Anti-Her2 CAR-NK92 Cells and Their Exosomes: Generation, Characterization, and Selective Cytotoxicity Against Her2-Positive Tumor Cells
Abstract
1. Introduction
2. Results
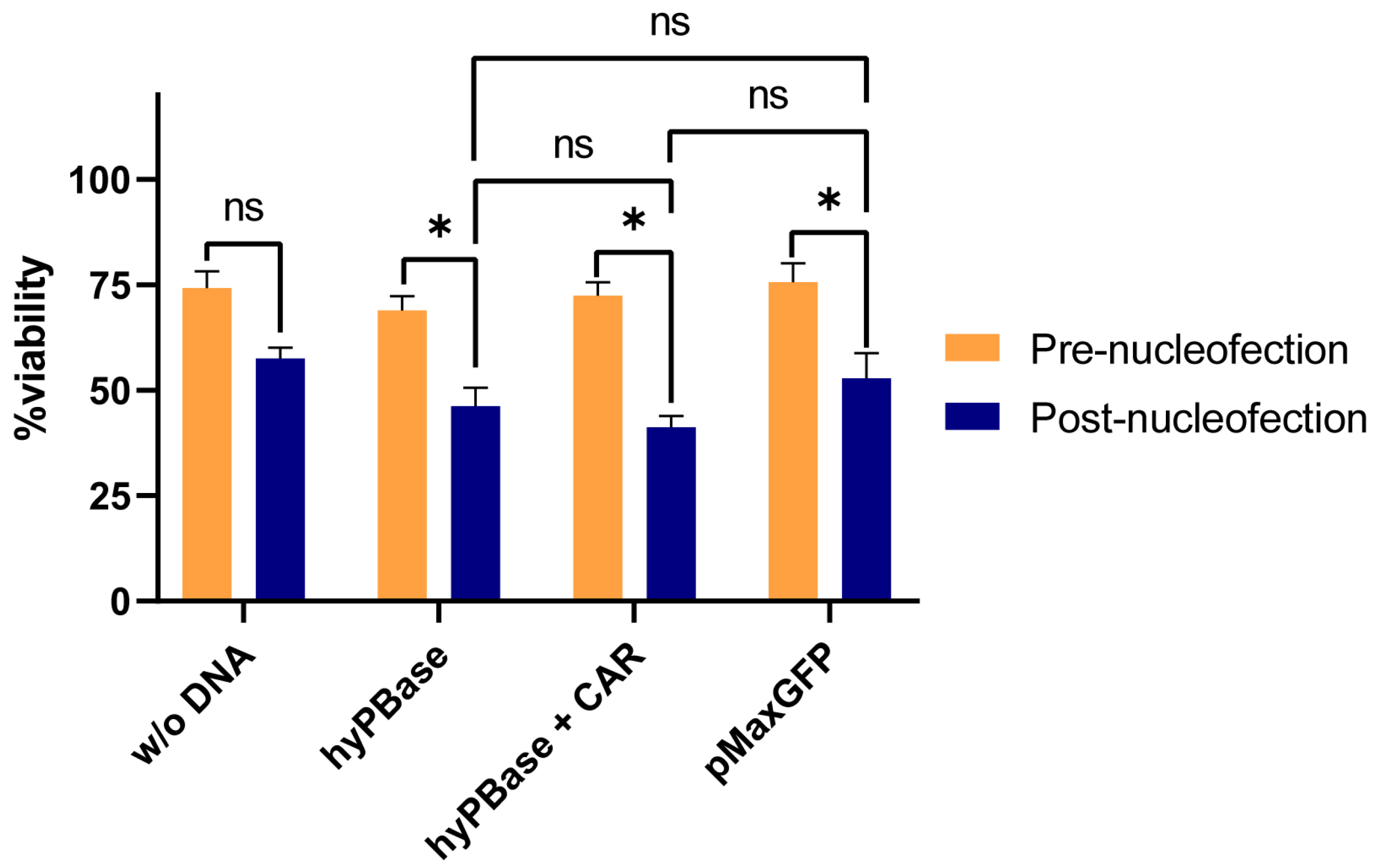
2.1. Plasmid Electroporation Does Not Significantly Affect NK92 Cell Viability
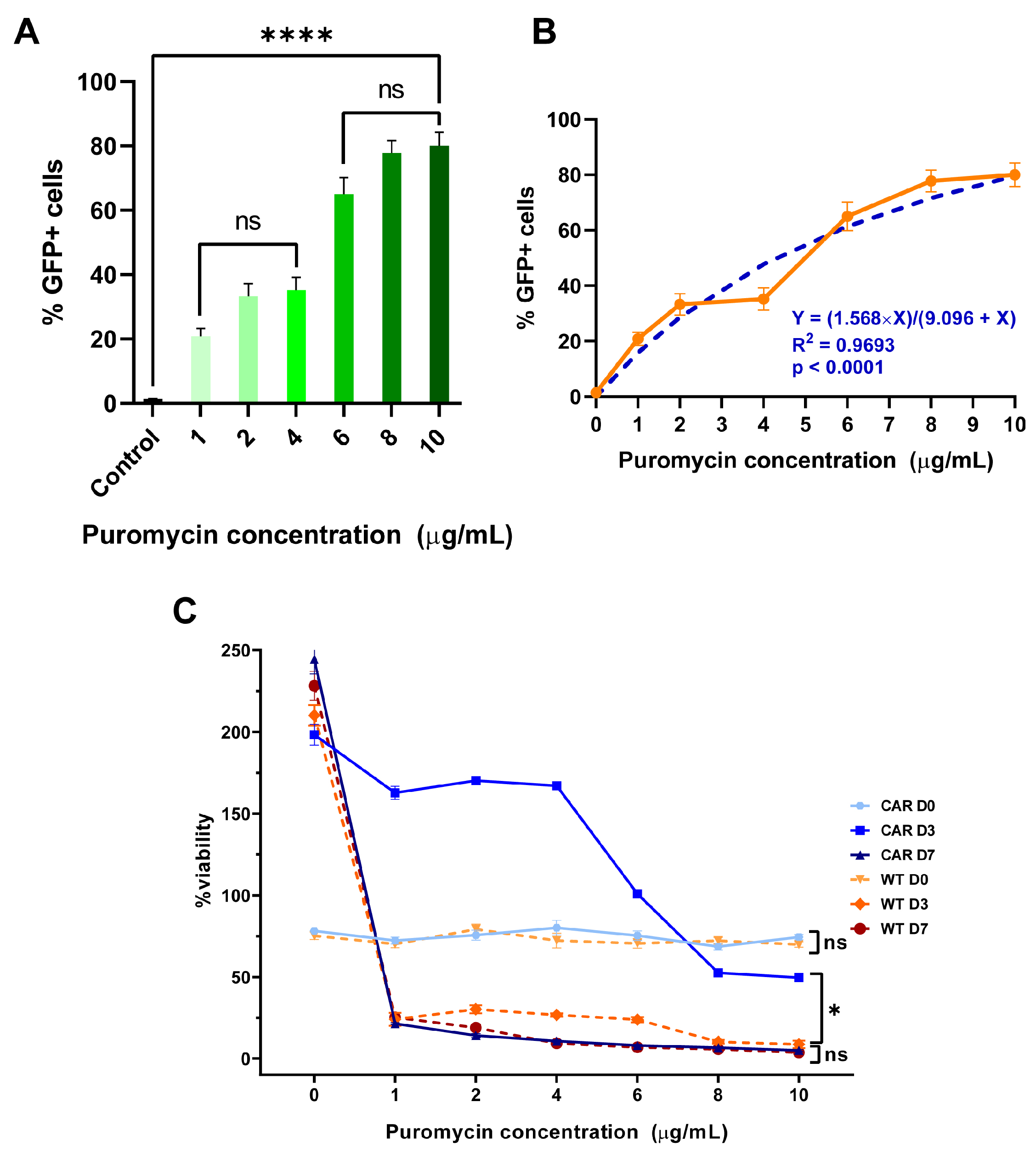
2.2. Puromycin Toxicity Curves
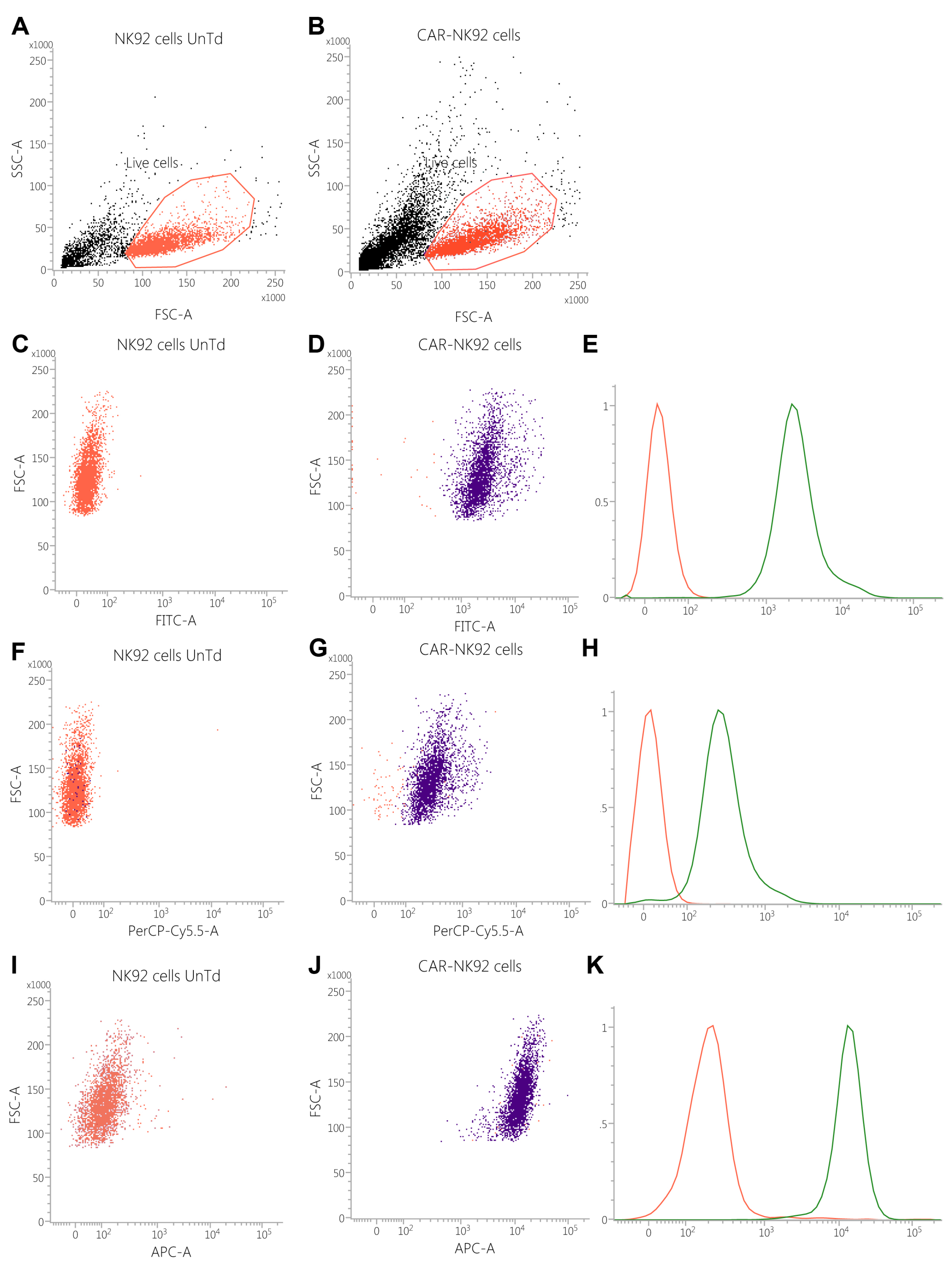
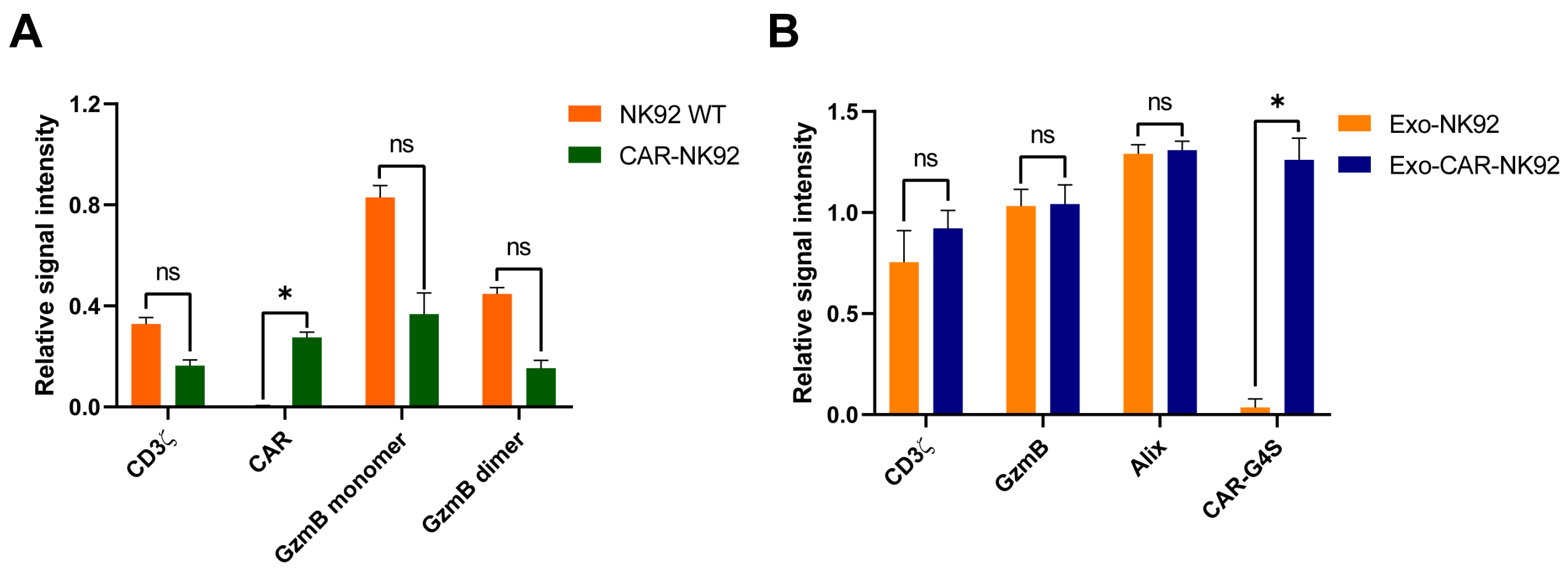
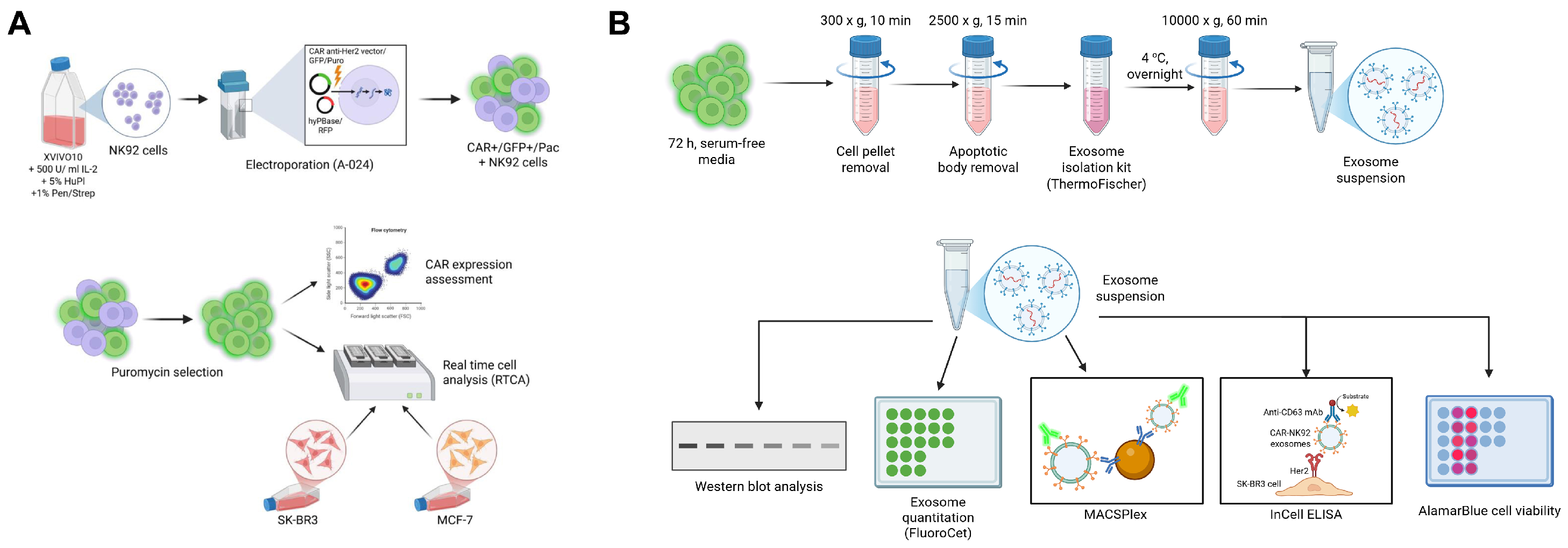
2.3. Transfected Cells Express GFP and CAR Molecules
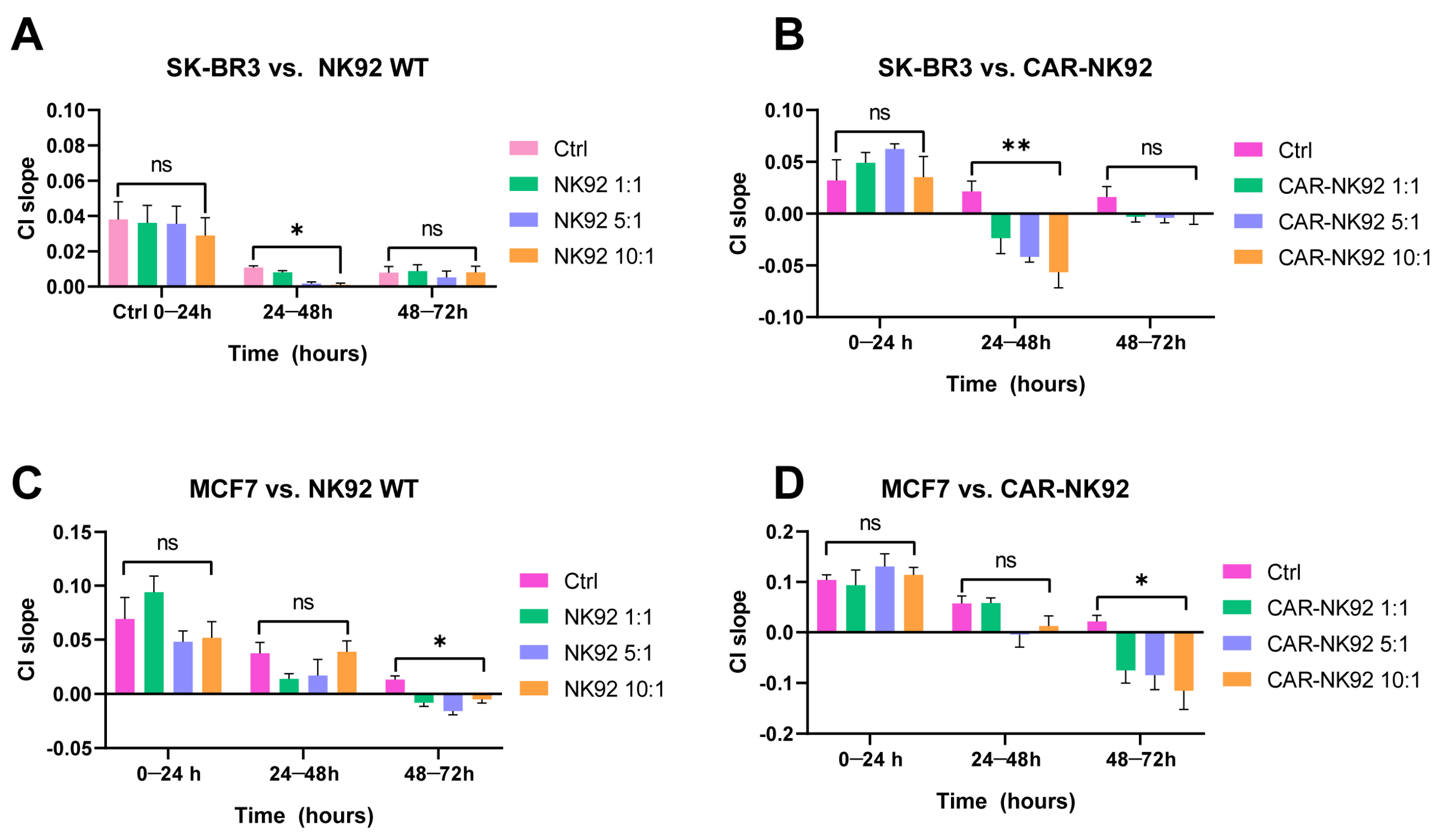
2.4. CAR-NK92 Exert Her2-Specific Cytotoxic Effects
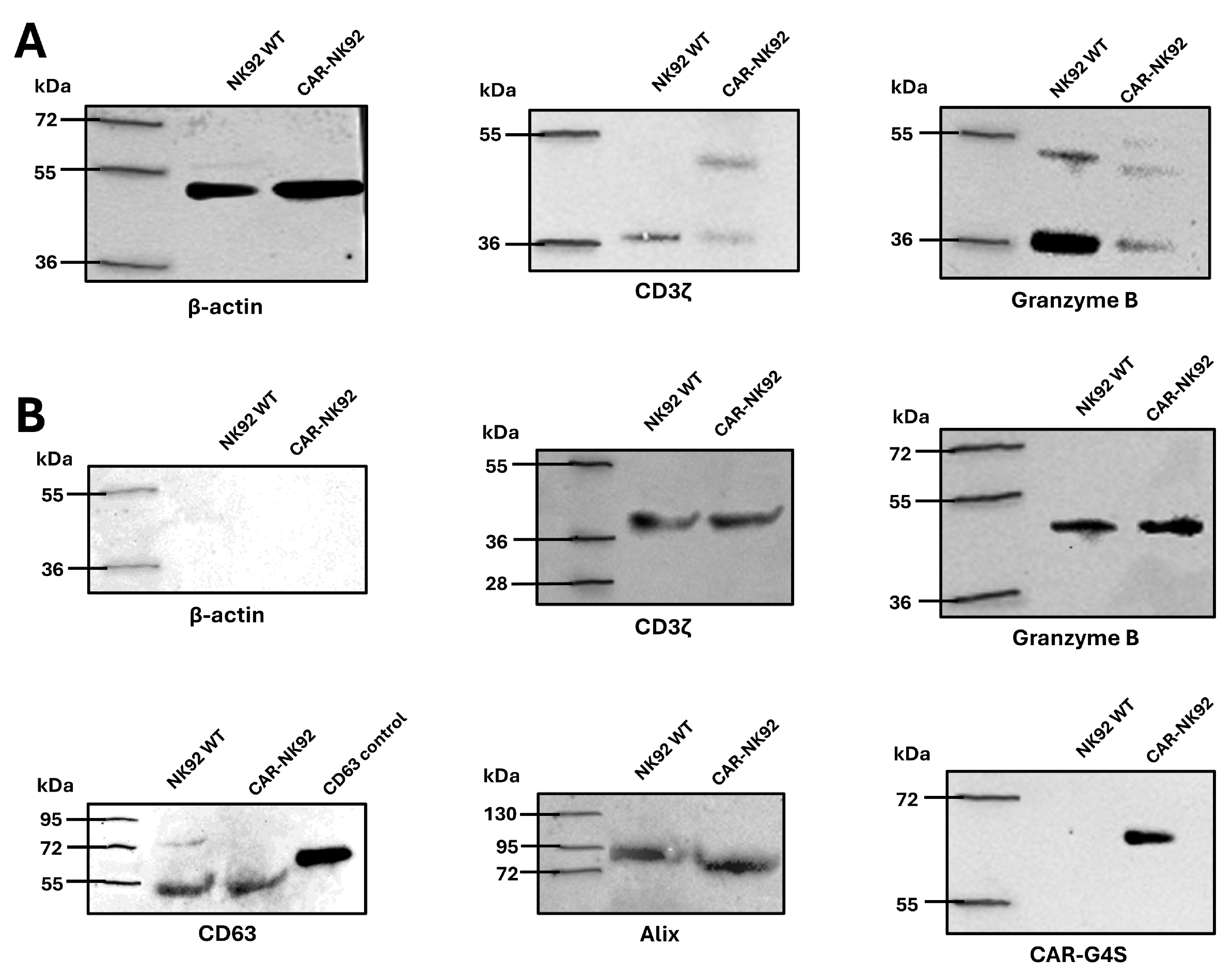
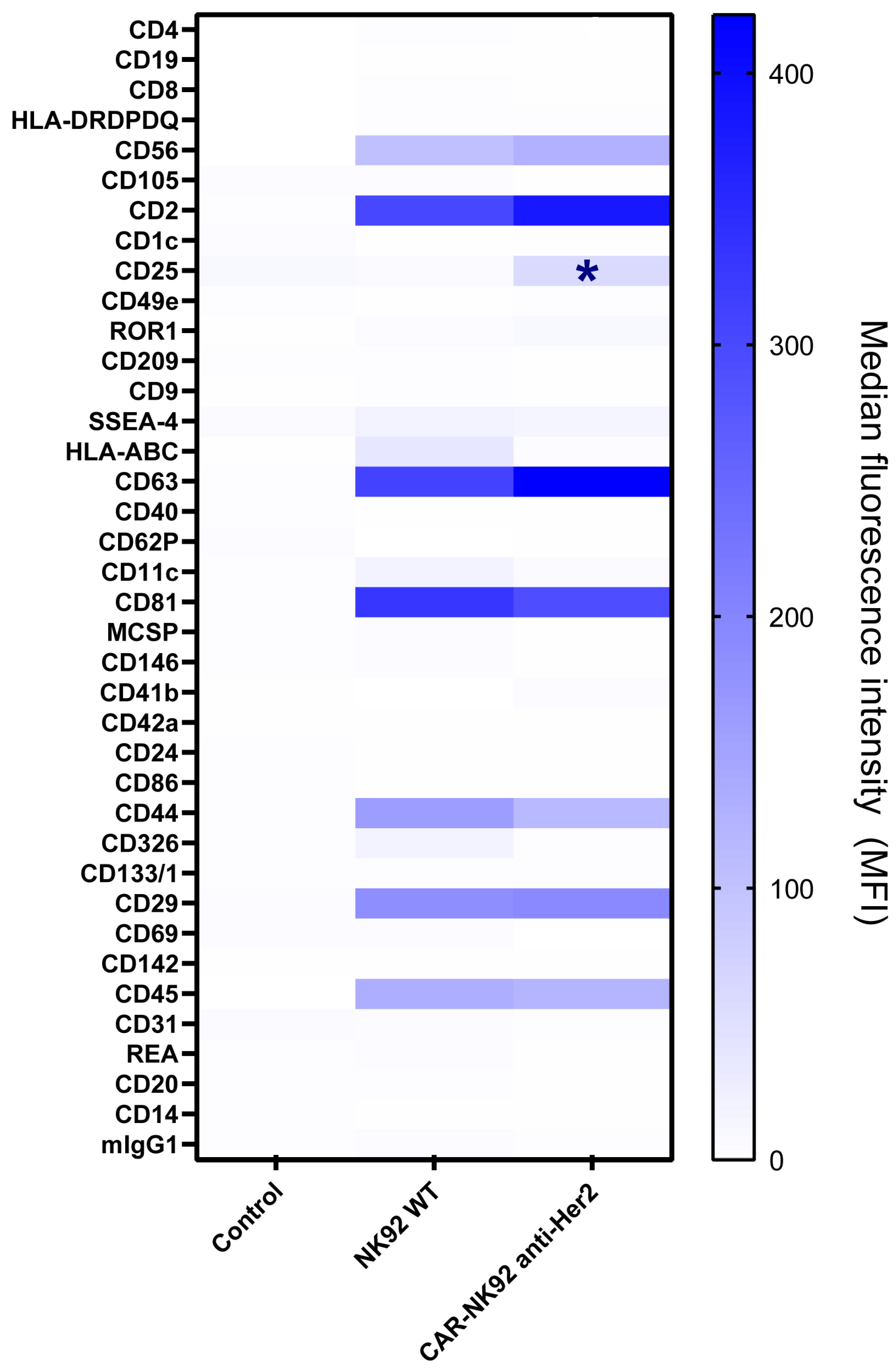
2.5. CAR-NK92-Derived Exosome Characterization
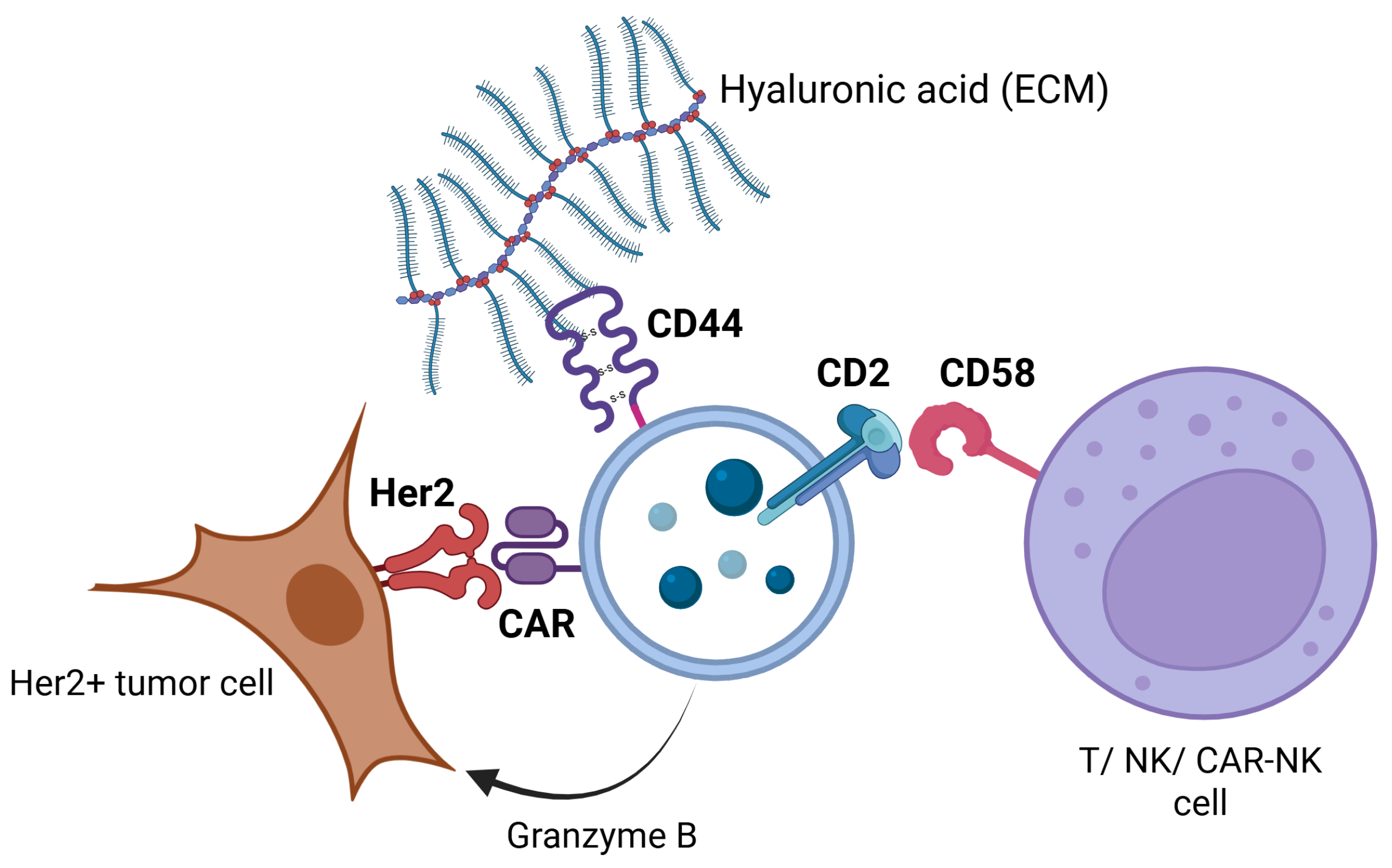
2.6. CAR-NK92-Exo Bind Her2 Molecules on SK-BR3 Cells
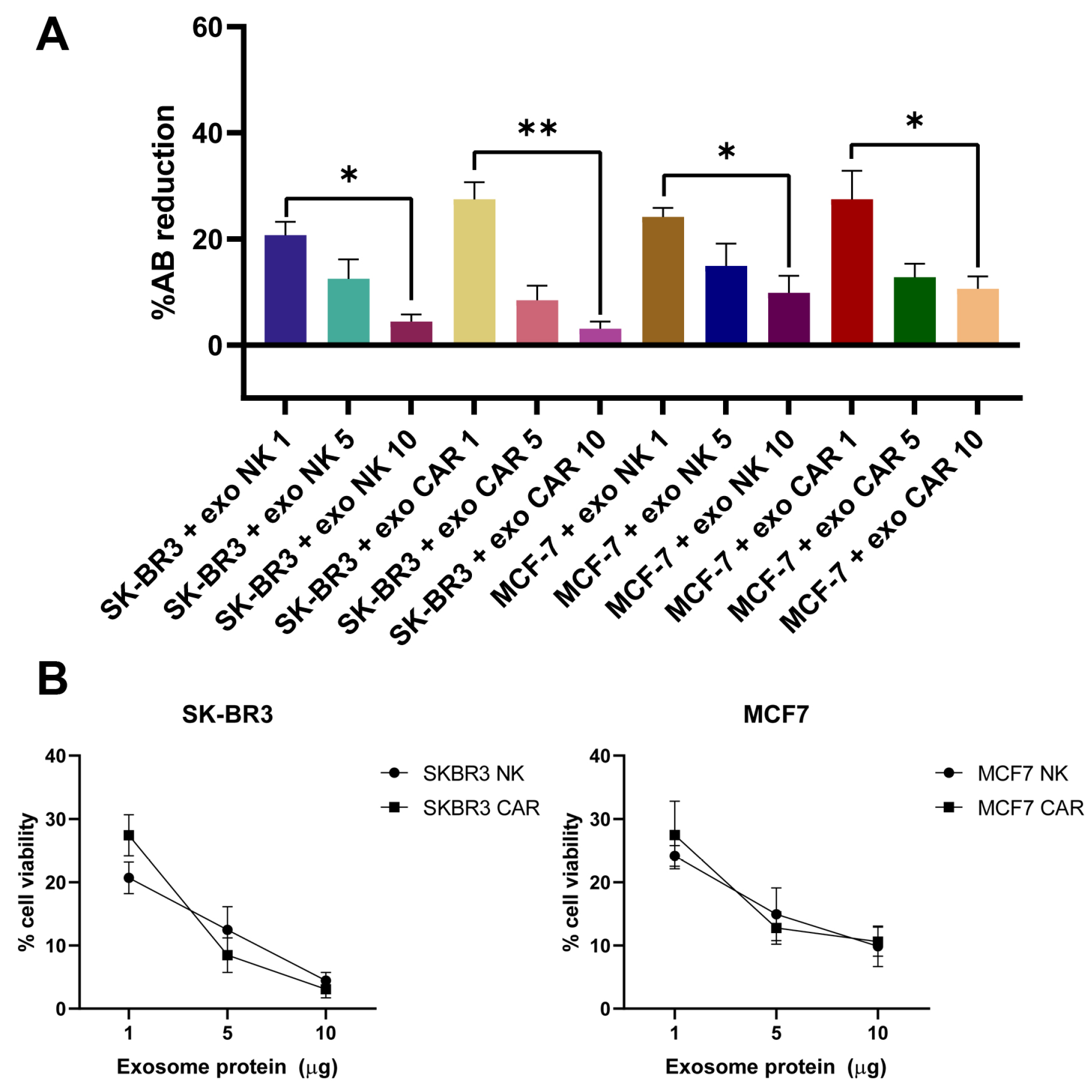
2.7. Exosomes from NK92 and CAR-NK92 Cells Exert Cytotoxic Effects
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Flow Cytometry Assessment of the Target Cells
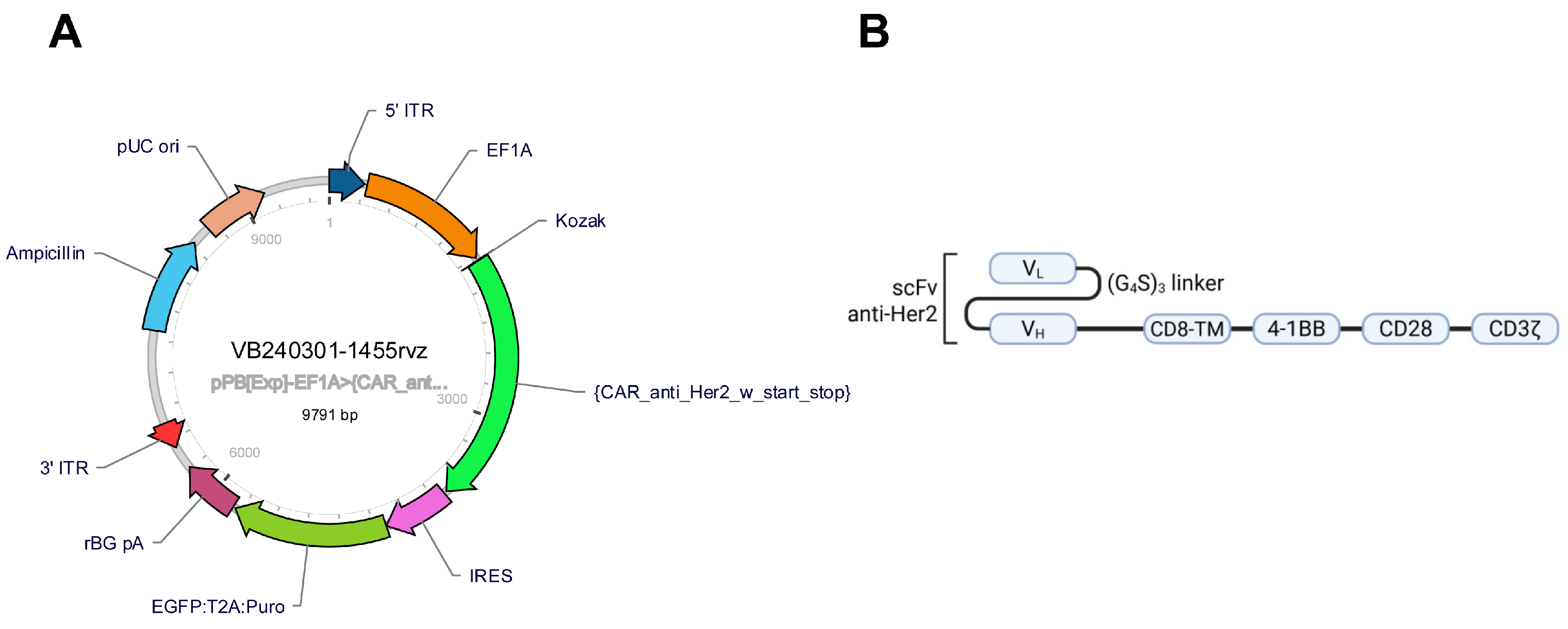
4.3. CAR Anti-Her2 Vector Design
4.4. NK-92 Cell Transfection
4.5. Puromycin Killing Curve
4.6. Transfection Efficiency and Cellular Viability Assessment
4.7. In Vitro Cytotoxicity Assay
4.8. Exosome Isolation
4.9. Identification of Exosomal Markers and the Presence of CAR on Exosomes
4.10. Her2-Specific Exosomal Binding Assessment
4.11. Exosomal Cytotoxic Effect Assessment
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aR2 | Adjusted Coefficient of Determination |
| CAR | Chimeric Antigen Receptor |
| CAR-NK | Chimeric Antigen Receptor-Engineered Natural Killer (cell) |
| CD3ζ | CD3 zeta chain (T-cell co-receptor signaling molecule) |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| D0, D3, D7 | Day 0, Day 3, Day 7 (timepoints) |
| DNA | Deoxyribonucleic Acid |
| df | Degrees of Freedom |
| E:T | Effector-to-Target (cell ratio) |
| (e)GFP | (Enhanced) Green Fluorescent Protein |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FACS | Fluorescence-Activated Cell Sorting |
| FBS | Fetal Bovine Serum |
| FSC | Forward Scatter (Flow Cytometry) |
| F | F-statistic (analysis of variance) |
| GVHD | Graft-versus-Host Disease |
| Her2 | Human Epidermal Growth Factor Receptor 2 |
| hyPBase | Hyperactive PiggyBac Transposase |
| ICOS | Inducible T-cell COStimulator |
| ILV | Intraluminal Vesicle |
