Potential Cutaneous Applications of Boesenbergia rotunda Extract Based on Its In Vitro Anti-Melanogenic and Anti-Fibroproliferative Properties
Abstract
1. Introduction
2. Results and Discussion
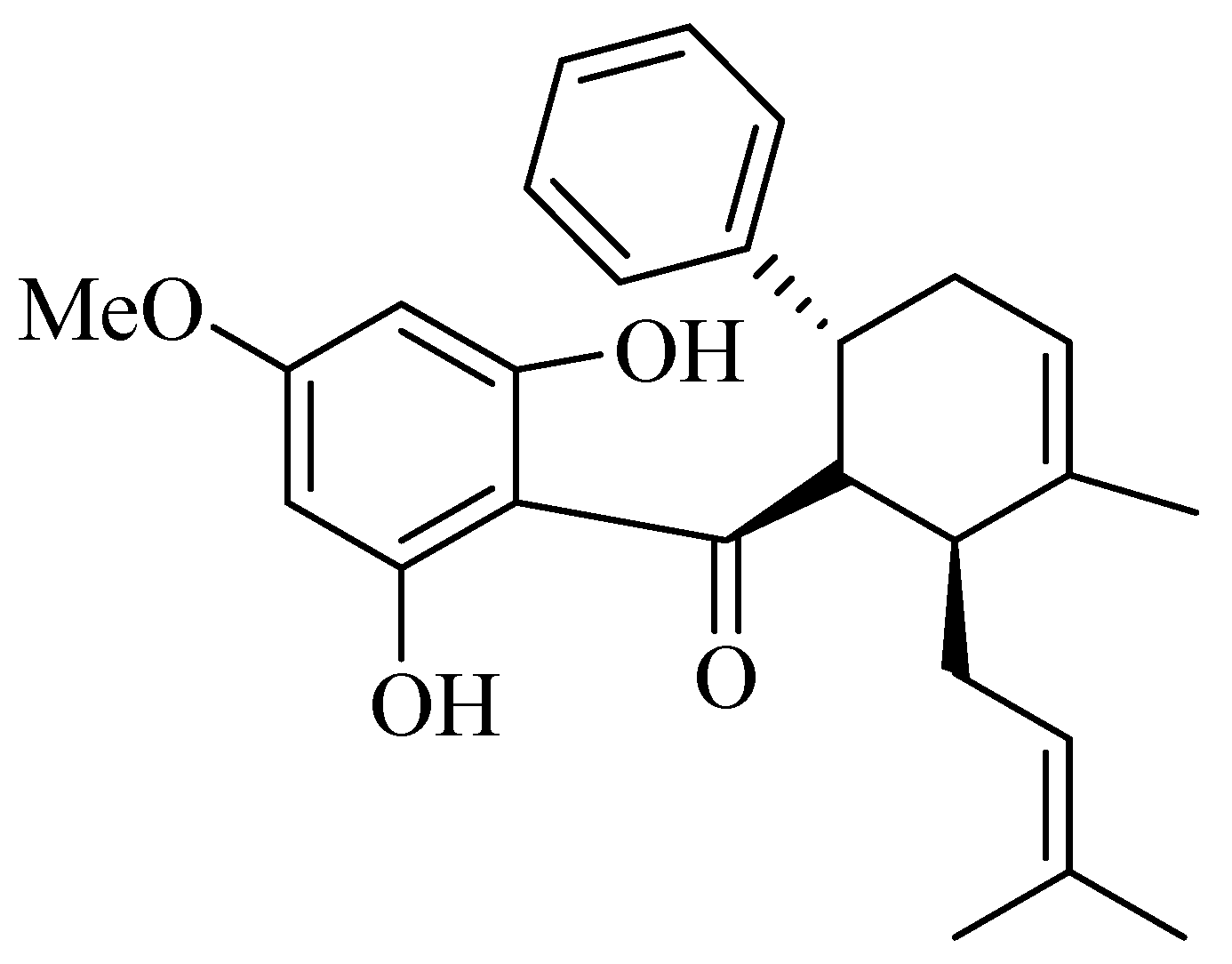
2.1. In Vitro Study of the Effect of Panduratin A and B. rotunda Extract on Cellular Melanogenesis Induced by UV Radiation
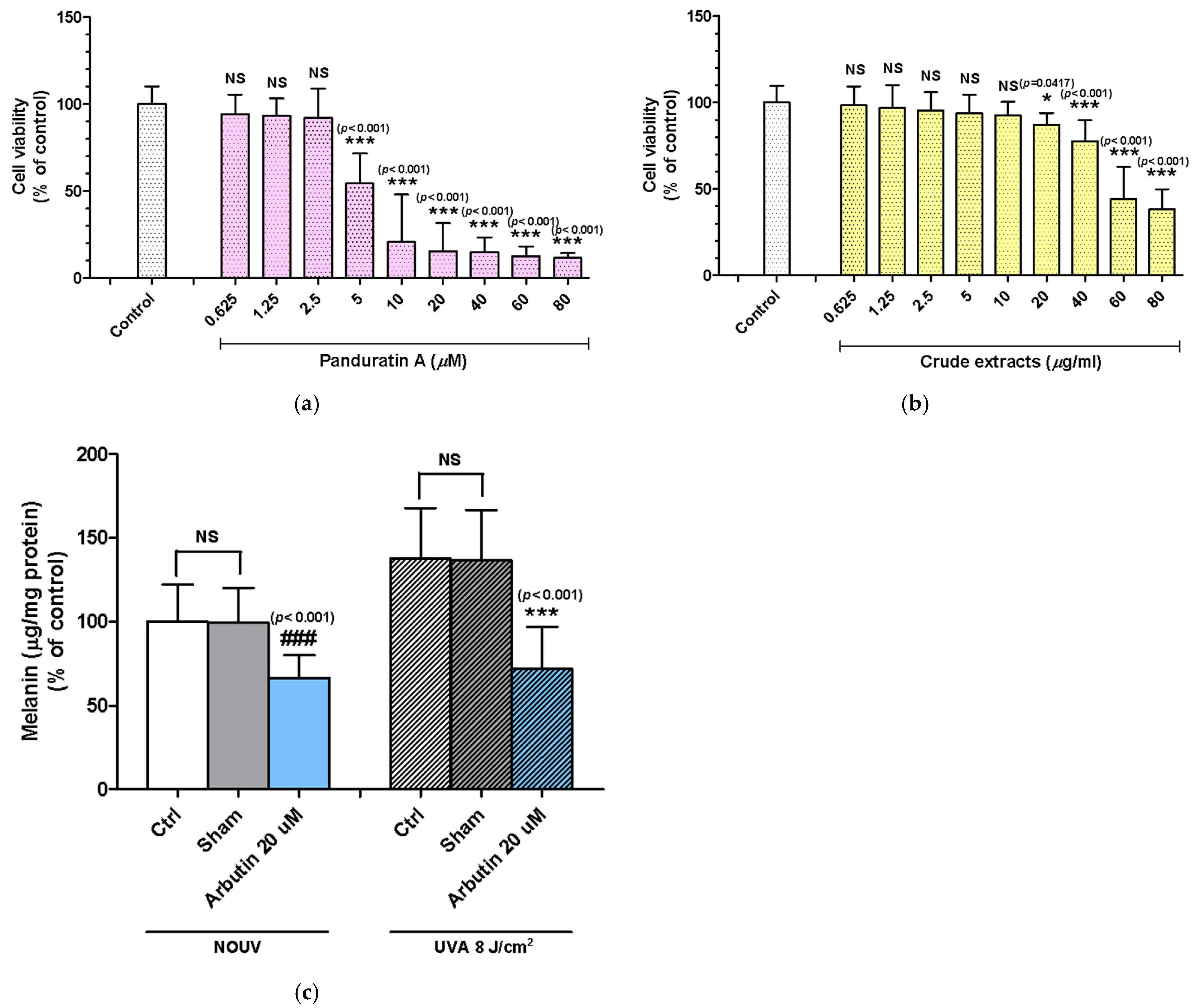
Cytotoxicity
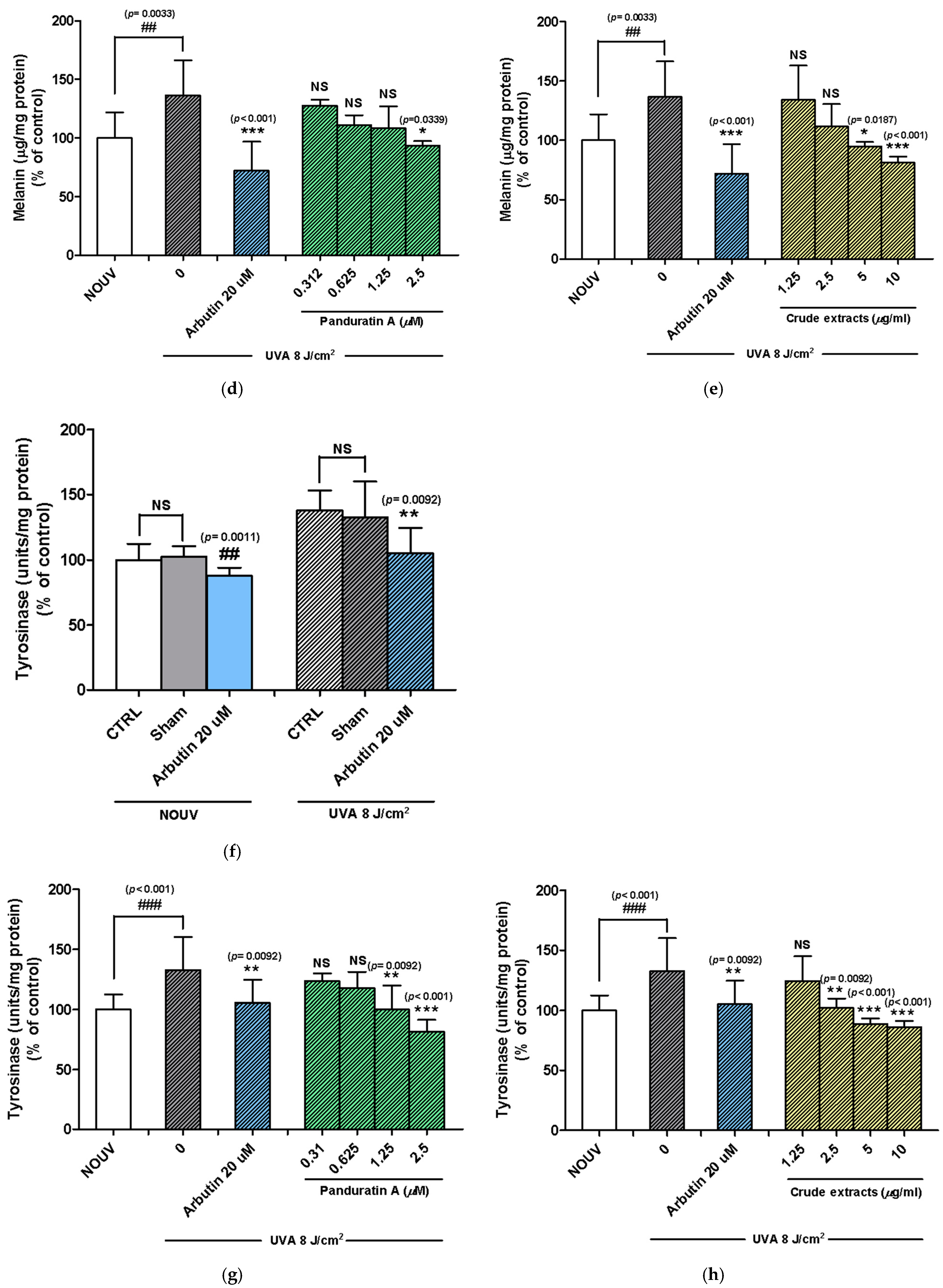
2.2. Panduratin A and Fingerroot Extract Inhibited UVA-Induced Melanin Content in B16F10 Cells
2.3. Panduratin A and Fingerroot Extract Inhibited UVA-Induced Tyrosinase Activity in B16F10 Cells
2.4. Summary of Study in Cellular Melanogenesis
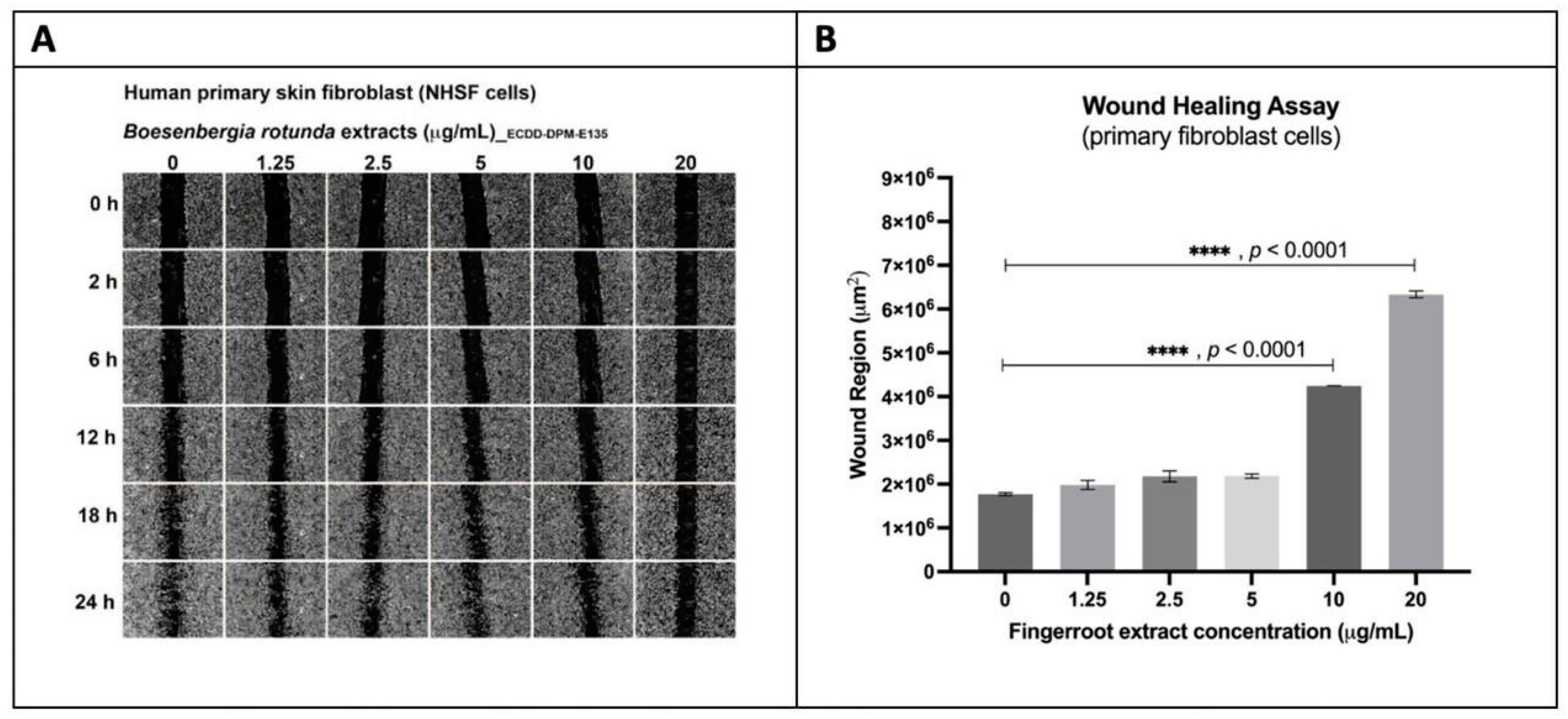
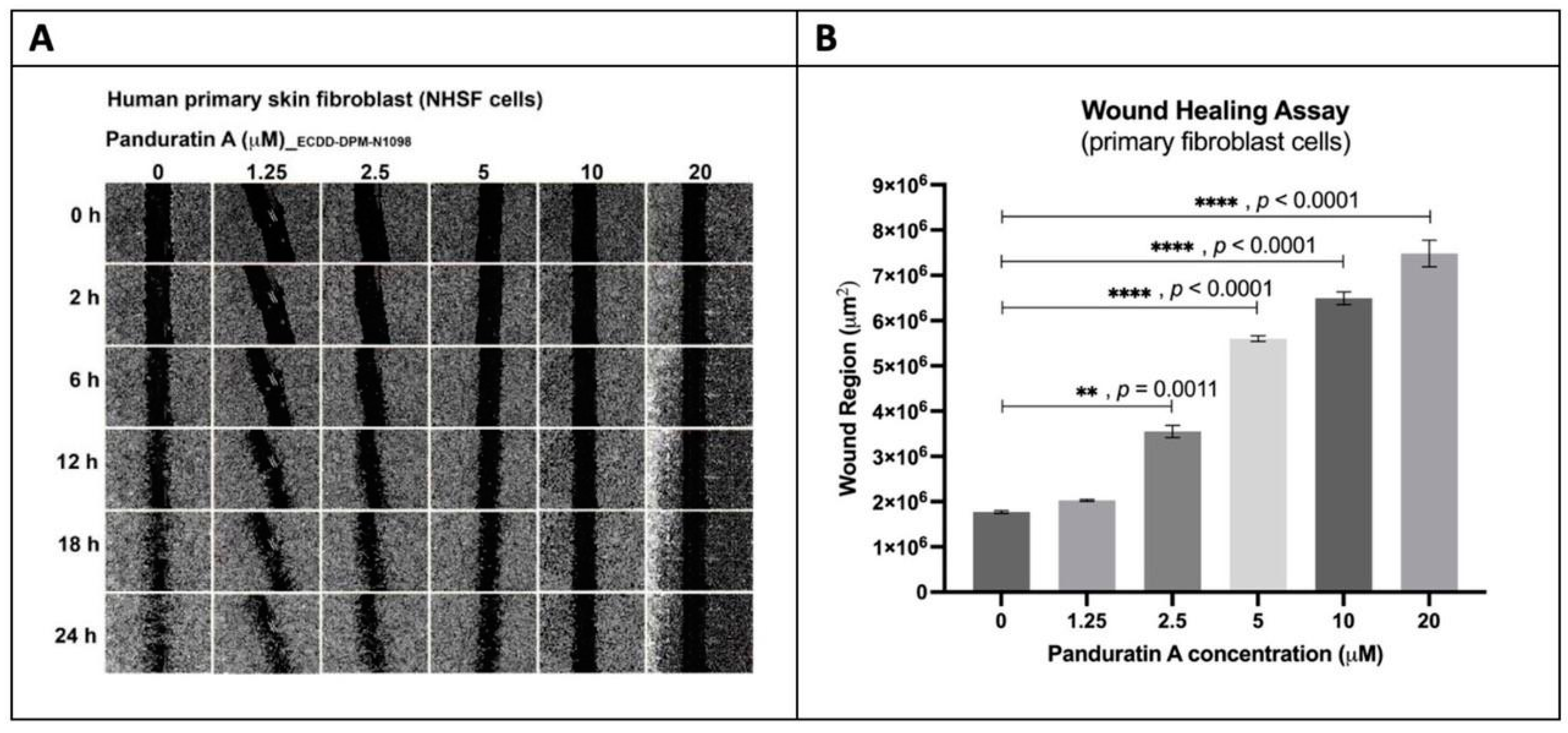
2.5. Evaluation of the Wound-Healing Effect
3. Materials and Methods
3.1. Crude Extract and Panduratin A Preparation
3.2. Cell Culture
3.2.1. B16F10 Mouse Melanoma Cells
3.2.2. Human Primary Fibroblasts
3.2.3. Cytotoxicity Test of Panduratin A and B. rotunda Extract on B16F10 Cells
3.2.4. Treatment of Cells with Phenolics and UVA Irradiation
3.2.5. Preparation of Total Cell Lysate
3.2.6. Melanin Content Assay
3.2.7. Tyrosinase Activity Assay
3.2.8. Protein Quantification and Normalization
3.2.9. In Vitro Wound Healing, Proliferation, and Migration Assay
