Cellular Metabolic Disorders in a Cohort of Patients with Sjogren’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Genetic Studies
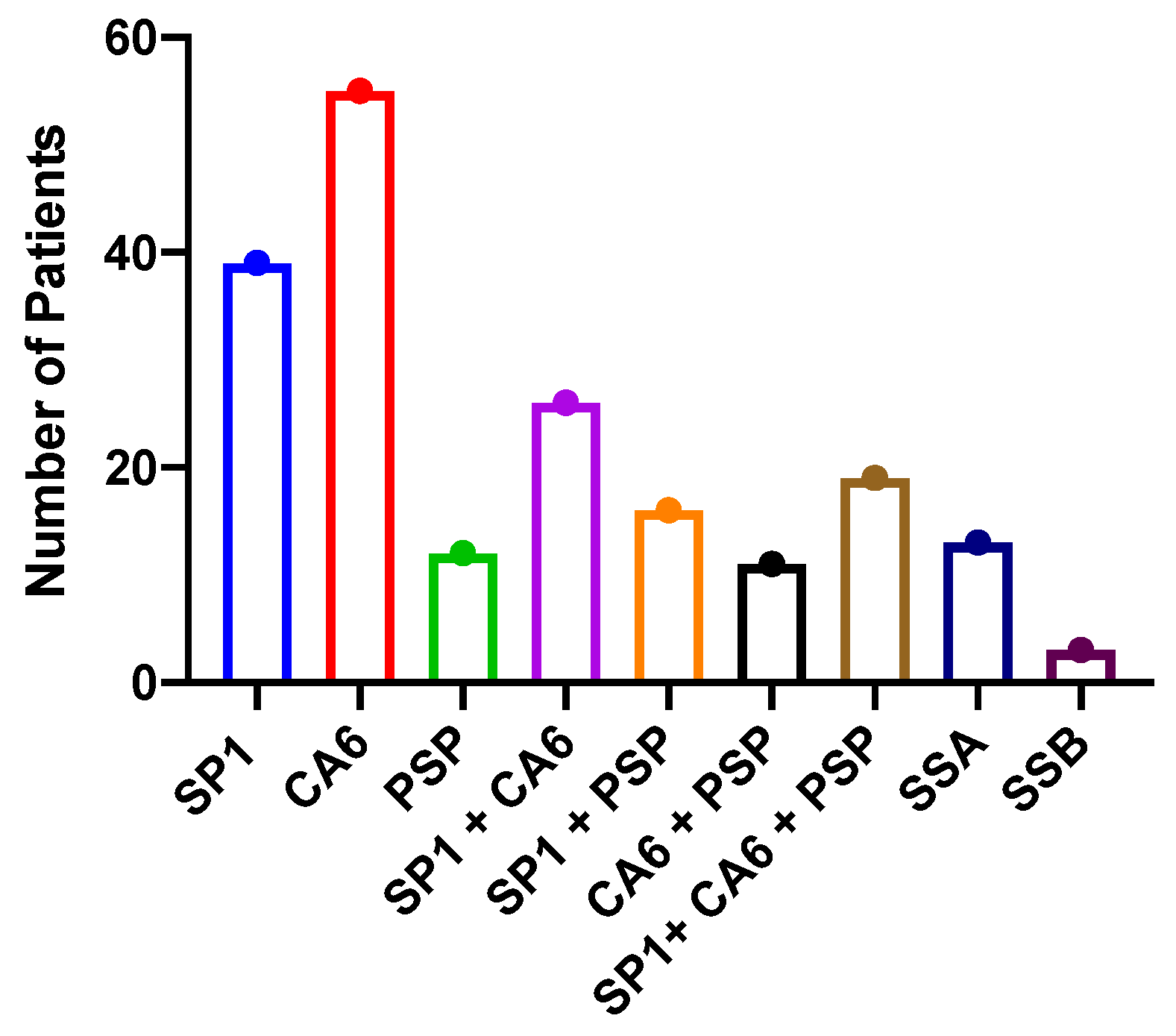
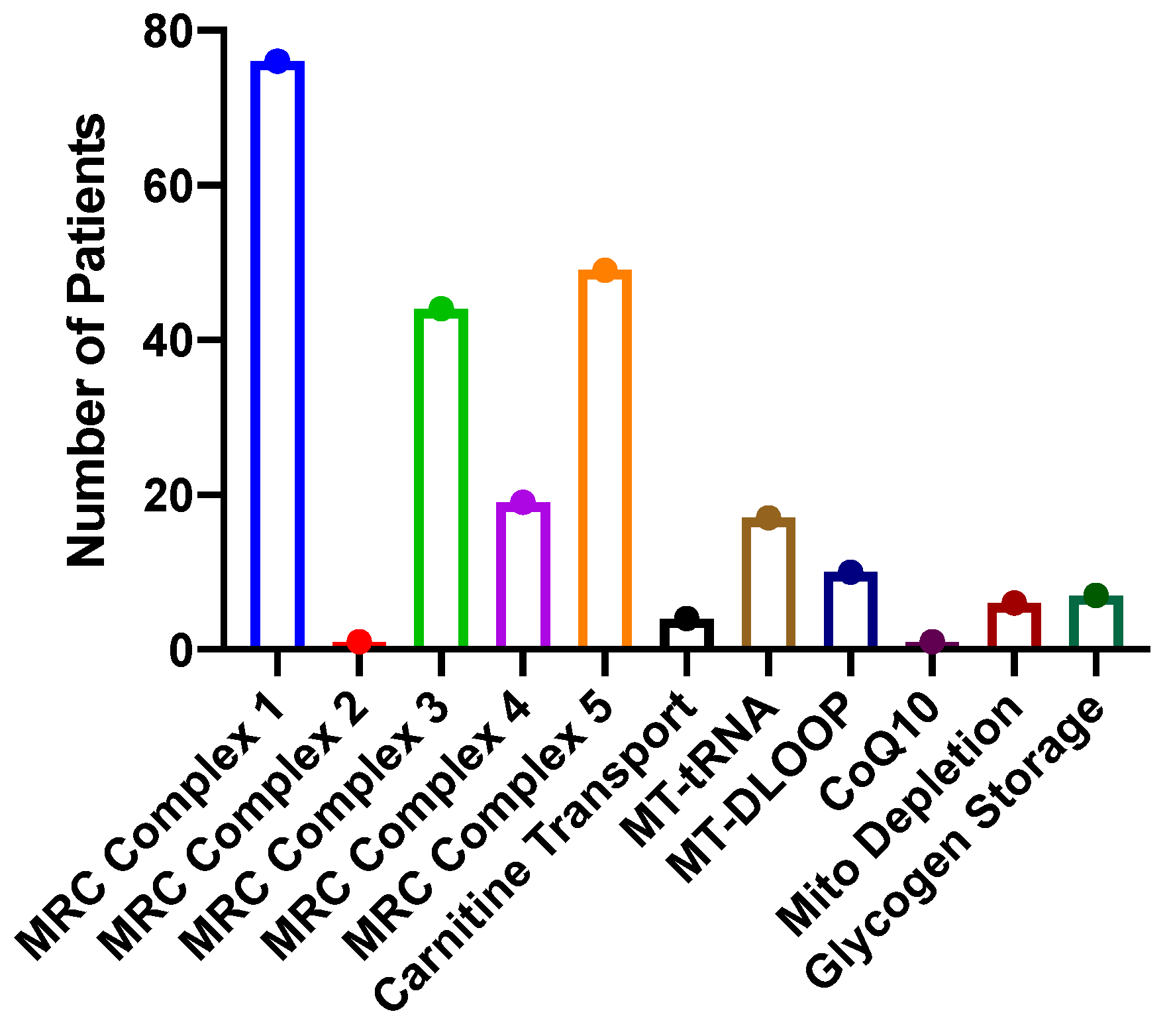
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanco, L.P.; Kaplan, M.J. Metabolic alterations of the immune system in the pathogenesis of autoimmune diseases. PLoS Biol. 2023, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.D.; Tse, H.M. Targeting Mitochondrial-Derived Reactive Oxygen Species in T Cell-Mediated Autoimmune Diseases. Front. Immunol. 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Clayton, S.A.; MacDonald, L.; Kurowska-Stolarska, M.; Clark, A.R. Mitochondria as Key Players in the Pathogenesis and Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 673916. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Berod, L.; Kamradt, T.; Sparwasser, T. Immunometabolism and autoimmunity. Immunol. Cell Biol. 2016, 94, 925–934. [Google Scholar] [CrossRef]
- Huang, N.; Perl, A. Metabolism as a Target for Modulation in Autoimmune Diseases. Trends Immunol. 2018, 39, 562–576. [Google Scholar] [CrossRef]
- Mubariki, R.; Vadasz, Z. The role of B cell metabolism in autoimmune diseases. Autoimmun. Rev. 2022, 21, 5. [Google Scholar] [CrossRef]
- Perl, A. Metabolic Control of Immune System Activation in Rheumatic Diseases. Arthritis Rheumatol. 2017, 69, 2259–2270. [Google Scholar] [CrossRef]
- Choi, S.C.; Titov, A.A.; Sivakumar, R.; Li, W.; Morel, L. Immune Cell Metabolism in Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2016, 18, 66. [Google Scholar] [CrossRef]
- Lightfoot, Y.L.; Blanco, L.P.; Kaplan, M.J. Metabolic abnormalities and oxidative stress in lupus. Curr. Opin. Rheumatol. 2017, 29, 442–449. [Google Scholar] [CrossRef]
- Monteith, A.J.; Miller, J.M.; Williams, J.M.; Voss, K.; Rathmell, J.C.; Crofford, L.J.; Skaar, E.P. Altered Mitochondrial Homeostasis during Systemic Lupus Erythematosus Impairs Neutrophil Extracellular Trap Formation Rendering Neutrophils Ineffective at Combating Staphylococcus aureus. J. Immunol. 2022, 208, 454–463. [Google Scholar] [CrossRef]
- Morel, L. Immunometabolism in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2017, 13, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.A.; Wilkinson, M.G.L.; Wincup, C. The Role of Immunometabolism in the Pathogenesis of Systemic Lupus Erythematosus. Front. Immunol. 2022, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.; Tsokos, G.C. T cell metabolism: New insights in systemic lupus erythematosus pathogenesis and therapy. Nat. Rev. Rheumatol. 2020, 16, 100–112. [Google Scholar] [CrossRef]
