Overexpression of Tetrahymena Cysteine Synthetase 1 Promotes Cadmium Removal by Biosynthesizing Cadmium Sulfide Quantum Dots in Escherichia coli
Abstract
:1. Introduction
2. Results
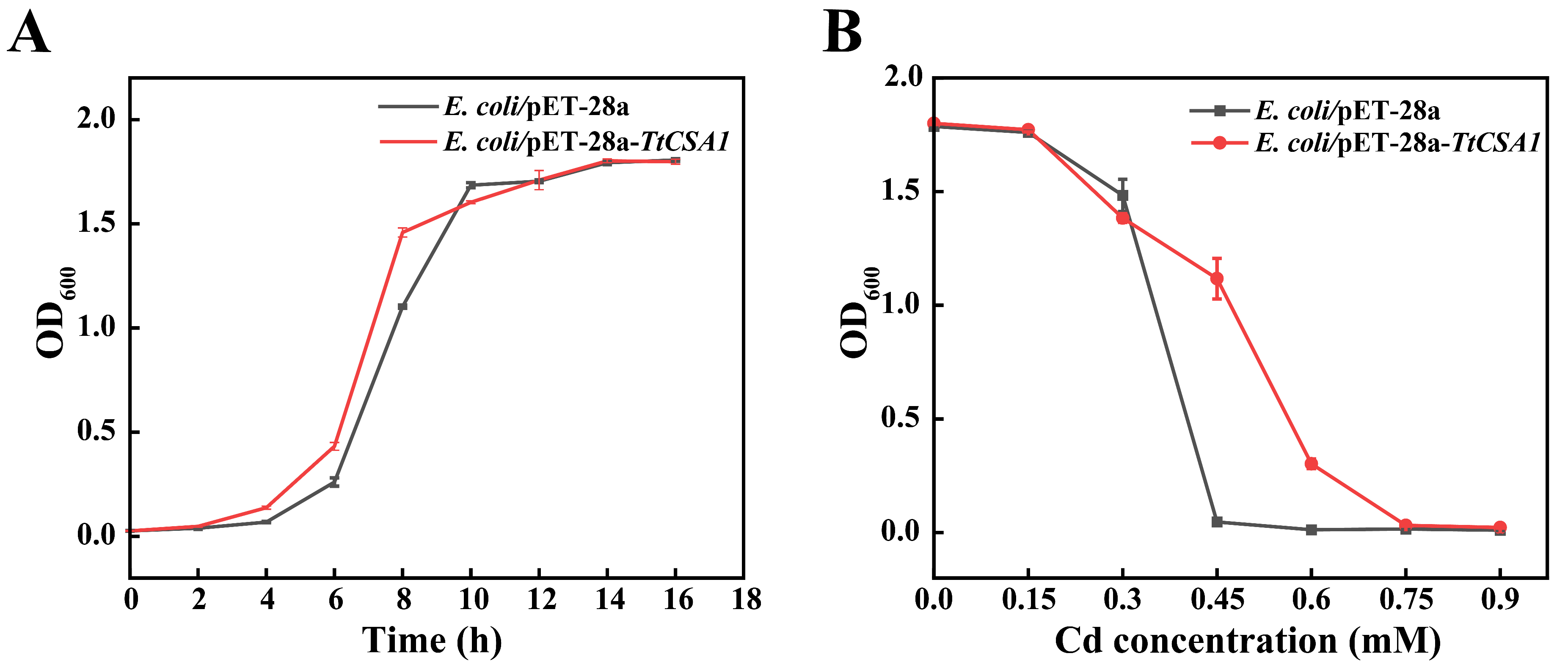
2.1. Overexpression of TtCsa1 Significantly Enhances Cadmium Resistance in E. coli
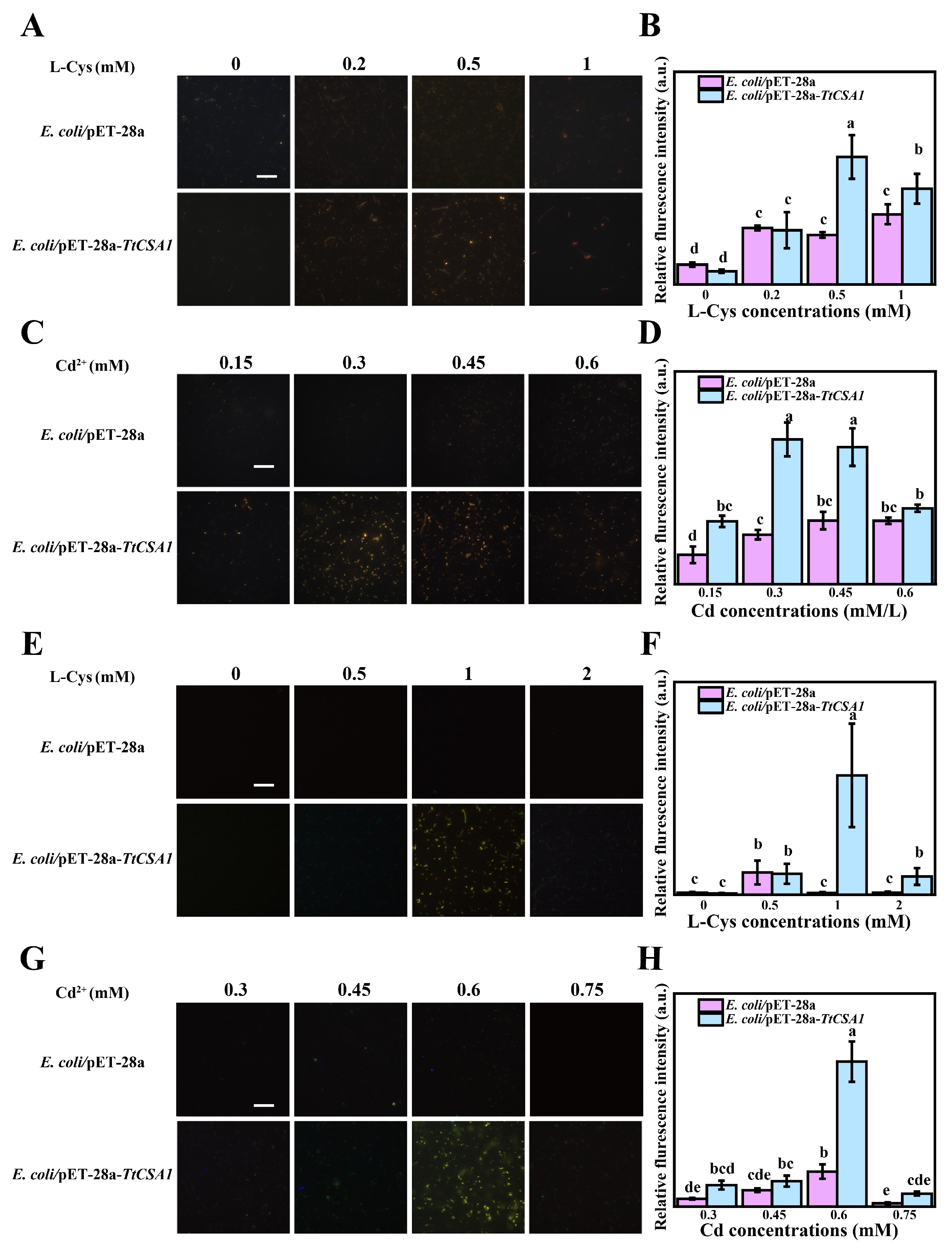