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wu, S.; Wu, H.; Liang, X.; Guo, D.; Zhuo, F. Comparison of the Efficacy of Melasma Treatments: A Network Meta-Analysis of Randomized Controlled Trials. Front. Med. 2021, 8, 713554. [Google Scholar] [CrossRef] [PubMed]
- Moolla, S.; Miller-Monthrope, Y. Dermatology: How to manage facial hyperpigmentation in skin of colour. Drugs Context 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Esposito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Update on Melasma-Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef]
- Ogawa, R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef]
- Tan, S.; Khumalo, N.; Bayat, A. Understanding Keloid Pathobiology from a Quasi-Neoplastic Perspective: Less of a Scar and More of a Chronic Inflammatory Disease with Cancer-Like Tendencies. Front. Immunol. 2019, 10, 1810. [Google Scholar] [CrossRef] [PubMed]
- Biazus Soares, G.; Mahmoud, O.; Yosipovitch, G. Pruritus in keloid scars: Mechanisms and treatments. Ital. J. Dermatol. Venerol. 2023, 158, 401–407. [Google Scholar] [CrossRef]
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006, 297, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Hawash, A.A.; Ingrasci, G.; Nouri, K.; Yosipovitch, G. Pruritus in Keloid Scars: Mechanisms and Treatments. Acta Derm. Venereol. 2021, 101, adv00582. [Google Scholar] [CrossRef]
- Unahabhokha, T.; Sucontphunt, A.; Nimmannit, U.; Chanvorachote, P.; Yongsanguanchai, N.; Pongrakhananon, V. Molecular signalings in keloid disease and current therapeutic approaches from natural based compounds. Pharm. Biol. 2015, 53, 457–463. [Google Scholar] [CrossRef]