| ITR | Inverted Terminal Repeat |
| mAb | Monoclonal Antibody |
| mRNA | Messenger RNA |
| miRNA | MicroRNA |
| MVB | Multivesicular Body |
| NK | Natural Killer (cell) |
| NK92 | NK92 cell line (human natural killer cell line) |
| ns | Not Significant |
| OD | Optical Density |
| PB | PiggyBac (transposon system) |
| p | p-value (probability value in statistical tests) |
| RAU | Relative Absorbance Units |
| RTCA | Real-Time Cell Analysis |
| scFv | Single-chain Variable Fragment |
| SD | Standard Deviation |
| SE | Standard Error |
| SEM | Standard Error of the Mean |
| SNARE | Soluble NSF Attachment Protein Receptor |
| SSE | Sum of Squared Errors |
| TCR | T Cell Receptor |
| WT | Wild-Type |
| VSV-G | Vesicular Stomatitis Virus Glycoprotein (used in viral pseudotyping) |
| LDL | Low-Density Lipoprotein |
References
- Choi, S.J.; Cho, H.; Yea, K.; Baek, M.C. Immune cell-derived small extracellular vesicles in cancer treatment. BMB Rep. 2022, 55, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sani, F.; Shojaei, S.; Tabatabaei, S.A.; Khorraminejad-Shirazi, M.; Latifi, M.; Sani, M.; Azarpira, N. CAR-T cell-derived exosomes: A new perspective for cancer therapy. Stem Cell Res. Ther. 2024, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Chuvin, N.; Valladeau-Guilemond, J.; Depil, S. Development of NK cell-based cancer immunotherapies through receptor engineering. Cell. Mol. Immunol. 2024, 21, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yu, J.; Caligiuri, M.A. Natural killer cell–based immunotherapy for cancer. J. Immunol. 2025, 214, 1444–1456. [Google Scholar] [CrossRef]
- Garg, P.; Pareek, S.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Next-Generation Immunotherapy: Advancing Clinical Applications in Cancer Treatment. J. Clin. Med. 2024, 13, 6537. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Francisco, N.M.; Zhang, Y.; Wu, M. The application of CAR-T cell therapy in hematological malignancies: Advantages and challenges. Acta Pharm. Sin. B 2018, 8, 539–551. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Zhang, X.; Aziz, A.U.R.; Wang, D. CAR-NK Cell Therapy: A Transformative Approach to Overcoming Oncological Challenges. Biomolecules 2024, 14, 1035. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.; Li, Z.; Hu, Y.; Wang, H. From CAR-T Cells to CAR-NK Cells: A Developing Immunotherapy Method for Hematological Malignancies. Front. Oncol. 2021, 11, 720501. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, J. Emerging roles of CAR-NK cell therapies in tumor immunotherapy: Current status and future directions. Cell. Death Discov. 2024, 10, 318. [Google Scholar] [CrossRef]
