Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea
Abstract
:1. Introduction
2. Results
2.1. Sequence Analysis and De Novo Assembly for Large Yellow Croaker
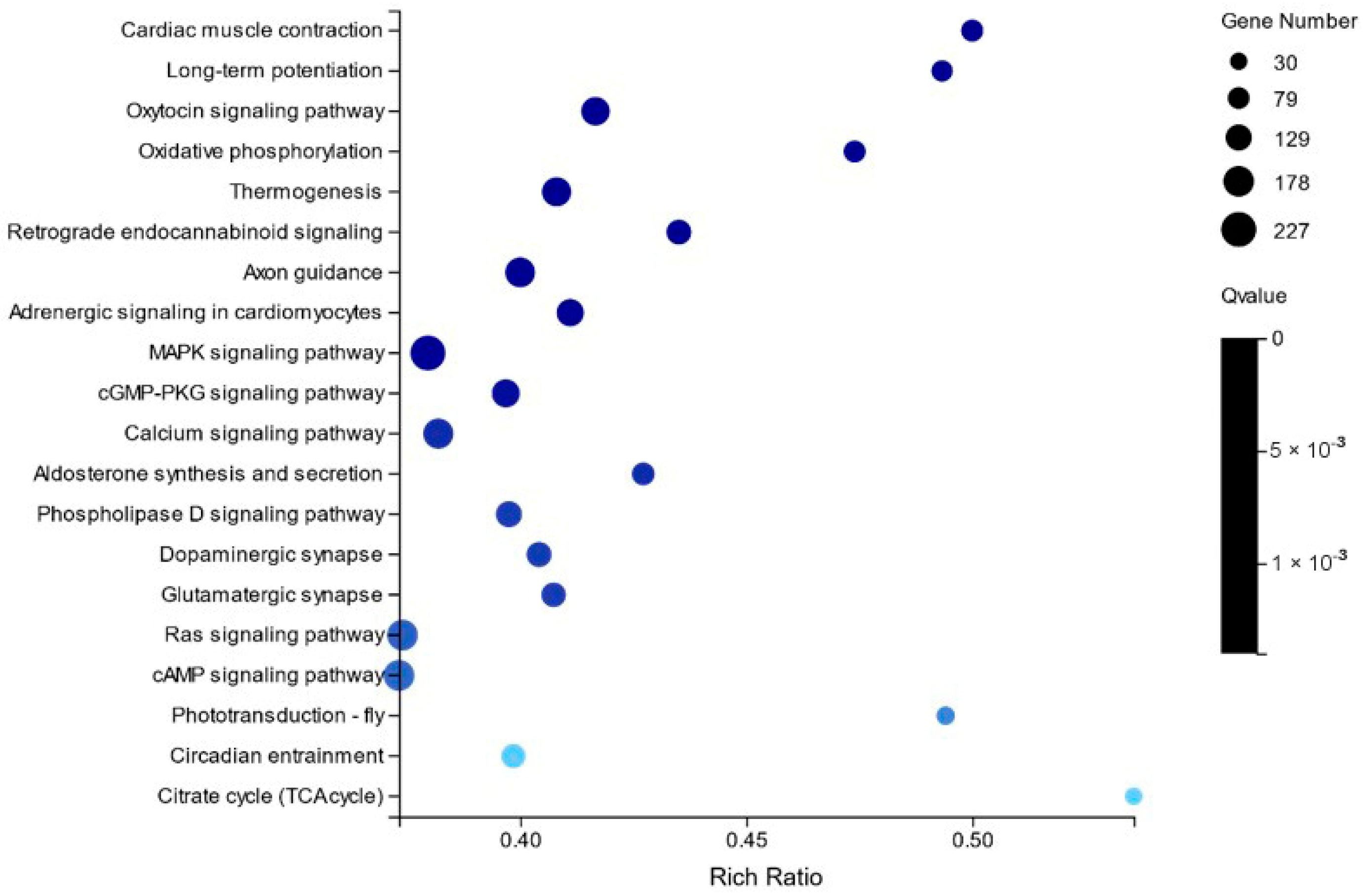
2.2. Differentially Expressed Gene Analysis
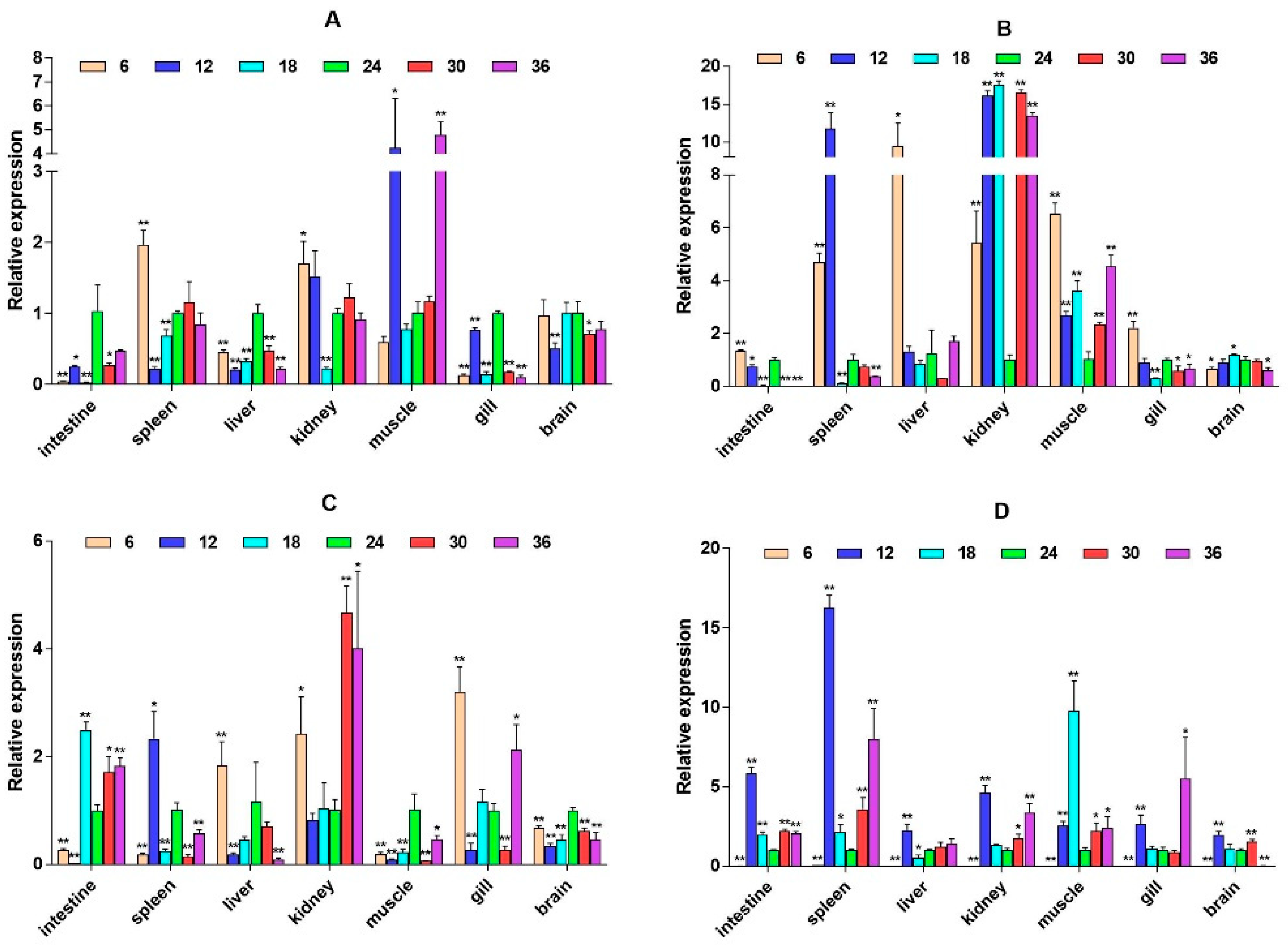
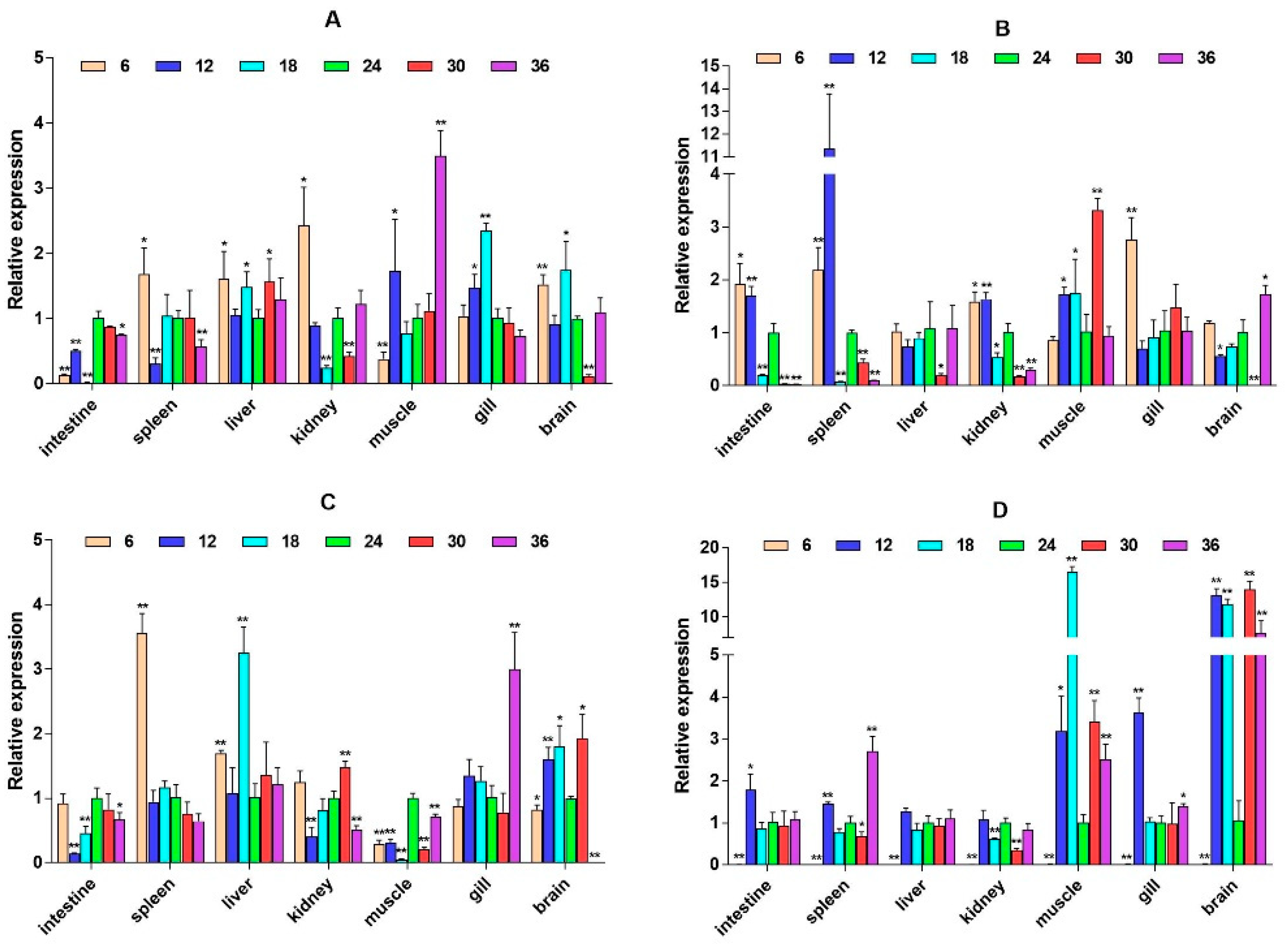
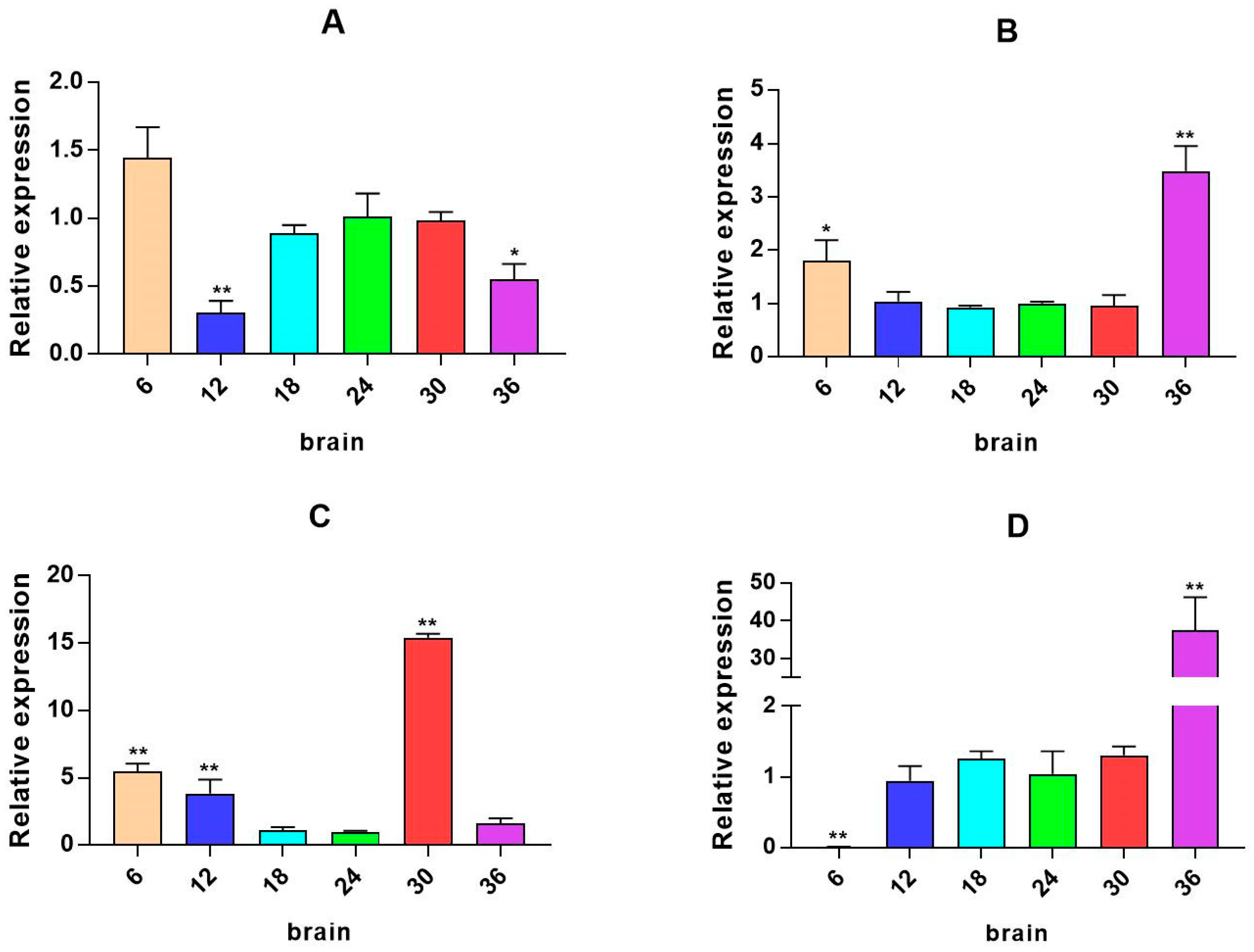
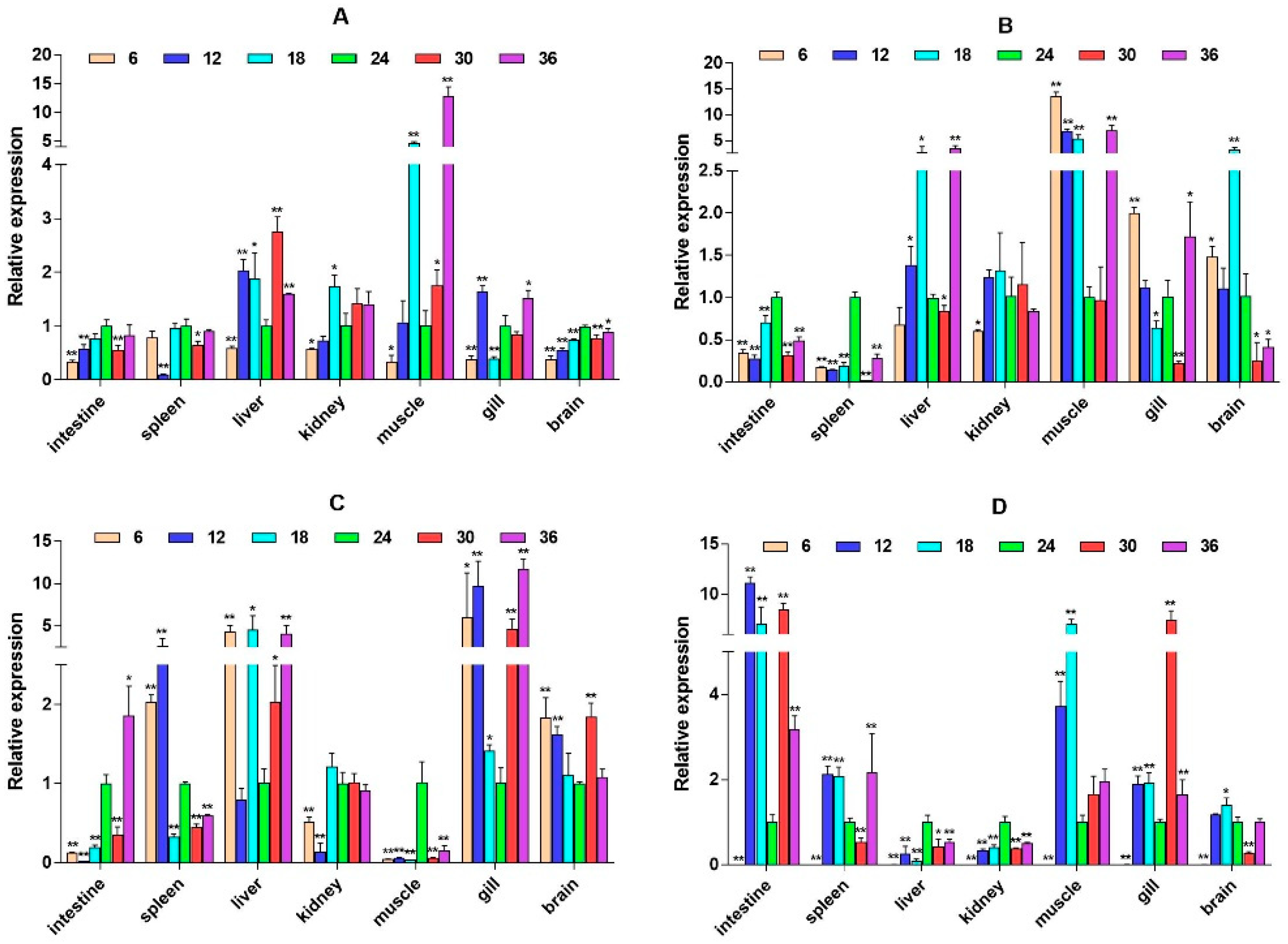
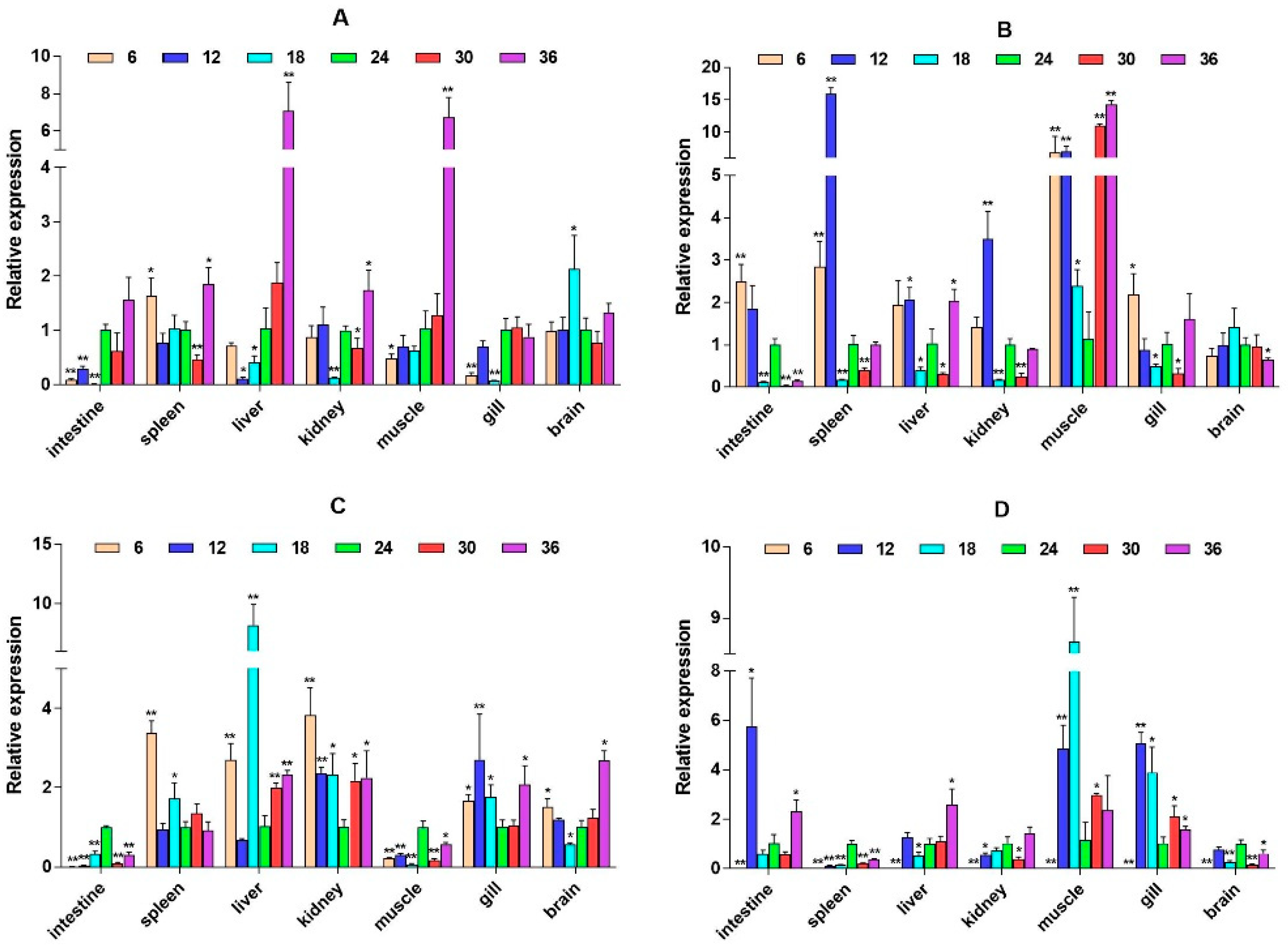
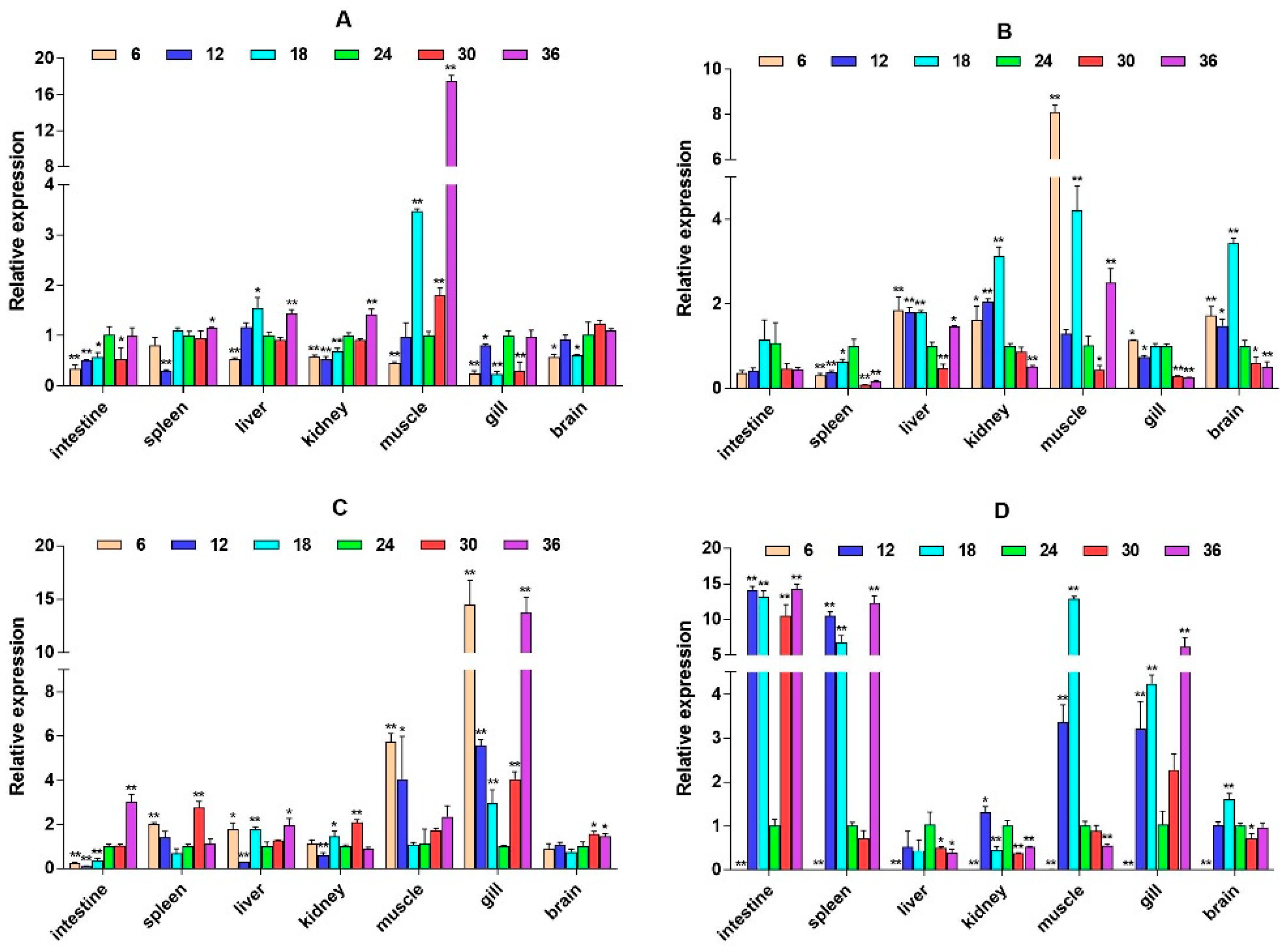
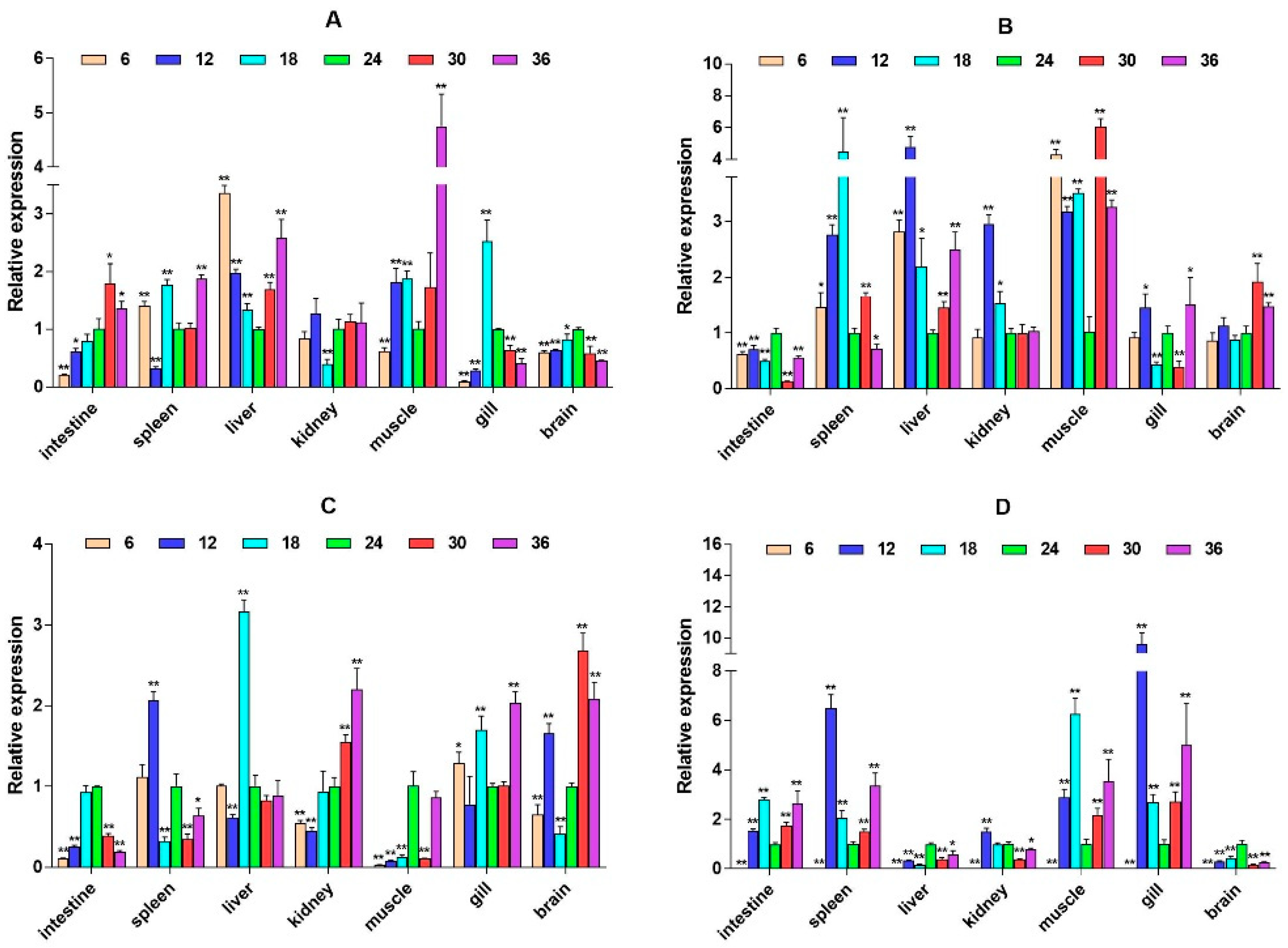
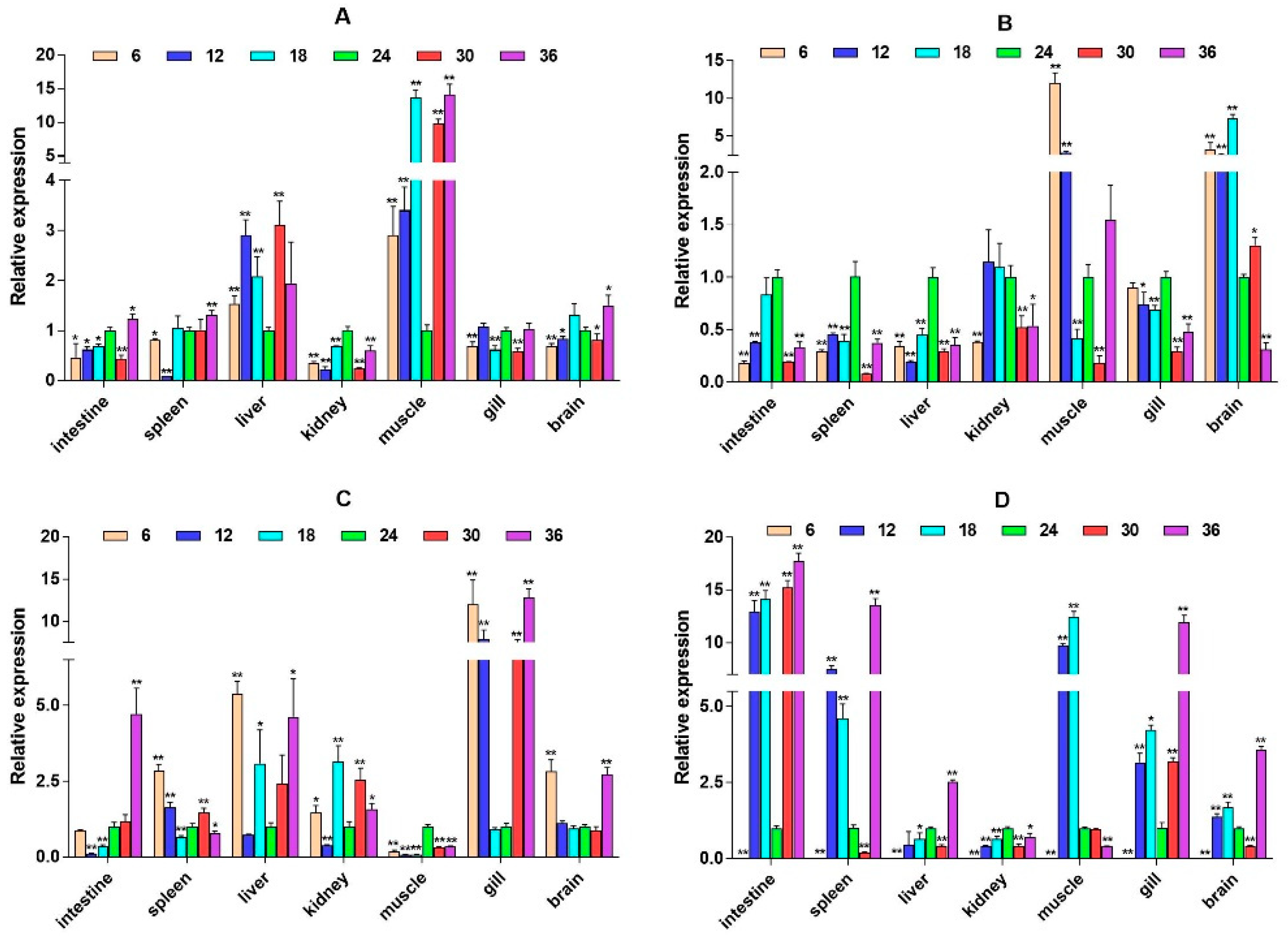
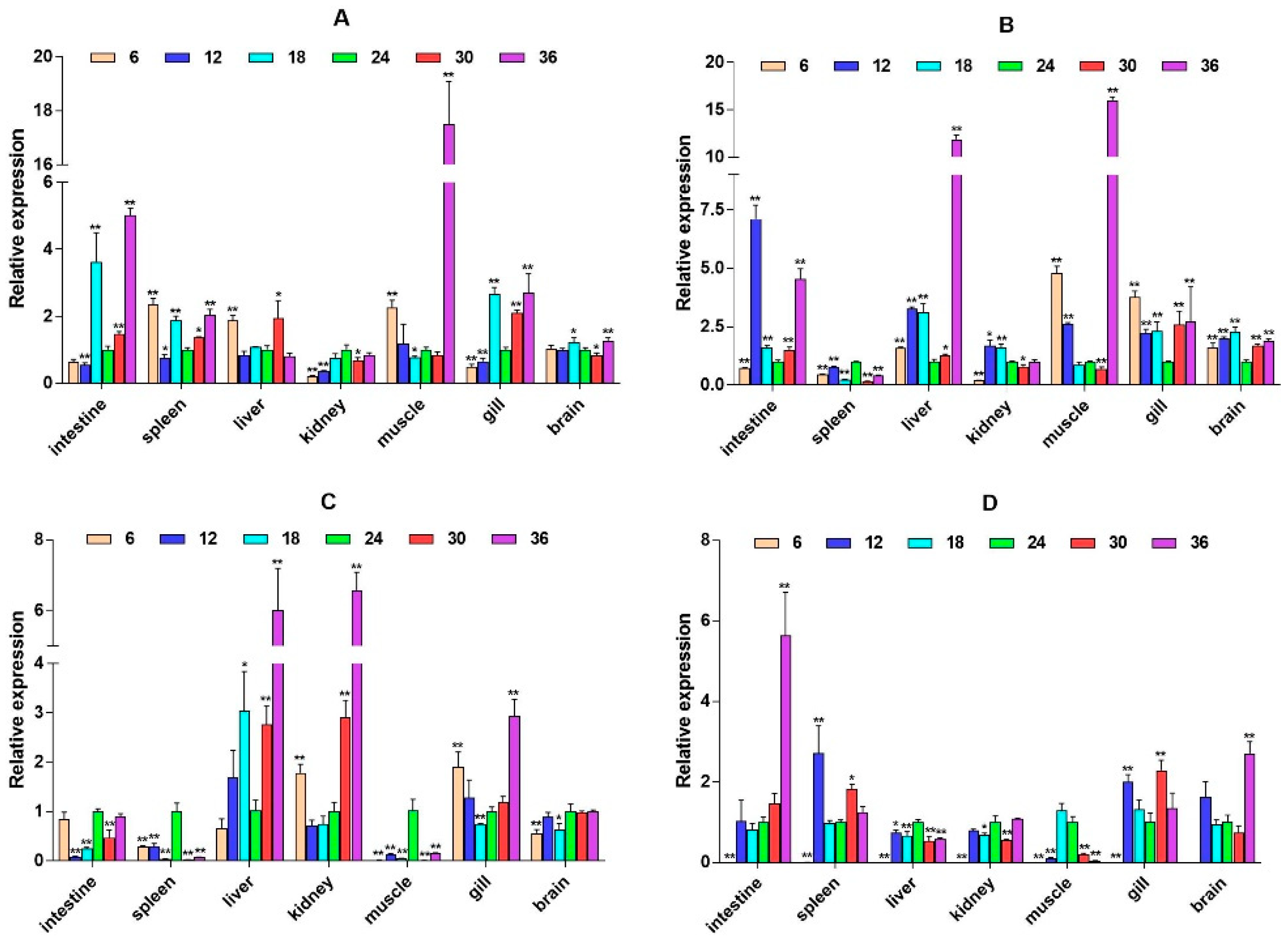
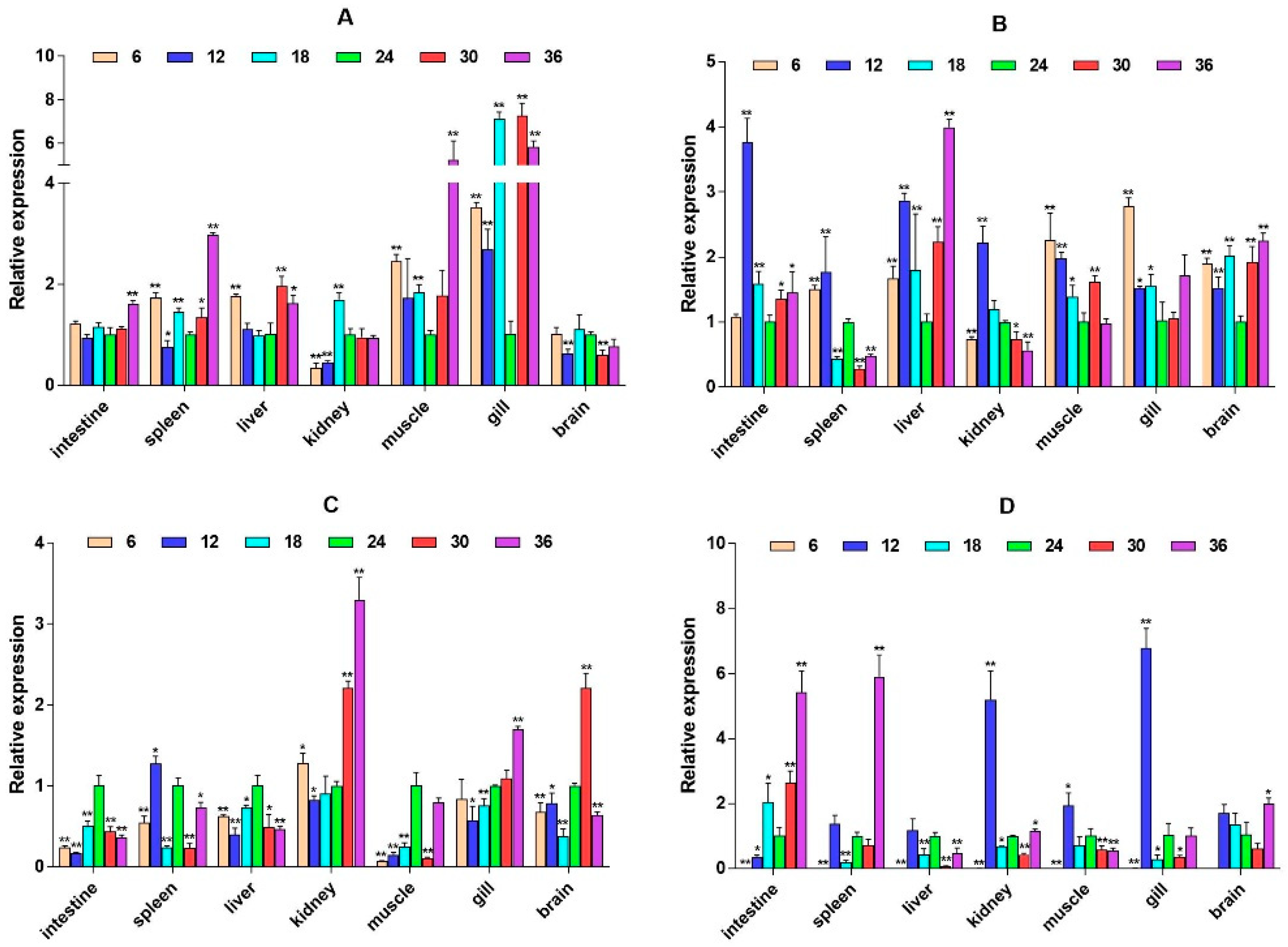
2.3. mRNA Expression Levels of HPI Axis and Immune-Related Genes
3. Discussion
4. Materials and Methods
4.1. Treatment of Fish Salinity Stress
4.2. RNA Extraction and Quantification
4.3. Quantitative Real-Time PCR Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 1–27. [Google Scholar] [CrossRef]
- Romero, L.M.; Butler, L.K. Endocrinology of Stress. Int. J. Comp. Psychol. 2007, 20, 89–95. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Shi, X.; Zhang, H.; Gao, Y.; Wang, M.; Kong, P.; Qiu, L.; Song, L. The modulation of catecholamines to the immune response against bacteria Vibrio anguillarum challenge in scallop Chlamys farreri. Fish Shellfish Immunol. 2011, 31, 1065–1071. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Miguez, J.M.; Soengas, J.L. Actions of growth hormone on carbohydrate metabolism and osmoregulation of rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2005, 141, 214–225. [Google Scholar] [CrossRef]
- Prunet, P.; Sandra, O.; Le Rouzic, P.; Marchand, O.; Laudet, V. Molecular characterization of the prolactin receptor in two fish species, tilapia Oreochromis niloticus and rainbow trout, Oncorhynchus mykiss: A comparative approach. Can. J. Physiol. Pharmacol. 2000, 78, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, E.; Hasegawa, S.; Mishiro, K.; Ando, H. Osmoregulatory responses of expression of vasotocin, isotocin, prolactin and growth hormone genes following hypoosmotic challenge in a stenohaline marine teleost, tiger puffer (Takifugu rubripes). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 353–359. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N.N.; Choi, C.Y. Profiles of hypothalamus-pituitary-interrenal axis gene expression in the parr and smolt stages of rainbow trout, Oncorhynchus mykiss: Effects of recombinant aquaporin 3 and seawater acclimation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 182, 14–21. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; González, A.A.; Suárez, R.A.; Galal-Khallaf, A.; Martos-Sitcha, J.A.; Ibrahim, H.M.; Martínez-Rodríguez, G.; Mancera, J.M. Molecular performance of Prl and Gh/Igf1 axis in the Mediterranean meager, Argyrosomus regius, acclimated to different rearing salinities. Fish Physiol. Biochem. 2017, 43, 203–216. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; Yúfera, M.; Martínez-Rodríguez, G.; Mancera, J.M. Molecular endocrine changes of Gh/Igf1 axis in gilthead sea bream (Sparus aurata L.) exposed to different environmental salinities during larvae to post-larvae stages. Fish Physiol. Biochem. 2016, 42, 1177–1186. [Google Scholar] [CrossRef]
- Gu, J.; Dai, S.; Liu, H.; Cao, Q.; Yin, S.; Lai, K.P.; Tse, W.K.F.; Wong, C.K.C.; Shi, H. Identification of immune-related genes in gill cells of Japanese eels (Anguilla japonica) in adaptation to water salinity changes. Fish Shellfish Immunol. 2018, 73, 288–296. [Google Scholar] [CrossRef]
- Wang, J.; He, R.Z.; Lu, G.L.; Luo, H.L.; Lu, D.Q.; Li, A.X. Vaccine-induced antibody level as the parameter of the influence of environmental salinity on vaccine efficacy in Nile tilapia. Fish Shellfish Immunol. 2018, 82, 522–530. [Google Scholar] [CrossRef]
- Suo, Y.T. Effects of Salinity Stress on Immune Damage and Modulation Effects of Dietary β-Glucan in Nile tilapia (Oreochromis niloticus). Master. Thesis, East China Normal University, Shanghai, China, 2018. [Google Scholar]
- Huang, W.Q.; Zhang, Y.; Ke, Q.Z.; Lin, P.H.; Chen, M.H.; Han, K.H. Growth traits research of the breeding group sub 2 generation of large yellow croaker (Larimichthys crocea). South China Fish. Sci. 2013, 9, 14–19. [Google Scholar]