- Bharadvaja, N.; Gautam, S.; Singh, H. Natural polyphenols: A promising bioactive compounds for skin care and cosmetics. Mol. Biol. Rep. 2023, 50, 1817–1828. [Google Scholar] [CrossRef]
- Shim, J.S.; Kwon, Y.Y.; Hwang, J.K. The effects of panduratin A isolated from Kaempferia pandurata on the expression of matrix metalloproteinase-1 and type-1 procollagen in human skin fibroblasts. Planta Med. 2008, 74, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Q.; Wang, X.; Jaisi, A.; Olatunji, O.J. Boesenbergia rotunda displayed anti-inflammatory, antioxidant and anti-apoptotic efficacy in doxorubicin-induced cardiotoxicity in rats. Sci. Rep. 2023, 13, 11398. [Google Scholar] [CrossRef]
- Wang, P.; Wen, C.; Olatunji, O.J. Anti-Inflammatory and Antinociceptive Effects of Boesenbergia rotunda Polyphenol Extract in Diabetic Peripheral Neuropathic Rats. J. Pain Res. 2022, 15, 779–788. [Google Scholar] [CrossRef]
- Listyawati, S.; Sismindari; Mubarika, S.; Murti, Y.B.; Ikawati, M. Anti-Proliferative Activity and Apoptosis Induction of an Ethanolic Extract of Boesenbergia pandurata (Roxb.) Schlecht. against HeLa and Vero Cell Lines. Asian Pac. J. Cancer Prev. 2016, 17, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Kongtaphan, K.; Onkoksoong, T.; Jaemsak, K.; Phadungrakwittaya, R.; Thaworn, A.; Akarasereenont, P.; Wongkajornsilp, A. Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Biol. Toxicol. 2010, 26, 103–116. [Google Scholar] [CrossRef]
