Antineoplastic Activity of Podophyllotoxin and Juniper Extracts Encapsulated in MPEG-b-PLA Diblock Copolymer Micelles in Cutaneous Squamous Carcinoma Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation, Drug Loading, and Physicochemical Characterization of Micelles
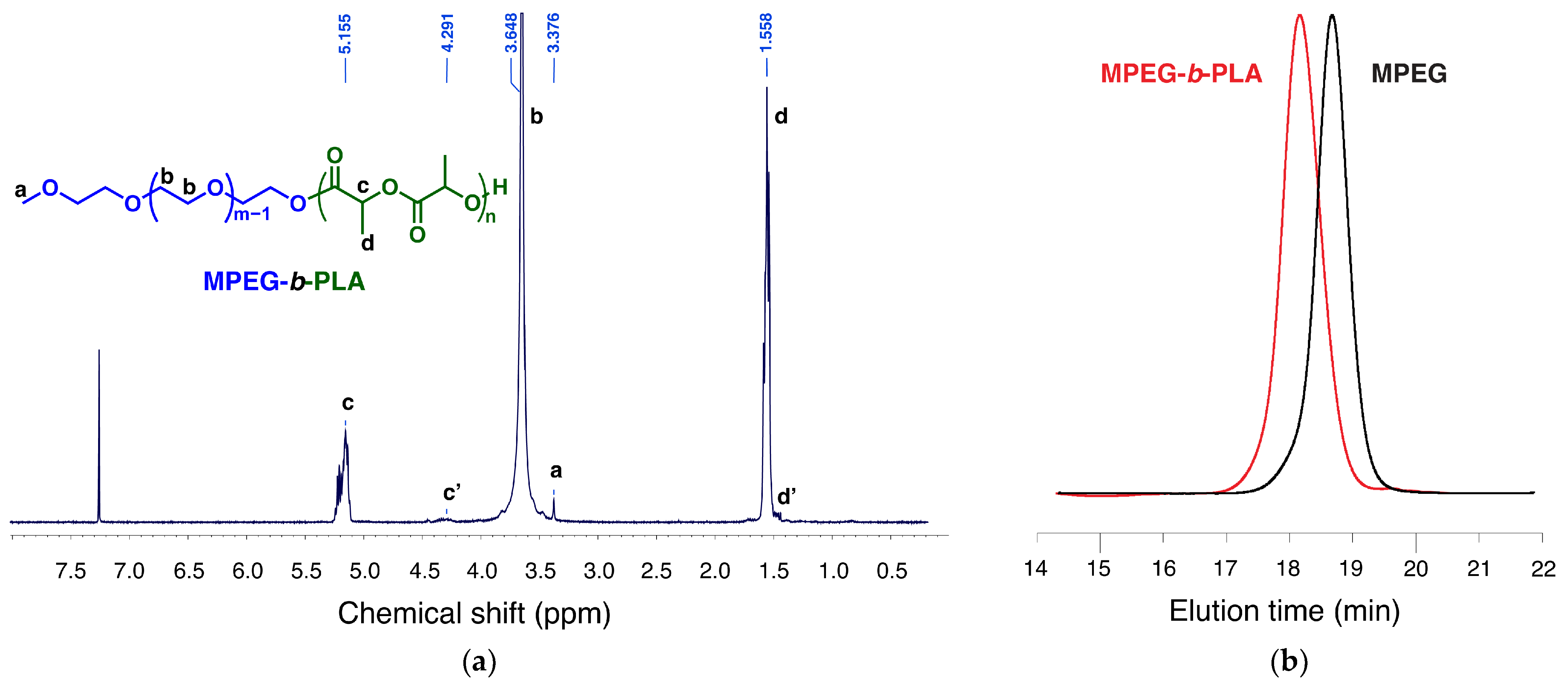
2.1.1. Copolymer Synthesis, Preparation, and Drug Loading of Micelles
2.1.2. Physicochemical Characterization of Micelles
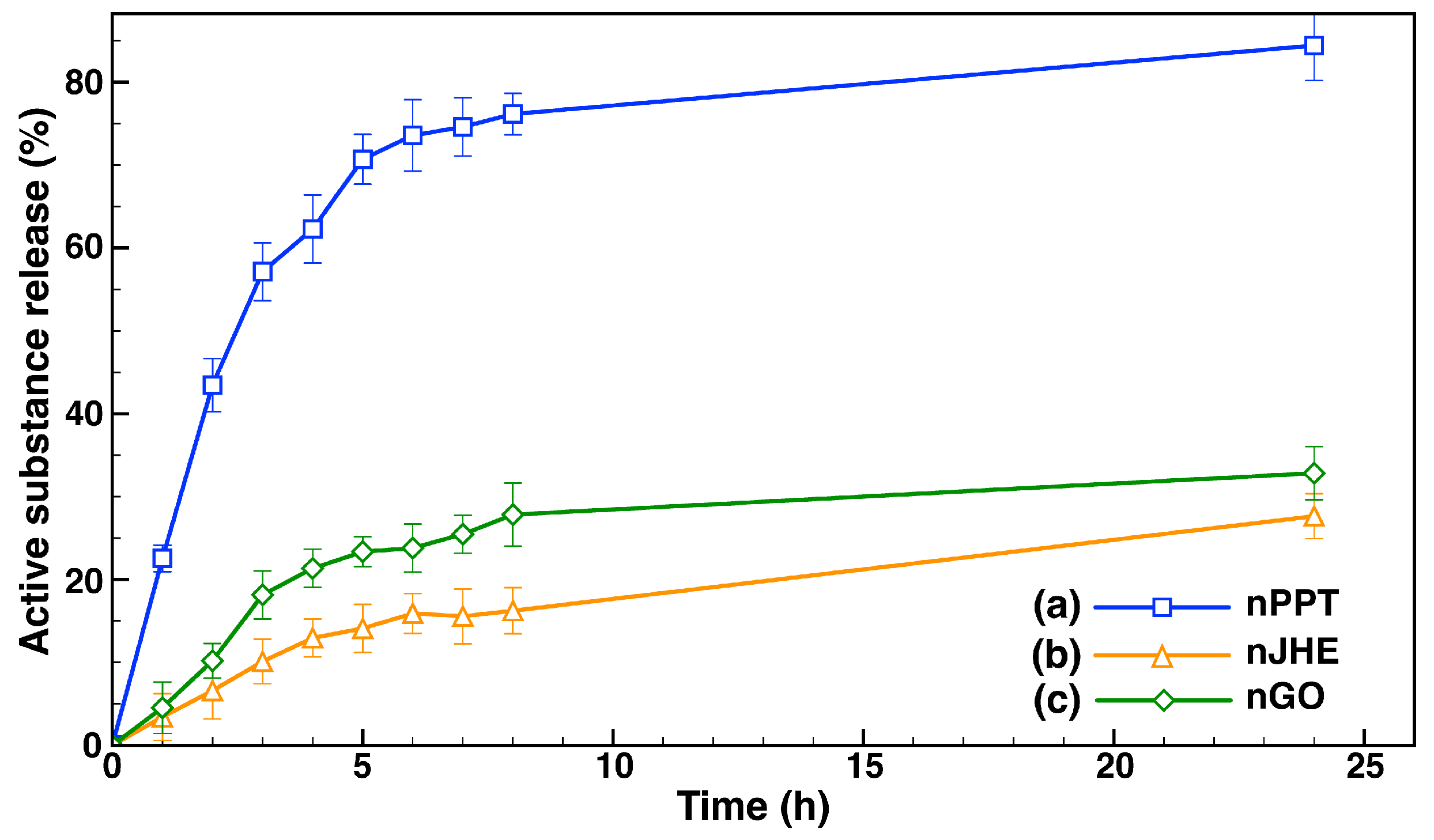
2.1.3. In Vitro Drug Release Evaluation
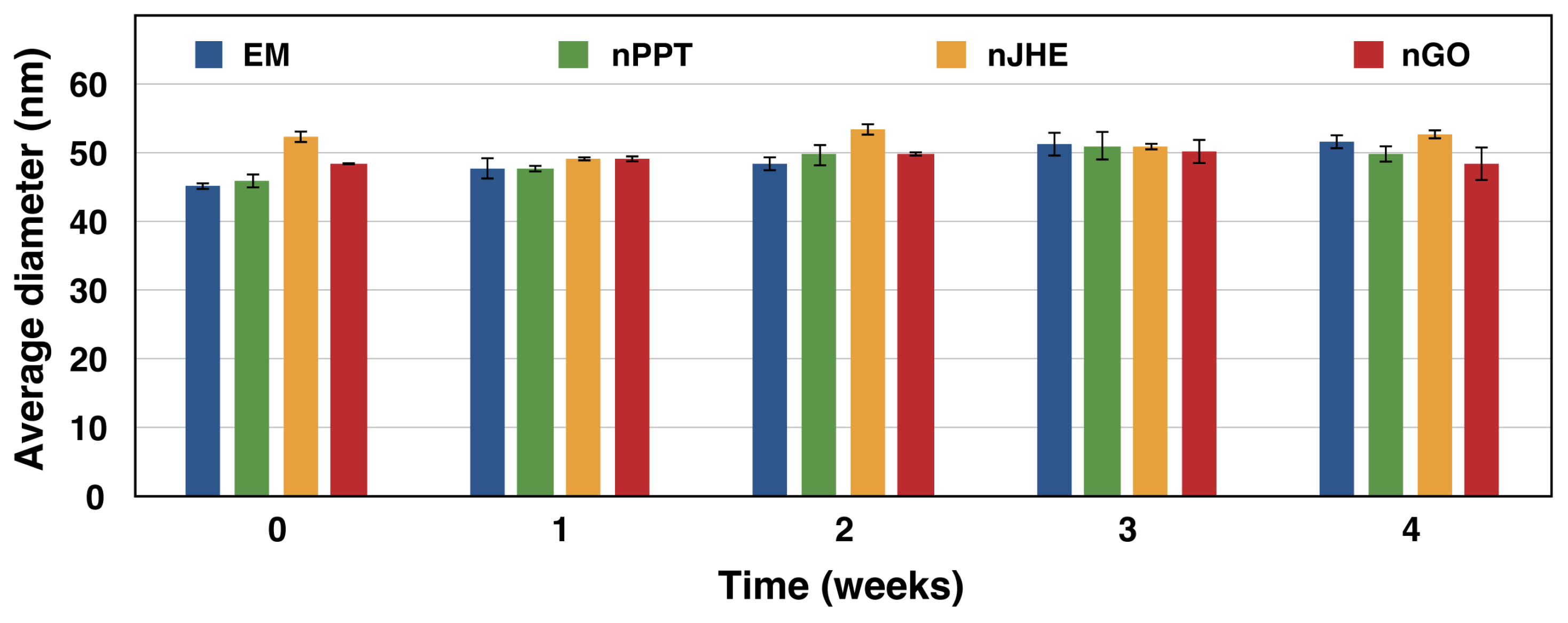
2.1.4. Stability Test
2.2. Mechanism of Antineoplastic Activity of Nanocarriers Loaded with Podophyllotoxin or Juniper Leaf Extracts
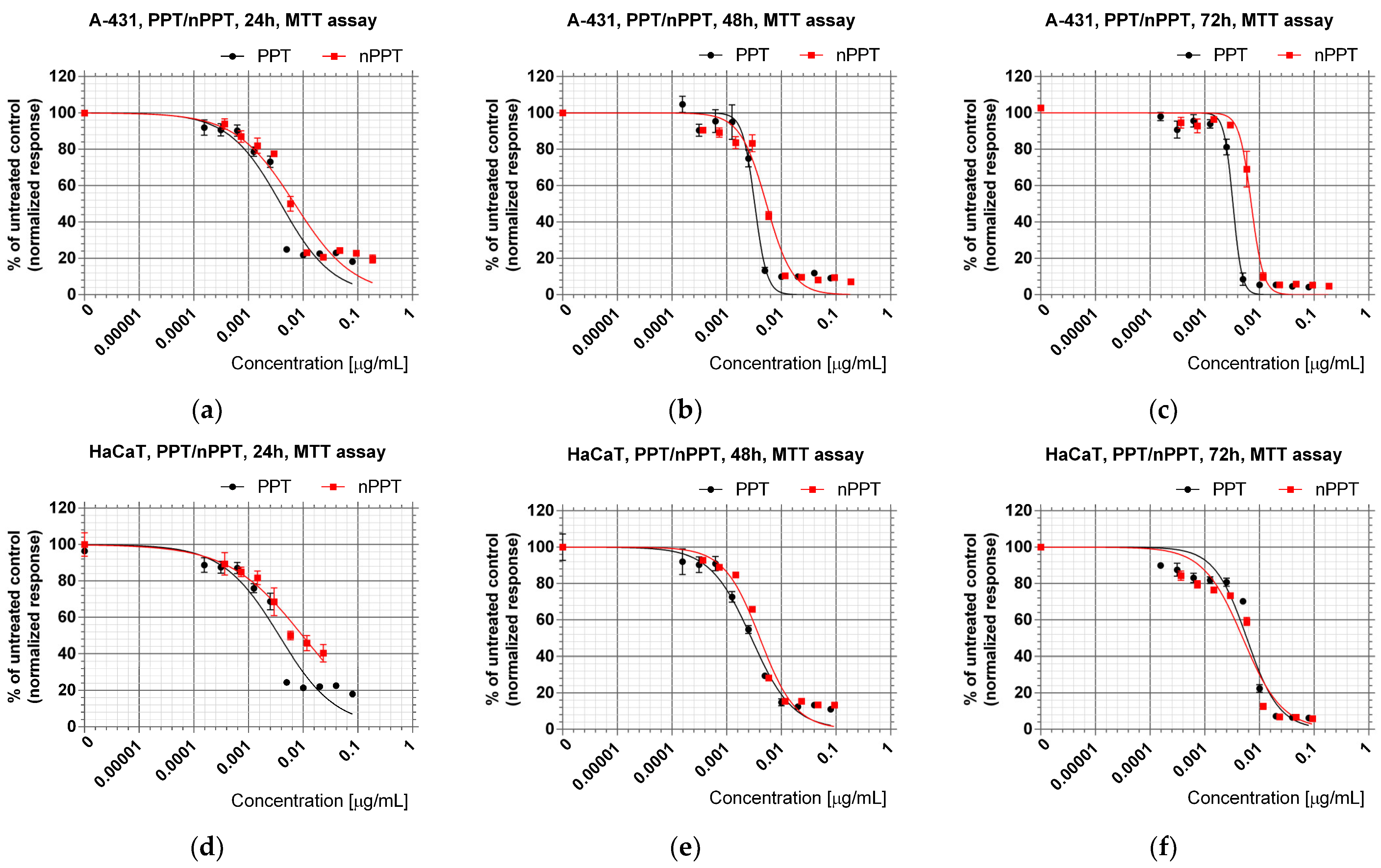
2.2.1. Cytotoxic Effects of PPT-Containing Bioactive Components Loaded in Nanocarriers
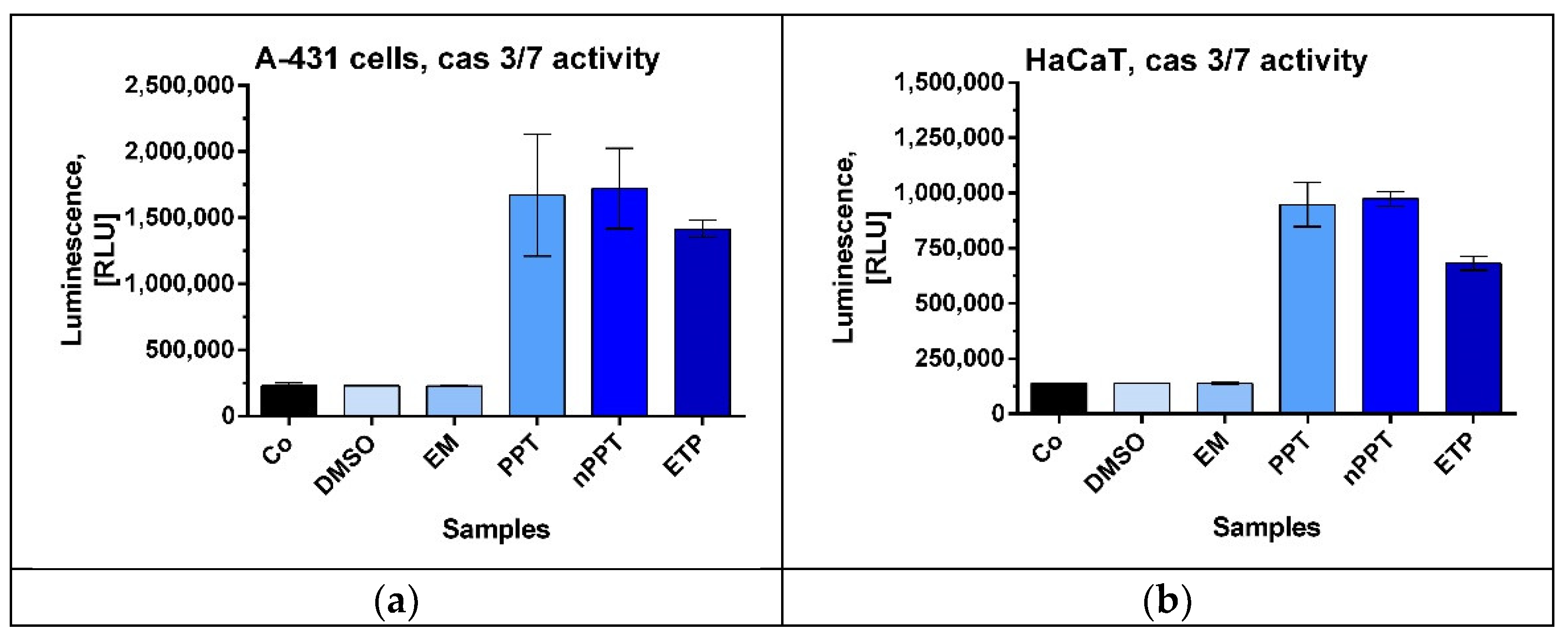
2.2.2. Activation of Caspase-3 and Caspase-7 as a Sign of Apoptosis Induction in A-431 and HaCaT Cells
2.2.3. Hoechst Assay for DNA Staining, Showing Apoptosis Induction in A-431 and HaCaT Cells Treated with PPT-Containing Bioactive Components
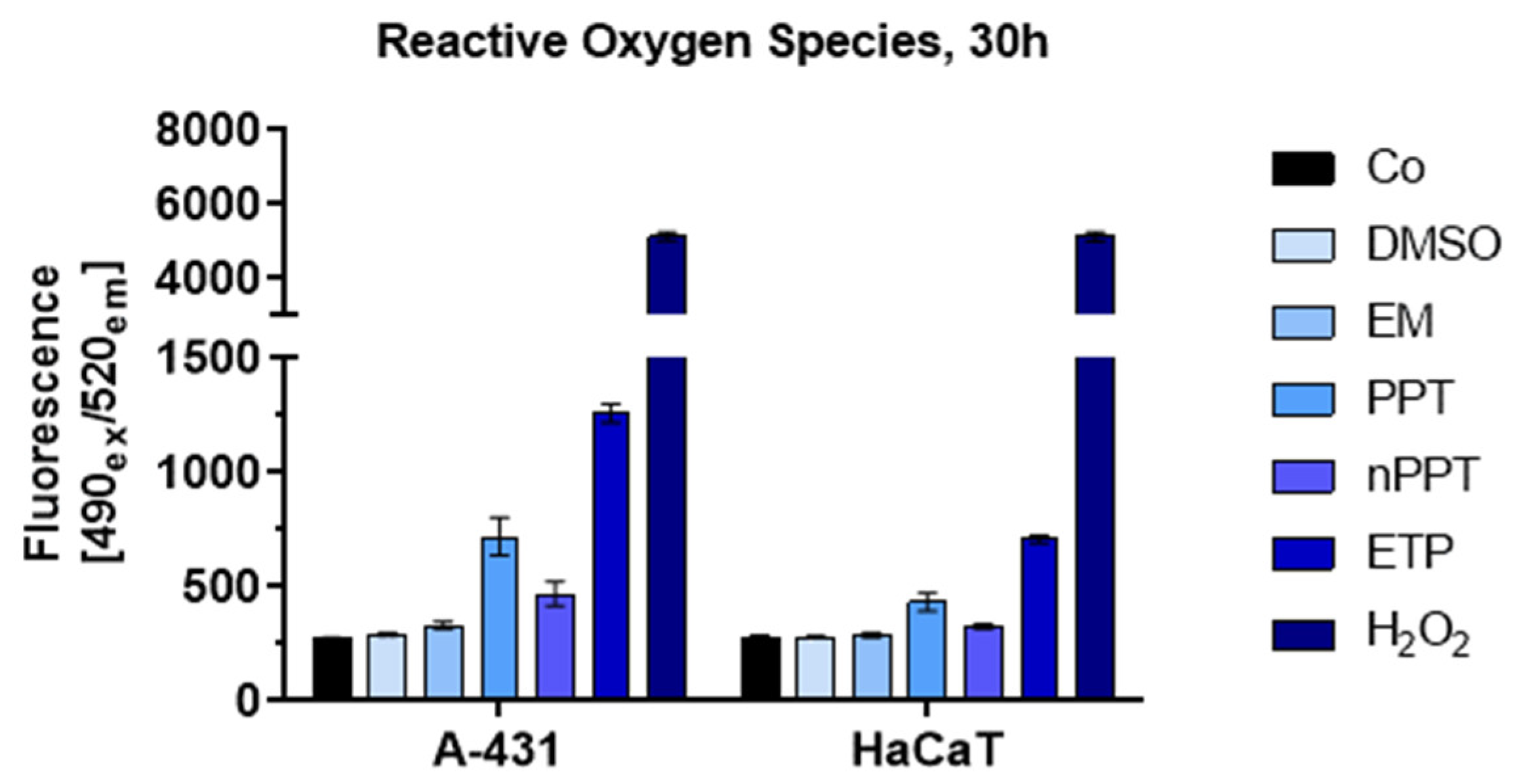
2.2.4. Rise in Intracellular Reactive Oxygen Species (ROS) Induced by PPT-Containing Bioactive Components
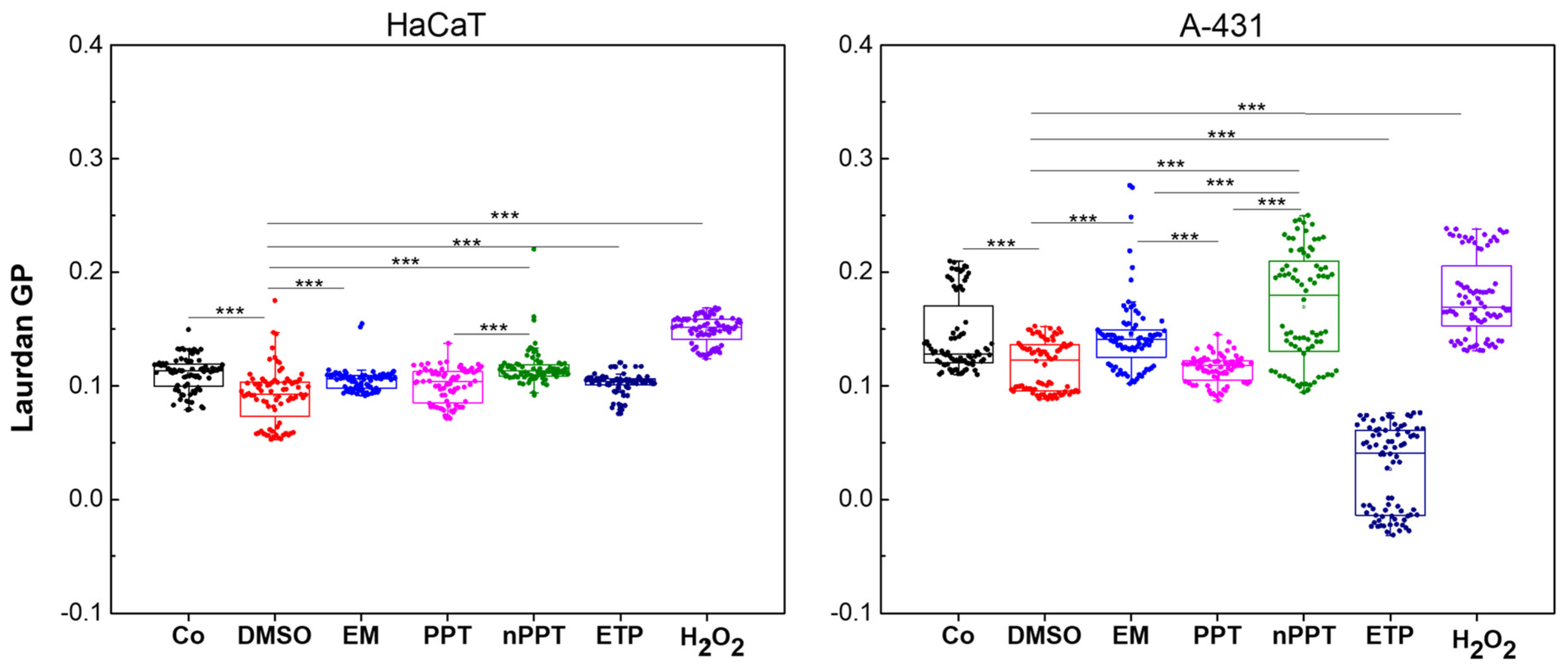
2.2.5. Membrane Lipid Order Assessment of PPT Versus nPPT in A-431 and HaCaT Cells
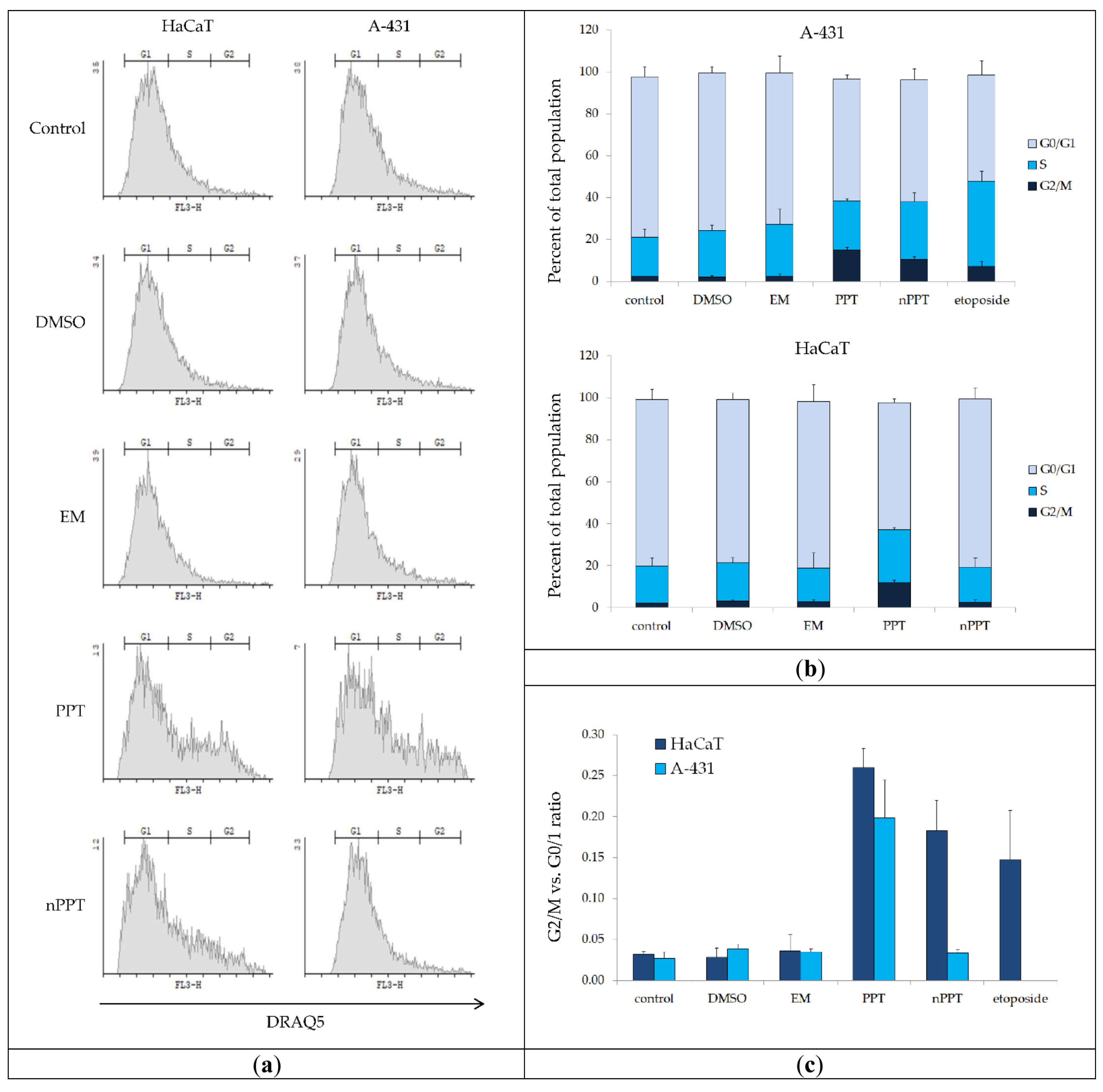
2.2.6. FACS Analysis of Cell Cycle Arrest in Treated A-431 and HaCaT Cells
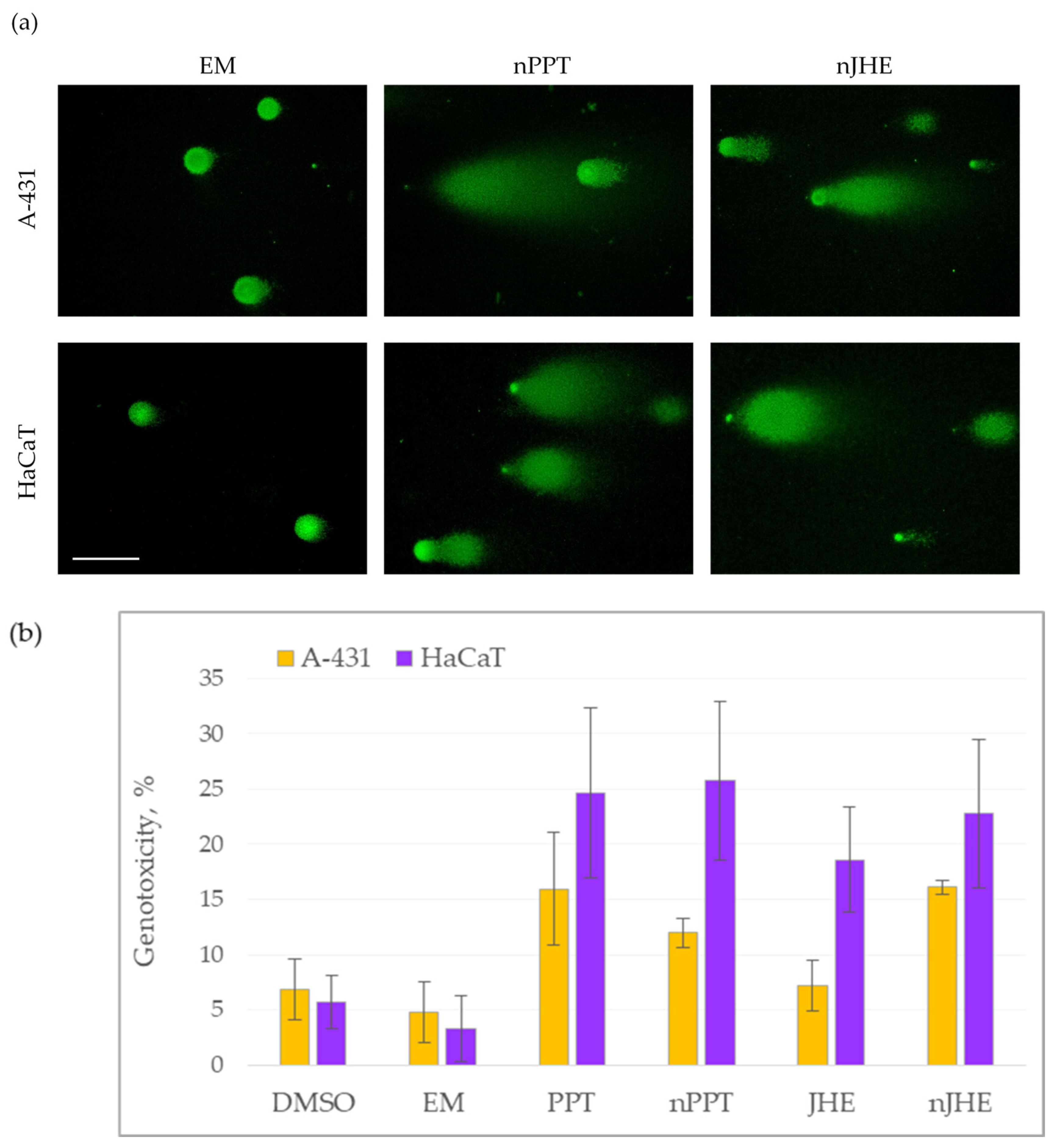
2.2.7. Measurement of Genotoxic Effects of Tested Compounds on A-431 and HaCaT Cells by Comet Assay
3. Materials and Methods
3.1. Chemicals
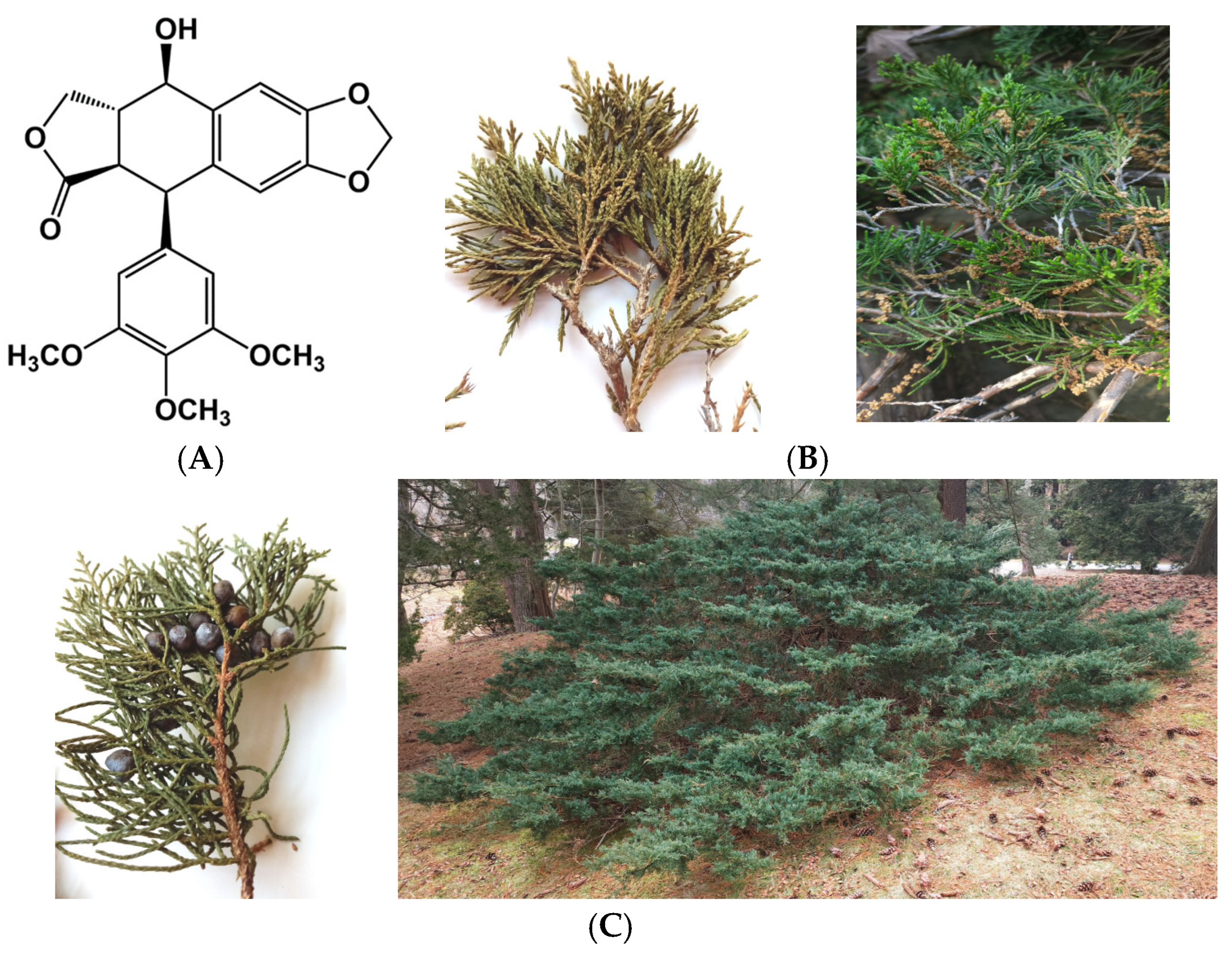
3.2. Plant Material and Preparation of Extracts
3.3. MPEG-b-PLA Block Copolymer Synthesis
3.4. Preparation of Micelles
3.5. Drug Loading of Micelles
3.6. In Vitro Drug Release Profiles
3.7. Characterization Methods
3.8. Cell Culture Conditions
3.9. MTT Assay for Antiproliferative Activity Determination
3.10. Hoechst Assay for DNA Staining During Apoptosis Induction in Cancer and Normal Cells
3.11. Caspase-3 and Caspase-7 Assays
3.12. Intracellular ROS Generation Assay
3.13. Membrane Lipid Order Assessment
3.14. Fluorescence-Activated Cell Sorting (FACS) for Cell Cycle Analysis
