Different Mechanisms in Doxorubicin-Induced Neurotoxicity: Impact of BRCA Mutations
Abstract
:1. Introduction
2. Breast Cancer Genes 1 and 2
2.1. BRCA1/2 in DSB Repair
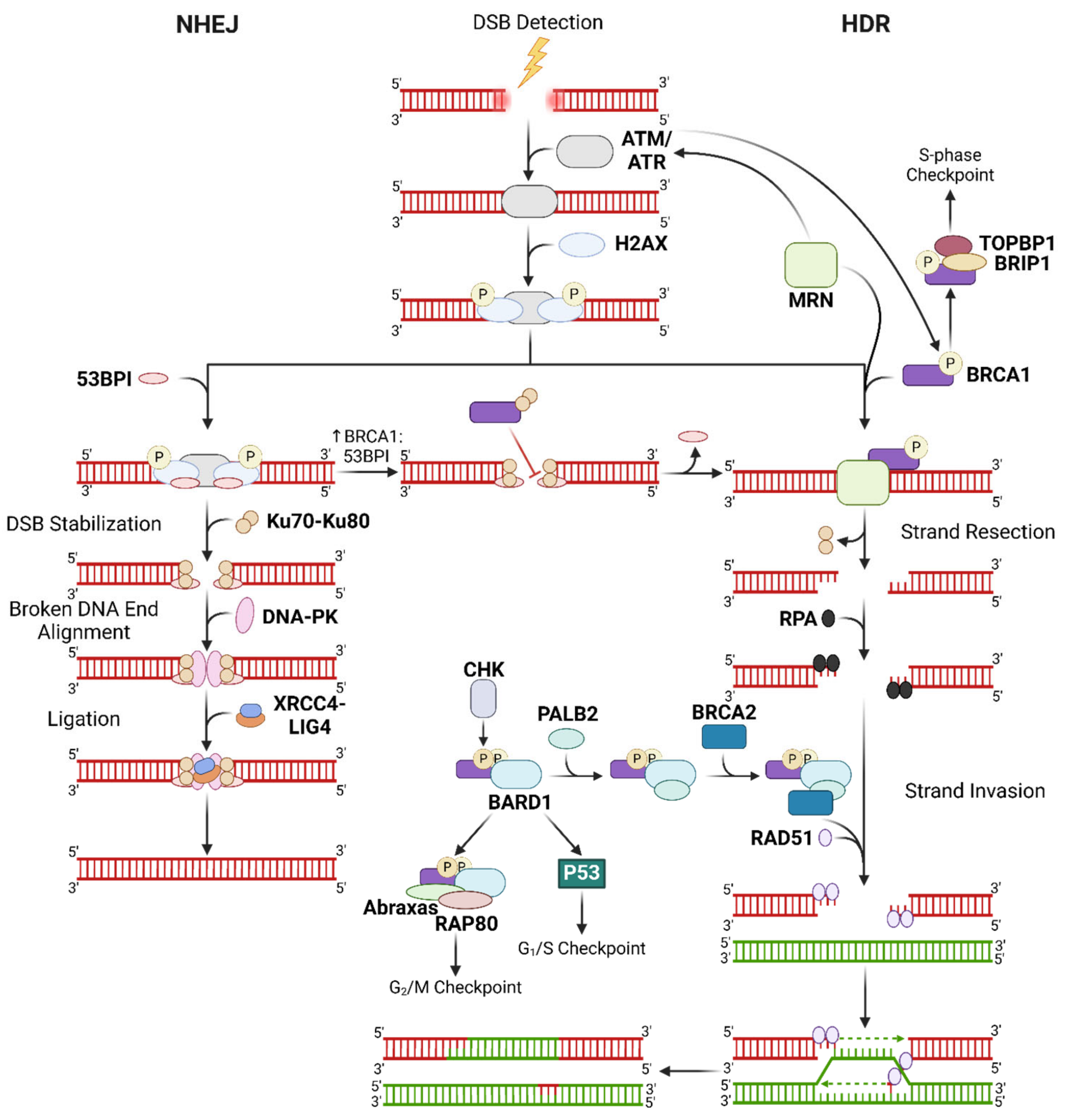
2.1.1. Mechanisms of DSB Repair
2.1.2. Role of BRCA1/2 in Modulating Oxidative Stress and Cellular Stress Reponses
3. Mechanisms of Direct DIN
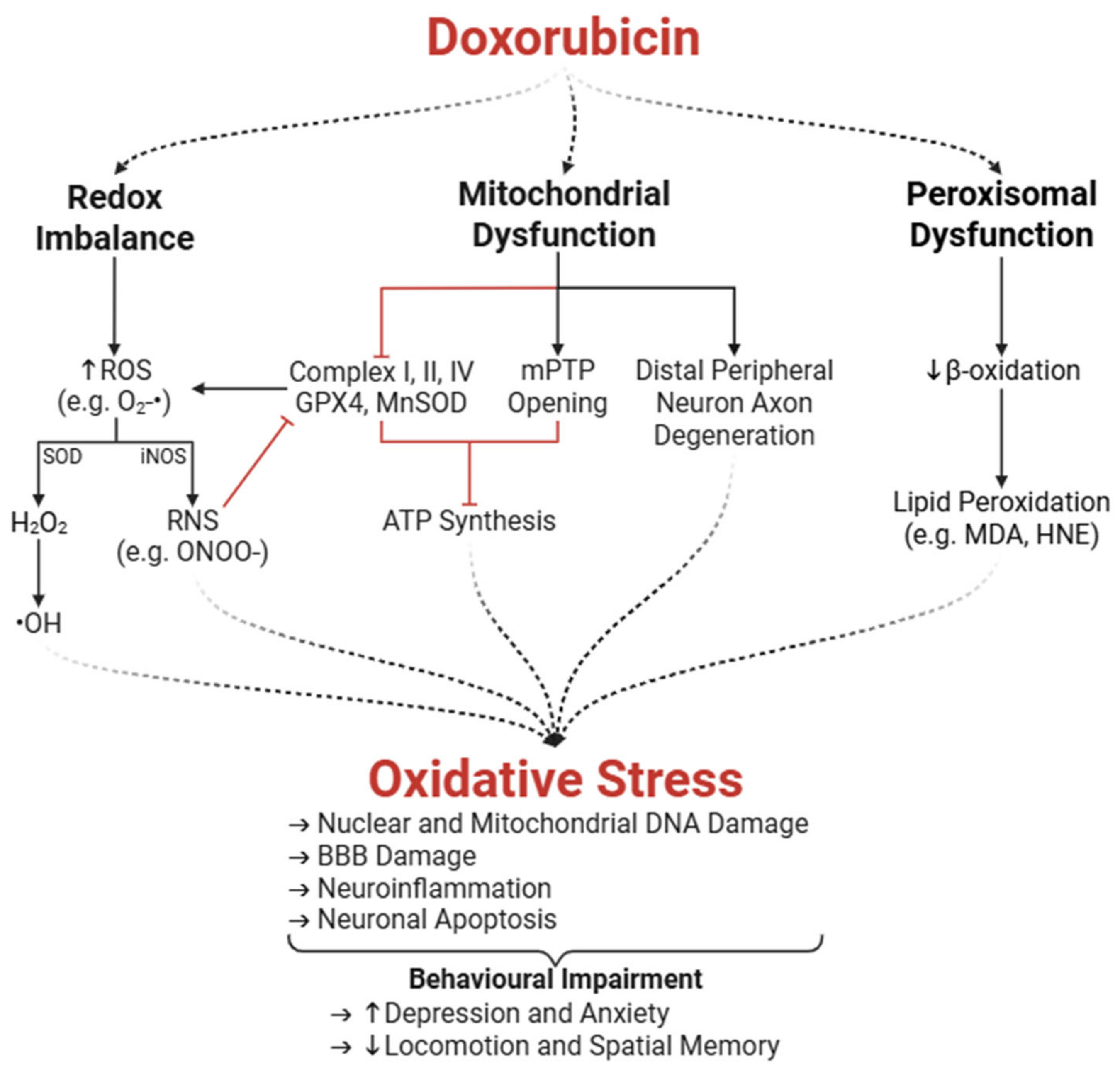
3.1. Oxidative Stress
3.1.1. Enzymatic Amplification of ROS and Redox Imbalance
3.1.2. Peroxisomal and Mitochondrial Dysfunction
3.1.3. Mitochondrial Dysfunction in the Peripheral Nervous System
3.1.4. Neurobehavioural Impairments Associated with Oxidative Stress
3.1.5. Therapeutic Interventions to Mitigate DIN-Associated Oxidative Stress
3.2. Impaired Neurogenesis and Altered Neural Cell Morphology
3.2.1. Dox-Mediated Impaired Neurogenesis
3.2.2. Dox-Induced Structural and Morphological Changes to Brain Tissue
3.3. Impaired Neurotransmitter Regulation
3.3.1. Dysregulation of Neurotransmitter Systems
3.3.2. Synaptic Dysplasia
3.4. Autophagic Dysregulation
3.4.1. Dox-Induced Autophagic Dysregulation in the Brain
3.4.2. Prospective Therapeutic Interventions
4. Mechanisms of Indirect DIN
4.1. Neuroinflammation
4.2. Impaired Metabolism
4.2.1. Impaired Cholesterol Metabolism and Apolipoprotein A-I Dysfunction
4.2.2. Impaired Amino Acid Metabolism
5. Endotheliotoxicity and BRCA1/2 in Cancer and Neurotoxicity
5.1. Endotheliotoxicity
5.1.1. Endothelial Dysfunction
5.1.2. Dox-Induced Endothelial Dysfunction, Endotheliotoxicity, and Neurotoxicity
5.2. DDR Impairments, Cancer, and Neurodegeneration
5.3. BRCA Mutation Screening, Doxorubicin, and Neurotoxicity
5.4. BRCA Mutations in DIN and Endotheliotoxicity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ongnok, B.; Khuanjing, T.; Chunchai, T.; Pantiya, P.; Kerdphoo, S.; Arunsak, B.; Nawara, W.; Jaiwongkam, T.; Apaijai, N.; Chattipakorn, N.; et al. Donepezil Protects Against Doxorubicin-Induced Chemobrain in Rats via Attenuation of Inflammation and Oxidative Stress Without Interfering With Doxorubicin Efficacy. Neurotherapeutics 2021, 18, 2107–2125. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Saupe, S.; Jäger, E.; Schmidt, M.; Kreienberg, R.; Müller, L.; Otremba, B.J.; Waldenmaier, D.; Dorn, J.; Warm, M.; et al. A Randomized Phase III Study Evaluating Pegylated Liposomal Doxorubicin versus Capecitabine as First-Line Therapy for Metastatic Breast Cancer: Results of the PELICAN Study. Breast Cancer Res. Treat. 2017, 161, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Cecere, S.C.; Di Napoli, M.; Cavaliere, C.; Tambaro, R.; Facchini, G.; Scaffa, C.; Losito, S.; Pizzolorusso, A.; Pignata, S. Clinical Trials with Pegylated Liposomal Doxorubicin in the Treatment of Ovarian Cancer. J. Drug Deliv. 2013, 2013, 898146. [Google Scholar] [CrossRef] [PubMed]
- Fukuokaya, W.; Kimura, T.; Miki, J.; Kimura, S.; Watanabe, H.; Bo, F.; Okada, D.; Aikawa, K.; Ochi, A.; Suzuki, K.; et al. Effectiveness of Intravesical Doxorubicin Immediately Following Resection of Primary Non-Muscle-Invasive Bladder Cancer: A Propensity Score-Matched Analysis. Clin. Genitourin. Cancer 2020, 18, e55–e61. [Google Scholar] [CrossRef]
- Al-Gallab, M.I.; Naddaf, L.A.; Kanan, M.R. The Management of Non-Invasive Bladder Tumours with Doxorubicin Intravesical Instillation after Transurethral Resection. Sultan Qaboos Univ. Med. J. 2009, 9, 53–58. [Google Scholar] [CrossRef]
- Cho, H.J.; Sim, N.S.; Shin, S.-J.; Yun, K.-H.; Lee, Y.H.; Jun, H.J.; Rha, S.Y.; Kim, H.S. Phase IB/II Trial of Durvalumab plus Doxorubicin Combination in Patients with Advanced Soft-Tissue Sarcoma. J. Clin. Oncol. 2024, 42, 11552. [Google Scholar] [CrossRef]
- Pisters, P.W.T.; Patel, S.R.; Prieto, V.G.; Thall, P.F.; Lewis, V.O.; Feig, B.W.; Hunt, K.K.; Yasko, A.W.; Lin, P.P.; Jacobson, M.G.; et al. Phase I Trial of Preoperative Doxorubicin-Based Concurrent Chemoradiation and Surgical Resection for Localized Extremity and Body Wall Soft Tissue Sarcomas. J. Clin. Oncol. 2004, 22, 3375–3380. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Walters, R.S.; Keating, M.J.; Smith, T.L.; O’Brien, S.; Estey, E.H.; Huh, Y.O.; Spinolo, J.; Dicke, K.; Barlogie, B. Results of the Vincristine, Doxorubicin, and Dexamethasone Regimen in Adults with Standard- and High-Risk Acute Lymphocytic Leukemia. J. Clin. Oncol. 1990, 8, 994–1004. [Google Scholar] [CrossRef]
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and Other Anthracyclines in Cancers: Activity, Chemoresistance and Its Overcoming. Mol. Asp. Med. 2023, 93, 101205. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA Torsion, and Chromatin Dynamics. Biochim. Biophys. Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Frisbee, J.C.; Singh, K.K. Different Mechanisms in Doxorubicin-Induced Cardiomyopathy: Impact of BRCA1 and BRCA2 Mutations. Hearts 2024, 5, 54–74. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different Roles in a Common Pathway of Genome Protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef]
- Incorvaia, L.; Badalamenti, G.; Novo, G.; Gori, S.; Cortesi, L.; Brando, C.; Cinieri, S.; Curigliano, G.; Ricciardi, G.R.; Toss, A.; et al. Anthracycline-Related Cardiotoxicity in Patients with Breast Cancer Harboring Mutational Signature of Homologous Recombination Deficiency (HRD). ESMO Open 2024, 9, 102196. [Google Scholar] [CrossRef]
- Singh, K.K.; Shukla, P.C.; Quan, A.; Al-Omran, M.; Lovren, F.; Pan, Y.; Brezden-Masley, C.; Ingram, A.J.; Stanford, W.L.; Teoh, H.; et al. BRCA1 Is a Novel Target to Improve Endothelial Dysfunction and Retard Atherosclerosis. J. Thorac. Cardiovasc. Surg. 2013, 146, 949–960.e4. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.C.; Singh, K.K.; Quan, A.; Al-Omran, M.; Teoh, H.; Lovren, F.; Cao, L.; Rovira, I.I.; Pan, Y.; Brezden-Masley, C.; et al. BRCA1 Is an Essential Regulator of Heart Function and Survival Following Myocardial Infarction. Nat. Commun. 2011, 2, 593. [Google Scholar] [CrossRef]
- Singh, K.K.; Shukla, P.C.; Quan, A.; Desjardins, J.-F.; Lovren, F.; Pan, Y.; Garg, V.; Gosal, S.; Garg, A.; Szmitko, P.E.; et al. BRCA2 Protein Deficiency Exaggerates Doxorubicin-Induced Cardiomyocyte Apoptosis and Cardiac Failure. J. Biol. Chem. 2012, 287, 6604–6614. [Google Scholar] [CrossRef]
- Bhinge, K.N.; Gupta, V.; Hosain, S.B.; Satyanarayanajois, S.D.; Meyer, S.A.; Blaylock, B.; Zhang, Q.-J.; Liu, Y.-Y. The Opposite Effects of Doxorubicin on Bone Marrow Stem Cells versus Breast Cancer Stem Cells Depend on Glucosylceramide Synthase. Int. J. Biochem. Cell Biol. 2012, 44, 1770–1778. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-Induced Cognitive Impairment: The Mechanistic Insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef] [PubMed]
- Ongnok, B.; Chattipakorn, N.; Chattipakorn, S.C. Doxorubicin and Cisplatin Induced Cognitive Impairment: The Possible Mechanisms and Interventions. Exp. Neurol. 2020, 324, 113118. [Google Scholar] [CrossRef]
- Berndt, U.; Leplow, B.; Schoenfeld, R.; Lantzsch, T.; Grosse, R.; Thomssen, C. Memory and Spatial Cognition in Breast Cancer Patients Undergoing Adjuvant Endocrine Therapy. Breast Care 2016, 11, 240–246. [Google Scholar] [CrossRef]
- Barzilai, A.; Biton, S.; Shiloh, Y. The Role of the DNA Damage Response in Neuronal Development, Organization and Maintenance. DNA Repair 2008, 7, 1010–1027. [Google Scholar] [CrossRef] [PubMed]