- Panich, U.; Tangsupa-a-nan, V.; Onkoksoong, T.; Kongtaphan, K.; Kasetsinsombat, K.; Akarasereenont, P.; Wongkajornsilp, A. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch. Pharm. Res. 2011, 34, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Mocan, T.; Cosgarea, R. The role of ultraviolet radiation and tyrosine stimulated melanogenesis in the induction of oxidative stress alterations in fair skin melanocytes. Exp. Oncol. 2009, 31, 200–208. [Google Scholar]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef]
- Yanti; Oh, H.I.; Anggakusuma; Hwang, J.K. Effects of panduratin A isolated from Kaempferia pandurata ROXB. on the expression of matrix metalloproteinase-9 by porphyromonas gingivalis supernatant-induced KB cells. Biol. Pharm. Bull. 2009, 32, 110–115. [Google Scholar] [CrossRef]
- Lai, S.L.; Cheah, S.C.; Wong, P.F.; Noor, S.M.; Mustafa, M.R. In vitro and in vivo anti-angiogenic activities of Panduratin A. PLoS ONE 2012, 7, e38103. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Qian, H.; Wang, C. Characterization of the skin keloid microenvironment. Cell Commun. Signal. 2023, 21, 207. [Google Scholar] [CrossRef]
- Rachmin, I.; Ostrowski, S.M.; Weng, Q.Y.; Fisher, D.E. Topical treatment strategies to manipulate human skin pigmentation. Adv. Drug Deliv. Rev. 2020, 153, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Kim, H.S.; Kim, H.K.; Kim, J.W.; Yoon, J.H.; Cho, Y.; Hwang, J.K. Inhibitory effect of panduratin A isolated from Kaempferia panduarata Roxb. on melanin biosynthesis. Phytother. Res. 2010, 24, 1600–1604. [Google Scholar] [CrossRef]
- Jamornwan, S.; Chokpanuwat, T.; Uppakara, K.; Soodvilai, S.; Saengsawang, W. Anti-Inflammatory Activity of Panduratin A against LPS-Induced Microglial Activation. Biomedicines 2022, 10, 2587. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Kwon, H.; Hwang, J.K. In vitro anti-inflammatory activity of panduratin A isolated from Kaempferia pandurata in RAW264.7 cells. Planta Med. 2003, 69, 1102–1108. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N.; et al. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef] [PubMed]
- Rukayadi, Y.; Han, S.; Yong, D.; Hwang, J.K. In vitro antibacterial activity of panduratin A against enterococci clinical isolates. Biol. Pharm. Bull. 2010, 33, 1489–1493. [Google Scholar] [CrossRef]
- Thadtapong, N.; Chaturongakul, S.; Napaswad, C.; Dubbs, P.; Soodvilai, S. Enhancing effect of natural adjuvant, panduratin A, on antibacterial activity of colistin against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2024, 14, 9863. [Google Scholar] [CrossRef]
