Setting Up a “Green” Extraction Protocol for Bioactive Compounds in Buckwheat Husk
Abstract
1. Introduction
2. Results
2.1. Profiling Bioactive Compounds in Various Extracts
2.2. Biological Activity
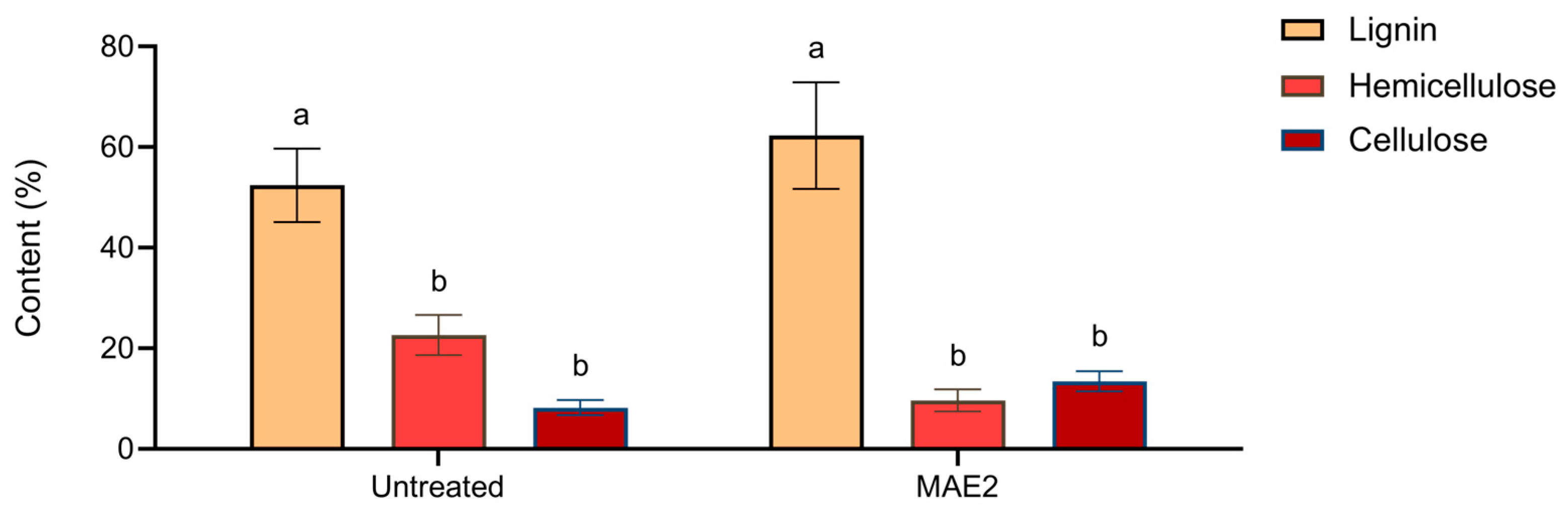
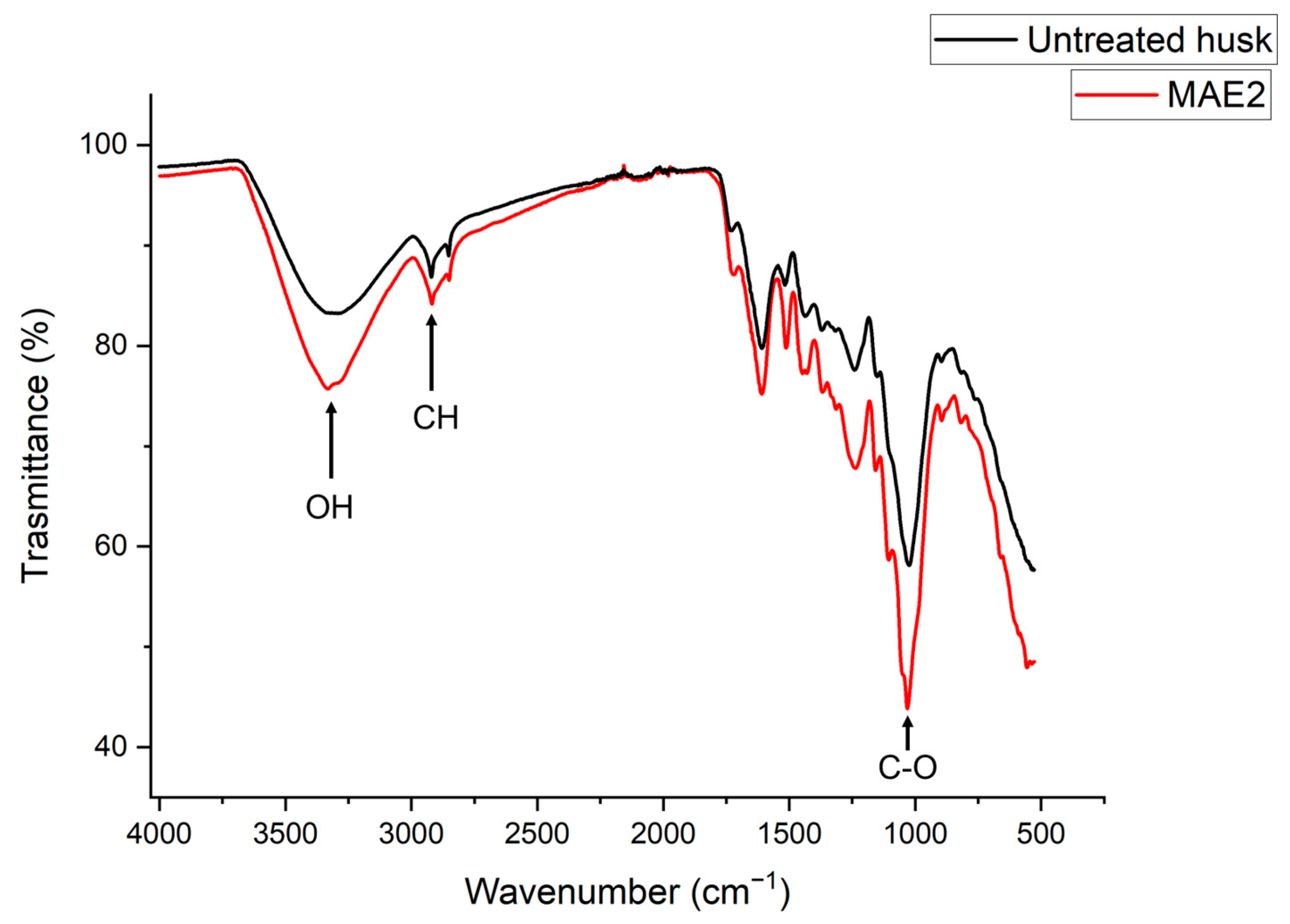
2.3. Effects of Treatment on Morphological Traits of Buckwheat Husk
3. Discussion
4. Materials and Methods
4.1. Buckwheat Materials
4.2. Extraction
4.2.1. Acidified Methanol Extraction
4.2.2. Ultrasound-Assisted Extraction
4.2.3. Microwave-Assisted Extraction [41]
4.2.4. Aqueous Acetic Acid Extraction
4.3. Characterization of the Extracts
4.3.1. TPC
4.3.2. RP-HPLC
4.4. Antioxidant Activity Assays
4.4.1. ABTS Assay
4.4.2. DPPH Assay
4.5. Caco-2 Cell Cultivation
4.6. MTT Viability Assay
4.7. Anti-Inflammatory Assay
4.7.1. Cell Incubation
4.7.2. RNA Extraction
4.7.3. Reverse Transcription PCR (RT-PCR)
4.7.4. Expression of IL-8 Measurement by Real-Time PCR Analysis (qPCR)
4.8. SEM, FTIR and Van Soest Analysis
4.8.1. SEM
4.8.2. FTIR
4.8.3. Van Soest
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijngaard, H.H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Chettry, U.; Chrungoo, N.K. Beyond the cereal box: Breeding buckwheat as a strategic crop for human nutrition. Plant Foods Hum. Nutr. 2021, 76, 399–409. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 17 July 2025).
