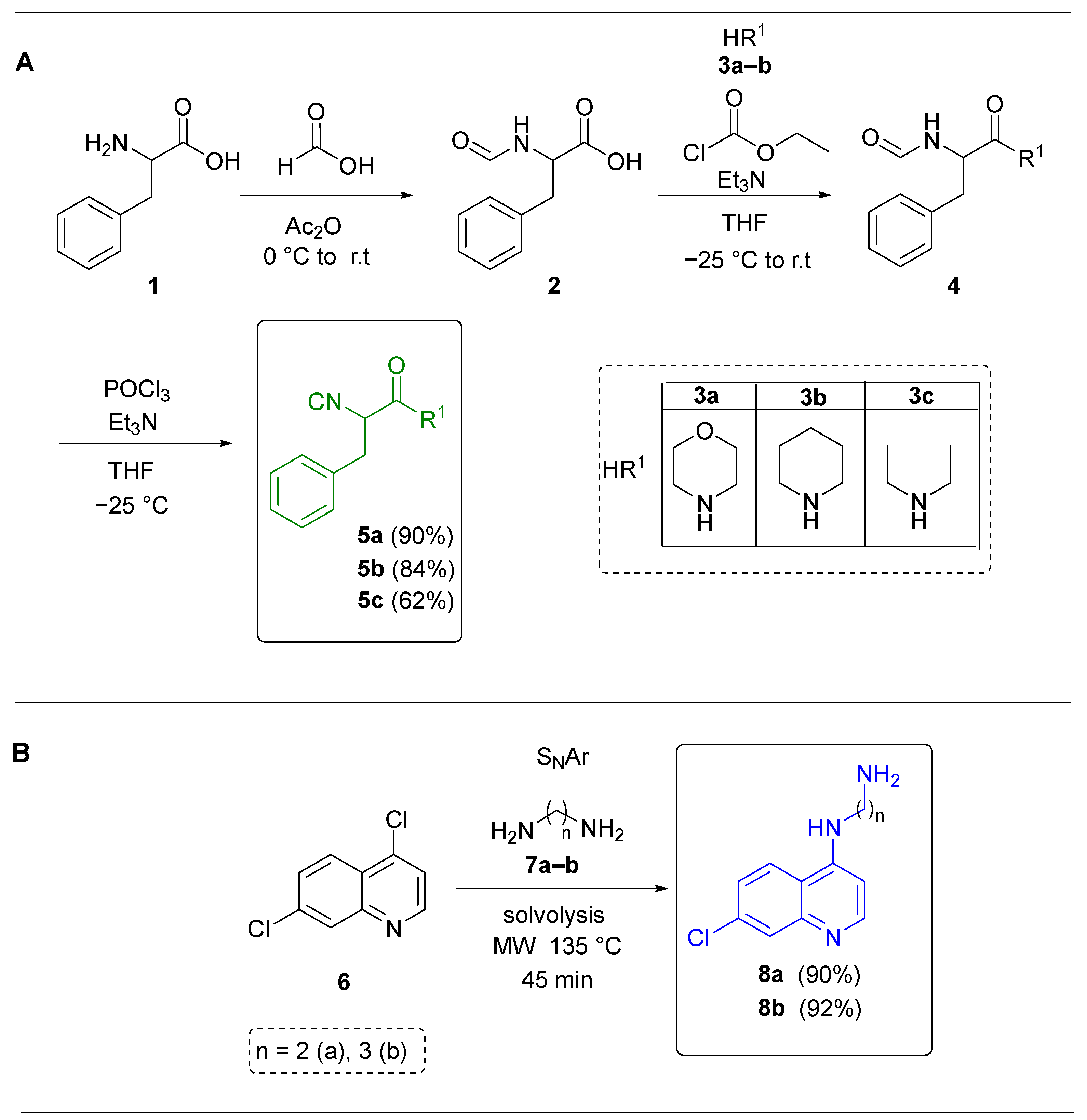



| Entry | Solvent | Conditions 1 Additives/T1 (°C) | Conditions 2 Additives/T2 (°C) | Yield (%) a |
|---|---|---|---|---|
| 1 | PhMe | Na2SO4 (anh.)/r.t (25) | InCl3 (15% mol) MW (70) | - |
| 2 | PhMe | Na2SO4 (anh.)/MW (80) | InCl3 (15% mol) MW (70) | - |
| 3 | MeOH | Na2SO4 (anh.)/MW (80) | InCl3 (15% mol) MW (70) | 8 |
| 4 | PhMe/MeOH 9:2 v/v | Na2SO4 (anh.)/MW (80) | InCl3 (15% mol) MW (70) | 14 |
| 5 | PhMe/MeOH 9:2 v/v | Na2SO4 (anh.)/MW (80) | InCl3 (15% mol) MW (80) | 19 |
| 6 | PhCl | Na2SO4 (anh.)/MW (80) | InCl3 (15% mol) MW (80) | 41 |
| 7 | PhCl | Na2SO4 (anh.)/MW (80) | Sc(OTf)3 (5% mol) MW (80) | 52 |
| 8 | PhCl | Na2SO4 (anh.)/MW (80) | Yb(OTf)3 (5% mol) MW (80) | 75 |

| Entry | Solvent | Conditions 3 T1 (°C) | Yield (%) a |
|---|---|---|---|
| 1 | PhMe/MeOH 9:2 | MW (70) | 79 |
| 2 | PhMe/MeOH 9:2 | MW (80) | 74 |
| 3 | PhCl | MW (80) | 94 |
| 19a | 19b | 19c | 19d | 19e | 19f | 19g | 19h | 19i | 19j | 19k | 19l | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (µM) 0 h | - | - | - | 6.74 * | 17.14 | 9.23 | 7.50 | - | 24.00 | 15.00 | - | 7.06 |
| IC50 (µM) 1 h | - | - | - | - | - | - | 6.98 | - | 5.29 * | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Carapia, R.E.; Hernández-López, R.; Alcaraz-Estrada, S.L.; Sarmiento-Silva, R.E.; García-Hernández, M.E.; Estrada-Toledo, N.V.; Padilla-Bernal, G.; Herrera-Zúñiga, L.D.; Garza, J.; Vargas, R.; et al. Multi-Component Synthesis of New Fluorinated-Pyrrolo[3,4-b]pyridin-5-ones Containing the 4-Amino-7-chloroquinoline Moiety and In Vitro–In Silico Studies Against Human SARS-CoV-2. Int. J. Mol. Sci. 2025, 26, 7651. https://doi.org/10.3390/ijms26157651