- Takeshima, Y.; Iwasaki, Y.; Fujio, K.; Yamamoto, K. Metabolism as a key regulator in the pathogenesis of systemic lupus erythematosus. Semin. Arthritis Rheum. 2019, 48, 1142–1145. [Google Scholar] [CrossRef]
- Zhang, C.X.; Wang, H.Y.; Yin, L.; Mao, Y.Y.; Zhou, W. Immunometabolism in the pathogenesis of systemic lupus erythematosus. J. Transl. Autoimmun. 2020, 3, 10. [Google Scholar] [CrossRef]
- Colafrancesco, S.; Simoncelli, E.; Priori, R.; Bombardieri, M. The pathogenic role of metabolism in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2023, 41, 2538–2546. [Google Scholar] [CrossRef]
- Luo, D.Y.; Li, L.; Wu, Y.C.; Yang, Y.; Ye, Y.L.; Hu, J.W.; Gao, Y.M.; Zeng, N.Y.; Fei, X.C.; Li, N.; et al. Mitochondria-related genes and metabolic profiles of innate and adaptive immune cells in primary Sjogren’s syndrome. Front. Immunol. 2023, 14, 16. [Google Scholar] [CrossRef]
- Apaydin, H.; Bicer, C.K.; Yurt, E.F.; Serdar, M.A.; Dogan, I.; Erten, S. Elevated Kynurenine Levels in Patients with Primary Sjogren’s Syndrome. Lab. Med. 2023, 54, 166–172. [Google Scholar] [CrossRef]
- Blokland, S.L.M.; Hillen, M.R.; Wichers, C.G.K.; Zimmermann, M.; Kruize, A.A.; Radstake, T.; Broen, J.C.A.; van Roon, J.A.G. Increased mTORC1 activation in salivary gland B cells and T cells from patients with Sjogren’s syndrome: mTOR inhibition as a novel therapeutic strategy to halt immunopathology? RMD Open 2019, 5, e000701. [Google Scholar] [CrossRef]
- Katsiougiannis, S.; Stergiopoulos, A.; Moustaka, K.; Havaki, S.; Samiotaki, M.; Stamatakis, G.; Tenta, R.; Skopouli, F.N. Salivary gland epithelial cell in Sjogren?s syndrome: Metabolic shift and altered mitochondrial morphology toward an innate immune cell function. J. Autoimmun. 2023, 136, 8. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Hu, J.; Wu, Y.; Yang, J.; Fan, H.; Li, L.; Luo, D.; Ye, Y.; Gao, Y.; et al. A Link Between Mitochondrial Dysfunction and the Immune Microenvironment of Salivary Glands in Primary Sjogren’s Syndrome. Front. Immunol. 2022, 13, 845209. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Castello, G.; Pallardo, F.V. Sjogren’s syndrome-associated oxidative stress and mitochondrial dysfunction: Prospects for chemoprevention trials. Free Radic. Res. 2013, 47, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Wadan, A.S.; Ahmed, M.A.; Ahmed, A.H.; Ellakwa, D.E.; Elmoghazy, N.H.; Gawish, A. The Interplay of Mitochondrial Dysfunction in Oral Diseases: Recent Updates in Pathogenesis and Therapeutic Implications. Mitochondrion 2024, 78, 21. [Google Scholar] [CrossRef]