- Sinkovič, L.; Kokalj Sinkovič, D.; Meglič, V. Milling fractions composition of common (Fagopyrum esculentum Moench) and Tartary (Fagopyrum tataricum (L.) Gaertn.) buckwheat. Food Chem. 2021, 365, 130459. [Google Scholar] [CrossRef]
- Pocienė, O.; Šlinkšienė, R. Studies on the Possibilities of Processing Buckwheat Husks and Ash in the Production of Environmentally Friendly Fertilizers. Agriculture 2022, 12, 193. [Google Scholar] [CrossRef]
- Lee, H.; Lim, T.; Kim, J.; Kim, R.H.; Hwang, K.T. Phenolics in Buckwheat Hull Extracts and Their Antioxidant Activities on Bulk Oil and Emulsions. J. Food Sci. 2022, 87, 2831–2846. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Tumbas, V.; Čanadanović-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) Grain and Fractions: Antioxidant Compounds and Activities. J. Food Sci. 2012, 77, C954–C959. [Google Scholar] [CrossRef]
- Kalinová, J.P.; Vrchotová, N.; Tříska, J. Phenolics levels in different parts of common buckwheat (Fagopyrum esculentum) achenes. J. Cereal Sci. 2019, 85, 243–248. [Google Scholar] [CrossRef]
- Wang, L.; Du, X.; Yue, D.; Chen, X. Catechin, rutin and quercetin in Quercus mongolica Fisch leaves exert inhibitory effects on multiple cancer cells. J. Food Biochem. 2022, 46, e14486. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Bioactive compounds in health and disease—Focus on rutin. Bioact. Compd. Health Dis. 2023, 6, 235. [Google Scholar] [CrossRef]
- Kan, J.; Cao, M.; Chen, C.; Gao, M.; Zong, S.; Zhang, J.; Liu, J.; Tang, C.; Jin, C. In Vitro Antioxidant and Lipid-Lowering Properties of Free and Bound Phenolic Compounds from Buckwheat Hulls. Food Biosci. 2023, 53, 102725. [Google Scholar] [CrossRef]