Blanco-Carapia RE, Hernández-López R, Alcaraz-Estrada SL, Sarmiento-Silva RE, García-Hernández ME, Estrada-Toledo NV, Padilla-Bernal G, Herrera-Zúñiga LD, Garza J, Vargas R, et al. Multi-Component Synthesis of New Fluorinated-Pyrrolo[3,4-b]pyridin-5-ones Containing the 4-Amino-7-chloroquinoline Moiety and In Vitro–In Silico Studies Against Human SARS-CoV-2. International Journal of Molecular Sciences. 2025; 26(15):7651. https://doi.org/10.3390/ijms26157651
Chicago/Turabian StyleBlanco-Carapia, Roberto E., Ricardo Hernández-López, Sofía L. Alcaraz-Estrada, Rosa Elena Sarmiento-Silva, Montserrat Elemi García-Hernández, Nancy Viridiana Estrada-Toledo, Gerardo Padilla-Bernal, Leonardo D. Herrera-Zúñiga, Jorge Garza, Rubicelia Vargas, and et al. 2025. "Multi-Component Synthesis of New Fluorinated-Pyrrolo[3,4-b]pyridin-5-ones Containing the 4-Amino-7-chloroquinoline Moiety and In Vitro–In Silico Studies Against Human SARS-CoV-2" International Journal of Molecular Sciences 26, no. 15: 7651. https://doi.org/10.3390/ijms26157651
APA StyleBlanco-Carapia, R. E., Hernández-López, R., Alcaraz-Estrada, S. L., Sarmiento-Silva, R. E., García-Hernández, M. E., Estrada-Toledo, N. V., Padilla-Bernal, G., Herrera-Zúñiga, L. D., Garza, J., Vargas, R., González-Zamora, E., & Islas-Jácome, A. (2025). Multi-Component Synthesis of New Fluorinated-Pyrrolo[3,4-b]pyridin-5-ones Containing the 4-Amino-7-chloroquinoline Moiety and In Vitro–In Silico Studies Against Human SARS-CoV-2. International Journal of Molecular Sciences, 26(15), 7651. https://doi.org/10.3390/ijms26157651