3.15. Comet Assay for DNA Fragmentation Detection
3.16. Data Processing and Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deep, A.; Kumar, D.; Bansal, N.; Narasimhan, B.; Marwaha, R.K.; Prabodh Chander Sharma, P.C. Understanding mechanistic aspects and therapeutic potential of natural substances as anticancer agents. Phytomed. Plus 2023, 3, 100418. [Google Scholar] [CrossRef]
- Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-UI-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef]
- Singh, A.; Choudhary, R.; Ganguly, S. Podophyllin in Dermatology: Revisiting a Historical Drug. Indian. Dermatol. Online J. 2022, 13, 167–171. [Google Scholar] [CrossRef]
- Ivanova, D.; Nedialkov, P.; Tashev, A.; Olech, M.; Nowak, R.; Ilieva, Y.; Kokanova-Nedialkova, Z.; Atanasova, T.; Angelov, G.; Najdenski, H. Junipers of Various Origins as Potential Sources of the Anticancer Drug Precursor Podophyllotoxin. Molecules 2021, 26, 5179. [Google Scholar] [CrossRef]
- Xu, Y.; He, Z.; Chen, L.; Wang, H. Podophyllotoxin derivatives targeting tubulin: An update (2017–2022). Drug Discov. Today 2023, 28, 103640. [Google Scholar] [CrossRef]
- Choi, J.Y.; Hong, W.G.; Cho, J.H.; Kim, E.M.; Kim, J.; Jung, C.; Hwang, S.; Um, H.; Park, J.K. Podophyllotoxin acetate triggers anticancer effects against non-small cell lung cancer cells by promoting cell death via cell cycle arrest, ER stress and autophagy. Int. J. Oncol. 2015, 47, 1257–1265. [Google Scholar] [CrossRef]
- Yoon, G.; Lee, M.-H.; Kwak, A.-W.; Oh, H.-N.; Cho, S.-S.; Choi, J.-S.; Liu, K.; Chae, J.-I.; Shim, J.-H. Podophyllotoxin Isolated from Podophyllum peltatum Induces G2/M Phase Arrest and Mitochondrial-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma Cells. Forests 2020, 11, 8. [Google Scholar] [CrossRef]
- Lee, S.O.; Joo, S.H.; Kwak, A.W.; Lee, M.H.; Seo, J.H.; Cho, S.S.; Yoon, G.; Chae, J.I.; Shim, J.H. Podophyllotoxin Induces ROS-Mediated Apoptosis and Cell Cycle Arrest in Human Colorectal Cancer Cells via pMAPK Signaling. Biomol. Ther. 2021, 29, 658–666. [Google Scholar] [CrossRef]
- Miranda-Vera, C.; Hernández, Á.P.; García-García, P.; Díez, D.; García, P.A.; Castro, M.Á. Podophyllotoxin: Recent Advances in the Development of Hybridization Strategies to Enhance Its Antitumoral Profile. Pharmaceutics 2023, 15, 2728. [Google Scholar] [CrossRef]