- Wang, G.L.; Zhu, J.L.; Jin, S. Molecular identification and phylogenetics of pathogenic vibrios in cultured large yellow croaker (Pseudosciaena crocea) Richardson. Oceanologia. Limnologia. Sinica 2008, 2, 162–167. [Google Scholar]
- Wang, Y.B.; Hu, Z.H.; Zhu, Y.H.; Chai, X.J. Effects of salinity on growth and non-specific immunity of Nibea japonica. J. Zhejiang Ocean. Univ. (Nat. Sci.) 2015, 34, 26–31. [Google Scholar]
- Kato, H.; Kawaguchi, M.; Inoue, S.; Hirai, K.; Furuya, H. The effects of beta-adrenoceptor antagonists on proinflammatory cytokine concentrations after subarachnoid hemorrhage in rats. Anesth. Analg. 2009, 108, 288–295. [Google Scholar] [CrossRef]
- Yin, F.; Wang, Y.Y.; Du, J.H.; Li, C.; Lu, Z.Z.; Han, C.; Zhang, Y.Y. Noncanonical cAMP pathway and p38 MAPK mediate β2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J. Mol. Cell Cardiol. 2006, 40, 384–393. [Google Scholar] [CrossRef]
- Chikazawa, M.; Sato, R. Identification of functional food factors as β2-adrenergic receptor agonists and their potential roles in skeletal muscle. J. Nutr. Sci. Vitaminol. 2018, 64, 68–74. [Google Scholar] [CrossRef]
- Sato, S.; Nomura, S.; Kawano, F.; Tanihata, J.; Tachiyashiki, K.; Imaizumi, K. Effects of the beta2-agonist clenbuterol on beta1- and beta2-adrenoceptor mRNA expressions of rat skeletal and left ventricle muscles. J. Pharmacol. Sci. 2008, 107, 393–400. [Google Scholar] [CrossRef]
- Li, W.S.; Chen, D.; Wong, A.O.; Lin, H.R. Molecular cloning, tissue distribution, and ontogeny of mRNA expression of growth hormone in orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2005, 144, 78–89. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, L.; Duan, D.; Zhang, H.; Wang, Y.; Li, W.; Lin, H. Growth hormone and two forms of insulin-like growth factors I in the giant grouper (Epinephelus lanceolatus): Molecular cloning and characterization of tissue distribution. Fish Physiol. Biochem. 2010, 36, 201–212. [Google Scholar] [CrossRef]
- Rousseau, K.; Dufour, S. Comparative aspects of GH and metabolic regulation in lower vertebrates. Neuroendocrinology 2007, 86, 165–174. [Google Scholar] [CrossRef]