- Pietrzak, D.; Zwolan, A.; Chmiel, M.; Adamczak, L.; Cegiełka, A.; Hać-Szymańczuk, E.; Ostrowska-Ligęza, E.; Florowski, T.; Oszmiański, J. The Effects of Extracts from Buckwheat Hulls on the Quality Characteristics of Chicken Meatballs During Refrigerated Storage. Appl. Sci. 2022, 12, 9612. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green Non-Conventional Techniques for the Extraction of Polyphenols from Agricultural Food By-Products: A Review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of High Added-Value Components from Food Wastes: Conventional, Emerging Technologies and Commercialized Applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Singh, N.; Patle, D.S.; Kumar, S. Microwave- and Ultrasonication-Based Intensified and Synergetic Approaches for Extraction of Bioactive Compounds from Pomegranate Peels: Parametric and Kinetic Studies. Ind. Eng. Chem. Res. 2024, 63, 9214–9224. [Google Scholar] [CrossRef]
- Stramarkou, M.; Missirli, T.-V.; Kyriakopoulou, K.; Papadaki, S.; Angelis-Dimakis, A.; Krokida, M. The Recovery of Bioactive Compounds from Olive Pomace Using Green Extraction Processes. Resources 2023, 12, 77. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A Comparative Study on Different Extraction Techniques to Recover Red Grape Pomace Polyphenols from Vinification Byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Hanula, M.; Wyrwisz, J.; Moczkowska, M.; Horbańczuk, O.K.; Pogorzelska-Nowicka, E.; Wierzbicka, A. Optimization of Microwave and Ultrasound Extraction Methods of Açai Berries in Terms of Highest Content of Phenolic Compounds and Antioxidant Activity. Appl. Sci. 2020, 10, 8325. [Google Scholar] [CrossRef]