- Rotolo, R.; Leuci, V.; Donini, C.; Cykowska, A.; Gammaitoni, L.; Medico, G.; Valabrega, G.; Aglietta, M.; Sangiolo, D. CAR-Based Strategies beyond T Lymphocytes: Integrative Opportunities for Cancer Adoptive Immunotherapy. Int. J. Mol. Sci. 2019, 20, 2839. [Google Scholar] [CrossRef]
- Niu, Z.; Wu, J.; Zhao, Q.; Zhang, J.; Zhang, P.; Yang, Y. CAR-based immunotherapy for breast cancer: Peculiarities, ongoing investigations, and future strategies. Front. Immunol. 2024, 15, 1385571. [Google Scholar] [CrossRef]
- Lei, W.; Liu, H.; Deng, W.; Chen, W.; Liang, Y.; Gao, W.; Yuan, X.; Guo, S.; Li, P.; Wang, J.; et al. Safety and feasibility of 4-1BB co-stimulated CD19-specific CAR-NK cell therapy in refractory/relapsed large B cell lymphoma: A phase 1 trial. Nat. Cancer 2025, 6, 786–800. [Google Scholar] [CrossRef]
- Khan, S.H.; Choi, Y.; Veena, M.; Lee, J.K.; Shin, D.S. Advances in CAR T cell therapy: Antigen selection, modifications, and current trials for solid tumors. Front. Immunol. 2025, 15, 1489827. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.J.; Sun, X.Y.; Huang, K.M.; Zhang, L.; Yang, Z.S.; Zou, D.D.; Wang, B.; Warnock, G.L.; Dai, L.-J.; Luo, J. Therapeutic potential of CAR-T cell-derived exosomes: A cell-free modality for targeted cancer therapy. Oncotarget 2015, 6, 44179. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Posey, A.D.; Shaw, C.; Wing, A.; Da, T.; Patel, P.R.; McGettigan, S.E.; Casado-Medrano, V.; Kawalekar, O.U.; Uribe-Herranz, M.; et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 2018, 3, e96976. [Google Scholar] [CrossRef] [PubMed]
- Weinkove, R.; George, P.; Dasyam, N.; McLellan, A.D. Selecting costimulatory domains for chimeric antigen receptors: Functional and clinical considerations. Clin. Transl. Immunol. 2019, 8, e1049. [Google Scholar] [CrossRef]
- Schönfeld, K.; Sahm, C.; Zhang, C.; Naundorf, S.; Brendel, C.; Odendahl, M.; Nowakowska, P.; Bönig, H.; Köhl, U.; Kloess, S.; et al. Selective Inhibition of Tumor Growth by Clonal NK Cells Expressing an ErbB2/HER2-Specific Chimeric Antigen Receptor. Mol. Ther. 2015, 23, 330–338. [Google Scholar] [CrossRef]