- Rosdianto, A.M.; Puspitasari, I.M.; Lesmana, R.; Levita, J. Inhibitory Activity of Boesenbergia rotunda (L.) Mansf. Rhizome towards the Expression of Akt and NF-KappaB p65 in Acetic Acid-Induced Wistar Rats. Evid. Based Complement. Altern. Med. 2020, 2020, 6940313. [Google Scholar] [CrossRef]
- Priya Mohanty, J.; Nath, L.K.; Bhuyan, N.; Mariappan, G. Evaluation of Antioxidant Potential of Kaempferia rotunda Linn. Indian J. Pharm. Sci. 2008, 70, 362–364. [Google Scholar] [CrossRef]
- Kaminski, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol. 2022, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yin, Z.; Yu, N.; Ou, S.; Wang, X.; Li, H.; Zhu, H. Tanshinone Alleviates UVA-induced Melanogenesis in Melanocytes via the Nrf2-regulated Antioxidant Defense Signaling Pathway. Curr. Mol. Med. 2024, 24, 1529–1539. [Google Scholar] [CrossRef]
- Panich, U.; Onkoksoong, T.; Limsaengurai, S.; Akarasereenont, P.; Wongkajornsilp, A. UVA-induced melanogenesis and modulation of glutathione redox system in different melanoma cell lines: The protective effect of gallic acid. J. Photochem. Photobiol. B 2012, 108, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef]
- Polefka, T.G.; Meyer, T.A.; Agin, P.P.; Bianchini, R.J. Effects of solar radiation on the skin. J. Cosmet. Dermatol. 2012, 11, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kohli, I.; Chaowattanapanit, S.; Mohammad, T.F.; Nicholson, C.L.; Fatima, S.; Jacobsen, G.; Kollias, N.; Lim, H.W.; Hamzavi, I.H. Synergistic effects of long-wavelength ultraviolet A1 and visible light on pigmentation and erythema. Br. J. Dermatol. 2018, 178, 1173–1180. [Google Scholar] [CrossRef]