- Bigotte, L.; Arvidson, B.; Olsson, Y. Cytofluorescence Localization of Adriamycin in the Nervous System. I. Distribution of the Drug in the Central Nervous System of Normal Adult Mice after Intravenous Injection. Acta Neuropathol. 1982, 57, 121–129. [Google Scholar] [CrossRef] [PubMed]
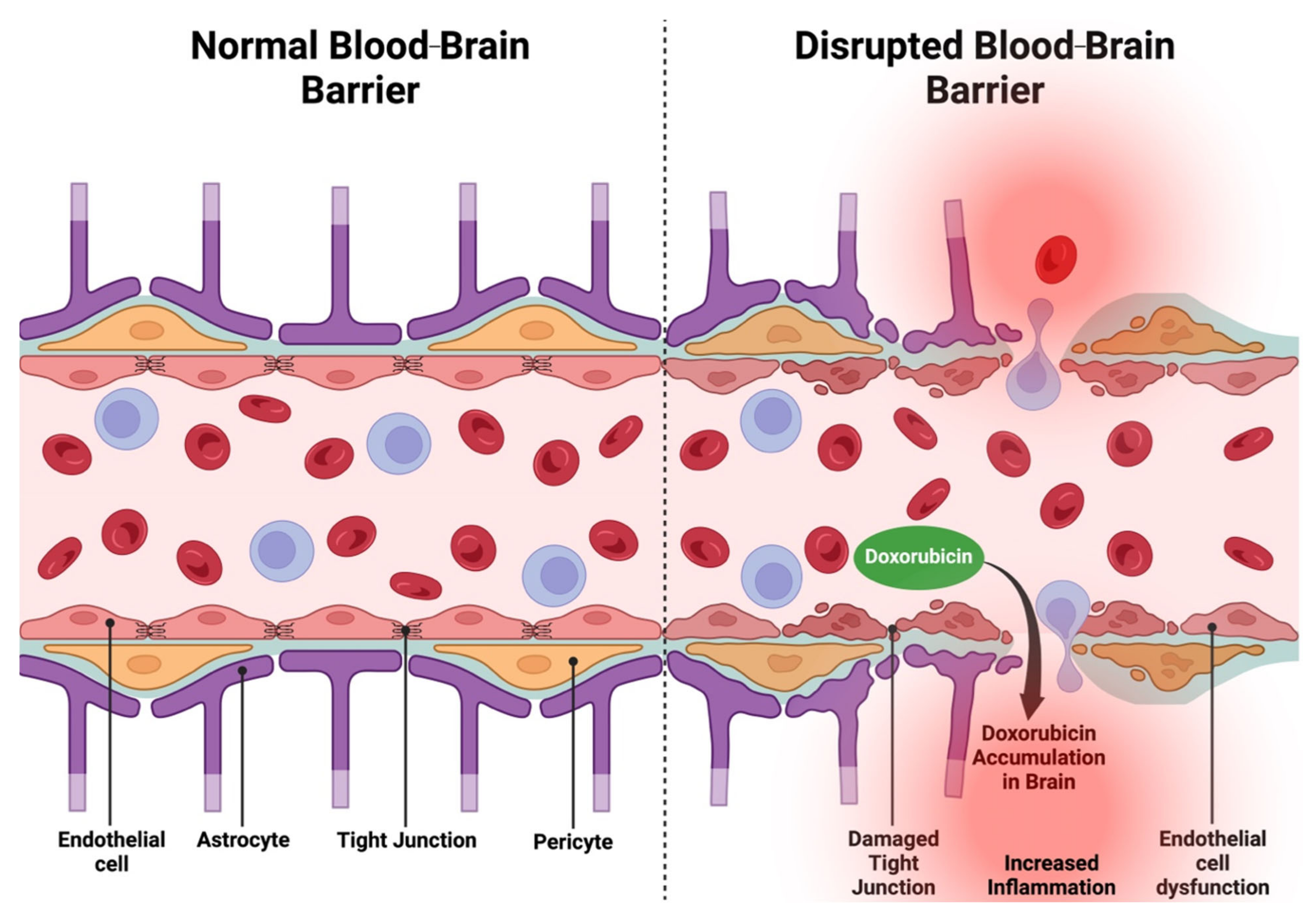
- Sardi, I.; la Marca, G.; Cardellicchio, S.; Giunti, L.; Malvagia, S.; Genitori, L.; Massimino, M.; de Martino, M.; Giovannini, M.G. Pharmacological Modulation of Blood-Brain Barrier Increases Permeability of Doxorubicin into the Rat Brain. Am. J. Cancer Res. 2013, 3, 424–432. [Google Scholar]
- Zhao, Y.L.; Du, J.; Kanazawa, H.; Sugawara, A.; Takagi, K.; Kitaichi, K.; Tatsumi, Y.; Takagi, K.; Hasegawa, T. Effect of Endotoxin on Doxorubicin Transport across Blood-Brain Barrier and P-Glycoprotein Function in Mice. Eur. J. Pharmacol. 2002, 445, 115–123. [Google Scholar] [CrossRef]
- Alsikhan, R.S.; Aldubayan, M.A.; Almami, I.S.; Alhowail, A.H. Protective Effect of Galantamine against Doxorubicin-Induced Neurotoxicity. Brain Sci. 2023, 13, 971. [Google Scholar] [CrossRef]
- Mohamed, R.H.; Karam, R.A.; Amer, M.G. Epicatechin Attenuates Doxorubicin-Induced Brain Toxicity: Critical Role of TNF-α, iNOS and NF-κB. Brain Res. Bull 2011, 86, 22–28. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood-Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Sardi, I.; Fantappiè, O.; la Marca, G.; Giovannini, M.G.; Iorio, A.L.; da Ros, M.; Malvagia, S.; Cardellicchio, S.; Giunti, L.; de Martino, M.; et al. Delivery of Doxorubicin across the Blood-Brain Barrier by Ondansetron Pretreatment: A Study in Vitro and in Vivo. Cancer Lett. 2014, 353, 242–247. [Google Scholar] [CrossRef]
- Wallez, Y.; Huber, P. Endothelial Adherens and Tight Junctions in Vascular Homeostasis, Inflammation and Angiogenesis. Biochim. Biophys. Acta 2008, 1778, 794–809. [Google Scholar] [CrossRef]
- Wilkinson, E.L.; Sidaway, J.E.; Cross, M.J. Cardiotoxic Drugs Herceptin and Doxorubicin Inhibit Cardiac Microvascular Endothelial Cell Barrier Formation Resulting in Increased Drug Permeability. Biol. Open 2016, 5, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.Z.; Chowdhury, B.; Al-Omran, M.; Teoh, H.; Hess, D.A.; Verma, S. Role of Endothelium in Doxorubicin-Induced Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 861–870. [Google Scholar] [CrossRef]
- Licht, T.; Sasson, E.; Bell, B.; Grunewald, M.; Kumar, S.; Kreisel, T.; Ben-Zvi, A.; Keshet, E. Hippocampal Neural Stem Cells Facilitate Access from Circulation via Apical Cytoplasmic Processes. Elife 2020, 9, e52134. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, S.; Wang, K.; Wang, X. Familial Breast Cancer: Disease Related Gene Mutations and Screening Strategies for Chinese Population. Front. Oncol. 2021, 11, 740227. [Google Scholar] [CrossRef]
- Kotsopoulos, J. BRCA Mutations and Breast Cancer Prevention. Cancers 2018, 10, 524. [Google Scholar] [CrossRef]
- Marmorstein, L.Y.; Ouchi, T.; Aaronson, S.A. The BRCA2 Gene Product Functionally Interacts with P53 and RAD51. Proc. Natl. Acad. Sci. USA 1998, 95, 13869–13874. [Google Scholar] [CrossRef]
- Griendling, K.K.; FitzGerald, G.A. Oxidative Stress and Cardiovascular Injury: Part II: Animal and Human Studies. Circulation 2003, 108, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Robert, G.; Wagner, J.R. ROS-Induced DNA Damage as an Underlying Cause of Aging. Adv. Geriatr. Med. Res. 2020, 2, e200024. [Google Scholar] [CrossRef]
- O’Driscoll, M. Diseases Associated with Defective Responses to DNA Damage. Cold Spring Harb. Perspect. Biol. 2012, 4, a012773. [Google Scholar] [CrossRef]
- Loughery, J.; Cox, M.; Smith, L.M.; Meek, D.W. Critical Role for P53-Serine 15 Phosphorylation in Stimulating Transactivation at P53-Responsive Promoters. Nucleic Acids Res. 2014, 42, 7666–7680. [Google Scholar] [CrossRef]
- Meyer, T.; Jahn, N.; Lindner, S.; Röhner, L.; Dolnik, A.; Weber, D.; Scheffold, A.; Köpff, S.; Paschka, P.; Gaidzik, V.I.; et al. Functional Characterization of BRCC3 Mutations in Acute Myeloid Leukemia with t(8;21)(Q22;Q22.1). Leukemia 2020, 34, 404–415. [Google Scholar] [CrossRef]
- Mao, Z.; Bozzella, M.; Seluanov, A.; Gorbunova, V. DNA Repair by Nonhomologous End Joining and Homologous Recombination during Cell Cycle in Human Cells. Cell Cycle 2008, 7, 2902–2906. [Google Scholar] [CrossRef]
- Lei, T.; Du, S.; Peng, Z.; Chen, L. Multifaceted Regulation and Functions of 53BP1 in NHEJ-mediated DSB Repair (Review). Int. J. Mol. Med. 2022, 50, 90. [Google Scholar] [CrossRef] [PubMed]
- Rappold, I.; Iwabuchi, K.; Date, T.; Chen, J. Tumor Suppressor P53 Binding Protein 1 (53BP1) Is Involved in DNA Damage-Signaling Pathways. J. Cell Biol. 2001, 153, 613–620. [Google Scholar] [CrossRef]
- Zhao, F.; Kim, W.; Kloeber, J.A.; Lou, Z. DNA End Resection and Its Role in DNA Replication and DSB Repair Choice in Mammalian Cells. Exp. Mol. Med. 2020, 52, 1705–1714. [Google Scholar] [CrossRef]
- Bunting, S.F.; Callén, E.; Wong, N.; Chen, H.-T.; Polato, F.; Gunn, A.; Bothmer, A.; Feldhahn, N.; Fernandez-Capetillo, O.; Cao, L.; et al. 53BP1 Inhibits Homologous Recombination in Brca1-Deficient Cells by Blocking Resection of DNA Breaks. Cell 2010, 141, 243–254. [Google Scholar] [CrossRef]
- Krasner, D.S.; Daley, J.M.; Sung, P.; Niu, H. Interplay between Ku and Replication Protein A in the Restriction of Exo1-Mediated DNA Break End Resection. J. Biol. Chem. 2015, 290, 18806–18816. [Google Scholar] [CrossRef]
- Achanta, G.; Pelicano, H.; Feng, L.; Plunkett, W.; Huang, P. Interaction of P53 and DNA-PK in Response to Nucleoside Analogues: Potential Role as a Sensor Complex for DNA Damage. Cancer Res. 2001, 61, 8723–8729. [Google Scholar]
- MacLachlan, T.K.; Takimoto, R.; El-Deiry, W.S. BRCA1 Directs a Selective P53-Dependent Transcriptional Response towards Growth Arrest and DNA Repair Targets. Mol. Cell. Biol. 2002, 22, 4280–4292. [Google Scholar] [CrossRef]
- Bae, I.; Fan, S.; Meng, Q.; Rih, J.K.; Kim, H.J.; Kang, H.J.; Xu, J.; Goldberg, I.D.; Jaiswal, A.K.; Rosen, E.M. BRCA1 Induces Antioxidant Gene Expression and Resistance to Oxidative Stress. Cancer Res. 2004, 64, 7893–7909. [Google Scholar] [CrossRef]
- Xu, P.; Liu, Q.; Xie, Y.; Shi, X.; Li, Y.; Peng, M.; Guo, H.; Sun, R.; Li, J.; Hong, Y.; et al. Breast Cancer Susceptibility Protein 1 (BRCA1) Rescues Neurons from Cerebral Ischemia/Reperfusion Injury through NRF2-Mediated Antioxidant Pathway. Redox Biol. 2018, 18, 158–172. [Google Scholar] [CrossRef]
- Gorrini, C.; Baniasadi, P.S.; Harris, I.S.; Silvester, J.; Inoue, S.; Snow, B.; Joshi, P.A.; Wakeham, A.; Molyneux, S.D.; Martin, B.; et al. BRCA1 Interacts with Nrf2 to Regulate Antioxidant Signaling and Cell Survival. J. Exp. Med. 2013, 210, 1529–1544. [Google Scholar] [CrossRef]
- Wang, C.; Gao, P.; Xu, J.; Liu, S.; Tian, W.; Liu, J.; Zhou, L. Natural Phytochemicals Prevent Side Effects in BRCA-Mutated Ovarian Cancer and PARP Inhibitor Treatment. Front. Pharmacol. 2022, 13, 1078303. [Google Scholar] [CrossRef]
- Ma, J.; Cai, H.; Wu, T.; Sobhian, B.; Huo, Y.; Alcivar, A.; Mehta, M.; Cheung, K.L.; Ganesan, S.; Kong, A.-N.T.; et al. PALB2 Interacts with KEAP1 to Promote NRF2 Nuclear Accumulation and Function. Mol. Cell Biol. 2012, 32, 1506–1517. [Google Scholar] [CrossRef]