- Ambrus, J.L.; Jacob, A.; Weisman, G.A.; He, J. Metabolic changes in the evolution of Sjogren’s syndrome in a mouse model. Clin. Exp. Rheumatol. 2018, 36, S301. [Google Scholar]
- Suresh, L.; Ambrus, J.; Shen, L.; Vishwanath, S. Metabolic Disorders Causing Fatigue in Sjogren’s Syndrome. Arthritis Rheumatol. 2014, 66, S1113. [Google Scholar]
- Bax, K.; Isackson, P.J.; Moore, M.; Ambrus, J.L. Carnitine Palmitoyl Transferase Deficiency in a University Immunology Practice. Curr. Rheumatol. Rep. 2020, 22, 8. [Google Scholar] [CrossRef]
- Barrera, M.J.; Aguilera, S.; Castro, I.; Carvajal, P.; Jara, D.; Molina, C.; Gonzalez, S.; Gonzalez, M.J. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: Potential role in Sjogren’s syndrome. Autoimmun. Rev. 2021, 20, 102867. [Google Scholar] [CrossRef]
- De Benedittis, G.; Latini, A.; Colafrancesco, S.; Priori, R.; Perricone, C.; Novelli, L.; Borgiani, P.; Ciccacci, C. Alteration of Mitochondrial DNA Copy Number and Increased Expression Levels of Mitochondrial Dynamics-Related Genes in Sjogren’s Syndrome. Biomedicines 2022, 10, 2699. [Google Scholar] [CrossRef]
- Kang, Y.; Sun, Y.; Zhang, Y.; Wang, Z. Cytochrome c is important in apoptosis of labial glands in primary Sjogren’s syndrome. Mol. Med. Rep. 2018, 17, 1993–1997. [Google Scholar] [CrossRef]
- Abdullah, M.; Vishwanath, S.; Elbalkhi, A.; Ambrus, J.L., Jr. Mitochondrial myopathy presenting as fibromyalgia: A case report. J. Med. Case Rep. 2012, 6, 55. [Google Scholar] [CrossRef]
- Ambrus, J.J.; Isackson, P.J.; Moore, M.; Butsch, J.; Balos, L. Investigating Fatigue and Exercise Inotolerance in a University Immunology Clinic. Arch. Rheumatol. Arthritis Res. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Montano, V.; Gruosso, F.; Simoncini, C.; Siciliano, G.; Mancuso, M. Clinical features of mtDNA-related syndromes in adulthood. Arch. Biochem. Biophys. 2021, 697, 108689. [Google Scholar] [CrossRef] [PubMed]
- Vivino, F.B.; Bunya, V.Y.; Massaro-Giordano, G.; Johr, C.R.; Giattino, S.L.; Schorpion, A.; Shafer, B.; Peck, A.; Sivils, K.; Rasmussen, A.; et al. Sjogren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clin. Immunol. 2019, 203, 81–121. [Google Scholar] [CrossRef] [PubMed]
- De Langhe, E.; Bossuyt, X.; Shen, L.; Malyavantham, K.; Ambrus, J.L.; Suresh, L. Evaluation of Autoantibodies in Patients with Primary and Secondary Sjogren’s Syndrome. Open Rheumatol. J. 2017, 11, 10–15. [Google Scholar] [CrossRef]