- Drent, E.; Poels, R.; Ruiter, R.; Van De Donk, N.W.C.J.; Zweegman, S.; Yuan, H.; de Bruijn, J.; Sadelain, M.; Lokhorst, H.M.; Groen, R.W.J.; et al. Combined CD28 and 4-1BB costimulation potentiates affinity-tuned chimeric antigen receptor-engineered t cells. Clin. Cancer Res. 2019, 25, 4014–4025. [Google Scholar] [CrossRef]
- Guedan, S.; Calderon, H.; Posey, A.D.; Maus, M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther. Methods Clin. Dev. 2019, 12, 145–156. [Google Scholar] [CrossRef]
- Savan, R.; Chan, T.; Young, H.A. Lentiviral Gene Transduction in Human and Mouse NK Cell Lines. In Natural Killer Cell Protocols; Humana Press: Totowa, NJ, USA, 2010; Volume 612, pp. 209–221. [Google Scholar]
- Allan, D.S.J.; Chakraborty, M.; Waller, G.C.; Hochman, M.J.; Poolcharoen, A.; Reger, R.N.; Childs, R.W. Systematic improvements in lentiviral transduction of primary human natural killer cells undergoing ex vivo expansion. Mol. Ther. Methods Clin. Dev. 2021, 20, 559–571. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.Y.; Choi, J.U.; Park, J.S.; Lee, S.M.; Kang, C.H.; Park, C.H. Optimized conditions for gene transduction into primary immune cells using viral vectors. Sci. Rep. 2023, 13, 12365. [Google Scholar] [CrossRef]
- Fus-Kujawa, A.; Mendrek, B.; Bajdak-Rusinek, K.; Diak, N.; Strzelec, K.; Gutmajster, E.; Janelt, K.; Kowalczuk, A.; Trybus, A.; Rozwadowska, P.; et al. Gene-repaired iPS cells as novel approach for patient with osteogenesis imperfecta. Front. Bioeng. Biotechnol. 2023, 11, 1205122. [Google Scholar] [CrossRef] [PubMed]
- Alsaggar, M.; Liu, D. Physical Methods for Gene Transfer. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2015; Volume 89, pp. 1–24. [Google Scholar]
- Lin, H.; Cheng, J.; Mu, W.; Zhou, J.; Zhu, L. Advances in Universal CAR-T Cell Therapy. Front. Immunol. 2021, 12, 744823. [Google Scholar] [CrossRef] [PubMed]
- Grabundzija, I.; Irgang, M.; Mátés, L.; Belay, E.; Matrai, J.; Gogol-Döring, A.; Kawakami, K.; Chen, W.; Ruiz, P.; Chuah, M.K.L.; et al. Comparative Analysis of Transposable Element Vector Systems in Human Cells. Mol. Ther. 2010, 18, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Tickner, J.A.; Urquhart, A.J.; Stephenson, S.A.; Richard, D.J.; O’Byrne, K.J. Functions and Therapeutic Roles of Exosomes in Cancer. Front. Oncol. 2014, 4, 127. [Google Scholar] [CrossRef]
- Lee, H.; Bae, K.; Baek, A.R.; Kwon, E.B.; Kim, Y.H.; Nam, S.W.; Lee, G.H.; Chang, Y. Glioblastoma-Derived Exosomes as Nanopharmaceutics for Improved Glioma Treatment. Pharmaceutics 2022, 14, 1002. [Google Scholar] [CrossRef]