- Motyka, S.; Jafernik, K.; Ekiert, H.; Sharifi-Rad, J.; Calina, D.; Al-Omari, B.; Szopa, A.; Cho, W.C. Podophyllotoxin and its derivatives: Potential anticancer agents of natural origin in cancer chemotherapy. Biomed. Pharmacother. 2023, 158, 114145. [Google Scholar] [CrossRef]
- Fan, H.Y.; Zhu, Z.L.; Xian, H.C.; Wang, H.F.; Chen, B.J.; Tang, Y.J.; Tang, Y.L.; Liang, X.H. Insight Into the Molecular Mechanism of Podophyllotoxin Derivatives as Anticancer Drugs. Front. Cell Dev. Biol. 2021, 9, 709075. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Biswasroy, P.; Sahu, A.; Sahu, D.K.; Ghosh, G.; Rath, G. Recent advances in herbal nanomedicines for cancer treatment. Curr. Mol. Pharmacol. 2021, 14, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Upaganlawar, A.; Polshettiwar, S.; Raut, S.; Tagalpallewar, A.; Pande, V. Effective Cancer Management: Inimitable Role of Phytochemical Based Nano-Formulations. Curr. Drug Metab. 2022, 23, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Pavan Kumar, V.; Harikrishnan, N. Nano-Phytoconstituents and its recent advancement in Anticancer efficacy. Res. J. Pharm. Technol. 2023, 16, 447–452. [Google Scholar] [CrossRef]
- Fatima, M.; Iqubal, M.K.; Iqubal, A.; Kaur, H.; Gilani, S.J.; Rahman, M.H.; Ahmadi, A.; Rizwanullah, M. Current Insight into the Therapeutic Potential of Phytocompounds and their Nanoparticle-Based Systems for Effective Management of Lung Cancer. Anticancer Agents Med. Chem. 2022, 22, 668–686. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Sakate, A.M.; Patil, O.B.; Manjappa, A.S.; Disouza, J.I. Podophyllotoxin-polyacrylic acid conjugate micelles: Improved anticancer efficacy against multidrug-resistant breast cancer. J. Egypt. Natl. Canc. Inst. 2020, 32, 42. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, S.; Zhang, D.; Deng, M.; Zhang, X.; Tang, Z.; Chen, X. A polypeptide based podophyllotoxin conjugate for the treatment of multi drug resistant breast cancer with enhanced efficiency and minimal toxicity. Acta Biomater. 2018, 73, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Shen, H.P.; Huang, X.; Jiang, X.H.; Jin, C.S.; Chu, Z.M. Effect of Podophyllotoxin Conjugated Stearic Acid Grafted Chitosan Oligosaccharide Micelle on Human Glioma Cells. J. Korean Neurosurg. Soc. 2020, 63, 698–706. [Google Scholar] [CrossRef]
