JEG-3 Trophoblast Cells Influence ILC-like Transformation of NK Cells In Vitro
Abstract
1. Introduction
2. Results
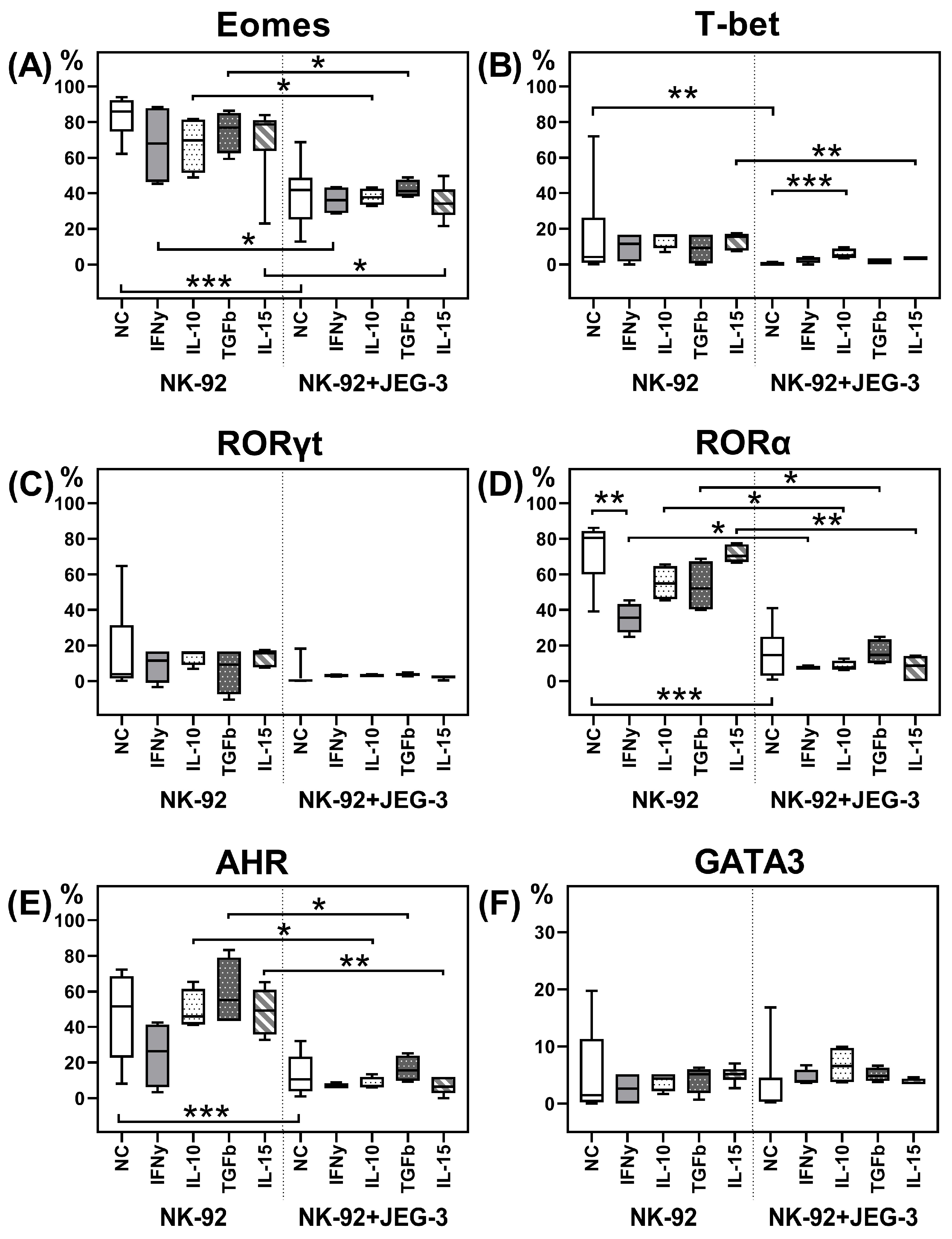
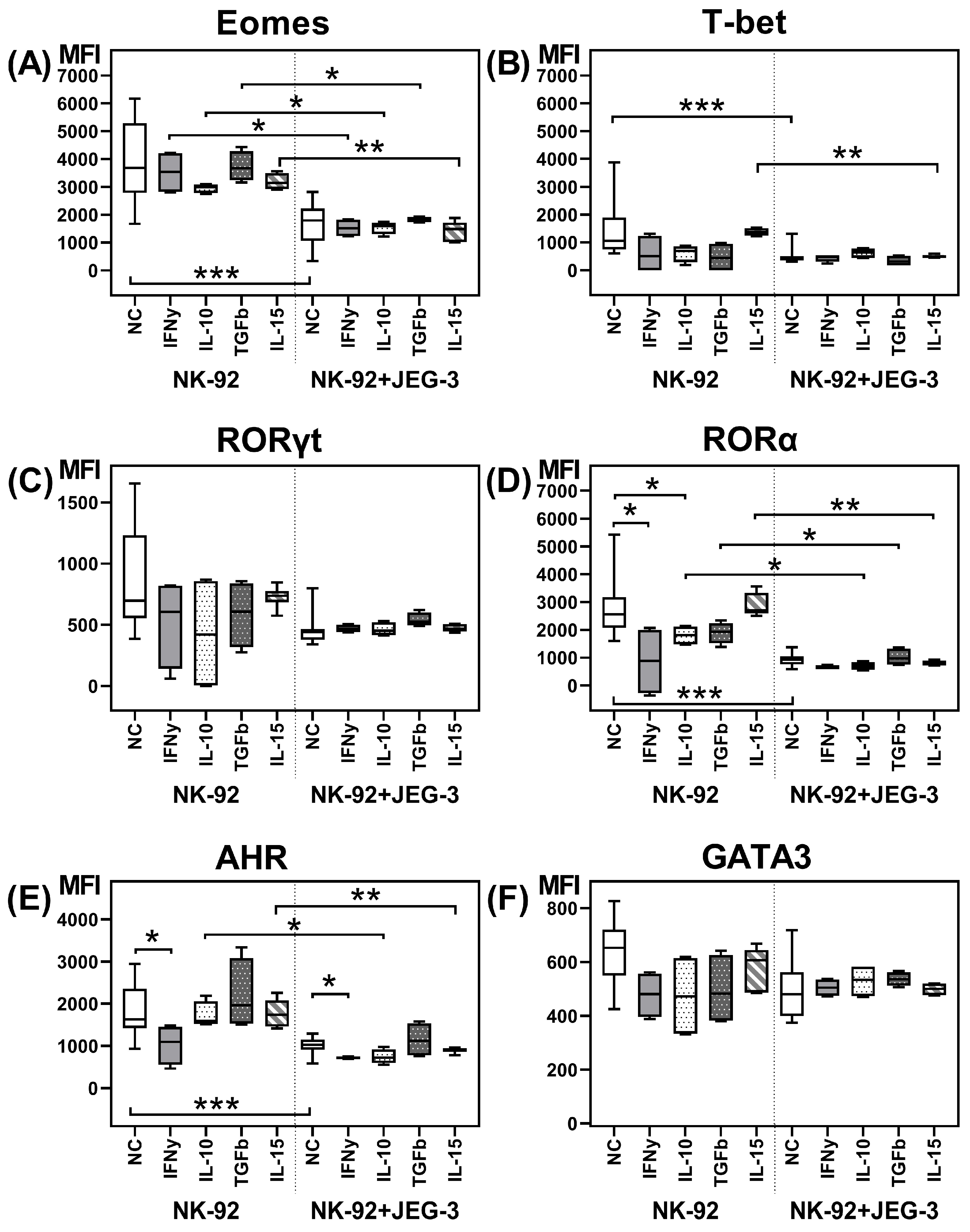
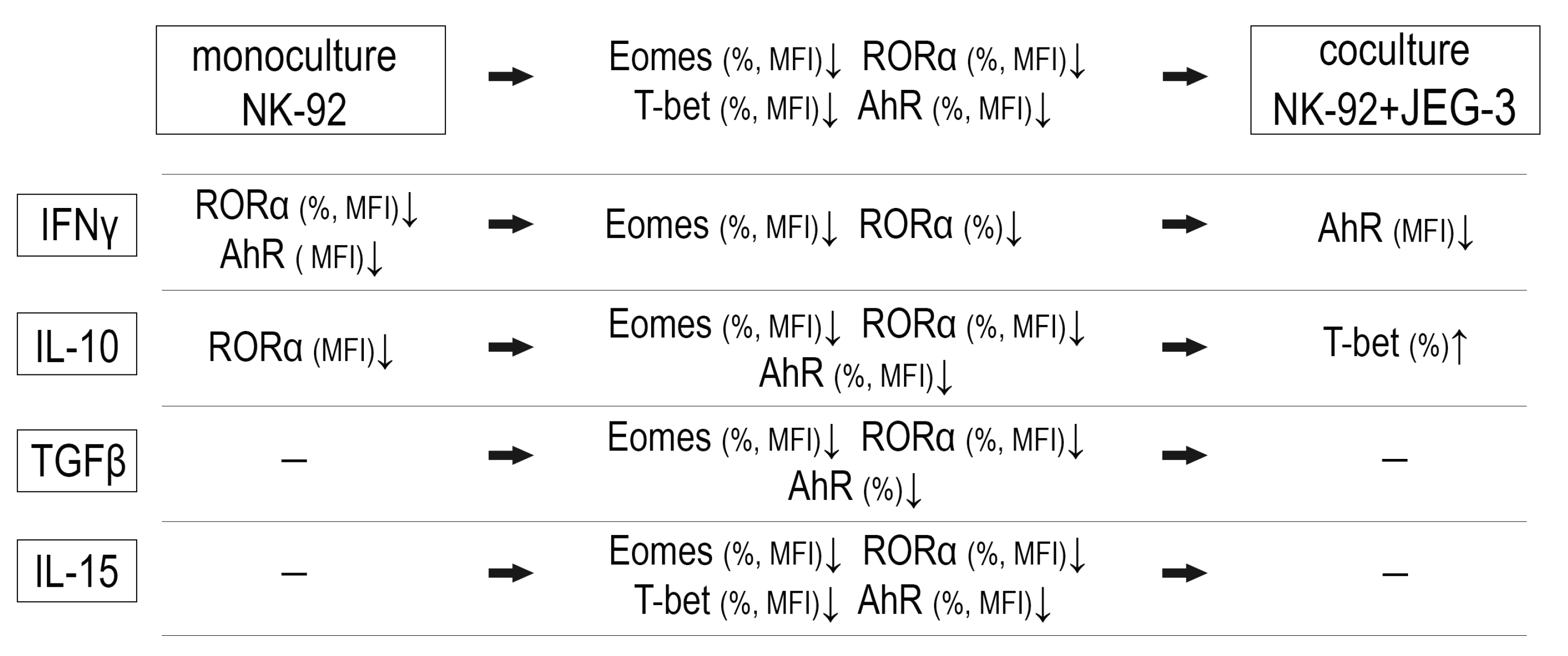
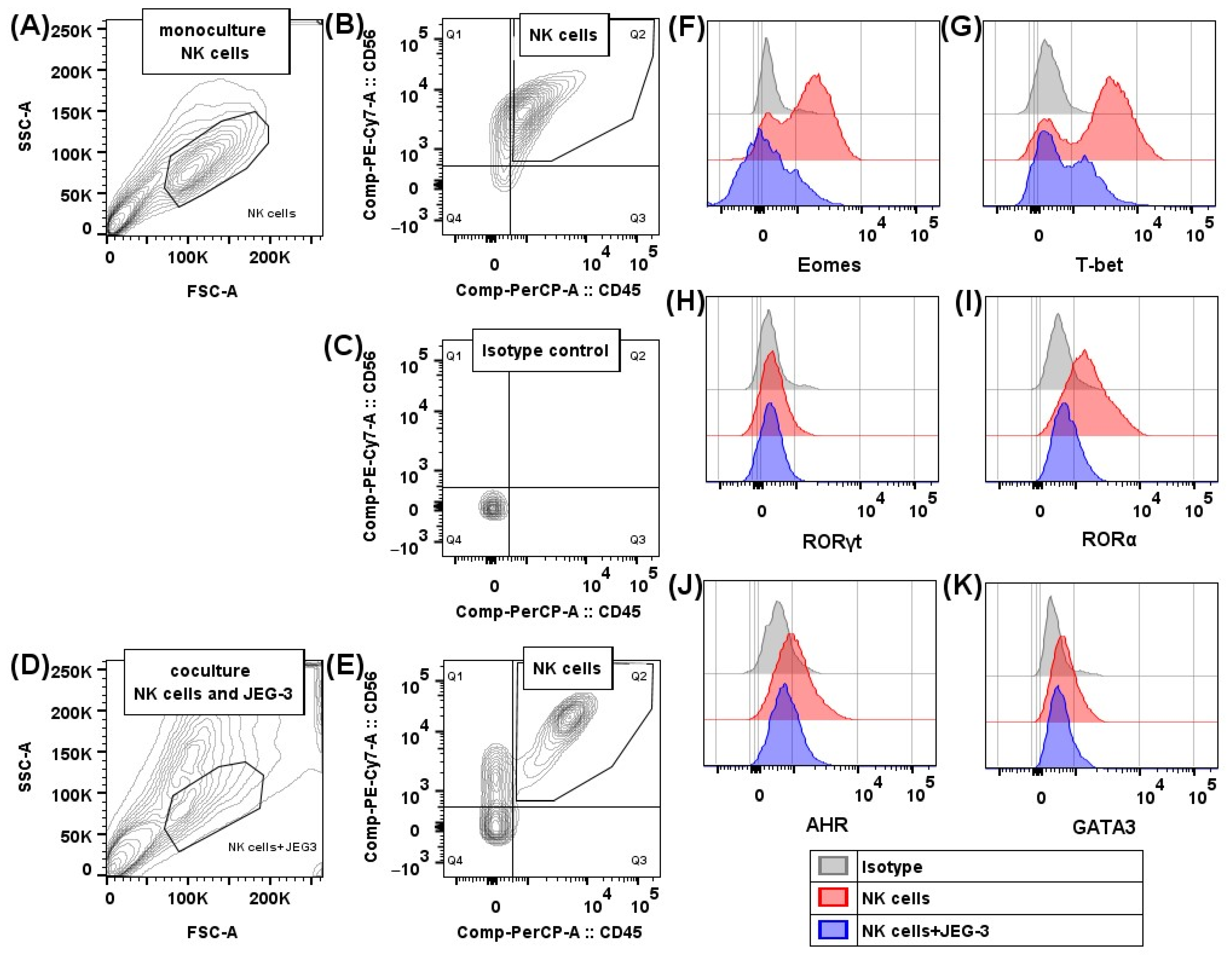
2.1. Transcription Factors in NK-92 Cells After Culturing with JEG-3 Trophoblast Cells
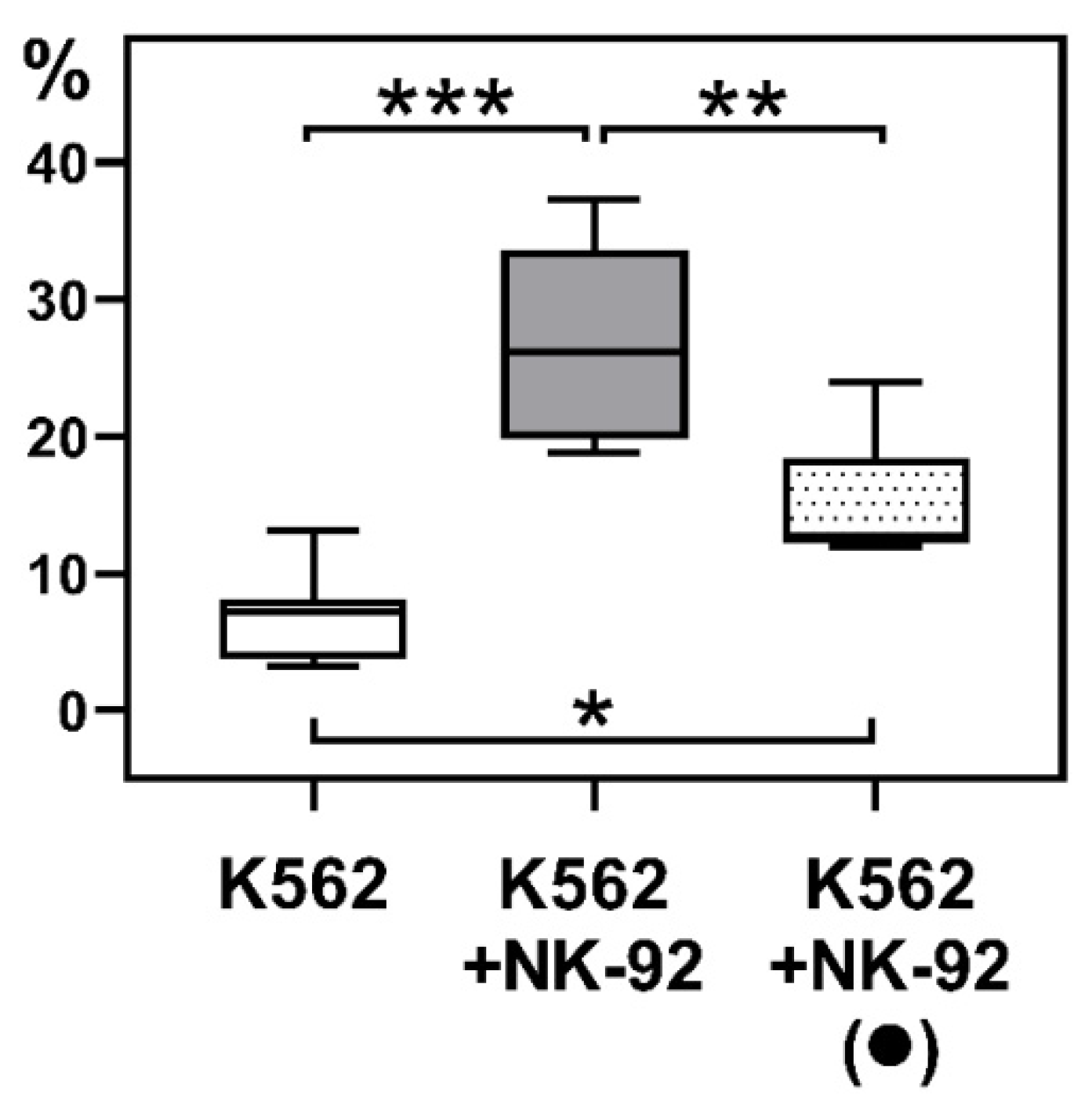
2.2. Cytotoxicity of NK-92 Cells Towards K-562 Cells After Culturing NK Cells with JEG-3 Trophoblast Cells
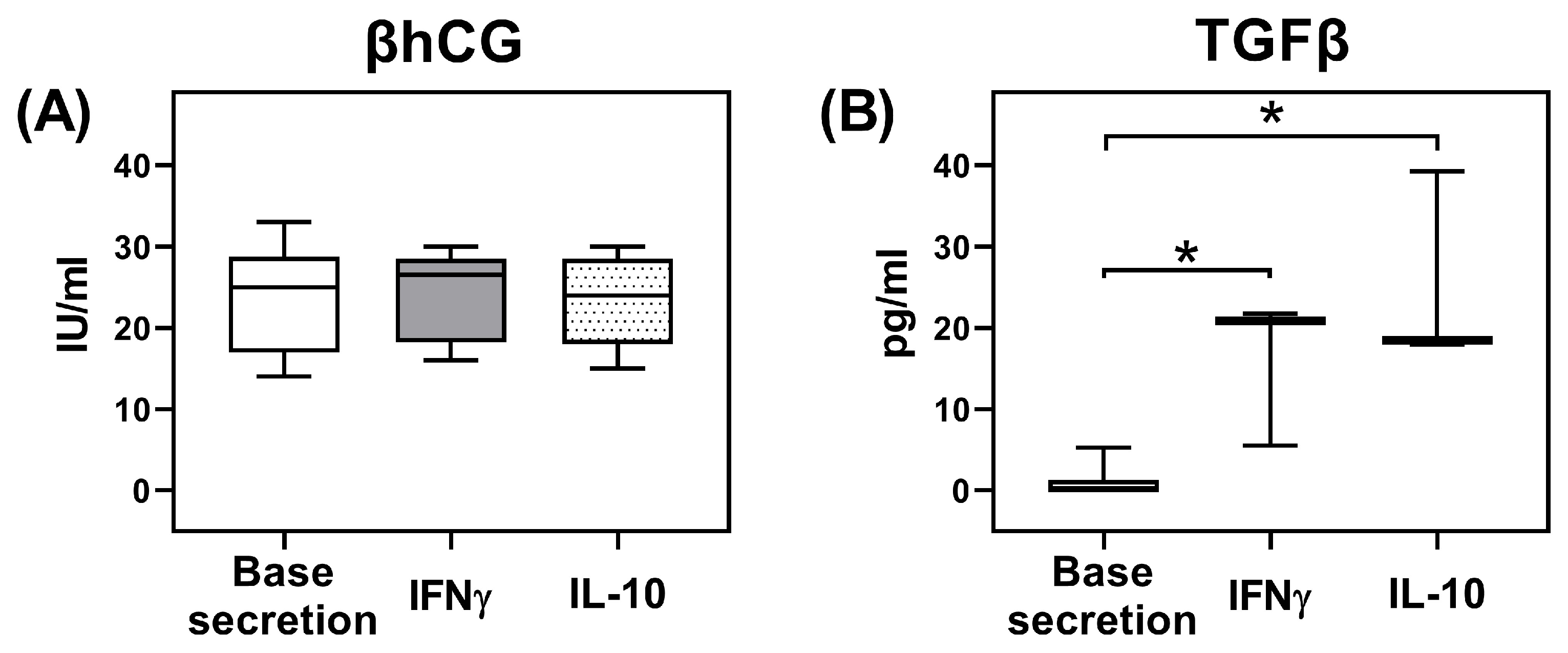
2.3. Secretion of TGFβ and β-Subunit of Human Chorionic Gonadotropin (βhCG) by JEG-3 Trophoblast Cells
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Inductors
4.3. Transcription Factors Evaluation in NK-92 Cells After Coculture with JEG-3 Trophoblast Cells
4.4. Cytotoxicity Assay of NK-92 Cells After Their Coculture with JEG-3 Trophoblast Cells
4.5. Secretion of TGFβ and β-Subunit of Human Chorionic Gonadotropin (βhCG) Evaluation by Trophoblast Cells of the JEG-3 Line
4.6. Data Statistical Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stokic-Trtica, V.; Diefenbach, A.; Klose, C.S.N. NK Cell Development in Times of Innate Lymphoid Cell Diversity. Front. Immunol. 2020, 11, 813. [Google Scholar] [CrossRef]
- Vacca, P.; Chiossone, L.; Mingari, M.C.; Moretta, L. Heterogeneity of NK Cells and Other Innate Lymphoid Cells in Human and Murine Decidua. Front. Immunol. 2019, 10, 170. [Google Scholar] [CrossRef]
- Kiekens, L.; Van Loocke, W.; Taveirne, S.; Wahlen, S.; Persyn, E.; Van Ammel, E.; De Vos, Z.; Matthys, P.; Van Nieuwerburgh, F.; Taghon, T.; et al. T-BET and EOMES Accelerate and Enhance Functional Differentiation of Human Natural Killer Cells. Front. Immunol. 2021, 12, 732511. [Google Scholar] [CrossRef]
- Zhang, J.; Le Gras, S.; Pouxvielh, K.; Faure, F.; Fallone, L.; Kern, N.; Moreews, M.; Mathieu, A.L.; Schneider, R.; Marliac, Q.; et al. Sequential actions of EOMES and T-BET promote stepwise maturation of natural killer cells. Nat. Commun. 2021, 12, 5446. [Google Scholar] [CrossRef]
- Cortez, V.S.; Colonna, M. Diversity and function of group 1 innate lymphoid cells. Immunol. Lett. 2016, 179, 19–24. [Google Scholar] [CrossRef]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. reviews. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Sun, X.-H.E. Innate Lymphoid Cells; Springer Nature Singapore Pte Ltd.: Singapore, 2022. [Google Scholar]