- Deng, C.-X. BRCA1: Cell Cycle Checkpoint, Genetic Instability, DNA Damage Response and Cancer Evolution. Nucleic Acids Res. 2006, 34, 1416–1426. [Google Scholar] [CrossRef]
- Aprelikova, O.N.; Fang, B.S.; Meissner, E.G.; Cotter, S.; Campbell, M.; Kuthiala, A.; Bessho, M.; Jensen, R.A.; Liu, E.T. BRCA1-Associated Growth Arrest Is RB-Dependent. Proc. Natl. Acad. Sci. USA 1999, 96, 11866–11871. [Google Scholar] [CrossRef]
- Ross, C.A.; Truant, R. DNA Repair: A Unifying Mechanism in Neurodegeneration. Nature 2017, 541, 34–35. [Google Scholar] [CrossRef]
- Naumann, M.; Pal, A.; Goswami, A.; Lojewski, X.; Japtok, J.; Vehlow, A.; Naujock, M.; Günther, R.; Jin, M.; Stanslowsky, N.; et al. Impaired DNA Damage Response Signaling by FUS-NLS Mutations Leads to Neurodegeneration and FUS Aggregate Formation. Nat. Commun. 2018, 9, 335. [Google Scholar] [CrossRef]
- Marques, L.; Johnson, A.A.; Stolzing, A. Doxorubicin Generates Senescent Microglia That Exhibit Altered Proteomes, Higher Levels of Cytokine Secretion, and a Decreased Ability to Internalize Amyloid β. Exp. Cell Res. 2020, 395, 112203. [Google Scholar] [CrossRef]
- Lavigne, M.C.; Malech, H.L.; Holland, S.M.; Leto, T.L. Genetic Requirement of P47phox for Superoxide Production by Murine Microglia. FASEB J. 2001, 15, 285–287. [Google Scholar] [CrossRef]
- Polidori, M.C.; Griffiths, H.R.; Mariani, E.; Mecocci, P. Hallmarks of Protein Oxidative Damage in Neurodegenerative Diseases: Focus on Alzheimer’s Disease. Amino Acids 2007, 32, 553–559. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Lopes, M.A.; Meisel, A.; Carvalho, F.D.; de Lourdes Bastos, M. Neuronal Nitric Oxide Synthase Is a Key Factor in Doxorubicin-Induced Toxicity to Rat-Isolated Cortical Neurons. Neurotox Res. 2011, 19, 14–22. [Google Scholar] [CrossRef]
- Ren, X.; Keeney, J.T.R.; Miriyala, S.; Noel, T.; Powell, D.K.; Chaiswing, L.; Bondada, S.; St. Clair, D.K.; Butterfield, D.A. The Triangle of Death of Neurons: Oxidative Damage, Mitochondrial Dysfunction, and Loss of Choline-Containing Biomolecules in Brains of Mice Treated with Doxorubicin. Advanced Insights into Mechanisms of Chemotherapy Induced Cognitive Impairment (“Chemobrain”) Involving TNF-α. Free. Radic. Biol. Med. 2019, 134, 1–8. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Dang, R.-L.; Tang, M.-M.; Cai, H.-L.; Li, H.-D.; Liao, D.-H.; He, X.; Cao, L.-J.; Xue, Y.; Jiang, P. Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Alleviates Doxorubicin-Induced Depressive-Like Behaviors and Neurotoxicity in Rats: Involvement of Oxidative Stress and Neuroinflammation. Nutrients 2016, 8, 243. [Google Scholar] [CrossRef]
- Keeney, J.T.R.; Förster, S.; Sultana, R.; Brewer, L.D.; Latimer, C.S.; Cai, J.; Klein, J.B.; Porter, N.M.; Butterfield, D.A. Dietary Vitamin D Deficiency in Rats from Middle to Old Age Leads to Elevated Tyrosine Nitration and Proteomics Changes in Levels of Key Proteins in Brain: Implications for Low Vitamin D-Dependent Age-Related Cognitive Decline. Free Radic. Biol. Med. 2013, 65, 324–334. [Google Scholar] [CrossRef]
- Liao, D.; Xiang, D.; Dang, R.; Xu, P.; Wang, J.; Han, W.; Fu, Y.; Yao, D.; Cao, L.; Jiang, P. Neuroprotective Effects of dl-3-n-Butylphthalide against Doxorubicin-Induced Neuroinflammation, Oxidative Stress, Endoplasmic Reticulum Stress, and Behavioral Changes. Oxidative Med. Cell. Longev. 2018, 2018, 9125601. [Google Scholar] [CrossRef]
- Conrad, M.; Friedmann Angeli, J.P. Glutathione Peroxidase 4 (Gpx4) and Ferroptosis: What’s so Special about It? Mol. Cell Oncol. 2015, 2, e995047. [Google Scholar] [CrossRef]
- Imosemi, I.O.; Owumi, S.E.; Arunsi, U.O. Biochemical and Histological Alterations of Doxorubicin-Induced Neurotoxicity in Rats: Protective Role of Luteolin. J. Biochem. Mol. Toxicol. 2022, 36, e22962. [Google Scholar] [CrossRef]
- Da-Silva, O.F.; Adelowo, A.R.; Babalola, A.A.; Ikeji, C.N.; Owoeye, O.; Rocha, J.B.T.; Adedara, I.A.; Farombi, E.O. Diphenyl Diselenide Through Reduction of Inflammation, Oxidative Injury and Caspase-3 Activation Abates Doxorubicin-Induced Neurotoxicity in Rats. Neurochem. Res. 2024, 49, 1076–1092. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Fu, Y.; Zhang, H.; Lai, Z.-Q.; Dong, Y.-F. Sophocarpine Alleviates Doxorubicin-Induced Heart Injury by Suppressing Oxidative Stress and Apoptosis. Sci. Rep. 2024, 14, 428. [Google Scholar] [CrossRef]
- Patra, R.C.; Swarup, D.; Dwivedi, S.K. Antioxidant Effects of Alpha Tocopherol, Ascorbic Acid and L-Methionine on Lead Induced Oxidative Stress to the Liver, Kidney and Brain in Rats. Toxicology 2001, 162, 81–88. [Google Scholar] [CrossRef]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of Peroxisomes in ROS/RNS-Metabolism: Implications for Human Disease. Biochim. Biophys. Acta 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Keeney, J.T.R.; Ren, X.; Warrier, G.; Noel, T.; Powell, D.K.; Brelsfoard, J.M.; Sultana, R.; Saatman, K.E.; St. Clair, D.K.; Butterfield, D.A. Doxorubicin-Induced Elevated Oxidative Stress and Neurochemical Alterations in Brain and Cognitive Decline: Protection by MESNA and Insights into Mechanisms of Chemotherapy-Induced Cognitive Impairment (“Chemobrain”). Oncotarget 2018, 9, 30324–30339. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, P.; Dang, R.; Guo, Y.; Li, G.; Qiao, Y.; Xie, R.; Liu, Y.; Jiang, P. The Involvement of Autophagic Flux in the Development and Recovery of Doxorubicin-Induced Neurotoxicity. Free Radic. Biol. Med. 2018, 129, 440–445. [Google Scholar] [CrossRef]
- Moruno-Manchon, J.F.; Uzor, N.-E.; Kesler, S.R.; Wefel, J.S.; Townley, D.M.; Nagaraja, A.S.; Pradeep, S.; Mangala, L.S.; Sood, A.K.; Tsvetkov, A.S. Peroxisomes Contribute to Oxidative Stress in Neurons during Doxorubicin-Based Chemotherapy. Mol. Cell Neurosci. 2018, 86, 65–71. [Google Scholar] [CrossRef]
- Tangpong, J.; Cole, M.P.; Sultana, R.; Joshi, G.; Estus, S.; Vore, M.; St Clair, W.; Ratanachaiyavong, S.; St Clair, D.K.; Butterfield, D.A. Adriamycin-Induced, TNF-Alpha-Mediated Central Nervous System Toxicity. Neurobiol. Dis. 2006, 23, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Alhowail, A.H.; Bloemer, J.; Majrashi, M.; Pinky, P.D.; Bhattacharya, S.; Yongli, Z.; Bhattacharya, D.; Eggert, M.; Woodie, L.; Buabeid, M.A.; et al. Doxorubicin-Induced Neurotoxicity Is Associated with Acute Alterations in Synaptic Plasticity, Apoptosis, and Lipid Peroxidation. Toxicol. Mech. Methods 2019, 29, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Dias-Carvalho, A.; Ferreira, M.; Reis-Mendes, A.; Ferreira, R.; de Lourdes Bastos, M.; Fernandes, E.; Sá, S.I.; Capela, J.P.; Carvalho, F.; Costa, V.M. Doxorubicin-Induced Neurotoxicity Differently Affects the Hippocampal Formation Subregions in Adult Mice. Heliyon 2024, 10, e31608. [Google Scholar] [CrossRef]
- Cardoso, S.; Santos, R.X.; Carvalho, C.; Correia, S.; Pereira, G.C.; Pereira, S.S.; Oliveira, P.J.; Santos, M.S.; Proença, T.; Moreira, P.I. Doxorubicin Increases the Susceptibility of Brain Mitochondria to Ca(2+)-Induced Permeability Transition and Oxidative Damage. Free Radic. Biol. Med. 2008, 45, 1395–1402. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St Clair, D.K. Manganese Superoxide Dismutase: Guardian of the Powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef]