- Xiang, C.; Fu, Y.; Hao, T.; Wei, L.; Liu, Y.; Fan, Z.C.; Guo, N.; Yu, P.; Teng, Y.O. Podophyllotoxin-loaded PEGylated E-selectin peptide conjugate targeted cancer site to enhance tumor inhibition and reduce side effect. Eur. J. Med. Chem. 2023, 260, 115780. [Google Scholar] [CrossRef]
- Zhu, K.J.; Lin, X.Z.; Yang, S.L. Preparation, characterization and properties of polylactide-poly(ethylene glycol) copolymers: A potential drug carrier. J. Appl. Polym. Sci. 1990, 39, 1–9. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soliman, G.M.; Al-Hajaj, N.; Sharma, R.; Maysinger, D.; Kakkar, A. Design and evaluation of multifunctional nanocarriers for selective delivery of coenzyme Q10 tomitochondria. Biomacromolecules 2011, 13, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, C.X.; Li, X.C.; Yuan, Z.; An, Y.L.; He, B.L. Biodegradable polylactide/poly(ethylene glycol)/polylactidetriblock copolymer micelles as anticancer drug carriers. J. Appl.Polym. Sci. 2001, 80, 1976–1982. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.D. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J.; Casey, L. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry 2002, 41, 10315–10322. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Karunanithi Nivedita, A.; Shoshan-Barmatz, V. Etoposide-induced cancer cell death: Roles of mitochondrial VDAC1 and calpain, and resistance mechanisms. Mol. Oncol. 2025. Epub ahead of print. [Google Scholar] [CrossRef]
- Liu, X.; Xie, X.; Ren, Y.; Shao, Z.; Zhang, N.; Li, L.; Ding, X.; Zhang, L. The role of necroptosis in disease and treatment. MedComm (2020) 2021, 2, 730–755. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef]
- Katsuki, Y.; Jeggo, P.A.; Uchihara, Y.; Takata, M.; Shibata, A. DNA double-strand break end resection: A critical relay point for determining the pathway of repair and signaling. Genome Instab. Dis. 2020, 1, 155–171. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Liu, L.; Zheng, C.; Wang, Y.; Chena, Y.; Wei, G. Podophyllotoxin–pterostilbene fused conjugates as potential multifunctional antineoplastic agents against human uveal melanoma cells. RSC Adv. 2017, 7, 10601–10608. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Staversky, R.J.; Finkelstein, J.N.; Keng, P.C. Activation of the G2 cell cycle checkpoint enhances survival of epithelial cells exposed to hyperoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L368–L375. [Google Scholar] [CrossRef] [PubMed]
- Merlino, G.T.; Xu, Y.H.; Ishii, S.; Clark, A.J.; Semba, K.; Toyoshima, K.; Yamamoto, T.; Pastan, I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science 1984, 224, 417–419. [Google Scholar] [CrossRef]
- Ge, G.; Wu, J.; Lin, Q. Effect of membrane fluidity on tyrosine kinase activity of reconstituted epidermal growth factor receptor. Biochem. Biophys. Res. Commun. 2001, 282, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Chitkara, D.; Mittal, A. Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers. Acta Pharm. Sin. B 2021, 11, 903–924. [Google Scholar] [CrossRef]
- Ruzzi, F.; Cappello, C.; Semprini, M.S.; Scalambra, L.; Angelicola, S.; Pittino, O.M.; Landuzzi, L.; Palladini, A.; Nanni, P.; Lollini, P.L. Lipid rafts, caveolae, and epidermal growth factor receptor family: Friends or foes? Cell Commun. Signal 2024, 22, 489. [Google Scholar] [CrossRef]
- Mineo, C.; Gill, G.N.; Anderson, R.G. Regulated migration of epidermal growth factor receptor from caveolae. J. Biol. Chem. 1999, 274, 30636–30643. [Google Scholar] [CrossRef]
- Busse, D.; Doughty, R.S.; Ramsey, T.T.; Russell, W.E.; Price, J.O.; Flanagan, W.M.; Shawver, L.K.; Arteaga, C.L. Reversible G(1) arrest induced by inhibition of the epidermal growth factor receptor tyrosine kinase requires up-regulation of p27(KIP1) independent of MAPK activity. J. Biol. Chem. 2000, 275, 6987–6995. [Google Scholar] [CrossRef]
- Tian, R.; Li, Y.; Gao, M. Shikonin causes cell-cycle arrest and induces apoptosis by regulating the EGFR-NF-κB signalling pathway in human epidermoid carcinoma A431 cells. Biosci. Rep. 2015, 35, e00189. [Google Scholar] [CrossRef]
- Shi, R.J.; Fan, H.Y.; Yu, X.H.; Tang, Y.L.; Jiang, J.; Liang, X.H. Advances of podophyllotoxin and its derivatives: Patterns and mechanisms. Biochem. Pharmacol. 2022, 200, 115039. [Google Scholar] [CrossRef]