- Everett, S.; Vishwanath, S.; Cavero, V.; Shen, L.; Suresh, L.; Malyavantham, K.; Lincoff-Cohen, N.; Ambrus, J.L., Jr. Analysis of novel Sjogren’s syndrome autoantibodies in patients with dry eyes. BMC Ophthalmol. 2017, 17, 20. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Chen, J.; Shao, M.; Zhang, R.; Liang, Y.; Zhang, X.; Zhang, X.; Zhang, Q.; Li, F.; et al. Tissue-Specific Autoantibodies Improve Diagnosis of Primary Sjogren’s Syndrome in the Early Stage and Indicate Localized Salivary Injury. J. Immunol. Res. 2019, 2019, 3642937. [Google Scholar] [CrossRef]
- Karakus, S.; Baer, A.N.; Akpek, E.K. Clinical Correlations of Novel Autoantibodies in Patients with Dry Eye. J. Immunol. Res. 2019, 2019, 7935451. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. The mitochondrial cocktail: Rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv. Drug Deliv. Rev. 2008, 60, 1561–1567. [Google Scholar] [CrossRef]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Vega, A.F.; de la Mata, M.; Pavon, A.D.; Alcocer-Gomez, E.; Calero, C.P.; Paz, M.V.; Alanis, M.; et al. Clinical applications of coenzyme Q10. Front. Biosci.-Landmark 2014, 19, 619–633. [Google Scholar] [CrossRef]
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Altern. Ther. Health Med. 2014, 20 (Suppl. 1), 18–25. [Google Scholar]
- Glover, E.I.; Martin, J.; Maher, A.; Thornhill, R.E.; Moran, G.R.; Tarnopolsky, M.A. A randomized trial of coenzyme Q(10) in mitochondrial disorders. Muscle Nerve 2010, 42, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, I.; Shadiack, E.; Ganetzky, R.D.; Falk, M.J. Mitochondrial medicine therapies: Rationale, evidence, and dosing guidelines. Curr. Opin. Pediatr. 2020, 32, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Prasad, S.K.; Banerjee, O.; Singh, S.; Banerjee, A.; Bose, A.; Pal, S.; Maji, B.K.; Mukherjee, S. Targeting mitochondria with folic acid and vitamin B-12 ameliorates nicotine mediated islet cell dysfunction. Environ. Toxicol. 2018, 33, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Avula, S.; Parikh, S.; Demarest, S.; Kurz, J.; Gropman, A. Treatment of Mitochondrial Disorders. Curr. Treat. Options Neurol. 2014, 16, 292. [Google Scholar] [CrossRef]
- Ezerina, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Bornstein, R.; Mulholland, M.T.; Sedensky, M.; Morgan, P.; Johnson, S.C. Glutamine metabolism in diseases associated with mitochondrial dysfunction. Mol. Cell. Neurosci. 2023, 126, 12. [Google Scholar] [CrossRef]
- Hannah, W.B.; Derks, T.G.J.; Drumm, M.L.; Gruenert, S.C.; Kishnani, P.S.; Vissing, J. Glycogen storage diseases. Nat. Rev. Dis. Primers 2023, 9, 23. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Beckemeyer, A.A.; Mendelsohn, N.J. The new era of Pompe disease: Advances in the detection, understanding of the phenotypic spectrum, pathophysiology, and management. Am. J. Med. Genet. Part C-Semin. Med. Genet. 2012, 160, 1–7. [Google Scholar] [CrossRef]