- Tangpong, J.; Cole, M.P.; Sultana, R.; Estus, S.; Vore, M.; St Clair, W.; Ratanachaiyavong, S.; St Clair, D.K.; Butterfield, D.A. Adriamycin-Mediated Nitration of Manganese Superoxide Dismutase in the Central Nervous System: Insight into the Mechanism of Chemobrain. J. Neurochem. 2007, 100, 191–201. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to Different Experimental Organ Systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-Induced Peripheral Neuropathy: What Do We Know about Mechanisms? Neurosci. Lett. 2015, 596, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Melli, G.; Taiana, M.; Camozzi, F.; Triolo, D.; Podini, P.; Quattrini, A.; Taroni, F.; Lauria, G. Alpha-Lipoic Acid Prevents Mitochondrial Damage and Neurotoxicity in Experimental Chemotherapy Neuropathy. Exp. Neurol. 2008, 214, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, C.; Eleftheriou, G. Neurotoxicity of Antineoplastic Drugs: Mechanisms, Susceptibility, and Neuroprotective Strategies. Adv. Med. Sci. 2020, 65, 265–285. [Google Scholar] [CrossRef]
- Merzoug, S.; Toumi, M.L.; Tahraoui, A. Quercetin Mitigates Adriamycin-Induced Anxiety- and Depression-like Behaviors, Immune Dysfunction, and Brain Oxidative Stress in Rats. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 921–933. [Google Scholar] [CrossRef]
- Kwatra, M.; Jangra, A.; Mishra, M.; Sharma, Y.; Ahmed, S.; Ghosh, P.; Kumar, V.; Vohora, D.; Khanam, R. Naringin and Sertraline Ameliorate Doxorubicin-Induced Behavioral Deficits Through Modulation of Serotonin Level and Mitochondrial Complexes Protection Pathway in Rat Hippocampus. Neurochem. Res. 2016, 41, 2352–2366. [Google Scholar] [CrossRef]
- Okudan, N.; Belviranlı, M.; Sezer, T. Potential Protective Effect of Coenzyme Q10 on Doxorubicin-Induced Neurotoxicity and Behavioral Disturbances in Rats. Neurochem. Res. 2022, 47, 1280–1289. [Google Scholar] [CrossRef]
- Ebrahim, N.A.; Elnagar, M.R.; El-Gamal, R.; Habotta, O.A.; Albadawi, E.A.; Albadrani, M.; Bahashwan, A.S.; Hassan, H.M. Melatonin Mitigates Doxorubicin Induced Chemo Brain in a Rat Model in a NRF2/P53-SIRT1 Dependent Pathway. Heliyon 2024, 10, e38081. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, J.; Jing, L.; Li, R.; Zhang, T.; Peng, S. Enhanced Toxicity and ROS Generation by Doxorubicin in Primary Cultures of Cardiomyocytes from Neonatal Metallothionein-I/II Null Mice. Toxicol. Vitr. 2010, 24, 1584–1591. [Google Scholar] [CrossRef]
- Tangpong, J.; Miriyala, S.; Noel, T.; Sinthupibulyakit, C.; Jungsuwadee, P.; St Clair, D.K. Doxorubicin-Induced Central Nervous System Toxicity and Protection by Xanthone Derivative of Garcinia Mangostana. Neuroscience 2011, 175, 292–299. [Google Scholar] [CrossRef]
- El-Shetry, E.S.; Ibrahim, I.A.; Kamel, A.M.; Abdelwahab, O.A. Quercetin Mitigates Doxorubicin-Induced Neurodegenerative Changes in the Cerebral Cortex and Hippocampus of Rats; Insights to DNA Damage, Inflammation, Synaptic Plasticity. Tissue Cell 2024, 87, 102313. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Fouad, G.; Ahmed, K.A. Neuroprotective Potential of Berberine Against Doxorubicin-Induced Toxicity in Rat’s Brain. Neurochem. Res. 2021, 46, 3247–3263. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Zhou, J.; Chen, T.; Liu, W.; Dong, W.; Qu, Y.; Jiang, X.; Ji, X.; Zhen, H.; Fei, Z. Neuroprotective Effect of Osthole against Acute Ischemic Stroke on Middle Cerebral Ischemia Occlusion in Rats. Brain Res. 2010, 1363, 206–211. [Google Scholar] [CrossRef]
- Ogawa, H.; Sasai, N.; Kamisako, T.; Baba, K. Effects of Osthol on Blood Pressure and Lipid Metabolism in Stroke-Prone Spontaneously Hypertensive Rats. J. Ethnopharmacol. 2007, 112, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Hosseinzadeh, L.; Moieni-Arya, M.; Mostafaie, A.; Mohammadi-Motlagh, H.-R. Osthole Attenuates Doxorubicin-Induced Apoptosis in PC12 Cells through Inhibition of Mitochondrial Dysfunction and ROS Production. Biomed. Res. Int. 2014, 2014, 156848. [Google Scholar] [CrossRef]
- Kohman, R.A.; Rhodes, J.S. Neurogenesis, Inflammation and Behavior. Brain Behav. Immun. 2013, 27, 22–32. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef]
- Christie, L.-A.; Acharya, M.M.; Parihar, V.K.; Nguyen, A.; Martirosian, V.; Limoli, C.L. Impaired Cognitive Function and Hippocampal Neurogenesis Following Cancer Chemotherapy. Clin. Cancer Res. 2012, 18, 1954–1965. [Google Scholar] [CrossRef]
- Sekeres, M.J.; Bradley-Garcia, M.; Martinez-Canabal, A.; Winocur, G. Chemotherapy-Induced Cognitive Impairment and Hippocampal Neurogenesis: A Review of Physiological Mechanisms and Interventions. Int. J. Mol. Sci. 2021, 22, 12697. [Google Scholar] [CrossRef]
- Alhowail, A.H.; Pinky, P.D.; Eggert, M.; Bloemer, J.; Woodie, L.N.; Buabeid, M.A.; Bhattacharya, S.; Jasper, S.L.; Bhattacharya, D.; Dhanasekaran, M.; et al. Doxorubicin Induces Dysregulation of AMPA Receptor and Impairs Hippocampal Synaptic Plasticity Leading to Learning and Memory Deficits. Heliyon 2021, 7, e07456. [Google Scholar] [CrossRef]
- Kitamura, Y.; Hattori, S.; Yoneda, S.; Watanabe, S.; Kanemoto, E.; Sugimoto, M.; Kawai, T.; Machida, A.; Kanzaki, H.; Miyazaki, I.; et al. Doxorubicin and Cyclophosphamide Treatment Produces Anxiety-like Behavior and Spatial Cognition Impairment in Rats: Possible Involvement of Hippocampal Neurogenesis via Brain-Derived Neurotrophic Factor and Cyclin D1 Regulation. Behav. Brain Res. 2015, 292, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Manchon, J.F.M.; Dabaghian, Y.; Uzor, N.-E.; Kesler, S.R.; Wefel, J.S.; Tsvetkov, A.S. Levetiracetam Mitigates Doxorubicin-Induced DNA and Synaptic Damage in Neurons. Sci. Rep. 2016, 6, 25705. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Kanemoto, E.; Sugimoto, M.; Machida, A.; Nakamura, Y.; Naito, N.; Kanzaki, H.; Miyazaki, I.; Asanuma, M.; Sendo, T. Influence of Nicotine on Doxorubicin and Cyclophosphamide Combination Treatment-Induced Spatial Cognitive Impairment and Anxiety-like Behavior in Rats. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ramalingayya, G.V.; Cheruku, S.P.; Nayak, P.G.; Kishore, A.; Shenoy, R.; Rao, C.M.; Krishnadas, N. Rutin Protects against Neuronal Damage in Vitro and Ameliorates Doxorubicin-Induced Memory Deficits in Vivo in Wistar Rats. Drug Des. Devel. Ther. 2017, 11, 1011–1026. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, J.; Chen, M.; Wang, T.; Yang, Z.; He, K.; Li, Y.; Wang, K.; Jiang, J.; Zhang, S. Protective Effect of Low-Dose Radiation on Doxorubicin-Induced Brain Injury in Mice. Arch. Biochem. Biophys. 2022, 729, 109390. [Google Scholar] [CrossRef]
- Bigotte, L.; Olsson, Y. Toxic Effects of Adriamycin on the Central Nervous System. Ultrastructural Changes in Some Circumventricular Organs of the Mouse after Intravenous Administration of the Drug. Acta Neuropathol. 1983, 61, 291–299. [Google Scholar] [CrossRef]
- Matusova, Z.; Hol, E.M.; Pekny, M.; Kubista, M.; Valihrach, L. Reactive Astrogliosis in the Era of Single-Cell Transcriptomics. Front. Cell Neurosci. 2023, 17, 1173200. [Google Scholar] [CrossRef]
- Deuker, L.; Doeller, C.F.; Fell, J.; Axmacher, N. Human Neuroimaging Studies on the Hippocampal CA3 Region—Integrating Evidence for Pattern Separation and Completion. Front. Cell Neurosci. 2014, 8, 64. [Google Scholar] [CrossRef]