- Olive, P.; Banáth, J. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Peycheva, E.; Georgieva, M.; Miloshev, G. Comparison Between Alkaline and Neutral Variants of Yeast Comet Assay. Biotechnol. Biotechnol. Equip. 2009, 23, 1090–1092. [Google Scholar] [CrossRef]
- Staneva, D.; Dimitrova, N.; Popov, B.; Alexandrova, A.; Georgieva, M.; Miloshev, G. Haberlea rhodopensis Extract Tunes the Cellular Response to Stress by Modulating DNA Damage, Redox Components, and Gene Expression. Int. J. Mol. Sci. 2023, 24, 15964. [Google Scholar] [CrossRef]
- Ilieva, Y.; Dimitrova, L.; Zaharieva, M.; Kaleva, M.; Alov, P.; Tsakovska, I.; Pencheva, T.; Pencheva-El Tibi, I.; Najdenski, H.; Pajeva, I. Cytotoxicity and Microbicidal Activity of Commonly Used Organic Solvents: A Comparative Study and Application to a Standardized Extract from Vaccinium macrocarpon. Toxics 2021, 9, 92. [Google Scholar] [CrossRef]
- Kalinova, R.G.; Dimitrov, I.V.; Ivanova, D.I.; Ilieva, Y.E.; Tashev, A.N.; Zaharieva, M.M.; Angelov, G.; Najdenski, H.M. Polycarbonate-Based Copolymer Micelles as Biodegradable Carriers of Anticancer Podophyllotoxin or Juniper Extracts. J. Funct. Biomater. 2024, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.L.; Luk, A.; Castagnola, J.; Cronin, M.; Mendelsohn, J. EGF induces cell cycle arrest of A431 human epidermoid carcinoma cells. J. Cell. Physiol. 1986, 127, 175–182. [Google Scholar] [CrossRef]
- Kim, H.S.; Oh, H.; Kwak, A.; Kim, E.; Lee, M.; Seo, J.; Cho, S.; Yoon, G.; Chae, J.; Shim, J. Deoxypodophyllotoxin Inhibits Cell Growth and Induces Apoptosis by Blocking EGFR and MET in Gefitinib-Resistant Non-Small Cell Lung Cancer. J. Microbiol. Biotechnol. 2021, 31, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.N.; Kwak, A.W.; Lee, M.H.; Kim, E.; Yoon, G.; Cho, S.S.; Liu, K.; Chae, J.I.; Shim, J.H. Targeted inhibition of c-MET by podophyllotoxin promotes caspase-dependent apoptosis and suppresses cell growth in gefitinib-resistant non-small cell lung cancer cells. Phytomedicine 2021, 80, 153355. [Google Scholar] [CrossRef]
- Wang, Z. Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef]
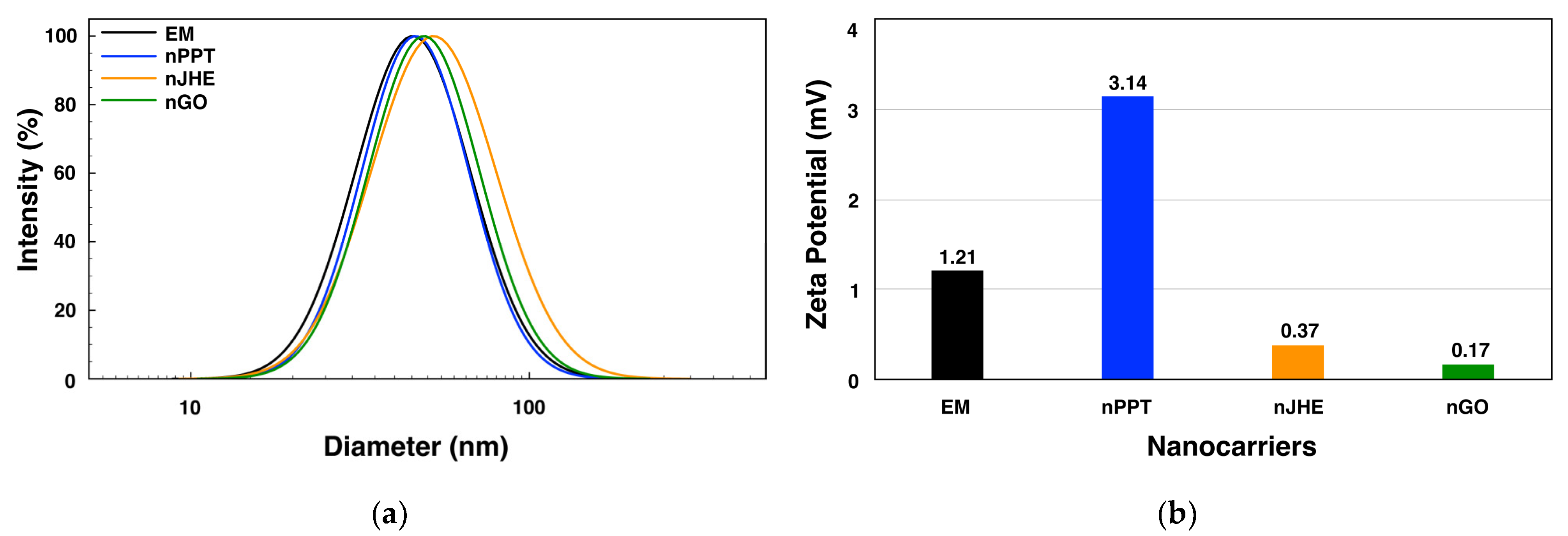
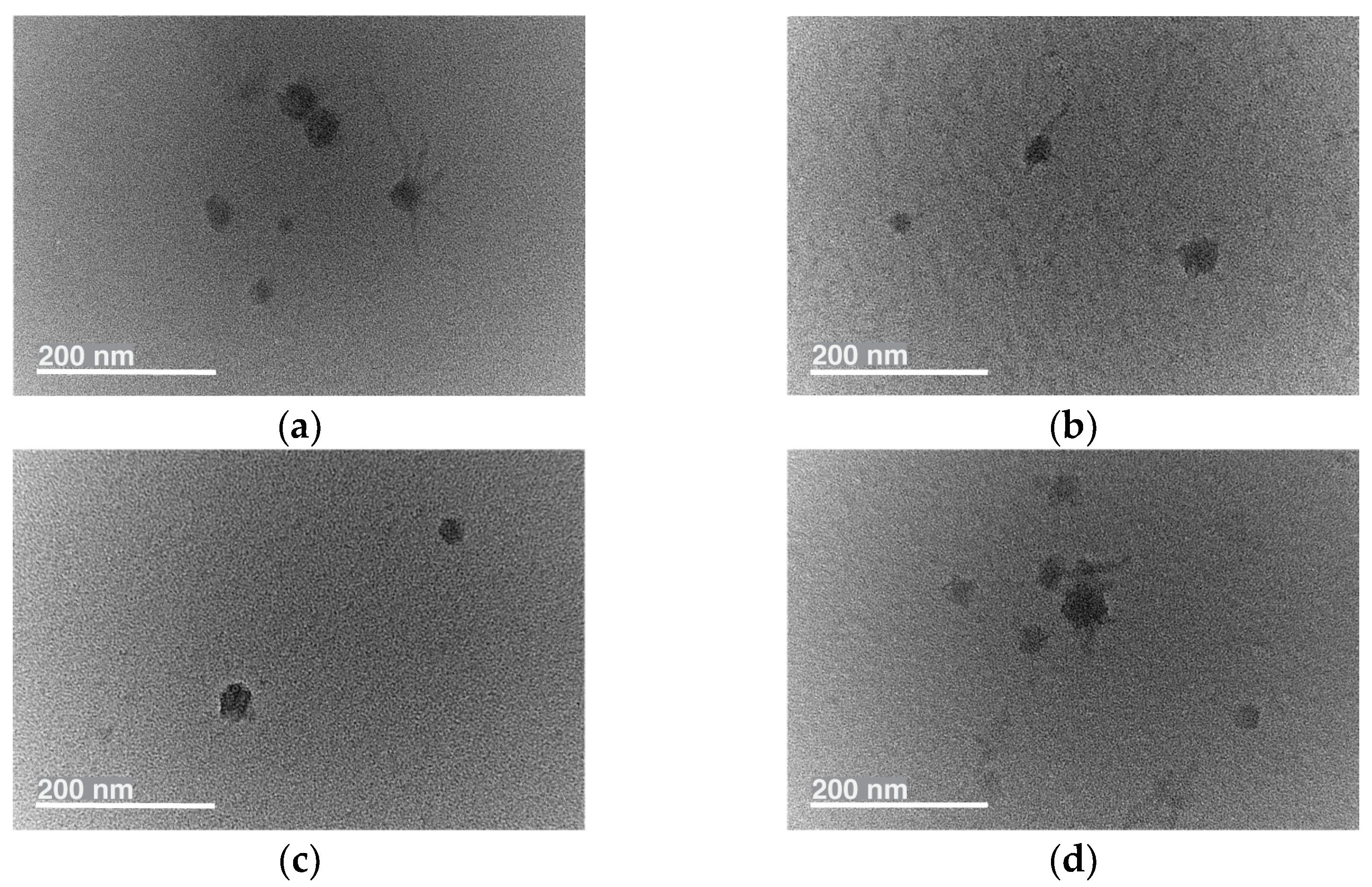
| Code | d a (nm) | PdIa | ζ a (mV) | EE b (%) | LC b (%) |
|---|---|---|---|---|---|