- Shpigelman, J.; Lao, F.S.; Yao, S.; Li, C.; Saito, T.; Sato-Kaneko, F.; Nolan, J.; Shukla, N.M.; Pu, M.; Messer, K.; et al. Generation and Application of a Reporter Cell Line for the Quantitative Screen of Extracellular Vesicle Release. Front. Pharmacol. 2021, 12, 668609. [Google Scholar] [CrossRef]
- Munson, P.; Shukla, A. Exosomes: Potential in Cancer Diagnosis and Therapy. Medicines 2015, 2, 310–327. [Google Scholar] [CrossRef]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef]
- Tao, B.; Du, R.; Zhang, X.; Jia, B.; Gao, Y.; Zhao, Y.; Liu, Y. Engineering CAR-NK cell derived exosome disguised nano-bombs for enhanced HER2 positive breast cancer brain metastasis therapy. J. Control. Release 2023, 363, 692–706. [Google Scholar] [CrossRef]
- Hu, D.; Yang, R.; Wang, G.; Li, H.; Fan, X.; Liang, G. Emerging Strategies to Overcome Current CAR-T Therapy Dilemmas—Exosomes Derived from CAR-T Cells. Int. J. Nanomed. 2024, 19, 2773–2791. [Google Scholar] [CrossRef]
- Pagotto, S.; Simeone, P.; Brocco, D.; Catitti, G.; De Bellis, D.; Vespa, S.; Di Pietro, N.; Marinelli, L.; Di Stefano, A.; Veschi, S.; et al. CAR-T-Derived Extracellular Vesicles: A Promising Development of CAR-T Anti-Tumor Therapy. Cancers 2023, 15, 1052. [Google Scholar] [CrossRef]
- Muntasell, A.; Costa-Garcia, M.; Vera, A.; Marina-Garcia, N.; Kirschning, C.J.; López-Botet, M. Priming of NK Cell Anti-Viral Effector Mechanisms by Direct Recognition of Human Cytomegalovirus. Front. Immunol. 2013, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Colamartino, A.B.L.; Lemieux, W.; Bifsha, P.; Nicoletti, S.; Chakravarti, N.; Sanz, J.; Roméro, H.; Selleri, S.; Béland, K.; Guiot, M.; et al. Efficient and Robust NK-Cell Transduction With Baboon Envelope Pseudotyped Lentivector. Front. Immunol. 2019, 10, 2873. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef] [PubMed]
- Batista Napotnik, T.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Lesueur, L.L.; Mir, L.M.; André, F.M. Overcoming the Specific Toxicity of Large Plasmids Electrotransfer in Primary Cells In Vitro. Mol. Ther. Nucleic Acids 2016, 5, e291. [Google Scholar] [CrossRef]
- Althaus, J.; Nilius-Eliliwi, V.; Maghnouj, A.; Döring, S.; Schroers, R.; Hudecek, M.; Hahn, S.A.; Mika, T. Cytotoxicity of CD19-CAR-NK92 cells is primarily mediated via perforin/granzyme pathway. Cancer Immunol. Immunother. 2023, 72, 2573–2583. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, J. The Chimeric Antigen Receptor Detection Toolkit. Front. Immunol. 2020, 11, 1770. [Google Scholar] [CrossRef]