- van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running Enhances Neurogenesis, Learning, and Long-Term Potentiation in Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- van Praag, H.; Kempermann, G.; Gage, F.H. Running Increases Cell Proliferation and Neurogenesis in the Adult Mouse Dentate Gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef]
- Mohamad, H.E.; Abo-Elmatty, D.M.; Wahba, N.S.; Shaheen, M.A.; Sakr, R.T.; Wahba, A.S. Infliximab and/or MESNA Alleviate Doxorubicin-Induced Alzheimer’s Disease-like Pathology in Rats: A New Insight into TNF-α/Wnt/β-Catenin Signaling Pathway. Life Sci. 2022, 301, 120613. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kim, J.-S.; Kim, J.; Kim, S.-H.; Kim, J.-C.; Kim, J.; Wang, H.; Shin, T.; Moon, C. Neurotoxicity of Methotrexate to Hippocampal Cells in Vivo and in Vitro. Biochem. Pharmacol. 2011, 82, 72–80. [Google Scholar] [CrossRef]
- Alhowail, A.H.; Aldubayan, M.A. Doxorubicin Impairs Cognitive Function by Upregulating AMPAR and NMDAR Subunit Expression and Increasing Neuroinflammation, Oxidative Stress, and Apoptosis in the Brain. Front. Pharmacol. 2023, 14, 1251917. [Google Scholar] [CrossRef]
- Pal, M.M. Glutamate: The Master Neurotransmitter and Its Implications in Chronic Stress and Mood Disorders. Front. Hum. Neurosci. 2021, 15, 722323. [Google Scholar] [CrossRef] [PubMed]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells 2023, 12, 1273. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Uryash, A.; Flores, V.; Adams, J.A.; Allen, P.D.; Lopez, J.R. Memory and Learning Deficits Are Associated With Ca2+ Dyshomeostasis in Normal Aging. Front. Aging Neurosci. 2020, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Thomas, T.C.; Beitchman, J.A.; Pomerleau, F.; Noel, T.; Jungsuwadee, P.; Butterfield, D.A.; Clair, D.K.S.; Vore, M.; Gerhardt, G.A. Acute Treatment with Doxorubicin Affects Glutamate Neurotransmission in the Mouse Frontal Cortex and Hippocampus. Brain Res. 2017, 1672, 10–17. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Todd, A.C.; Hardingham, G.E. The Regulation of Astrocytic Glutamate Transporters in Health and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9607. [Google Scholar] [CrossRef]
- Su, Z.; Leszczyniecka, M.; Kang, D.; Sarkar, D.; Chao, W.; Volsky, D.J.; Fisher, P.B. Insights into Glutamate Transport Regulation in Human Astrocytes: Cloning of the Promoter for Excitatory Amino Acid Transporter 2 (EAAT2). Proc. Natl. Acad. Sci. USA 2003, 100, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, L.; Zuo, Z. N-Acetylcysteine Reverses Existing Cognitive Impairment and Increased Oxidative Stress in Glutamate Transporter Type 3 Deficient Mice. Neuroscience 2012, 220, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ezeriņa, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459.e4. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Foster, J.B.; Lin, C.-L.G. Glutamate Transporter EAAT2: Regulation, Function, and Potential as a Therapeutic Target for Neurological and Psychiatric Disease. Cell Mol. Life Sci. 2015, 72, 3489–3506. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- El-Agamy, S.E.; Abdel-Aziz, A.K.; Wahdan, S.; Esmat, A.; Azab, S.S. Astaxanthin Ameliorates Doxorubicin-Induced Cognitive Impairment (Chemobrain) in Experimental Rat Model: Impact on Oxidative, Inflammatory, and Apoptotic Machineries. Mol. Neurobiol. 2018, 55, 5727–5740. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Mohammed, H.S. Protective Effect of Nanocurcumin against Neurotoxicity Induced by Doxorubicin in Rat’s Brain. Neurotoxicology 2021, 85, 1–9. [Google Scholar] [CrossRef]
- Garcia-Ratés, S.; Greenfield, S. Cancer and Neurodegeneration: Two Sides, Same Coin? Oncotarget 2017, 8, 22307–22308. [Google Scholar] [CrossRef]
- Onganer, P.U.; Djamgoz, M.B.A.; Whyte, K.; Greenfield, S.A. An Acetylcholinesterase-Derived Peptide Inhibits Endocytic Membrane Activity in a Human Metastatic Breast Cancer Cell Line. Biochim. Biophys. Acta 2006, 1760, 415–420. [Google Scholar] [CrossRef]
- Greenfield, S. Discovering and Targeting the Basic Mechanism of Neurodegeneration: The Role of Peptides from the C-Terminus of Acetylcholinesterase: Non-Hydrolytic Effects of Ache: The Actions of Peptides Derived from the C-Terminal and Their Relevance to Neurodegeneration. Chem. Biol. Interact. 2013, 203, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Lim, I.; Joung, H.-Y.; Yu, A.R.; Shim, I.; Kim, J.S. PET Evidence of the Effect of Donepezil on Cognitive Performance in an Animal Model of Chemobrain. Biomed. Res. Int. 2016, 2016, 6945415. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Chen, K.; Shih, J.C. Monoamine Oxidase Inactivation: From Pathophysiology to Therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Simillion, C.; Kruzliak, P.; Sabo, J.; Heller, M. Doxorubicin Affects Expression of Proteins of Neuronal Pathways in MCF-7 Breast Cancer Cells. Cancer Genom. Proteom. 2015, 12, 347–358. [Google Scholar]
- Ugalde-Triviño, L.; Díaz-Guerra, M. PSD-95: An Effective Target for Stroke Therapy Using Neuroprotective Peptides. Int. J. Mol. Sci. 2021, 22, 12585. [Google Scholar] [CrossRef]
- Vallejo, D.; Codocedo, J.F.; Inestrosa, N.C. Posttranslational Modifications Regulate the Postsynaptic Localization of PSD-95. Mol. Neurobiol. 2017, 54, 1759–1776. [Google Scholar] [CrossRef]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Oxidative Stress and Modification of Synaptic Proteins in Hippocampus after Traumatic Brain Injury. Free Radic. Biol. Med. 2008, 45, 443–452. [Google Scholar] [CrossRef]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef]
- Bu, S.; Joseph, J.J.; Nguyen, H.C.; Ehsan, M.; Rasheed, B.; Singh, A.; Qadura, M.; Frisbee, J.C.; Singh, K.K. MicroRNA miR-378-3p Is a Novel Regulator of Endothelial Autophagy and Function. J. Mol. Cell Cardiol. Plus 2023, 3, 100027. [Google Scholar] [CrossRef]
- Singh, A.; Ravendranathan, N.; Frisbee, J.C.; Singh, K.K. Complex Interplay between DNA Damage and Autophagy in Disease and Therapy. Biomolecules 2024, 14, 922. [Google Scholar] [CrossRef]
- Van Limbergen, J.; Stevens, C.; Nimmo, E.R.; Wilson, D.C.; Satsangi, J. Autophagy: From Basic Science to Clinical Application. Mucosal Immunol. 2009, 2, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Myerowitz, R.; Puertollano, R.; Raben, N. Impaired Autophagy: The Collateral Damage of Lysosomal Storage Disorders. EBioMedicine 2021, 63, 103166. [Google Scholar] [CrossRef] [PubMed]
- Moruno-Manchon, J.F.; Uzor, N.-E.; Kesler, S.R.; Wefel, J.S.; Townley, D.M.; Nagaraja, A.S.; Pradeep, S.; Mangala, L.S.; Sood, A.K.; Tsvetkov, A.S. TFEB Ameliorates the Impairment of the Autophagy-Lysosome Pathway in Neurons Induced by Doxorubicin. Aging 2016, 8, 3507–3519. [Google Scholar] [CrossRef]
- Liao, D.; Shangguan, D.; Wu, Y.; Chen, Y.; Liu, N.; Tang, J.; Yao, D.; Shi, Y. Curcumin Protects against Doxorubicin Induced Oxidative Stress by Regulating the Keap1-Nrf2-ARE and Autophagy Signaling Pathways. Psychopharmacology 2023, 240, 1179–1190. [Google Scholar] [CrossRef]
- Song, W.; Wang, F.; Lotfi, P.; Sardiello, M.; Segatori, L. 2-Hydroxypropyl-β-Cyclodextrin Promotes Transcription Factor EB-Mediated Activation of Autophagy: Implications for Therapy. J. Biol. Chem. 2014, 289, 10211–10222. [Google Scholar] [CrossRef]