- Eberl, G.; Di Santo, J.P.; Vivier, E. The brave new world of innate lymphoid cells. Nat. Immunol. 2015, 16, 1–5. [Google Scholar] [CrossRef]
- Favaro, R.R.; Phillips, K.; Delaunay-Danguy, R.; Ujcic, K.; Markert, U.R. Emerging Concepts in Innate Lymphoid Cells, Memory, and Reproduction. Front. Immunol. 2022, 13, 824263. [Google Scholar] [CrossRef]
- Wang, P.; Liang, T.; Zhan, H.; Zhu, M.; Wu, M.; Qian, L.; Zhou, Y.; Ni, F. Unique metabolism and protein expression signature in human decidual NK cells. Front. Immunol. 2023, 14, 1136652. [Google Scholar] [CrossRef] [PubMed]
- de Mendonca Vieira, R.; Meagher, A.; Crespo, A.C.; Kshirsagar, S.K.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Human Term Pregnancy Decidual NK Cells Generate Distinct Cytotoxic Responses. J. Immunol. 2020, 204, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.J.; Huang, S.J.; Chen, C.P.; Huang, Y.; Xu, J.; Faramarzi, S.; Kayisli, O.; Kayisli, U.; Koopman, L.; Smedts, D.; et al. Decidual cell regulation of natural killer cell-recruiting chemokines: Implications for the pathogenesis and prediction of preeclampsia. Am. J. Pathol. 2013, 183, 841–856. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, X.; Zhang, L.; Liu, J.; Shao, X.; Li, Y.X.; Wang, Y.L. Uterine decidual niche modulates the progressive dedifferentiation of spiral artery vascular smooth muscle cells during human pregnancydagger. Biol. Reprod. 2021, 104, 624–637. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, S.; Zhou, Q.; Li, X. Trophoblast-derived interleukin 9 mediates immune cell conversion and contributes to maternal-fetal tolerance. J. Reprod. Immunol. 2021, 148, 103379. [Google Scholar] [CrossRef]
- Eikmans, M.; van der Keur, C.; Anholts, J.D.H.; Drabbels, J.J.M.; van Beelen, E.; de Sousa Lopes, S.M.C.; van der Hoorn, M.L. Primary Trophoblast Cultures: Characterization of HLA Profiles and Immune Cell Interactions. Front. Immunol. 2022, 13, 814019. [Google Scholar] [CrossRef]
- Zhuang, B.M.; Cao, D.D.; Li, T.X.; Liu, X.F.; Lyu, M.M.; Wang, S.D.; Cui, X.Y.; Wang, L.; Chen, X.L.; Lin, X.L.; et al. Single-cell characterization of self-renewing primary trophoblast organoids as modeling of EVT differentiation and interactions with decidual natural killer cells. BMC Genom. 2023, 24, 618. [Google Scholar] [CrossRef]