- Abass, N.Y.; Elwakil, H.E.; Hemeida, A.A.; Abdelsalam, N.R.; Ye, Z.; Su, B.; Alsaqufi, A.S.; Weng, C.C.; Trudeau, V.L.; Dunham, R.A. Genotype–environment interactions for survival at low and sub-zero temperatures at varying salinity for channel catfish, hybrid catfish and transgenic channel catfish. Aquaculture 2016, 458, 140–148. [Google Scholar] [CrossRef]
- Marano, R.J.; Ben-Jonathan, N. Minireview: Extrapituitary prolactin: An update on the distribution, regulation, and functions. Mol. Endocrinol. 2014, 28, 622–633. [Google Scholar] [CrossRef]
- Makino, K.; Onuma, T.A.; Kitahashi, T.; Ando, H.; Ban, M.; Urano, A. Expression of hormone genes and osmoregulation in homing chum salmon: A minireview. Gen. Comp. Endocrinol. 2007, 152, 304–309. [Google Scholar] [CrossRef]
- Onuma, T.; Kitahashi, T.; Taniyama, S.; Saito, D.; Ando, H.; Urano, A. Changes in expression of genes encoding gonadotropin subunits and growth hormone/prolactin/somatolactin family hormones during final maturation and freshwater adaptation in prespawning chum salmon. Endocrine 2003, 20, 23–33. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Ataie-Kachoie, P.; Pourgholami, M.H.; Richardson, D.R.; Morris, D.L. Gene of the month: Interleukin 6 (IL-6). J. Clin. Pathol. 2014, 67, 932–937. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Wen, C.; Lv, M.; Gan, N.; Zhou, H.; Zhang, A.; Yang, K. Molecular characterization of grass carp interleukin-6 receptor and the agonistic activity of its soluble form in head kidney leucocytes. Fish Shellfish Immunol. 2019, 86, 1072–1080. [Google Scholar] [CrossRef]
- Engelsma, M.Y.; Huising, M.O.; van Muiswinkel, W.B.; Flik, G.; Kwang, J.; Savelkoul, H.F.; Verburg-van Kemenade, B.L. Neuroendocrine-immune interactions in fish: A role for interleukin-1. Vet. Immunol. Immunopathol. 2002, 87, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Thulasitha, W.S.; Umasuthan, N.; Whang, I.; Lim, B.S.; Jung, H.B.; Noh, J.K.; Lee, J. A CXC chemokine gene, CXCL12, from rock bream, Oplegnathus fasciatus: Molecular characterization and transcriptional profile. Fish Shellfish Immunol. 2015, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Foord, S.M.; Bonner, T.I.; Neubig, R.R.; Rosser, E.M.; Pin, J.P.; Davenport, A.P.; Spedding, M.; Harmar, A.J. International union of pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 2005, 57, 279–288. [Google Scholar] [CrossRef]