- Habbas, S.; Santello, M.; Becker, D.; Stubbe, H.; Zappia, G.; Liaudet, N.; Klaus, F.R.; Kollias, G.; Fontana, A.; Pryce, C.R.; et al. Neuroinflammatory TNFα Impairs Memory via Astrocyte Signaling. Cell 2015, 163, 1730–1741. [Google Scholar] [CrossRef]
- Brás, J.P.; Bravo, J.; Freitas, J.; Barbosa, M.A.; Santos, S.G.; Summavielle, T.; Almeida, M.I. TNF-Alpha-Induced Microglia Activation Requires miR-342: Impact on NF-kB Signaling and Neurotoxicity. Cell Death Dis. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Keeney, J.T.R.; Miriyala, S.; Noel, T.; Moscow, J.A.; St Clair, D.K.; Butterfield, D.A. Superoxide Induces Protein Oxidation in Plasma and TNF-α Elevation in Macrophage Culture: Insights into Mechanisms of Neurotoxicity Following Doxorubicin Chemotherapy. Cancer Lett. 2015, 367, 157–161. [Google Scholar] [CrossRef]
- Geng, C.; Cui, C.; Wang, C.; Lu, S.; Zhang, M.; Chen, D.; Jiang, P. Systematic Evaluations of Doxorubicin-Induced Toxicity in Rats Based on Metabolomics. ACS Omega 2021, 6, 358–366. [Google Scholar] [CrossRef]
- Lal, R.; Dharavath, R.N.; Chopra, K. Alpha-Lipoic Acid Ameliorates Doxorubicin-Induced Cognitive Impairments by Modulating Neuroinflammation and Oxidative Stress via NRF-2/HO-1 Signaling Pathway in the Rat Hippocampus. Neurochem. Res. 2023, 48, 2476–2489. [Google Scholar] [CrossRef]
- Aluise, C.D.; Miriyala, S.; Noel, T.; Sultana, R.; Jungsuwadee, P.; Taylor, T.J.; Cai, J.; Pierce, W.M.; Vore, M.; Moscow, J.A.; et al. 2-Mercaptoethane Sulfonate Prevents Doxorubicin-Induced Plasma Protein Oxidation and TNF-α Release: Implications for the Reactive Oxygen Species-Mediated Mechanisms of Chemobrain. Free Radic. Biol. Med. 2011, 50, 1630–1638. [Google Scholar] [CrossRef]
- Shao, B.; Tang, C.; Heinecke, J.W.; Oram, J.F. Oxidation of Apolipoprotein A-I by Myeloperoxidase Impairs the Initial Interactions with ABCA1 Required for Signaling and Cholesterol Export. J. Lipid Res. 2010, 51, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Guo, L.; Deng, X.; Yang, F.; Tian, Y.; Liu, P.; Xu, F.; Zhang, Z.; Huang, Y. Attenuation of Doxorubicin-Induced Oxidative Damage in Rat Brain by Regulating Amino Acid Homeostasis with Astragali Radix. Amino Acids 2021, 53, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Guo, L.; Yu, X.; Liu, P.; Yu, Y.; Kong, X.; Yu, X.; Zephania, H.M.; Liu, P.; Huang, Y. Identification of Region-Specific Amino Acid Signatures for Doxorubicin-Induced Chemo Brain. Amino Acids 2023, 55, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA Imbalance Following Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 27. [Google Scholar] [CrossRef]
- Ramalingayya, G.; Nayak, P.; Shenoy, R.; Mallik, S.; Gourishetti, K.; Hussain, S.; Rao, C.; Nandakumar, K. Naringin Ameliorates Doxorubicin-Induced Neurotoxicity In Vitro and Cognitive Dysfunction In Vivo. Phcog. Mag. 2018, 14, 197. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and Molecular Mechanisms of the Blood-Brain Barrier Dysfunction in Neurodegenerative Diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef]
- Bu, S.; Nguyen, H.C.; Nikfarjam, S.; Michels, D.C.R.; Rasheed, B.; Maheshkumar, S.; Singh, S.; Singh, K.K. Endothelial Cell-Specific Loss of eNOS Differentially Affects Endothelial Function. PLoS ONE 2022, 17, e0274487. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The Blood-Brain Barrier in Aging and Neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, L.; Qiao, Y.; Zhou, Q.; Li, H.; Chen, S.; Yin, D.; Huang, Q.; He, M. Doxorubicin Induces Endotheliotoxicity and Mitochondrial Dysfunction via ROS/eNOS/NO Pathway. Front. Pharmacol. 2019, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Liu, H.; Zhang, J.; Huang, J.; Zhu, C.; Lu, Y.; Shi, Y.; Wang, Y. Ursolic Acid Prevents Doxorubicin-Induced Cardiac Toxicity in Mice through eNOS Activation and Inhibition of eNOS Uncoupling. J. Cell. Mol. Med. 2019, 23, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, K.-P.; Hornung, V. Molecular Mechanisms and Cellular Functions of cGAS-STING Signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Wang, S.; Kotamraju, S.; Konorev, E.; Kalivendi, S.; Joseph, J.; Kalyanaraman, B. Activation of Nuclear Factor-kappaB during Doxorubicin-Induced Apoptosis in Endothelial Cells and Myocytes Is pro-Apoptotic: The Role of Hydrogen Peroxide. Biochem. J. 2002, 367, 729–740. [Google Scholar] [CrossRef]
- Graziani, S.; Scorrano, L.; Pontarin, G. Transient Exposure of Endothelial Cells to Doxorubicin Leads to Long-Lasting Vascular Endothelial Growth Factor Receptor 2 Downregulation. Cells 2022, 11, 210. [Google Scholar] [CrossRef]
- Luu, A.Z.; Luu, V.Z.; Chowdhury, B.; Kosmopoulos, A.; Pan, Y.; Al-Omran, M.; Quan, A.; Teoh, H.; Hess, D.A.; Verma, S. Loss of Endothelial Cell-Specific Autophagy-Related Protein 7 Exacerbates Doxorubicin-Induced Cardiotoxicity. Biochem. Biophys. Rep. 2021, 25, 100926. [Google Scholar] [CrossRef]
- Dhulkifle, H.; Therachiyil, L.; Hasan, M.H.; Sayed, T.S.; Younis, S.M.; Korashy, H.M.; Yalcin, H.C.; Maayah, Z.H. Inhibition of Cytochrome P450 Epoxygenase Promotes Endothelium-to-Mesenchymal Transition and Exacerbates Doxorubicin-Induced Cardiovascular Toxicity. Mol. Biol. Rep. 2024, 51, 859. [Google Scholar] [CrossRef]
- Deng, C.-X.; Wang, R.-H. Roles of BRCA1 in DNA Damage Repair: A Link between Development and Cancer. Hum. Mol. Genet. 2003, 12, R113–R123. [Google Scholar] [CrossRef]
- O’Donovan, P.J.; Livingston, D.M. BRCA1 and BRCA2: Breast/Ovarian Cancer Susceptibility Gene Products and Participants in DNA Double-Strand Break Repair. Carcinogenesis 2010, 31, 961–967. [Google Scholar] [CrossRef]
- Baretta, Z.; Mocellin, S.; Goldin, E.; Olopade, O.I.; Huo, D. Effect of BRCA Germline Mutations on Breast Cancer Prognosis: A Systematic Review and Meta-Analysis. Medicine 2016, 95, e4975. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Frost, D.; Barrowdale, D.; Evans, D.G.; Bancroft, E.; Adlard, J.; Ahmed, M.; Barwell, J.; Brady, A.F.; Brewer, C.; et al. Prostate Cancer Risks for Male BRCA1 and BRCA2 Mutation Carriers: A Prospective Cohort Study. Eur. Urol. 2020, 77, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Yadav, S.; Ogunleye, F.; Zakalik, D. Male BRCA Mutation Carriers: Clinical Characteristics and Cancer Spectrum. BMC Cancer 2018, 18, 179. [Google Scholar] [CrossRef]
- Hagemeister, F.B.; Buzdar, A.U.; Luna, M.A.; Blumenschein, G.R. Causes of Death in Breast Cancer: A Clinicopathologic Study. Cancer 1980, 46, 162–167. [Google Scholar] [CrossRef]
- Madabhushi, R.; Pan, L.; Tsai, L.-H. DNA Damage and Its Links to Neurodegeneration. Neuron 2014, 83, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Nouspikel, T. DNA Repair in Differentiated Cells: Some New Answers to Old Questions. Neuroscience 2007, 145, 1213–1221. [Google Scholar] [CrossRef]
- Fortini, P.; Dogliotti, E. Mechanisms of Dealing with DNA Damage in Terminally Differentiated Cells. Mutat. Res. 2010, 685, 38–44. [Google Scholar] [CrossRef]
- Suberbielle, E.; Djukic, B.; Evans, M.; Kim, D.H.; Taneja, P.; Wang, X.; Finucane, M.; Knox, J.; Ho, K.; Devidze, N.; et al. DNA Repair Factor BRCA1 Depletion Occurs in Alzheimer Brains and Impairs Cognitive Function in Mice. Nat. Commun. 2015, 6, 8897. [Google Scholar] [CrossRef]
- Li, D.; Bi, F.-F.; Chen, N.-N.; Cao, J.-M.; Sun, W.-P.; Zhou, Y.-M.; Li, C.-Y.; Yang, Q. A Novel Crosstalk between BRCA1 and Sirtuin 1 in Ovarian Cancer. Sci. Rep. 2014, 4, 6666. [Google Scholar] [CrossRef]