- Huhn, O.; Ivarsson, M.A.; Gardner, L.; Hollinshead, M.; Stinchcombe, J.C.; Chen, P.; Shreeve, N.; Chazara, O.; Farrell, L.E.; Theorell, J.; et al. Distinctive phenotypes and functions of innate lymphoid cells in human decidua during early pregnancy. Nat. Commun. 2020, 11, 381. [Google Scholar] [CrossRef]
- Mendes, J.; Areia, A.L.; Rodrigues-Santos, P.; Santos-Rosa, M.; Mota-Pinto, A. Innate Lymphoid Cells in Human Pregnancy. Front. Immunol. 2020, 11, 551707. [Google Scholar] [CrossRef]
- Vacca, P.; Montaldo, E.; Croxatto, D.; Loiacono, F.; Canegallo, F.; Venturini, P.L.; Moretta, L.; Mingari, M.C. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol. 2015, 8, 254–264. [Google Scholar] [CrossRef]
- Xu, Y.; Romero, R.; Miller, D.; Silva, P.; Panaitescu, B.; Theis, K.R.; Arif, A.; Hassan, S.S.; Gomez-Lopez, N. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am. J. Reprod. Immunol. 2018, 79, e12820. [Google Scholar] [CrossRef]
- Mendes, J.; Rodrigues-Santos, P.; Areia, A.L.; Almeida, J.S.; Alves, V.; Santos-Rosa, M.; Mota-Pinto, A. Type 2 and type 3 innate lymphoid cells at the maternal-fetal interface: Implications in preterm birth. BMC Immunol. 2021, 22, 28. [Google Scholar] [CrossRef]
- Doisne, J.M.; Balmas, E.; Boulenouar, S.; Gaynor, L.M.; Kieckbusch, J.; Gardner, L.; Hawkes, D.A.; Barbara, C.F.; Sharkey, A.M.; Brady, H.J.; et al. Composition, Development, and Function of Uterine Innate Lymphoid Cells. J. Immunol. 2015, 195, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.; Kiss, E.A.; Schwierzeck, V.; Ebert, K.; Hoyler, T.; d’Hargues, Y.; Goppert, N.; Croxford, A.L.; Waisman, A.; Tanriver, Y.; et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature 2013, 494, 261–265. [Google Scholar] [CrossRef]
- Li, Y.; Lopez, G.E.; Lindner, P.N.; Parrella, L.; Larson, M.; Sun, Y.; Stanic, A.K. The role of RORgammat at maternal-fetal interface during murine pregnancy. Am. J. Reprod. Immunol. 2020, 84, e13250. [Google Scholar] [CrossRef]
- Jia, W.; Ma, L.; Yu, X.; Wang, F.; Yang, Q.; Wang, X.; Fan, M.; Gu, Y.; Meng, R.; Wang, J.; et al. Human CD56(+)CD39(+) dNK cells support fetal survival through controlling trophoblastic cell fate: Immune mechanisms of recurrent early pregnancy loss. Natl. Sci. Rev. 2024, 11, nwae142. [Google Scholar] [CrossRef]