- Sarikonda, G.; Mathieu, M.; Natalia, M.; Pahuja, A.; Xue, Q.; Pierog, P.L.; Trampont, P.C.; Decman, V.; Reynolds, S.; Hanafi, L.-A.; et al. Best practices for the development, analytical validation and clinical implementation of flow cytometric methods for chimeric antigen receptor T cell analyses. Cytom. B Clin. Cytom. 2021, 100, 79–91. [Google Scholar] [CrossRef]
- Schindler, K.; Eva Ruppel, K.; Müller, C.; Koehl, U.; Fricke, S.; Schmiedel, D. Linker-specific monoclonal antibodies present a simple and reliable detection method for scFv-based CAR NK cells. Mol. Ther. Methods Clin. Dev. 2024, 32, 101328. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, O.A.; Fong, L.K.; Ishiyama, K.; DeGrado, W.F.; Lanier, L.L. The CD3ζ adaptor structure determines functional differences between human and mouse CD16 Fc receptor signaling. J. Exp. Med. 2022, 219, e20220022. [Google Scholar] [CrossRef]
- Aharon, A.; Horn, G.; Bar-Lev, T.H.; Zagagi Yohay, E.; Waks, T.; Levin, M.; Unger, N.D.; Avivi, I.; Levin, A.G. Extracellular Vesicles Derived from Chimeric Antigen Receptor-T Cells: A Potential Therapy for Cancer. Hum. Gene. Ther. 2021, 32, 1224–1241. [Google Scholar] [CrossRef]
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Fan, X.; Lin, F.; et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019, 10, 4355. [Google Scholar] [CrossRef]
- Yang, P.; Cao, X.; Cai, H.; Feng, P.; Chen, X.; Zhu, Y.; Yang, Y.; An, W.; Yang, Y.; Jie, J. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell. Immunol. 2021, 360, 104262. [Google Scholar] [CrossRef]
- Kim, H.Y.; Min, H.K.; Song, H.W.; Yoo, A.; Lee, S.; Kim, K.P.; Park, J.-O.; Choi, Y.H.; Choi, E. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: An in vivo study in subcutaneous and orthotopic animal models. Drug Deliv. 2022, 29, 2897–2911. [Google Scholar] [CrossRef]
- Di Pace, A.; Tumino, N.; Besi, F.; Alicata, C.; Conti, L.; Munari, E.; Maggi, E.; Vacca, P.; Moretta, L. Characterization of Human NK Cell-Derived Exosomes: Role of DNAM1 Receptor in Exosome-Mediated Cytotoxicity against Tumor. Cancers 2020, 12, 661. [Google Scholar] [CrossRef]
- Cochran, A.M.; Kornbluth, J. Extracellular Vesicles From the Human Natural Killer Cell Line NK3.3 Have Broad and Potent Anti-Tumor Activity. Front. Cell. Dev. Biol. 2021, 9, 698639. [Google Scholar] [CrossRef]