- Kley, R.A.; Tarnopolsky, M.A.; Vorgerd, M. Creatine treatment in muscle disorders: A meta-analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 2008, 79, 366–367. [Google Scholar] [CrossRef]
- Llavero, F.; Sastre, A.A.; Montoro, M.L.; Galvez, P.; Lacerda, H.M.; Parada, L.A.; Zugaza, J.L. McArdle Disease: New Insights into Its Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2019, 20, 5919. [Google Scholar] [CrossRef] [PubMed]
- Meena, N.K.; Raben, N. Pompe Disease: New Developments in an Old Lysosomal Storage Disorder. Biomolecules 2020, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Vissing, J.; Haller, R.G. The effect of oral sucrose on exercise tolerance in patients with McArdle’s disease. N. Engl. J. Med. 2003, 349, 2503–2509. [Google Scholar] [CrossRef]
- Raben, N.; Wong, A.; Ralston, E.; Myerowitz, R. Autophagy and mitochondria in Pompe disease: Nothing is so new as what has long been forgotten. Am. J. Med. Genet. Part C-Semin. Med. Genet. 2012, 160, 13–21. [Google Scholar] [CrossRef]
- Mishra, K.; Kakhlon, O. Mitochondrial Dysfunction in Glycogen Storage Disorders (GSDs). Biomolecules 2024, 14, 1096. [Google Scholar] [CrossRef]
- Hogendorf, A.; Zielinski, M.; Constantinou, M.; Smigiel, R.; Wierzba, J.; Wyka, K.; Wedrychowicz, A.; Jakubiuk-Tomaszuk, A.; Budzynska, E.; Piotrowicz, M.; et al. Immune Dysregulation in Patients with Chromosome 18q Deletions-Searching for Putative Loci for Autoimmunity and Immunodeficiency. Front. Immunol. 2021, 12, 742834. [Google Scholar] [CrossRef]
- Panimolle, F.; Tiberti, C.; Spaziani, M.; Riitano, G.; Lucania, G.; Anzuini, A.; Lenzi, A.; Gianfrilli, D.; Sorice, M.; Radicioni, A.F. Non-organ-specific autoimmunity in adult 47,XXY Klinefelter patients and higher-grade X-chromosome aneuploidies. Clin. Exp. Immunol. 2021, 205, 316–325. [Google Scholar] [CrossRef]
- Ryo, K.; Yamada, H.; Nakagawa, Y.; Tai, Y.; Obara, K.; Inoue, H.; Mishima, K.; Saito, I. Possible involvement of oxidative stress in salivary gland of patients with Sjogren’s syndrome. Pathobiology 2006, 73, 252–260. [Google Scholar] [CrossRef]
- Norheim, K.B.; Le Hellard, S.; Nordmark, G.; Harboe, E.; Goransson, L.; Brun, J.G.; Wahren-Herlenius, M.; Jonsson, R.; Omdal, R. A possible genetic association with chronic fatigue in primary Sjogren’s syndrome: A candidate gene study. Rheumatol. Int. 2014, 34, 191–197. [Google Scholar] [CrossRef]