- Lu, H.; Jin, L.P.; Huang, H.L.; Ha, S.Y.; Yang, H.L.; Chang, R.Q.; Li, D.J.; Li, M.Q. Trophoblast-derived CXCL12 promotes CD56(bright) CD82(-) CD29(+) NK cell enrichment in the decidua. Am. J. Reprod. Immunol. 2020, 83, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yun, S.; Ryu, B.J.; Han, A.R.; Lee, S.K. Trophoblasts regulate natural killer cells via control of interleukin-15 receptor signaling. Am. J. Reprod. Immunol. 2017, 78, e12628. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, Y.; Tao, Y.; Piao, H.; Wang, X.; Luan, X.; Du, M.; Li, D. Involvement of the JAK-STAT pathway in collagen regulation of decidual NK cells. Am. J. Reprod. Immunol. 2017, 78, e12769. [Google Scholar] [CrossRef]
- Yang, S.L.; Tan, H.X.; Niu, T.T.; Li, D.J.; Wang, H.Y.; Li, M.Q. Kynurenine promotes the cytotoxicity of NK cells through aryl hydrocarbon receptor in early pregnancy. J. Reprod. Immunol. 2021, 143, 103270. [Google Scholar] [CrossRef] [PubMed]
- Vacca, P.; Vitale, C.; Munari, E.; Cassatella, M.A.; Mingari, M.C.; Moretta, L. Human Innate Lymphoid Cells: Their Functional and Cellular Interactions in Decidua. Front. Immunol. 2018, 9, 1897. [Google Scholar] [CrossRef]
- Mikhailova, V.; Khokhlova, E.; Grebenkina, P.; Salloum, Z.; Nikolaenkov, I.; Markova, K.; Davidova, A.; Selkov, S.; Sokolov, D. NK-92 cells change their phenotype and function when cocultured with IL-15, IL-18 and trophoblast cells. Immunobiology 2021, 226, 152125. [Google Scholar] [CrossRef]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J.; et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, A.; Alicata, C.; Tumino, N.; Ingegnere, T.; Loiacono, F.; Mingari, M.C.; Moretta, L.; Vacca, P. An Anti-inflammatory microRNA Signature Distinguishes Group 3 Innate Lymphoid Cells From Natural Killer Cells in Human Decidua. Front. Immunol. 2020, 11, 133. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.L.; Zhao, S.J.; Lin, X.X.; Liao, A.H. IL-10: A bridge between immune cells and metabolism during pregnancy. J. Reprod. Immunol. 2022, 154, 103750. [Google Scholar] [CrossRef]
- Robinette, M.L.; Colonna, M. Innate lymphoid cells and the MHC. HLA 2016, 87, 5–11. [Google Scholar] [CrossRef]
- Li, B.W.S.; Stadhouders, R.; de Bruijn, M.J.W.; Lukkes, M.; Beerens, D.; Brem, M.D.; KleinJan, A.; Bergen, I.; Vroman, H.; Kool, M.; et al. Group 2 Innate Lymphoid Cells Exhibit a Dynamic Phenotype in Allergic Airway Inflammation. Front. Immunol. 2017, 8, 1684. [Google Scholar] [CrossRef]