- St-Denis-Bissonnette, F.; Cummings, S.E.; Qiu, S.; Stalker, A.; Muradia, G.; Mehic, J.; Mediratta, K.; Kaczmarek, S.; Burger, D.; Lee, S.-H.; et al. A clinically relevant large-scale biomanufacturing workflow to produce natural killer cells and natural killer cell-derived extracellular vesicles for cancer immunotherapy. J. Extracell. Vesicles 2023, 12, e12387. [Google Scholar] [CrossRef]
- Samara, A.; Anbar, M.; Shapira, S.; Zemlyansky, A.; Zozovsky, A.; Raanani, P.; Granot, G.; Rozovski, U. Using natural killer cell-derived exosomes as a cell-free therapy for leukemia. Hematol. Oncol. 2023, 41, 487–498. [Google Scholar] [CrossRef]
- Dosil, S.G.; Lopez-Cobo, S.; Rodriguez-Galan, A.; Fernandez-Delgado, I.; Ramirez-Huesca, M.; Milan-Rois, P.; Castellanos, M.; Somoza, A.; Gómez, M.J.; Reyburn, H.T.; et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. eLife 2022, 11, e76319. [Google Scholar] [CrossRef]
- Mu, W.; Xu, Y.; Gu, P.; Wang, W.; Li, J.; Ge, Y.; Wang, H. Exosomal CD44 Cooperates with Integrin α6β4 to Support Organotropic Metastasis via Regulating Tumor Cell Motility and Target Host Cell Activation. Engineering 2021, 7, 1413–1423. [Google Scholar] [CrossRef]
- Hazawa, M.; Tomiyama, K.; Saotome-Nakamura, A.; Obara, C.; Yasuda, T.; Gotoh, T.; Tanaka, I.; Yakumaru, H.; Ishihara, H.; Tajima, K. Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem. Biophys. Res. Commun. 2014, 446, 1165–1171. [Google Scholar] [CrossRef]
- Liu, L.L.; Landskron, J.; Ask, E.H.; Enqvist, M.; Sohlberg, E.; Traherne, J.A.; Hammer, Q.; Goodridge, J.P.; Larsson, S.; Jayaraman, J.; et al. Critical Role of CD2 Co-stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep. 2016, 15, 1088–1099. [Google Scholar] [CrossRef]
- Calvo, V.; Izquierdo, M. T Lymphocyte and CAR-T Cell-Derived Extracellular Vesicles and Their Applications in Cancer Therapy. Cells 2022, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/neu Oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Torres, M.B.; Spetz, M.R.; Wang, R.; Peng, L.; Tian, M.; Dower, C.M.; Nguyen, R.; Sun, M.; Tai, C.-H.; et al. CAR T cells targeting tumor-associated exons of glypican 2 regress neuroblastoma in mice. Cell Rep. Med. 2021, 2, 100297. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.F.; Purdon, T.J.; van Leeuwen, D.G.; Lopez, A.V.; Curran, K.J.; Daniyan, A.F.; Brentjenf, R.J. CD40 Ligand-Modified Chimeric Antigen Receptor T Cells Enhance Antitumor Function by Eliciting an Endogenous Antitumor Response. Cancer Cell 2019, 35, 473–488. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, H.T.; Wang, S.; Huang, S.L.; Zheng, Y.; Wang, C.Q.; Hu, B.-Y.; Qin, W.; Zou, T.-T.; Fu, Y.; et al. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef]