- Vassart, G.; Costagliola, S. G protein-coupled receptors: Mutations and endocrine diseases. Nat. Rev. Endocrinol. 2011, 7, 362–372. [Google Scholar] [CrossRef]
- Shao, C.; Niu, Y.; Rastas, P.; Liu, Y.; Xie, Z.; Li, H.; Wang, L.; Jiang, Y.; Tai, S.; Tian, Y. Genome-wide SNP identification for the construction of a high-resolution genetic map of Japanese flounder (Paralichthys olivaceus): Applications to QTL mapping of Vibrio anguillarum disease resistance and comparative genomic analysis. DNA Res. 2015, 22, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Nie, P.; Thompson, K.D.; Adams, A.; Wang, T.; Secombes, C.J.; Zou, J. The search for the IFN-γ receptor in fish: Functional and expression analysis of putative binding and signalling chains in rainbow trout Oncorhynchus mykiss. Dev. Comp. Immunol. 2009, 33, 920–931. [Google Scholar] [CrossRef]
- Sun, B.; Greiner-Tollersrud, L.; Koop, B.F.; Robertsen, B. Atlantic salmon possesses two clusters of type I interferon receptor genes on different chromosomes, which allows for a larger repertoire of interferon receptors than in zebrafish and mammals. Dev. Comp. Immunol. 2014, 47, 275–286. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Schneider, W.M.; Rice, C.M. Interferons and viruses: An evolutionary arms race of molecular interactions. Trends Immunol. 2015, 36, 124–138. [Google Scholar] [CrossRef]
- Yao, C.L.; Kong, P.; Huang, X.N.; Wang, Z.Y. Molecular cloning and expression of IRF1 in large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol. 2010, 28, 654–660. [Google Scholar] [CrossRef]
- Yabu, T.; Hirose, H.; Hirono, I.; Katagiri, T.; Aoki, T.; Yamamoto, E. Molecular cloning of a novel interferon regulatory factor in Japanese flounder, Paralichthys olivaceus. Mol. Mar. Biol. Biotechnol. 1998, 7, 138–144. [Google Scholar]