| EM | 45.45± 0.61 | 0.169 | 1.21± 3.40 | - | - |
| nPPT | 46.00 ± 1.06 | 0.145 | 3.14 ± 3.79 | 98 | 8.9 |
| nJHE | 51.69 ± 0.93 | 0.205 | 0.37 ± 1.91 | 74 | 7.4 |
| nGO | 48.68 ± 0.16 | 0.166 | 0.17 ± 0.65 | 83 | 8.3 |
| Cell Lines and Parameters | Treatment Time of the Cell Lines with the Corresponding Bioactive Components | |||||
|---|---|---|---|---|---|---|
| A-431 Parameter | PPT | nPPT | ||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Hillslope | −0.9109 | −3.904 | −5.357 | −0.8062 | −1.810 | −3.941 |
| R2 | 0.9173 | 0.9641 | 0.9872 | 0.9225 | 0.9668 | 0.9844 |
| IC50 [µg/mL] | 0.003904 | 0.003250 | 0.003272 | 0.007020 | 0.005323 | 0.007071 |
| CI 95% | 0.003131–0.004868 | 0.002971–0.003555 | 0.003069–0.003490 | 0.005682–0.008673 | 0.004648–0.006095 | 0.006663–0.007504 |
| A-431 Parameter | JHE | nJHE | ||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Hillslope | −4.147 | −5.815 | −6.145 | −2.090 | −1.948 | −4.518 |
| R2 | 0.9393 | 0.9659 | 0.9774 | 0.9386 | 0.9298 | 0.9665 |
| IC50 [µg/mL] | 0.7649 | 0.6909 | 0.7419 | 0.5012 | 0.3895 | 0.4612 |
| CI 95% | 0.6904–0.8442 | 0.6218–0.7617 | 0.6822–0.8211 | 0.4375–0.5712 | 0.3282–0.4550 | 0.4291–0.4971 |
| HaCaT Parameter | PPT | nPPT | ||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Hillslope | −0.6353 | −1.138 | −2.232 | −0.6441 | −1.322 | −1.156 |
| R2 | 0.9641 | 0.9728 | 0.9418 | 0.9348 | 0.9573 | 0.9364 |
| IC50 [µg/mL] | 0.006400 | 0.002831 | 0.005868 | 0.009696 | 0.004048 | 0.004963 |
| CI 95% | 0.005588–0.007330 | 0.002520–0.003181 | 0.004896–0.007032 | 0.007945–0.01183 | 0.003530–0.004641 | 0.004167–0.005910 |
| HaCaT Parameter | JHE | nJHE | ||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Hillslope | −0.7868 | −1.407 | −2.101 | −0.7532 | −1.374 | −1.264 |
| R2 | 0.9090 | 0.9790 | 0.9587 | 0.9395 | 0.9537 | 0.9398 |
| IC50 [µg/mL] | 0.9809 | 0.2724 | 0.3145 | 0.4451 | 0.1597 | 0.1686 |
| CI 95% | 0.7972–1.228 | 0.2479–0.2992 | 0.2793–0.3524 | 0.3745–0.5318 | 0.1361–0.1858 | 0.1407–0.2002 |
| EM [μg/mL] | A-431 Epidermoid Carcinoma % Viable Cells | HaCaT Normal Keratinocytes % Viable Cells | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 1 | 89 | 84 | 93 | 100 | 96 | 85 |
| 5 | 85 | 83 | 92 | 87 | 84 | 78 |
| 10 | 86 | 82 | 88 | 85 | 77 | 67 |