- Amaya-Uribe, L.; Rojas, M.; Azizi, G.; Anaya, J.M.; Gershwin, M.E. Primary immunodeficiency and autoimmunity: A comprehensive review. J. Autoimmun. 2019, 99, 52–72. [Google Scholar] [CrossRef]
- Maslinska, M.; Kostyra-Grabczak, K. The role of virus infections in Sjogren’s syndrome. Front. Immunol. 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, G.; Cappelli, S.; Consolini, R. Autoimmunity in Primary Immunodeficiency Disorders: An Updated Review on Pathogenic and Clinical Implications. J. Clin. Med. 2021, 10, 4729. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Sawalha, A.H. The Role of Oxidative Stress in Epigenetic Changes Underlying Autoimmunity. Antioxid. Redox Signal. 2022, 36, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Smolkin, R.M.; Chowdhury, P.; Fernandez, K.C.; Kim, Y.; Cols, M.; Alread, W.; Yen, W.F.; Hu, W.; Wang, Z.M.; et al. Distinct metabolic requirements regulate B cell activation and germinal center responses. Nat. Immunol. 2023, 24, 1358–1369. [Google Scholar] [CrossRef]
- Tomaszewicz, M.; Ronowska, A.; Zielinski, M.; Jankowska-Kulawy, A.; Trzonkowski, P. T regulatory cells metabolism: The influence on functional properties and treatment potential. Front. Immunol. 2023, 14, 11. [Google Scholar] [CrossRef]
- Abboud, G.; Choi, S.C.; Zhang, X.J.; Park, Y.P.; Kanda, N.; Zeumer-Spataro, L.; Terrell, M.; Teng, X.Y.; Nundel, K.; Shlomchik, M.J.; et al. Glucose Requirement of Antigen-Specific Autoreactive B Cells and CD4+ T Cells. J. Immunol. 2023, 210, 377–388. [Google Scholar] [CrossRef]
- Dimeloe, S.; Burgener, A.V.; Grahlert, J.; Hess, C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology 2017, 150, 35–44. [Google Scholar] [CrossRef]
- Yin, Y.; Choi, S.C.; Xu, Z.; Perry, D.J.; Seay, H.; Croker, B.P.; Sobel, E.S.; Brusko, T.M.; Morel, L. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 2015, 7, 274ra218. [Google Scholar] [CrossRef]
- Yu, H.Y.; Jacquelot, N.; Belz, G.T. Metabolic features of innate lymphoid cells. J. Exp. Med. 2022, 219, 15. [Google Scholar] [CrossRef]
- Shiraz, A.K.; Panther, E.J.; Reilly, C.M. Altered Germinal-Center Metabolism in B Cells in Autoimmunity. Metabolites 2022, 12, 40. [Google Scholar] [CrossRef]