- Wang, T.; Secombes, C.J. Rainbow trout suppressor of cytokine signalling (SOCS)-1, 2 and 3: Molecular identification, expression and modulation. Mol. Immunol. 2008, 45, 1449–1457. [Google Scholar] [CrossRef]
- Thanasaksiri, K.; Hirono, I.; Kondo, H. Identification and expression analysis of suppressors of cytokine signaling (SOCS) of Japanese flounder Paralichthys olivaceus. Fish Shellfish Immunol. 2016, 58, 145–152. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Q.; Nie, P.; Secombes, C.J. Identification of suppressor of cytokine signalling (SOCS) 6, 7, 9 and CISH in rainbow trout Oncorhynchus mykiss and analysis of their expression in relation to other known trout SOCS. Fish Shellfish Immunol. 2010, 29, 656–667. [Google Scholar] [CrossRef]
- Philip, A.M.; Daniel Kim, S.; Vijayan, M.M. Cortisol modulates the expression of cytokines and suppressors of cytokine signaling (SOCS) in rainbow trout hepatocytes. Dev. Comp. Immunol. 2012, 38, 360–367. [Google Scholar] [CrossRef]
- Kolmus, K.; Tavernier, J.; Gerlo, S. β2-Adrenergic receptors in immunity and inflammation: Stressing NF-kB. Brain Behav. Immun. 2015, 45, 297–310. [Google Scholar] [CrossRef]
- Barnett Jr, C.C.; Moore, E.E.; Partrick, D.A.; Silliman, C.C. β-adrenergic stimulation down-regulates neutrophil priming for superoxide generation, but not elastase release. J. Surg. Res. 1997, 70, 166–170. [Google Scholar] [CrossRef]
- Haskó, G.; Szabó, C.; Németh, Z.H.; Salzman, A.L.; Vizi, E.S. Stimulation of β-adrenoceptors inhibits endotoxin-induced IL-12 production in normal and IL-10 deficient mice. J. Neuroimmunol. 1998, 88, 57–61. [Google Scholar] [CrossRef]
- Szelényi, J.; Kiss, J.P.; Vizi, E.S. Differential involvement of sympathetic nervous system and immune system in the modulation of TNF-α production by α2- and β-adrenoceptors in mice. J. Neuroimmunol. 2000, 103, 34–40. [Google Scholar] [CrossRef]
- Van der Poll, T.; Lowry, S.F. Epinephrine inhibits endotoxin-induced IL-1β production: Roles of tumor necrosis factor-α and IL-10. Am. J. Physiol.-Reg. I 1997, 273, R1885–R1890. [Google Scholar] [CrossRef]