- Yu, M.; Wang, B.; Xin, G.; Li, W. Response Surface Method Was Used To Optimize the Ultrasonic-Assisted Extraction of Flavonoids from Crinum asiaticum. Saudi J. Biol. Sci. 2019, 26, 2079–2084. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic Assisted Aqueous Extraction of Catechin and Gallic Acid from Syzygium cumini Seed Kernel and Evaluation of Total Phenolic, Flavonoid Contents and Antioxidant Activity. Chem. Eng. Process. Process Intensif. 2020, 156, 107841. [Google Scholar] [CrossRef]
- Bouaoudia-Madi, N.; Boulekbache-Makhlouf, L.; Madani, K.; Silva, A.M.S.; Dairi, S.; Oukhmanou-Bensidhoum, S.; Cardoso, S.M. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Myrtus communis L. Pericarp. Antioxidants 2019, 8, 205. [Google Scholar] [CrossRef]
- Baltacıoğlu, C.; Baltacıoğlu, H.; Okur, İ.; Yetişen, M.; Alpas, H. Recovery of Phenolic Compounds from Peach Pomace Using Conventional Solvent Extraction and Different Emerging Techniques. J. Food Sci. 2024, 89, 1672–1683. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Expósito-Almellón, X.; García, P.; Pando, M.E.; Borrás-Linares, I.; Lozano-Sánchez, J. Extraction, Characterization, and Bioactivity of Phenolic Compounds—A Case on Hibiscus Genera. Foods 2023, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D.V. Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials. Materials 2023, 16, 7351. [Google Scholar] [CrossRef] [PubMed]
- Antón, M.; Aranibar, C.; Dusso, D.; Moyano, L.; Aguirre, A.; Borneo, R. Exploring Green Extraction Methods to Obtain Polyphenols from Partially Defatted Chia (Salvia hispanica L.) Flour. Expl. Foods Foodomics 2023, 1, 221–234. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Cherkashina, N.; Kuprieva, O.; Pushkarskaya, D.; Kashibadze, N.; Shrubchenko, L. Modification of Buckwheat Husk Powder and Creation of Composite Material on Its Basis. Phys. Scr. 2024, 99, 105921. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; González-Paramás, A.M.; Santos-Buelga, C. Latest Advances in Green Extraction of Polyphenols from Plants, Foods and Food By-Products. Molecules 2024, 30, 55. [Google Scholar] [CrossRef]