- Enomoto, Y.; Li, P.; Jenkins, L.M.; Anastasakis, D.; Lyons, G.C.; Hafner, M.; Leonard, W.J. Cytokine-enhanced cytolytic activity of exosomes from NK Cells. Cancer Gene Ther. 2021, 29, 734–749. [Google Scholar] [CrossRef]
- Portillo, A.L.; Hogg, R.; Poznanski, S.M.; Rojas, E.A.; Cashell, N.J.; Hammill, J.A.; Chew, M.V.; Shenouda, M.M.; Ritchie, T.M.; Cao, Q.T.; et al. Expanded human NK cells armed with CAR uncouple potent anti-tumor activity from off-tumor toxicity against solid tumors. iScience 2021, 24, 102619. [Google Scholar] [CrossRef]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef]

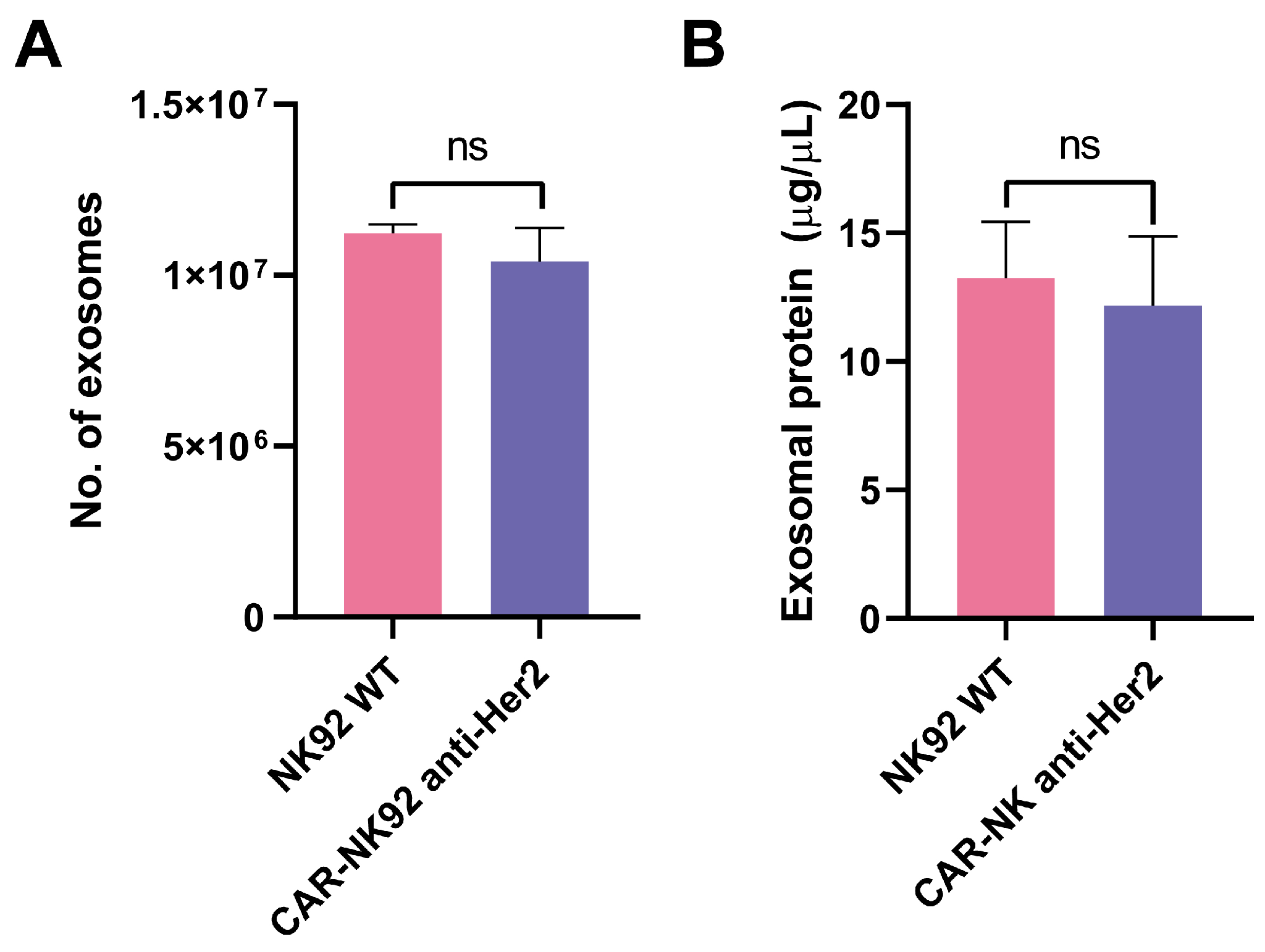

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tîrziu, A.; Bojin, F.M.; Gavriliuc, O.I.; Buzan, R.M.; Zbîrcea, L.E.; Grijincu, M.; Păunescu, V. Anti-Her2 CAR-NK92 Cells and Their Exosomes: Generation, Characterization, and Selective Cytotoxicity Against Her2-Positive Tumor Cells. Int. J. Mol. Sci. 2025, 26, 7648. https://doi.org/10.3390/ijms26157648
Tîrziu A, Bojin FM, Gavriliuc OI, Buzan RM, Zbîrcea LE, Grijincu M, Păunescu V. Anti-Her2 CAR-NK92 Cells and Their Exosomes: Generation, Characterization, and Selective Cytotoxicity Against Her2-Positive Tumor Cells. International Journal of Molecular Sciences. 2025; 26(15):7648. https://doi.org/10.3390/ijms26157648
Chicago/Turabian StyleTîrziu, Alexandru, Florina Maria Bojin, Oana Isabella Gavriliuc, Roxana Maria Buzan, Lauriana Eunice Zbîrcea, Manuela Grijincu, and Virgil Păunescu. 2025. "Anti-Her2 CAR-NK92 Cells and Their Exosomes: Generation, Characterization, and Selective Cytotoxicity Against Her2-Positive Tumor Cells" International Journal of Molecular Sciences 26, no. 15: 7648. https://doi.org/10.3390/ijms26157648
APA StyleTîrziu, A., Bojin, F. M., Gavriliuc, O. I., Buzan, R. M., Zbîrcea, L. E., Grijincu, M., & Păunescu, V. (2025). Anti-Her2 CAR-NK92 Cells and Their Exosomes: Generation, Characterization, and Selective Cytotoxicity Against Her2-Positive Tumor Cells. International Journal of Molecular Sciences, 26(15), 7648. https://doi.org/10.3390/ijms26157648