- Kaneko, M.; Imaizumi, K.; Saito, A.; Kanemoto, S.; Asada, R.; Matsuhisa, K.; Ohtake, Y. ER Stress and Disease: Toward Prevention and Treatment. Biol. Pharm. Bull. 2017, 40, 1337–1343. [Google Scholar] [CrossRef]
- Wezyk, M.; Zekanowski, C. Role of BRCA1 in Neuronal Death in Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Wezyk, M.; Szybinska, A.; Wojsiat, J.; Szczerba, M.; Day, K.; Ronnholm, H.; Kele, M.; Berdynski, M.; Peplonska, B.; Fichna, J.P.; et al. Overactive BRCA1 Affects Presenilin 1 in Induced Pluripotent Stem Cell-Derived Neurons in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 175–202. [Google Scholar] [CrossRef] [PubMed]
- Mano, T.; Nagata, K.; Nonaka, T.; Tarutani, A.; Imamura, T.; Hashimoto, T.; Bannai, T.; Koshi-Mano, K.; Tsuchida, T.; Ohtomo, R.; et al. Neuron-Specific Methylome Analysis Reveals Epigenetic Regulation and Tau-Related Dysfunction of BRCA1 in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2017, 114, E9645–E9654. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Stoltzner, S.; Lai, F.; Su, J.; Nixon, R.A. Morphological and Biochemical Assessment of DNA Damage and Apoptosis in Down Syndrome and Alzheimer Disease, and Effect of Postmortem Tissue Archival on TUNEL. Neurobiol. Aging 2000, 21, 511–524. [Google Scholar] [CrossRef]
- Su, J.H.; Satou, T.; Anderson, A.J.; Cotman, C.W. Up-Regulation of Bcl-2 Is Associated with Neuronal DNA Damage in Alzheimer’s Disease. Neuroreport 1996, 7, 437–440. [Google Scholar] [CrossRef]
- Nakanishi, A.; Minami, A.; Kitagishi, Y.; Ogura, Y.; Matsuda, S. BRCA1 and P53 Tumor Suppressor Molecules in Alzheimer’s Disease. Int. J. Mol. Sci. 2015, 16, 2879–2892. [Google Scholar] [CrossRef]
- Schapira, A.H.; Jenner, P. Etiology and Pathogenesis of Parkinson’s Disease. Mov. Disord. 2011, 26, 1049–1055. [Google Scholar] [CrossRef]
- Gandhi, S.; Wood, N.W. Molecular Pathogenesis of Parkinson’s Disease. Hum. Mol. Genet. 2005, 14, 2749–2755. [Google Scholar] [CrossRef]
- Illuzzi, J.L.; Vickers, C.A.; Kmiec, E.B. Modifications of P53 and the DNA Damage Response in Cells Expressing Mutant Form of the Protein Huntingtin. J. Mol. Neurosci. 2011, 45, 256–268. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Sohn, Y.K.; Ganju, N.; Wands, J.R. P53- and CD95-Associated Apoptosis in Neurodegenerative Diseases. Lab. Investig. 1998, 78, 401–411. [Google Scholar]
- Sawa, A. Alteration of Gene Expression in Down’s Syndrome (DS) Brains: Its Significance in Neurodegeneration. In Protein Expression in Down Syndrome Brain; Springer: Vienna, Austria, 2001; pp. 361–371. [Google Scholar] [CrossRef]
- Martin, L.J. P53 Is Abnormally Elevated and Active in the CNS of Patients with Amyotrophic Lateral Sclerosis. Neurobiol. Dis. 2000, 7, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Eve, D.J.; Dennis, J.S.; Citron, B.A. Transcription Factor P53 in Degenerating Spinal Cords. Brain Res. 2007, 1150, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Herold, S.; Kumar, P.; Wichert, S.P.; Kretzschmar, B.; Bähr, M.; Rossner, M.J.; Hein, K. Neurodegeneration in Autoimmune Optic Neuritis Is Associated with Altered APP Cleavage in Neurons and Up-Regulation of P53. PLoS ONE 2015, 10, e0138852. [Google Scholar] [CrossRef]
- Rossi, S.; Motta, C.; Studer, V.; Macchiarulo, G.; Volpe, E.; Barbieri, F.; Ruocco, G.; Buttari, F.; Finardi, A.; Mancino, R.; et al. Interleukin-1β Causes Excitotoxic Neurodegeneration and Multiple Sclerosis Disease Progression by Activating the Apoptotic Protein P53. Mol. Neurodegener. 2014, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Bowles, E.J.A.; Walker, R.L.; Anderson, M.L.; Dublin, S.; Crane, P.K.; Larson, E.B. Risk of Alzheimer’s Disease or Dementia Following a Cancer Diagnosis. PLoS ONE 2017, 12, e0179857. [Google Scholar] [CrossRef]
- Nudelman, K.N.H.; Risacher, S.L.; West, J.D.; McDonald, B.C.; Gao, S.; Saykin, A.J. Alzheimer’s Disease Neuroimaging Initiative Association of Cancer History with Alzheimer’s Disease Onset and Structural Brain Changes. Front. Physiol. 2014, 5, 423. [Google Scholar] [CrossRef]
- Kesler, S.R.; Rao, V.; Ray, W.J.; Rao, A. Alzheimer’s Disease Neuroimaging Initiative Probability of Alzheimer’s Disease in Breast Cancer Survivors Based on Gray-Matter Structural Network Efficiency. Alzheimer’s Dement. 2017, 9, 67–75. [Google Scholar] [CrossRef]
- Klus, P.; Cirillo, D.; Botta Orfila, T.; Gaetano Tartaglia, G. Neurodegeneration and Cancer: Where the Disorder Prevails. Sci. Rep. 2015, 5, 15390. [Google Scholar] [CrossRef]
- Hima Bindu, A.; Aliya, S.; Jeevani, T. Genetic and Degenerative Neurological Disorders ? An Emphasis on Alzheimer’s, the Mystery. J. Genet. Syndr. Gene Ther. 2011, 2, 109. [Google Scholar] [CrossRef]
- Du, L.; Pertsemlidis, A. Cancer and Neurodegenerative Disorders: Pathogenic Convergence through microRNA Regulation. J. Mol. Cell Biol. 2011, 3, 176–180. [Google Scholar] [CrossRef]
- Ariga, H. Common Mechanisms of Onset of Cancer and Neurodegenerative Diseases. Biol. Pharm. Bull 2015, 38, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, J.B.; Milton, R.; King, A.; Edwards, D. People with Dementia: What Is Known about Their Experience of Cancer Treatment and Cancer Treatment Outcomes? A Systematic Review. Psychooncology 2016, 25, 1137–1146. [Google Scholar] [CrossRef]
- Huang, H.-K.; Hsieh, J.-G.; Hsieh, C.-J.; Wang, Y.-W. Do Cancer Patients with Dementia Receive Less Aggressive Treatment in End-of-Life Care? A Nationwide Population-Based Cohort Study. Oncotarget 2017, 8, 63596–63604. [Google Scholar] [CrossRef] [PubMed]
- Potapova, A.; Hoffman, A.M.; Godwin, A.K.; Al-Saleem, T.; Cairns, P. Promoter Hypermethylation of the PALB2 Susceptibility Gene in Inherited and Sporadic Breast and Ovarian Cancer. Cancer Res. 2008, 68, 998–1002. [Google Scholar] [CrossRef]
- Thorstenson, Y.R.; Roxas, A.; Kroiss, R.; Jenkins, M.A.; Yu, K.M.; Bachrich, T.; Muhr, D.; Wayne, T.L.; Chu, G.; Davis, R.W.; et al. Contributions of ATM Mutations to Familial Breast and Ovarian Cancer. Cancer Res. 2003, 63, 3325–3333. [Google Scholar] [PubMed]
- Renwick, A.; Thompson, D.; Seal, S.; Kelly, P.; Chagtai, T.; Ahmed, M.; North, B.; Jayatilake, H.; Barfoot, R.; Spanova, K.; et al. ATM Mutations That Cause Ataxia-Telangiectasia Are Breast Cancer Susceptibility Alleles. Nat. Genet. 2006, 38, 873–875. [Google Scholar] [CrossRef]
- Yang, X.; Song, H.; Leslie, G.; Engel, C.; Hahnen, E.; Auber, B.; Horváth, J.; Kast, K.; Niederacher, D.; Turnbull, C.; et al. Ovarian and Breast Cancer Risks Associated With Pathogenic Variants in RAD51C and RAD51D. J. Natl. Cancer Inst. 2020, 112, 1242–1250. [Google Scholar] [CrossRef]
- Moyer, C.L.; Ivanovich, J.; Gillespie, J.L.; Doberstein, R.; Radke, M.R.; Richardson, M.E.; Kaufmann, S.H.; Swisher, E.M.; Goodfellow, P.J. Rare BRIP1 Missense Alleles Confer Risk for Ovarian and Breast Cancer. Cancer Res. 2020, 80, 857–867. [Google Scholar] [CrossRef]
- Shimelis, H.; LaDuca, H.; Hu, C.; Hart, S.N.; Na, J.; Thomas, A.; Akinhanmi, M.; Moore, R.M.; Brauch, H.; Cox, A.; et al. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. J. Natl. Cancer Inst. 2018, 110, 855–862. [Google Scholar] [CrossRef]