- Álvarez-Romero, M.; Ruíz-Rodríguez, A.; Barbero, G.F.; Vázquez-Espinosa, M.; El-Mansouri, F.; Brigui, J.; Palma, M. Comparison between Ultrasound- and Microwave-Assisted Extraction Methods to Determine Phenolic Compounds in Barley (Hordeum vulgare L.). Foods 2023, 12, 2638. [Google Scholar] [CrossRef]
- Amancio de Jesus, R.; da Silva, W.R.; Wisniewski, A.; de Andrade Nascimento, L.F.; Blank, A.F.; Alves, D.; de Andrade Wartha, E.R.S.; de Lima Nogueira, P.C.; de Souza Moraes, V.R.S.; de Souza Lima, E. Microwave and Ultrasound Extraction of Antioxidant Phenolic Compounds from Lantana Camara Linn. Leaves: Optimization, Comparative Study, and FT-Orbitrap MS Analysis. Phytochem. Anal. 2024, 35, 889–902. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-Assisted Extraction of Phenolics from Pomegranate Peels: Optimization, Kinetics, and Comparison with Ultrasounds Extraction. Chem. Eng. Process. Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Wang, S.; Gao, X.; Zhang, X. Research Progress on Extraction and Detection Technologies of Flavonoid Compounds in Foods. Foods 2024, 13, 628. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Choi, E.; Kim, C.; Sung, J.; Kim, Y.; Seo, D.; Choi, H.; Choi, Y.; Kum, J.; Park, J. Contribution of Flavonoids to the Antioxidant Properties of Common and Tartary Buckwheat. J. Cereal Sci. 2015, 68, 181–186. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Z.; Liu, Z.; Liu, J.; Piao, C.; Liu, D. Purification and Identification of Buckwheat Hull Flavonoids and Its Comparative Evaluation on Antioxidant and Cytoprotective Activity In Vitro. Food Sci. Nutr. 2020, 8, 3882–3892. [Google Scholar] [CrossRef]