- Salama, S.M.; Ibrahim, I.A.A.; Shahzad, N.; Al-Ghamdi, S.; Ayoub, N.; AlRashdi, A.S.; Abdulla, M.A.; Salehen, N.; Bilgen, M. Hepatoprotectivity of Panduratin A against liver damage: In vivo demonstration with a rat model of cirrhosis induced by thioacetamide. APMIS 2018, 126, 710–721. [Google Scholar] [CrossRef]
- Salama, S.M.; AlRashdi, A.S.; Abdulla, M.A.; Hassandarvish, P.; Bilgen, M. Protective activity of Panduratin A against thioacetamide-induced oxidative damage: Demonstration with in vitro experiments using WRL-68 liver cell line. BMC Complement. Altern. Med. 2013, 13, 279. [Google Scholar] [CrossRef]
- Cobbold, C.A. The role of nitric oxide in the formation of keloid and hypertrophic lesions. Med. Hypotheses 2001, 57, 497–502. [Google Scholar] [CrossRef]
- Yun, J.M.; Kweon, M.H.; Kwon, H.; Hwang, J.K.; Mukhtar, H. Induction of apoptosis and cell cycle arrest by a chalcone panduratin A isolated from Kaempferia pandurata in androgen-independent human prostate cancer cells PC3 and DU145. Carcinogenesis 2006, 27, 1454–1464. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salakshna, N.; Thanasarnaksorn, W.; Kanjanasirirat, P.; Jearawuttanakul, K.; Chabang, N.; Rangkansenee, N.; Panich, U.; Thanachaiphiwat, S.; Borwornpinyo, S.; Hongeng, S. Potential Cutaneous Applications of Boesenbergia rotunda Extract Based on Its In Vitro Anti-Melanogenic and Anti-Fibroproliferative Properties. Int. J. Mol. Sci. 2025, 26, 4319. https://doi.org/10.3390/ijms26094319