- Hostager, B.S.; Bishop, G.A. CD40-mediated activation of the NF-kB2 pathway. Front. Immunol. 2013, 4, 376. [Google Scholar] [CrossRef]
- Verburg-van Kemenade, B.M.; Ribeiro, C.M.; Chadzinska, M. Neuroendocrine-immune interaction in fish: Differential regulation of phagocyte activity by neuroendocrine factors. Gen. Comp. Endocrinol. 2011, 172, 31–38. [Google Scholar] [CrossRef]
- Wang, P.; Gao, C.; Guo, N.; Zhang, S.D.; Wang, W.; Yao, L.P.; Zhang, J.; Efferth, T.; Fu, Y.J. 2′-O-Galloylhyperin isolated from Pyrola incarnata fisch. Attenuates LPS-induced inflammatory response by activation of SIRT1/Nrf2 and inhibition of the NF-kB pathways in vitro and vivo. Front. Pharmacol. 2018, 9, 679. [Google Scholar] [CrossRef]
- Dejkhamron, P.; Thimmarayappa, J.; Kotlyarevska, K.; Sun, J.; Lu, C.; Bonkowski, E.L.; Denson, L.A.; Menon, R.K. Lipopolysaccharide (LPS) directly suppresses growth hormone receptor (GHR) expression through MyD88-dependent and -independent Toll-like receptor-4/MD2 complex signaling pathways. Mol. Cell Endocrinol. 2007, 274, 35–42. [Google Scholar] [CrossRef]
- Batista, C.R.; Figueiredo, M.A.; Almeida, D.V.; Romano, L.A.; Marins, L.F. Impairment of the immune system in GH-overexpressing transgenic zebrafish (Danio rerio). Fish Shellfish Immunol. 2014, 36, 519–524. [Google Scholar] [CrossRef]
- Batista, C.R.; Figueiredo, M.A.; Almeida, D.V.; Romano, L.A.; Marins, L.F. Effects of somatotrophic axis (GH/GHR) double transgenesis on structural and molecular aspects of the zebrafish immune system. Fish Shellfish Immunol. 2015, 45, 725–732. [Google Scholar] [CrossRef]
- Reinhardt-Poulin, P.; McLean, J.R.; Deslauriers, Y.; Gorman, W.; Cabat, S.; Rouabhia, M. The use of silver-stained “comets” to visualize DNA damage and repair in normal and Xeroderma pigmentosum fibroblasts after exposure to simulated solar radiation. Photochem. Photobiol. 2000, 71, 422–425. [Google Scholar] [CrossRef]
- Vidal, O.M.; Merino, R.; Rico-Bautista, E.; Fernandez-Perez, L.; Chia, D.J.; Woelfle, J.; Ono, M.; Lenhard, B.; Norstedt, G. In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol. Endocrinol. 2007, 21, 293–311. [Google Scholar] [CrossRef]