- Park, B.I.; Kim, J.; Lee, K.; Lim, T.; Hwang, K.T. Flavonoids in common and tartary buckwheat hull extracts and antioxidant activity of the extracts against lipids in mayonnaise. J. Food Sci. Technol. 2019, 56, 2712–2720. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In Vivo Antioxidant Activity of Grape, Pomace and Wine from Three Red Varieties Grown in Argentina: Its Relationship to Phenolic Profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Authur, D.A.; Ma, H. Ultrasound-, Subcritical Water- and Ultrasound-Assisted Subcritical Water-Derived Tartary Buckwheat Polyphenols Show Superior Antioxidant Activity and Cytotoxicity in Human Liver Carcinoma Cells. Food Res. Int. 2020, 137, 109598. [Google Scholar] [CrossRef]
- Tsubaki, S.; Nakauchi, M.; Ozaki, Y.; Azuma, J. Microwave Heating for Solubilization of Polysaccharide and Polyphenol from Soybean Residue (Okara). Food Sci. Technol. Res. 2009, 15, 307–314. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.; Gómez-Caravaca, A.M.; Di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Hayasaka, S.; Chi, Z.-L.; Cui, H.-S.; Hayasaka, Y. Effect of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) on IL-6, IL-8, and MCP-1 Expression in Human Retinal Pigment Epithelial Cell Line. Curr. Eye Res. 2005, 30, 1105–1111. [Google Scholar] [CrossRef]
- Li, J.; Moran, T.; Swanson, E.; Julian, C.; Harris, J.; Bonen, D.K.; Hedl, M.; Nicolae, D.L.; Abraham, C.; Cho, J.H. Regulation of IL-8 and IL-1 Expression in Crohn’s Disease Associated NOD2/CARD15 Mutations. Hum. Mol. Genet. 2004, 13, 1715–1725. [Google Scholar] [CrossRef]
- Bottero, V.; Imbert, V.; Freiin, C.; Formento, J.-L.; Peyron, J.-F. Monitoring NF-ΚB Transactivation Potential via Real-Time PCR Quantification of IκB-α Gene Expression. Mol. Diagn. 2003, 7, 187–194. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