Salakshna N, Thanasarnaksorn W, Kanjanasirirat P, Jearawuttanakul K, Chabang N, Rangkansenee N, Panich U, Thanachaiphiwat S, Borwornpinyo S, Hongeng S. Potential Cutaneous Applications of Boesenbergia rotunda Extract Based on Its In Vitro Anti-Melanogenic and Anti-Fibroproliferative Properties. International Journal of Molecular Sciences. 2025; 26(9):4319. https://doi.org/10.3390/ijms26094319
Chicago/Turabian StyleSalakshna, Nuntida, Wilai Thanasarnaksorn, Phongthon Kanjanasirirat, Kedchin Jearawuttanakul, Napason Chabang, Noppawan Rangkansenee, Uraiwan Panich, Saowalak Thanachaiphiwat, Suparerk Borwornpinyo, and Suradej Hongeng. 2025. "Potential Cutaneous Applications of Boesenbergia rotunda Extract Based on Its In Vitro Anti-Melanogenic and Anti-Fibroproliferative Properties" International Journal of Molecular Sciences 26, no. 9: 4319. https://doi.org/10.3390/ijms26094319
APA StyleSalakshna, N., Thanasarnaksorn, W., Kanjanasirirat, P., Jearawuttanakul, K., Chabang, N., Rangkansenee, N., Panich, U., Thanachaiphiwat, S., Borwornpinyo, S., & Hongeng, S. (2025). Potential Cutaneous Applications of Boesenbergia rotunda Extract Based on Its In Vitro Anti-Melanogenic and Anti-Fibroproliferative Properties. International Journal of Molecular Sciences, 26(9), 4319. https://doi.org/10.3390/ijms26094319