- Boonen, R.A.C.M.; Wiegant, W.W.; Celosse, N.; Vroling, B.; Heijl, S.; Kote-Jarai, Z.; Mijuskovic, M.; Cristea, S.; Solleveld-Westerink, N.; van Wezel, T.; et al. Functional Analysis Identifies Damaging CHEK2 Missense Variants Associated with Increased Cancer Risk. Cancer Res. 2022, 82, 615–631. [Google Scholar] [CrossRef]
- Prakash, R.; Zhang, Y.; Feng, W.; Jasin, M. Homologous Recombination and Human Health: The Roles of BRCA1, BRCA2, and Associated Proteins. Cold Spring Harb. Perspect. Biol. 2015, 7, a016600. [Google Scholar] [CrossRef] [PubMed]
- Plun-Favreau, H.; Lewis, P.A.; Hardy, J.; Martins, L.M.; Wood, N.W. Cancer and Neurodegeneration: Between the Devil and the Deep Blue Sea. PLoS Genet. 2010, 6, e1001257. [Google Scholar] [CrossRef]
- Hoch, N.C.; Hanzlikova, H.; Rulten, S.L.; Tétreault, M.; Komulainen, E.; Ju, L.; Hornyak, P.; Zeng, Z.; Gittens, W.; Rey, S.A.; et al. XRCC1 Mutation Is Associated with PARP1 Hyperactivation and Cerebellar Ataxia. Nature 2017, 541, 87–91. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Deneen, B.; Tzeng, S.-F. BRCA1/BRCA2-Containing Complex Subunit 3 Controls Oligodendrocyte Differentiation by Dynamically Regulating Lysine 63-Linked Ubiquitination. Glia 2019, 67, 1775–1792. [Google Scholar] [CrossRef] [PubMed]
- Szybińska, A.; Leśniak, W. P53 Dysfunction in Neurodegenerative Diseases—The Cause or Effect of Pathological Changes? Aging Dis. 2017, 8, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.R.; Ghafouri, M.; Mukerjee, R.; Bagashev, A.; Chabrashvili, T.; Sawaya, B.E. Role of P53 in Neurodegenerative Diseases. Neurodegener Dis. 2012, 9, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mandal, P.; Ganguly, S.; Jana, D.; Ayaz, A.; Banerjee, A.; Chouhan, R.; Sarkar, D.K. Decreased Expression of BRCA2 Accelerates Sporadic Breast Cancer Progression. Indian J. Surg. Oncol. 2015, 6, 378–383. [Google Scholar] [CrossRef]
- Loredo-Pozos, G.; Chiquete, E.; Oceguera-Villanueva, A.; Panduro, A.; Siller-López, F.; Ramos-Márquez, M.E. Expression Profile of BRCA1 and BRCA2 Genes in Premenopausal Mexican Women with Breast Cancer: Clinical and Immunohistochemical Correlates. Med. Oncol. 2009, 26, 269–275. [Google Scholar] [CrossRef]
- Melchor, L.; Benítez, J. The Complex Genetic Landscape of Familial Breast Cancer. Hum. Genet. 2013, 132, 845–863. [Google Scholar] [CrossRef]
- Yoshida, R. Hereditary Breast and Ovarian Cancer (HBOC): Review of Its Molecular Characteristics, Screening, Treatment, and Prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef]
- Mai, P.L.; Chatterjee, N.; Hartge, P.; Tucker, M.; Brody, L.; Struewing, J.P.; Wacholder, S. Potential Excess Mortality in BRCA1/2 Mutation Carriers beyond Breast, Ovarian, Prostate, and Pancreatic Cancers, and Melanoma. PLoS ONE 2009, 4, e4812. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, S.M. The BRCAness Landscape of Cancer. Cells 2022, 11, 3877. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, W.; Cheng, C.-T.; Ren, X.; Somlo, G.; Fong, M.Y.; Chin, A.R.; Li, H.; Yu, Y.; Xu, Y.; et al. TGFβ Induces “BRCAness” and Sensitivity to PARP Inhibition in Breast Cancer by Regulating DNA-Repair Genes. Mol. Cancer Res. 2014, 12, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nguyen, H.; Michels, D.; Bazinet, H.; Matkar, P.N.; Liu, Z.; Esene, L.; Adam, M.; Bugyei-Twum, A.; Mebrahtu, E.; et al. BReast CAncer Susceptibility Gene 2 Deficiency Exacerbates Oxidized LDL-induced DNA Damage and Endothelial Apoptosis. Physiol. Rep. 2020, 8, e14481. [Google Scholar] [CrossRef]
- Komirishetty, P.; Areti, A.; Yerra, V.G.; Ruby, P.K.; Sharma, S.S.; Gogoi, R.; Sistla, R.; Kumar, A. PARP Inhibition Attenuates Neuroinflammation and Oxidative Stress in Chronic Constriction Injury Induced Peripheral Neuropathy. Life Sci. 2016, 150, 50–60. [Google Scholar] [CrossRef]
- Nasr, M.M.; Wahdan, S.A.; El-Naga, R.N.; Salama, R.M. Neuroprotective Effect of Empagliflozin against Doxorubicin-Induced Chemobrain in Rats: Interplay between SIRT-1/MuRF-1/PARP-1/NLRP3 Signaling Pathways and Enhanced Expression of miRNA-34a and LncRNA HOTAIR. Neurotoxicology 2024, 105, 216–230. [Google Scholar] [CrossRef]
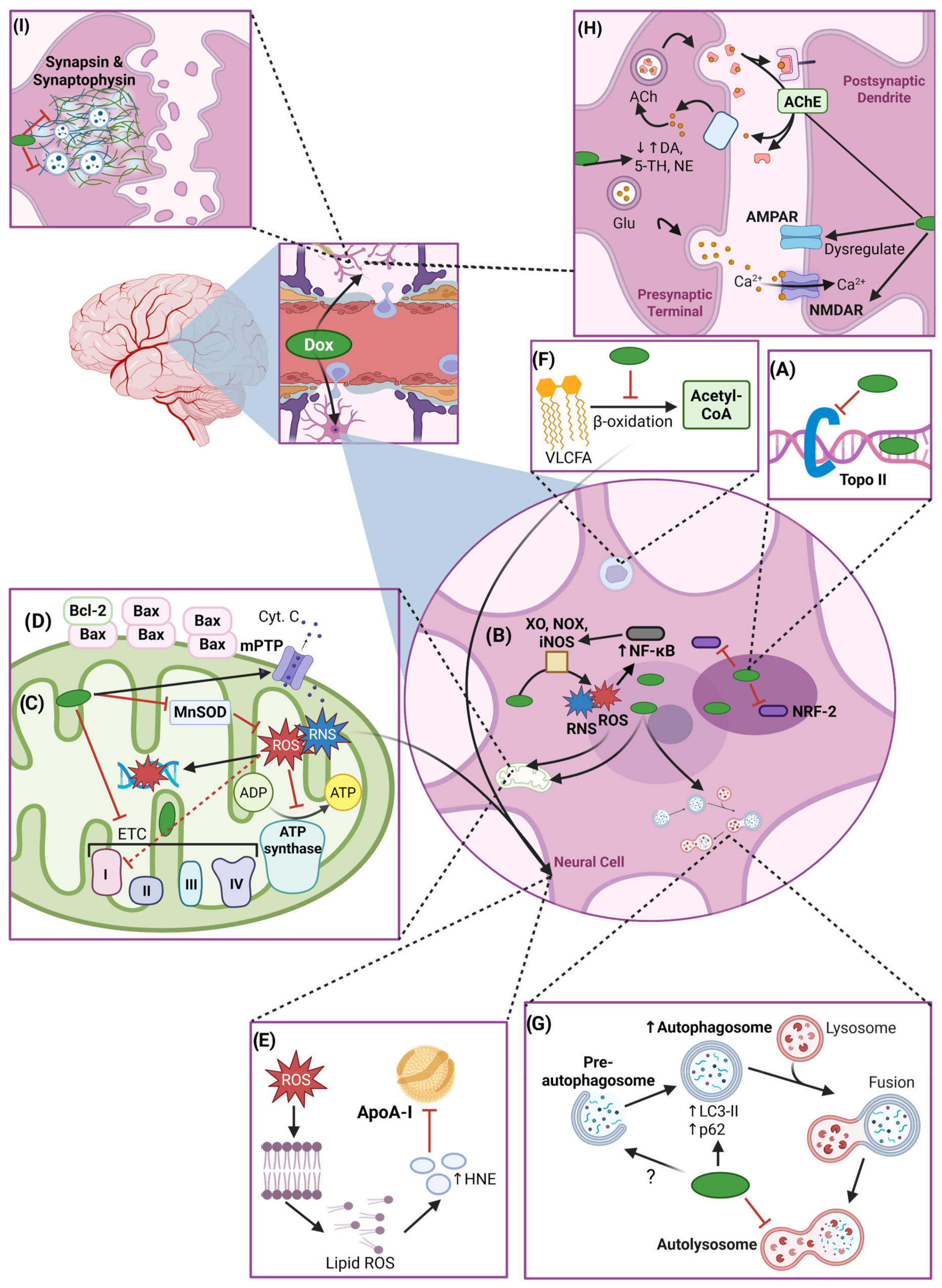
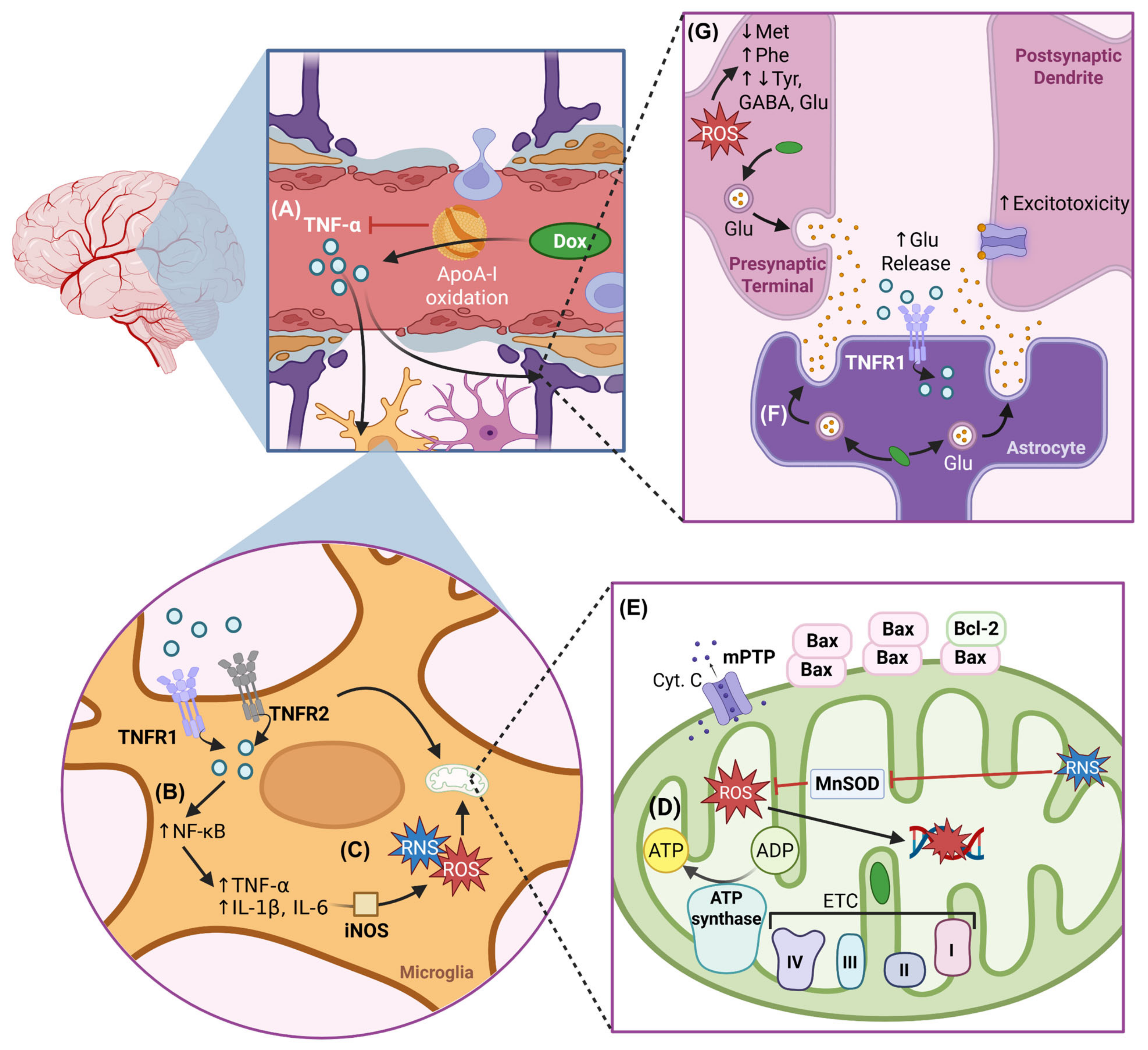
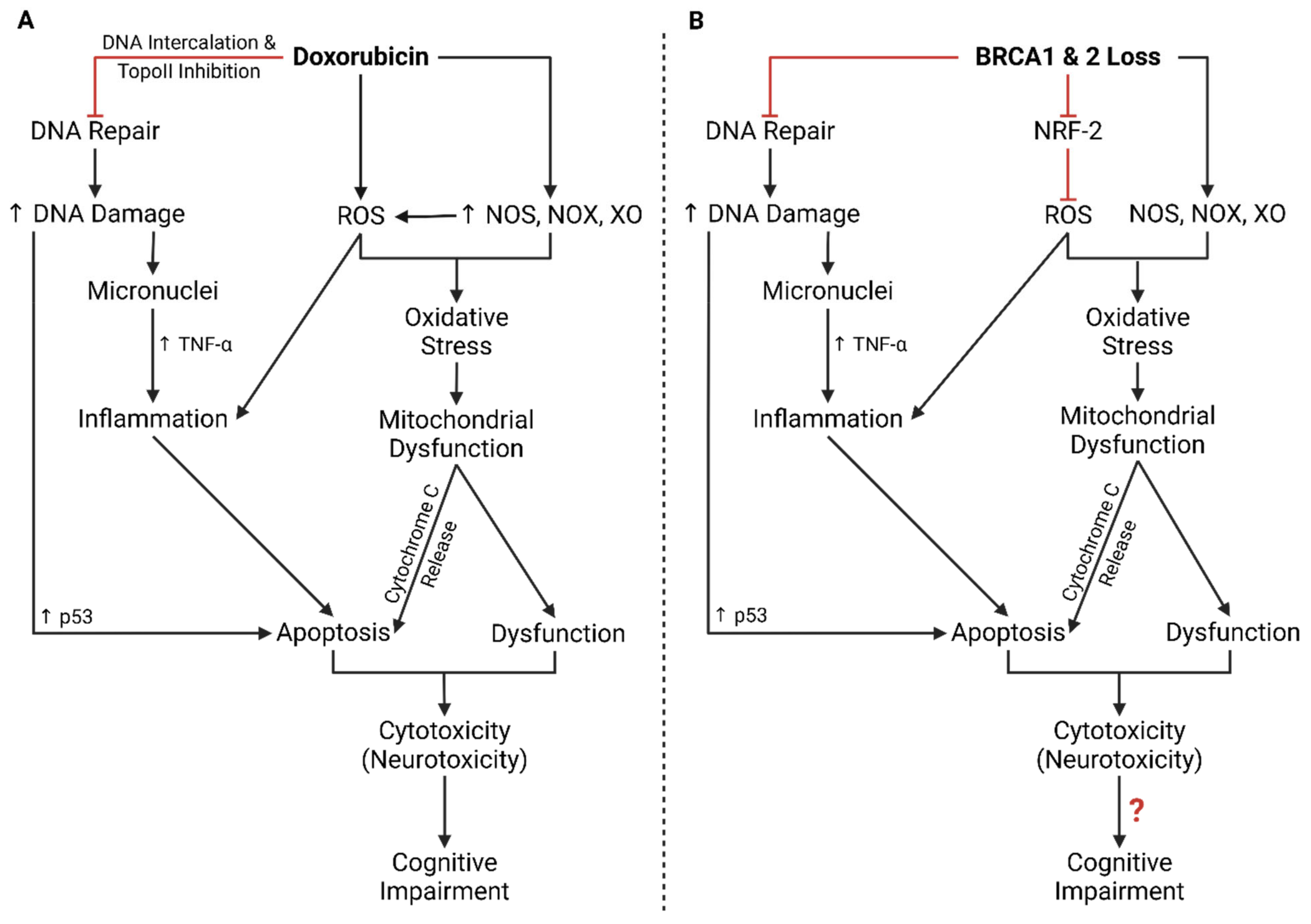
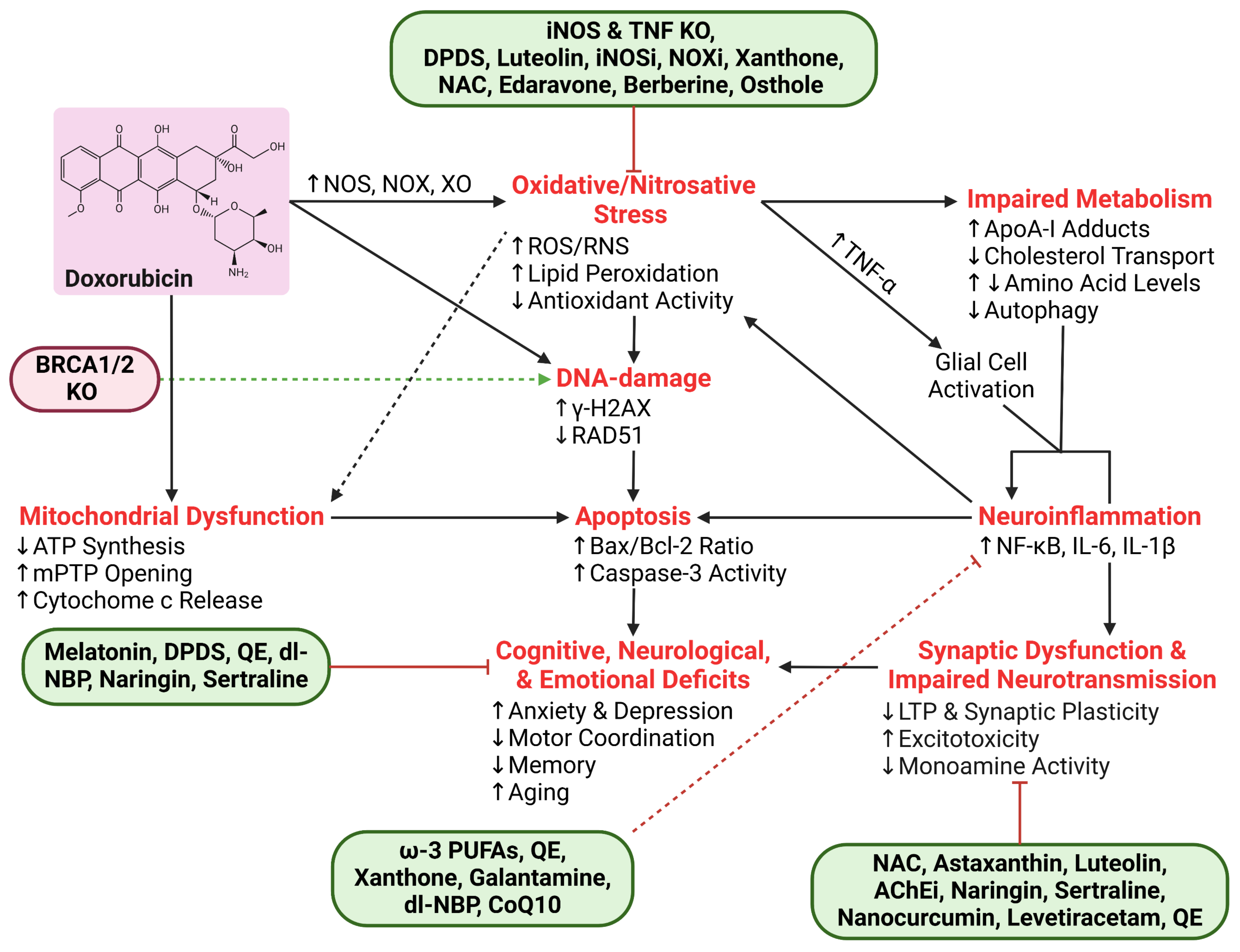
| Process | Protein/Molecule | Effect of Dox | Study Type |
|---|---|---|---|
| Oxidative Stress | NRF-2 | Reduced NRF-2 protein expression and impaired antioxidant functions. | In vivo [154] |
| ROS and RNS | Increased production of ROS and RNS via iNOS, XO, and NOX activity. | In vivo [69,71,74,83,86,90] In vitro [68] | |
| Mitochondrial Dysfunction | mPTP | mPTP opening and subsequent cytochrome c release. | In vivo [87] |
| Neurotransmitter Dysregulation | AMPAR/NMDAR | Altered AMPAR and NMDAR function and Ca2+ influx. | In vivo [110,123] |
| AChE | Altered AChE activity. | In vivo [137,138] | |
| Monoamine Neurotransmitters | ROS-mediated reduction in monoamine levels. | In vivo [96] | |
| Synaptic Dysplasia | Synapsin and Synaptophysin | Decreased synapsin and synaptophysin expression, dendritic spine loss, and synaptic dysfunction. | In vivo [101,147] In vitro [112] |
| Autophagy | LC3-II | Increased LC3-II levels: impaired autophagosome turnover. | In vivo [83] In vitro [153] |
| p62 | Elevated p62 levels: impaired autophagosome degradation. | In vivo [83] In vitro [153] | |
| Beclin-1 | Reduced expression of Beclin-1: impaired pre-autophagosome formation. | In vivo [83] In vitro [153] | |
| Neuroinflammation | TNF-α | Systemic TNF-α elevation and subsequent microglia and astrocyte activation. | In vivo [72,83,123,156,157] In vitro [157] |
| IL-1β and IL-6 | Dox-induced upregulation of IL-1β and IL-6 via NF-κB pathway. | In vivo [70,83] | |
| ApoA-I | Dox-induced ApoA-I oxidization and impaired TNF-α regulation. | In vivo [90,158,161] Clinical study [161] | |
| Metabolism | Cholesterol | Disrupted cholesterol metabolism: altered membrane composition and impaired neuronal function. | In vivo [158,159] |
| Amino Acid | Altered amino acid levels and dysregulated neurotransmitter synthesis and homeostasis. | In vivo [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, K.S.; Singh, A.; Marwaha, G.S.; Ravendranathan, N.; Sandhu, I.S.; Kim, K.; Singh, E.; Frisbee, J.C.; Singh, K.K. Different Mechanisms in Doxorubicin-Induced Neurotoxicity: Impact of BRCA Mutations. Int. J. Mol. Sci. 2025, 26, 4736. https://doi.org/10.3390/ijms26104736
Bhatt KS, Singh A, Marwaha GS, Ravendranathan N, Sandhu IS, Kim K, Singh E, Frisbee JC, Singh KK. Different Mechanisms in Doxorubicin-Induced Neurotoxicity: Impact of BRCA Mutations. International Journal of Molecular Sciences. 2025; 26(10):4736. https://doi.org/10.3390/ijms26104736
Chicago/Turabian StyleBhatt, Kriti S., Aman Singh, Gursharan S. Marwaha, Naresh Ravendranathan, Inderbir S. Sandhu, Kristen Kim, Eesha Singh, Jefferson C. Frisbee, and Krishna K. Singh. 2025. "Different Mechanisms in Doxorubicin-Induced Neurotoxicity: Impact of BRCA Mutations" International Journal of Molecular Sciences 26, no. 10: 4736. https://doi.org/10.3390/ijms26104736
APA StyleBhatt, K. S., Singh, A., Marwaha, G. S., Ravendranathan, N., Sandhu, I. S., Kim, K., Singh, E., Frisbee, J. C., & Singh, K. K. (2025). Different Mechanisms in Doxorubicin-Induced Neurotoxicity: Impact of BRCA Mutations. International Journal of Molecular Sciences, 26(10), 4736. https://doi.org/10.3390/ijms26104736