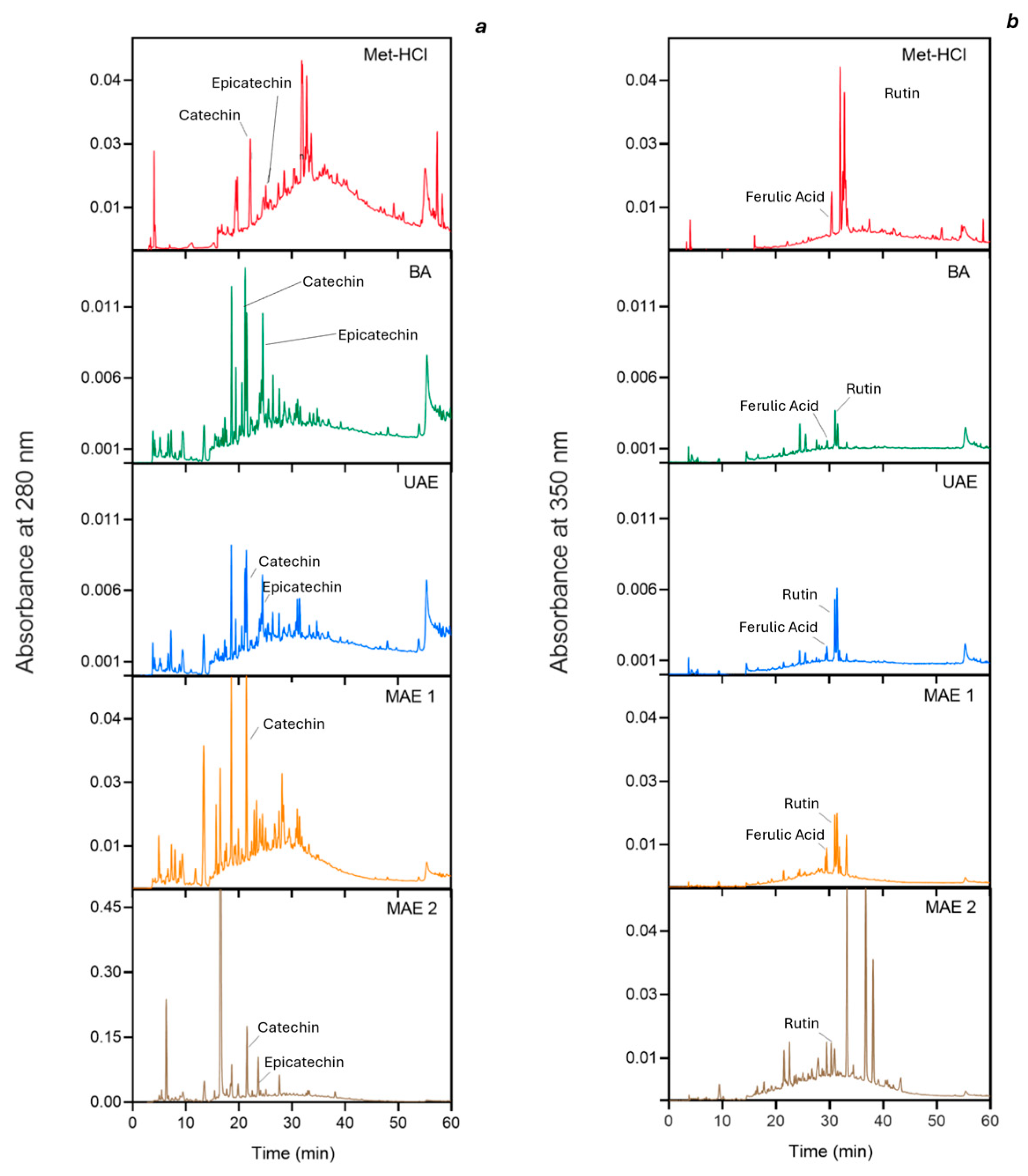
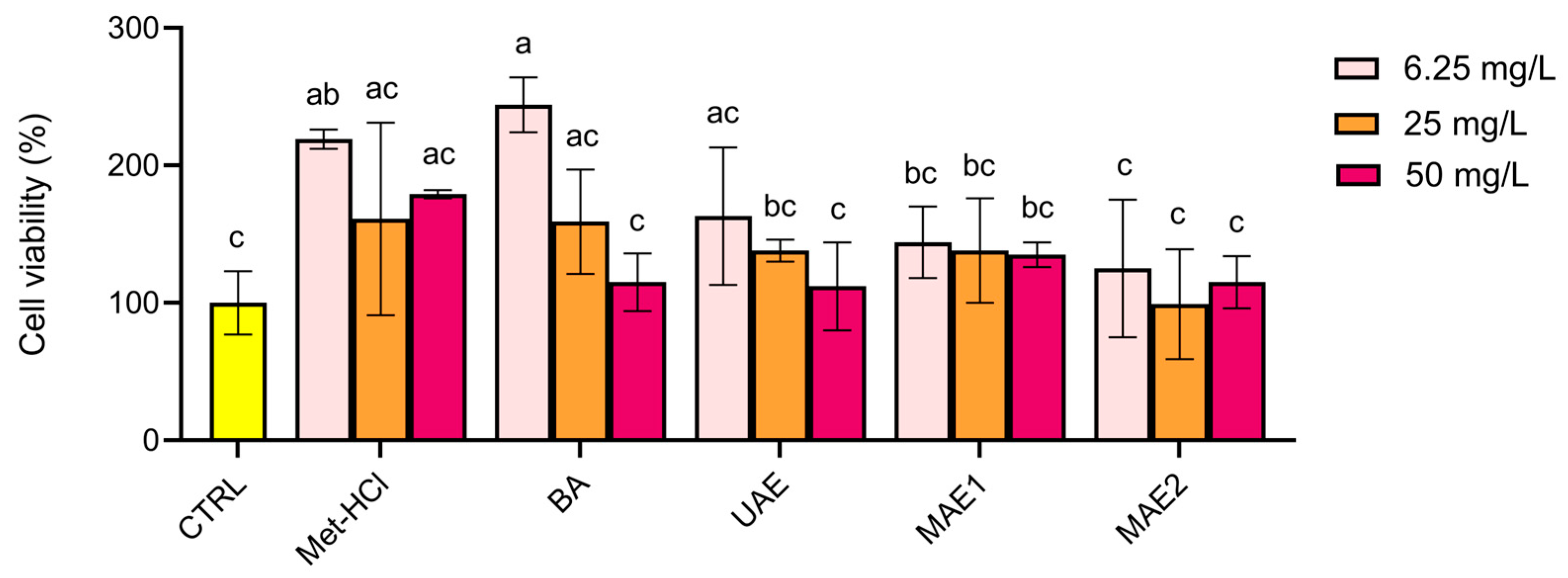
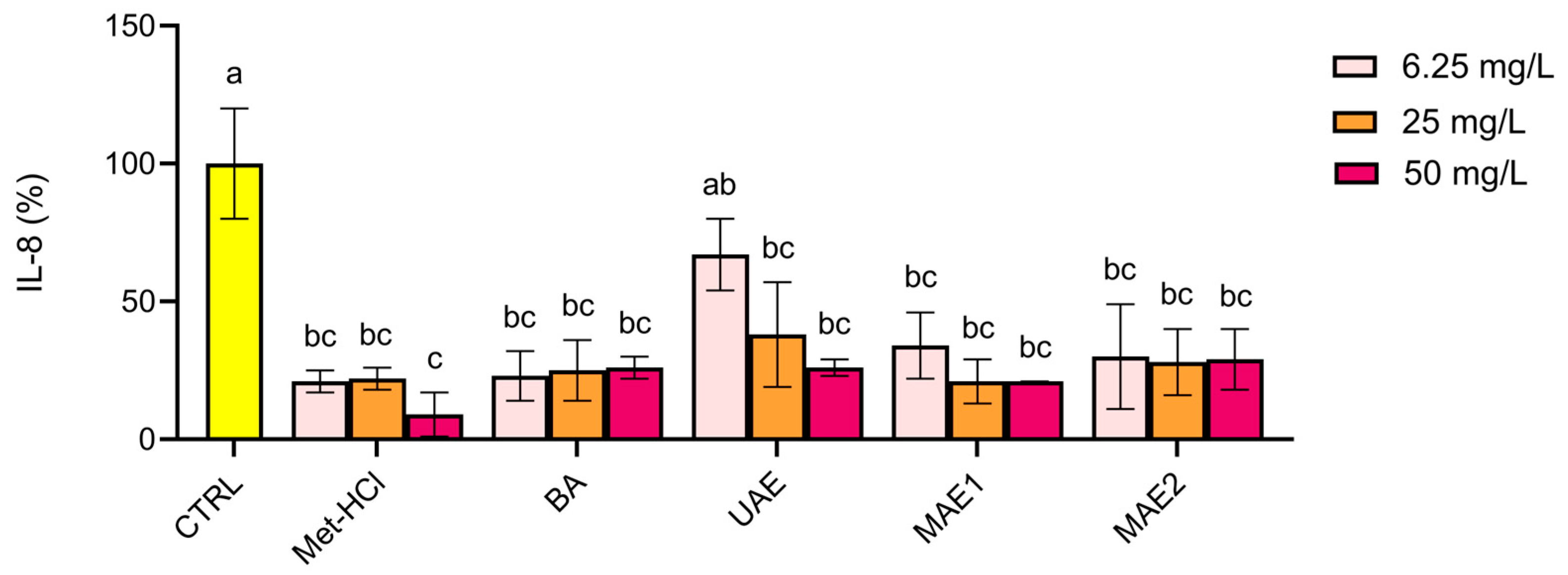
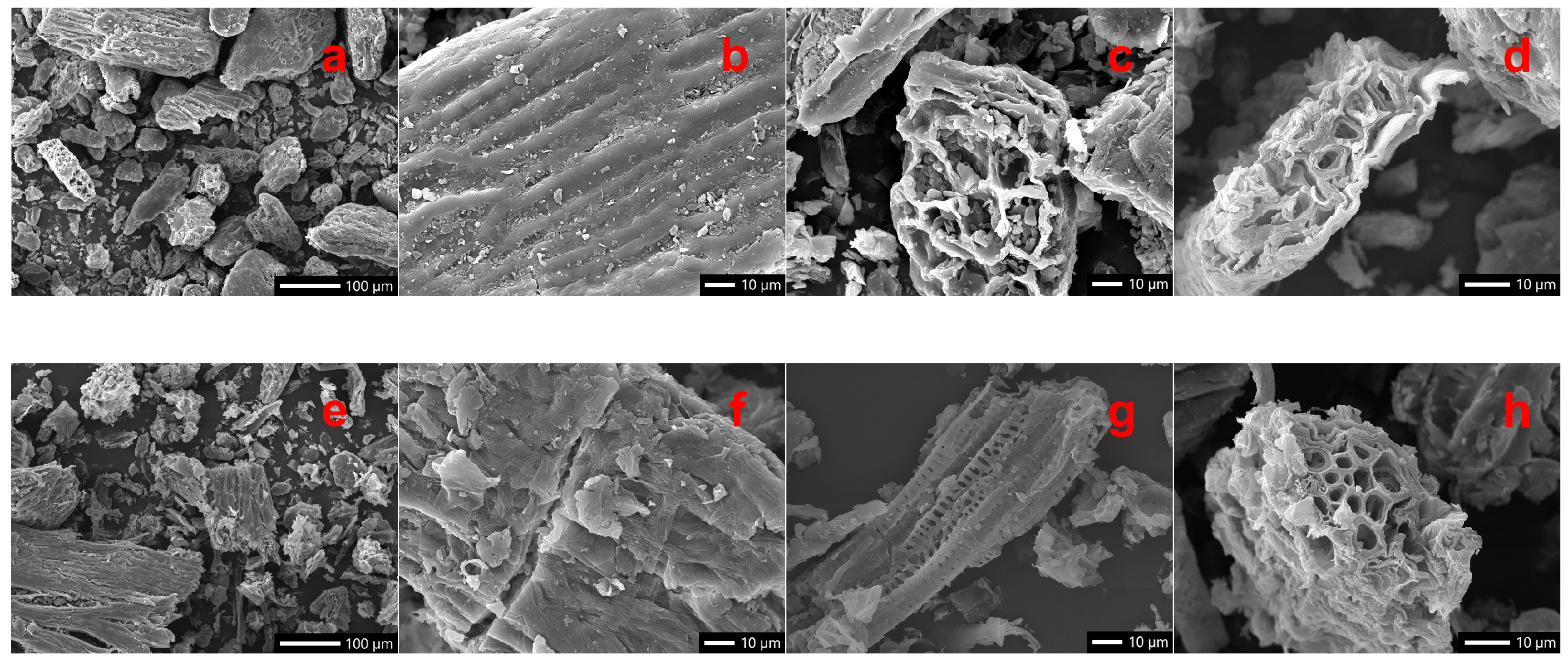
| Extraction Method | TPC (mg GAE/g) | ABTS (mmol TE/g GAE) | DPPH (mmol TE/g GAE) |
|---|---|---|---|
| Met-HCl | 9.68 ± 0.13 b | 9.00 ± 0.02 b | 8.34 ± 0.05 a |
| BA | 1.00 ± 0.01 d | 8.01 ± 0.25 c | 5.31 ± 0.04 c |
| UAE | 1.11 ± 0.02 d | 8.02 ± 0.08 c | 6.38 ± 0.03 b |
| MAE1 | 7.15 ± 0.05 c | 9.52 ± 0.17 a | 4.90 ± 0.21 c |
| MAE2 | 13.90 ± 0.09 a | 9.14 ± 0.02 b | 7.56 ± 0.63 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, A.R.; Ghidotti, F.G.; Barbiroli, A.; Scarafoni, A.; Limbo, S.; Iametti, S. Setting Up a “Green” Extraction Protocol for Bioactive Compounds in Buckwheat Husk. Int. J. Mol. Sci. 2025, 26, 7407. https://doi.org/10.3390/ijms26157407
Speranza AR, Ghidotti FG, Barbiroli A, Scarafoni A, Limbo S, Iametti S. Setting Up a “Green” Extraction Protocol for Bioactive Compounds in Buckwheat Husk. International Journal of Molecular Sciences. 2025; 26(15):7407. https://doi.org/10.3390/ijms26157407
Chicago/Turabian StyleSperanza, Anna R., Francesca G. Ghidotti, Alberto Barbiroli, Alessio Scarafoni, Sara Limbo, and Stefania Iametti. 2025. "Setting Up a “Green” Extraction Protocol for Bioactive Compounds in Buckwheat Husk" International Journal of Molecular Sciences 26, no. 15: 7407. https://doi.org/10.3390/ijms26157407
APA StyleSperanza, A. R., Ghidotti, F. G., Barbiroli, A., Scarafoni, A., Limbo, S., & Iametti, S. (2025). Setting Up a “Green” Extraction Protocol for Bioactive Compounds in Buckwheat Husk. International Journal of Molecular Sciences, 26(15), 7407. https://doi.org/10.3390/ijms26157407