- Matha, L.; Krabbendam, L.; Martinez Hoyer, S.; Heesters, B.A.; Golebski, K.; Kradolfer, C.; Ghaedi, M.; Ma, J.; Stadhouders, R.; Bachert, C.; et al. Human CD127 negative ILC2s show immunological memory. J. Exp. Med. 2024, 221, e20231827. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Cunha, S.C.; Goncalves, D.; Mendes, A.; Braga, J.; Correia-da-Silva, G.; Teixeira, N.A. Decidual NK cell-derived conditioned medium from miscarriages affects endometrial stromal cell decidualisation: Endocannabinoid anandamide and tumour necrosis factor-alpha crosstalk. Hum. Reprod. 2020, 35, 265–274. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamamoto, T.; Yamazaki, M.; Murase, T.; Matsuno, T.; Chishima, F. Natural Cytotoxicity Receptors in Decidua Natural Killer Cells of Term Normal Pregnancy. J. Pregnancy 2018, 2018, 4382084. [Google Scholar] [CrossRef] [PubMed]
- Plevyak, M.; Hanna, N.; Mayer, S.; Murphy, S.; Pinar, H.; Fast, L.; Ekerfelt, C.; Ernerudh, J.; Berg, G.; Matthiesen, L.; et al. Deficiency of decidual IL-10 in first trimester missed abortion: A lack of correlation with the decidual immune cell profile. Am. J. Reprod. Immunol. 2002, 47, 242–250. [Google Scholar] [CrossRef]
- Yamamoto, M.; Fukui, A.; Mai, C.; Saeki, S.; Takayama, R.; Wakimoto, Y.; Yamaya, A.; Kwak-Kim, J.; Shibahara, H. Evaluation of NKp46 expression and cytokine production of decidual NK cells in women with recurrent pregnancy loss. Reprod. Med. Biol. 2022, 21, e12478. [Google Scholar] [CrossRef]
- Yang, H.G.; Kang, M.C.; Kim, T.Y.; Hwang, I.; Jin, H.T.; Sung, Y.C.; Eom, K.S.; Kim, S.W. Discovery of a novel natural killer cell line with distinct immunostimulatory and proliferative potential as an alternative platform for cancer immunotherapy. J. Immunother. Cancer 2019, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, A.; Poehlmann, T.G.; Busch, S.; Schleussner, E.; Markert, U.R. Signal transducer and activator of transcription 3 (STAT3) and Suppressor of Cytokine Signaling (SOCS3) balance controls cytotoxicity and IL-10 expression in decidual-like natural killer cell line NK-92. Am. J. Reprod. Immunol. 2011, 66, 329–335. [Google Scholar] [CrossRef]