- Olavarria, V.H.; Figueroa, J.E.; Mulero, V. Prolactin-induced activation of phagocyte NADPH oxidase in the teleost fish gilthead seabream involves the phosphorylation of p47phox by protein kinase C. Dev. Comp. Immunol. 2012, 36, 216–221. [Google Scholar] [CrossRef]
- Paredes, M.; Gonzalez, K.; Figueroa, J.; Montiel-Eulefi, E. Immunomodulatory effect of prolactin on Atlantic salmon (Salmo salar) macrophage function. Fish Physiol. Biochem. 2013, 39, 1215–1221. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]


| Sample | Total Raw Reads (M) | Total Clean Reads (M) | Total Clean Bases (Gb) | Clean Reads Q20 (%) | Clean Reads Q30 (%) | Clean Reads Ratio (%) |
|---|---|---|---|---|---|---|
| HB1 | 43.82 | 43.37 | 6.51 | 98.11 | 94.62 | 98.97 |
| HB2 | 43.82 | 43.4 | 6.51 | 97.88 | 93.97 | 99.05 |
| HB3 | 43.82 | 43.38 | 6.51 | 97.96 | 94.18 | 99 |
| LB1 | 45.57 | 42.69 | 6.4 | 96.82 | 88.07 | 93.66 |
| LB2 | 43.82 | 43.41 | 6.51 | 97.83 | 93.8 | 99.07 |
| LB3 | 43.82 | 43.42 | 6.51 | 97.87 | 93.93 | 99.08 |
| NB1 | 43.82 | 43.38 | 6.51 | 98 | 94.31 | 98.99 |
| NB2 | 43.82 | 43.43 | 6.51 | 97.97 | 94.19 | 99.11 |
| NB3 | 43.82 | 43.37 | 6.51 | 98 | 94.32 | 98.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Lou, Z.; Feng, H.; Zhang, Y.; Li, H.; Chu, W.; Xue, L. Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea. Int. J. Mol. Sci. 2025, 26, 4298. https://doi.org/10.3390/ijms26094298
Cheng J, Lou Z, Feng H, Zhang Y, Li H, Chu W, Xue L. Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea. International Journal of Molecular Sciences. 2025; 26(9):4298. https://doi.org/10.3390/ijms26094298
Chicago/Turabian StyleCheng, Jia, Zhengjia Lou, Huijie Feng, Yu Zhang, Honghui Li, Wuying Chu, and Liangyi Xue. 2025. "Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea" International Journal of Molecular Sciences 26, no. 9: 4298. https://doi.org/10.3390/ijms26094298
APA StyleCheng, J., Lou, Z., Feng, H., Zhang, Y., Li, H., Chu, W., & Xue, L. (2025). Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea. International Journal of Molecular Sciences, 26(9), 4298. https://doi.org/10.3390/ijms26094298