| 20 | 72 | 75 | 83 | 79 | 72 | 62 |
| 40 | 71 | 67 | 66 | 64 | 64 | 51 |
| Sample | HaCaT | A-431 |
|---|---|---|
| Untreated control cells |  | 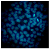 |
| DMSO |  |  |
| EM, 5 μg/mL |  | 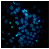 |
| JHE, 0.4 μg/mL |  | 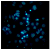 |
| nJHE, 0.4 μg/mL |  |  |
| PPT, 0.006 μg/mL | 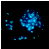 | 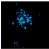 |
| nPPT, 0.006 μg/mL |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinova, R.G.; Dimitrov, I.V.; Ilieva, Y.; Iliev, D.B.; Miloshev, G.A.; Staneva, D.N.; Zaharieva, M.M.; Nesheva, A.; Staneva, G.; Ivanova, D.I.; et al. Antineoplastic Activity of Podophyllotoxin and Juniper Extracts Encapsulated in MPEG-b-PLA Diblock Copolymer Micelles in Cutaneous Squamous Carcinoma Cells. Int. J. Mol. Sci. 2025, 26, 5167. https://doi.org/10.3390/ijms26115167
Kalinova RG, Dimitrov IV, Ilieva Y, Iliev DB, Miloshev GA, Staneva DN, Zaharieva MM, Nesheva A, Staneva G, Ivanova DI, et al. Antineoplastic Activity of Podophyllotoxin and Juniper Extracts Encapsulated in MPEG-b-PLA Diblock Copolymer Micelles in Cutaneous Squamous Carcinoma Cells. International Journal of Molecular Sciences. 2025; 26(11):5167. https://doi.org/10.3390/ijms26115167
Chicago/Turabian StyleKalinova, Radostina G., Ivaylo V. Dimitrov, Yana Ilieva, Dimitar B. Iliev, George A. Miloshev, Dessislava N. Staneva, Maya M. Zaharieva, Aleksandrina Nesheva, Galya Staneva, Diana I. Ivanova, and et al. 2025. "Antineoplastic Activity of Podophyllotoxin and Juniper Extracts Encapsulated in MPEG-b-PLA Diblock Copolymer Micelles in Cutaneous Squamous Carcinoma Cells" International Journal of Molecular Sciences 26, no. 11: 5167. https://doi.org/10.3390/ijms26115167
APA StyleKalinova, R. G., Dimitrov, I. V., Ilieva, Y., Iliev, D. B., Miloshev, G. A., Staneva, D. N., Zaharieva, M. M., Nesheva, A., Staneva, G., Ivanova, D. I., Angelov, G., & Najdenski, H. M. (2025). Antineoplastic Activity of Podophyllotoxin and Juniper Extracts Encapsulated in MPEG-b-PLA Diblock Copolymer Micelles in Cutaneous Squamous Carcinoma Cells. International Journal of Molecular Sciences, 26(11), 5167. https://doi.org/10.3390/ijms26115167