- Lee, C.L.; Vijayan, M.; Wang, X.; Lam, K.K.W.; Koistinen, H.; Seppala, M.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; Chiu, P.C.N. Glycodelin-A stimulates the conversion of human peripheral blood CD16-CD56bright NK cell to a decidual NK cell-like phenotype. Hum. Reprod. 2019, 34, 689–701. [Google Scholar] [CrossRef]
- Hu, Y.; Eastabrook, G.; Tan, R.; MacCalman, C.D.; Dutz, J.P.; von Dadelszen, P. Decidual NK cell-derived conditioned medium enhances capillary tube and network organization in an extravillous cytotrophoblast cell line. Placenta 2010, 31, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Eastabrook, G.D.; Hu, Y.; Tan, R.; Dutz, J.P.; Maccalman, C.D.; von Dadelszen, P. Decidual NK cell-derived conditioned medium (dNK-CM) mediates VEGF-C secretion in extravillous cytotrophoblasts. Am. J. Reprod. Immunol. 2012, 67, 101–111. [Google Scholar] [CrossRef]
- Ma, L.; Li, G.; Cao, G.; Zhu, Y.; Du, M.R.; Zhao, Y.; Wang, H.; Liu, Y.; Yang, Y.; Li, Y.X.; et al. dNK cells facilitate the interaction between trophoblastic and endothelial cells via VEGF-C and HGF. Immunol. Cell Biol. 2017, 95, 695–704. [Google Scholar] [CrossRef]
- Cortez, V.S.; Cervantes-Barragan, L.; Robinette, M.L.; Bando, J.K.; Wang, Y.; Geiger, T.L.; Gilfillan, S.; Fuchs, A.; Vivier, E.; Sun, J.C.; et al. Transforming Growth Factor-beta Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands. Immunity 2016, 44, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Hawke, L.G.; Mitchell, B.Z.; Ormiston, M.L. TGF-beta and IL-15 Synergize through MAPK Pathways to Drive the Conversion of Human NK Cells to an Innate Lymphoid Cell 1-like Phenotype. J. Immunol. 2020, 204, 3171–3181. [Google Scholar] [CrossRef]
- Grebenkina, P.V.; Mikhailova, V.A.; Tyschuk, E.V.; Marko, O.B.; Yukhina, V.A.; Gulina, A.M.; Bespalova, O.N.; Selkov, S.A.; Sokolov, D.I. Regulation of natural killer cells cytotoxicity by trophoblast cells: Proteins and receptors. Res. Results Biomed. 2025, 11, 142–163. [Google Scholar] [CrossRef]
- Sun, J.; Yang, M.; Ban, Y.; Gao, W.; Song, B.; Wang, Y.; Zhang, Y.; Shao, Q.; Kong, B.; Qu, X. Tim-3 Is Upregulated in NK Cells during Early Pregnancy and Inhibits NK Cytotoxicity toward Trophoblast in Galectin-9 Dependent Pathway. PLoS ONE 2016, 11, e0147186. [Google Scholar] [CrossRef]