- Buck, M.D.; O’Sullivan, D.; Pearce, E.L. T cell metabolism drives immunity. J. Exp. Med. 2015, 212, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Chakraborty, D.; Malik, S.; Mann, S.; Agnihotri, P.; Monu, M.; Kumar, V.; Biswas, S. Alpha-Taxilin: A Potential Diagnosis and Therapeutics Target in Rheumatoid Arthritis Which Interacts with Key Glycolytic Enzymes Associated with Metabolic Shifts in Fibroblast-Like Synoviocytes. J. Inflamm. Res. 2024, 17, 10027–10045. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Suresh, L.; Malyavantham, K.; Kowal, P.; Xuan, J.X.; Lindemann, M.J.; Ambrus, J.L. Different Stages of Primary Sjogren’s Syndrome Involving Lymphotoxin and Type 1 IFN. J. Immunol. 2013, 191, 608–613. [Google Scholar] [CrossRef]
- Shen, L.; Gao, C.; Suresh, L.; Xian, Z.; Song, N.; Chaves, L.D.; Yu, M.; Ambrus, J.L., Jr. Central role for marginal zone B cells in an animal model of Sjogren’s syndrome. Clin. Immunol. 2016, 168, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; He, J.; Peck, A.; Jamil, A.; Bunya, V.; Alexander, J.; JL, A.J. Metabolic changes during evolution of Sjogren’s in both an animal model and human patients. Heliyon 2025, 11, e41082. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Jasek, M.C.; Malyavantham, K.; Suresh, L.; Ambrus, J.L.; Pardo, D. Two Year Results with IgG, IgA and IgM Antibody Specific to SP-1, PSP and CA-6 early Novel Antigens Compared to Classic Biomarkers in 2306 Dry Eye Patients. Scand. J. Immunol. 2015, 81, 348–349. [Google Scholar]
- Suresh, L.; Malyavantham, K.; Shen, L.; Ambrus, J.L. Investigation of novel autoantibodies in Sjogren’s syndrome utilizing Sera from the Sjogren’s international collaborative clinical alliance cohort. BMC Ophthalmol. 2015, 15, 38. [Google Scholar] [CrossRef]
- Shen, L.; Kapsogeorgou, E.K.; Yu, M.X.; Suresh, L.; Malyavantham, K.; Tzioufas, A.G.; Ambrus, J.L. Evaluation of salivary gland protein 1 antibodies in patients with primary and secondary Sjogren’s syndrome. Clin. Immunol. 2014, 155, 42–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrus, J.L.; Jacob, A.; Shukla, A.A. Cellular Metabolic Disorders in a Cohort of Patients with Sjogren’s Disease. Int. J. Mol. Sci. 2025, 26, 4668. https://doi.org/10.3390/ijms26104668
Ambrus JL, Jacob A, Shukla AA. Cellular Metabolic Disorders in a Cohort of Patients with Sjogren’s Disease. International Journal of Molecular Sciences. 2025; 26(10):4668. https://doi.org/10.3390/ijms26104668
Chicago/Turabian StyleAmbrus, Julian L., Alexander Jacob, and Abhay A. Shukla. 2025. "Cellular Metabolic Disorders in a Cohort of Patients with Sjogren’s Disease" International Journal of Molecular Sciences 26, no. 10: 4668. https://doi.org/10.3390/ijms26104668
APA StyleAmbrus, J. L., Jacob, A., & Shukla, A. A. (2025). Cellular Metabolic Disorders in a Cohort of Patients with Sjogren’s Disease. International Journal of Molecular Sciences, 26(10), 4668. https://doi.org/10.3390/ijms26104668