- Chiossone, L.; Vacca, P.; Orecchia, P.; Croxatto, D.; Damonte, P.; Astigiano, S.; Barbieri, O.; Bottino, C.; Moretta, L.; Mingari, M.C. In vivo generation of decidual natural killer cells from resident hematopoietic progenitors. Haematologica 2014, 99, 448–457. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Y.H.; Piao, H.L.; Zhou, W.J.; Zhang, D.; Fu, Q.; Wang, S.C.; Li, D.J.; Du, M.R. CD56(bright)CD25+ NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. Cell. Mol. Immunol. 2015, 12, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for study of human embryo implantation: Choice of cell lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef]
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994, 8, 652–658. [Google Scholar] [PubMed]
- Han, M.; Jiang, Y.; Lao, K.; Xu, X.; Zhan, S.; Wang, Y.; Hu, X. sHLA-G involved in the apoptosis of decidual natural killer cells following Toxoplasma gondii infection. Inflammation 2014, 37, 1718–1727. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E.O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 1999, 189, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, F.; Kajiwara, M. Relation of natural killer cell line NK-92-mediated cytolysis (NK-92-lysis) with the surface markers of major histocompatibility complex class I antigens, adhesion molecules, and Fas of target cells. Oncol. Res. 1998, 10, 483–489. [Google Scholar]
- Melsted, W.N.; Matzen, S.H.; Andersen, M.H.; Hviid, T.V.F. The choriocarcinoma cell line JEG-3 upregulates regulatory T cell phenotypes and modulates pro-inflammatory cytokines through HLA-G. Cell. Immunol. 2018, 324, 14–23. [Google Scholar] [CrossRef]
- Persson, G.; Bork, J.B.S.; Isgaard, C.; Larsen, T.G.; Bordoy, A.M.; Bengtsson, M.S.; Hviid, T.V.F. Cytokine stimulation of the choriocarcinoma cell line JEG-3 leads to alterations in the HLA-G expression profile. Cell. Immunol. 2020, 352, 104110. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Spitz, B.; Hanssens, M.; Luyten, C.; Pijnenborg, R. Differential effects of inducers of syncytialization and apoptosis on BeWo and JEG-3 choriocarcinoma cells. Hum. Reprod. 2006, 21, 193–201. [Google Scholar] [CrossRef]
- Poloski, E.; Oettel, A.; Ehrentraut, S.; Luley, L.; Costa, S.D.; Zenclussen, A.C.; Schumacher, A. JEG-3 Trophoblast Cells Producing Human Chorionic Gonadotropin Promote Conversion of Human CD4+FOXP3- T Cells into CD4+FOXP3+ Regulatory T Cells and Foster T Cell Suppressive Activity. Biol. Reprod. 2016, 94, 106. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.O.; Bridson, W.E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J. Clin. Endocrinol. Metab. 1971, 32, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gao, R.; Qing, P.; Zeng, X.; Liao, X.; Cheng, M.; Qin, L.; Liu, Y. Single-cell transcriptome analyses reveal disturbed decidual homoeostasis in obstetric antiphospholipid syndrome. Ann. Rheum. Dis. 2024, 83, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M. Interleukin-15 in Outcomes of Pregnancy. Int. J. Mol. Sci. 2021, 22, 11094. [Google Scholar] [CrossRef]
- Tauber, Z.; Chroma, K.; Baranova, R.; Cizkova, K. The expression patterns of IL-1beta and IL-10 and their relation to CYP epoxygenases in normal human placenta. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2021, 236, 151671. [Google Scholar] [CrossRef]
- Fang, L.; Yan, Y.; Gao, Y.; Wu, Z.; Wang, Z.; Yang, S.; Cheng, J.C.; Sun, Y.P. TGF-beta1 inhibits human trophoblast cell invasion by upregulating kisspeptin expression through ERK1/2 but not SMAD signaling pathway. Reprod. Biol. Endocrinol. 2022, 20, 22. [Google Scholar] [CrossRef]
- Bazhenov, D.; Mikhailova, V.; Nikolaenkov, I.; Markova, K.; Salloum, Z.; Kogan, I.; Gzgzyan, A.; Selkov, S.; Sokolov, D. The uteroplacental contact zone cytokine influence on NK cell cytotoxicity to trophoblasts. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, 151671. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, V.; Grebenkina, P.; Selkov, S.; Sokolov, D. JEG-3 Trophoblast Cells Influence ILC-like Transformation of NK Cells In Vitro. Int. J. Mol. Sci. 2025, 26, 3687. https://doi.org/10.3390/ijms26083687
Mikhailova V, Grebenkina P, Selkov S, Sokolov D. JEG-3 Trophoblast Cells Influence ILC-like Transformation of NK Cells In Vitro. International Journal of Molecular Sciences. 2025; 26(8):3687. https://doi.org/10.3390/ijms26083687
Chicago/Turabian StyleMikhailova, Valentina, Polina Grebenkina, Sergey Selkov, and Dmitry Sokolov. 2025. "JEG-3 Trophoblast Cells Influence ILC-like Transformation of NK Cells In Vitro" International Journal of Molecular Sciences 26, no. 8: 3687. https://doi.org/10.3390/ijms26083687
APA StyleMikhailova, V., Grebenkina, P., Selkov, S., & Sokolov, D. (2025). JEG-3 Trophoblast Cells Influence ILC-like Transformation of NK Cells In Vitro. International Journal of Molecular Sciences, 26(8), 3687. https://doi.org/10.3390/ijms26083687