2.2. TtCsa1 and Cysteine Enhance Biomineralization of Cd in E. coli
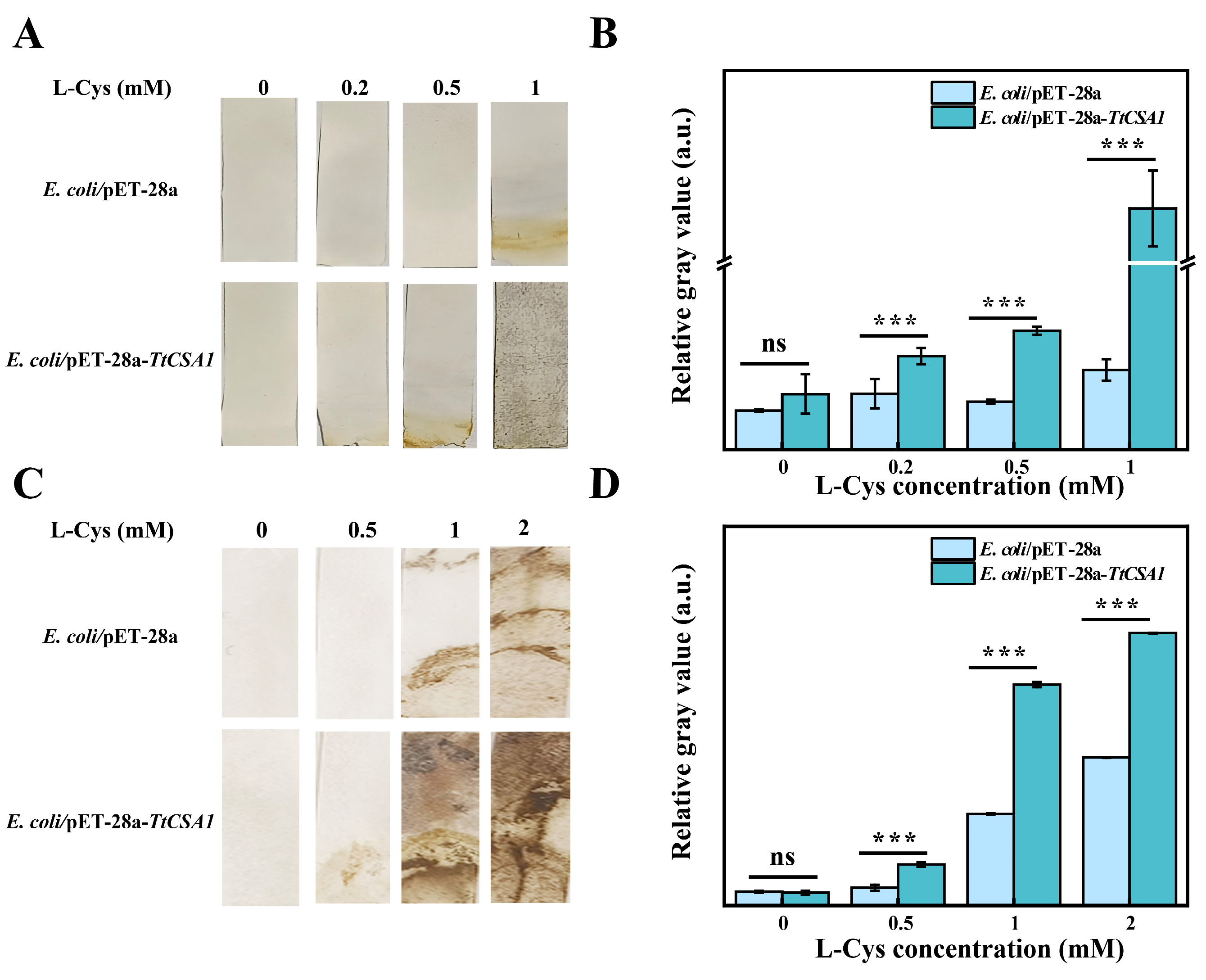
2.3. E. coli/pET-28a-TtCSA1 Produces More H2S upon the Addition of Cysteine
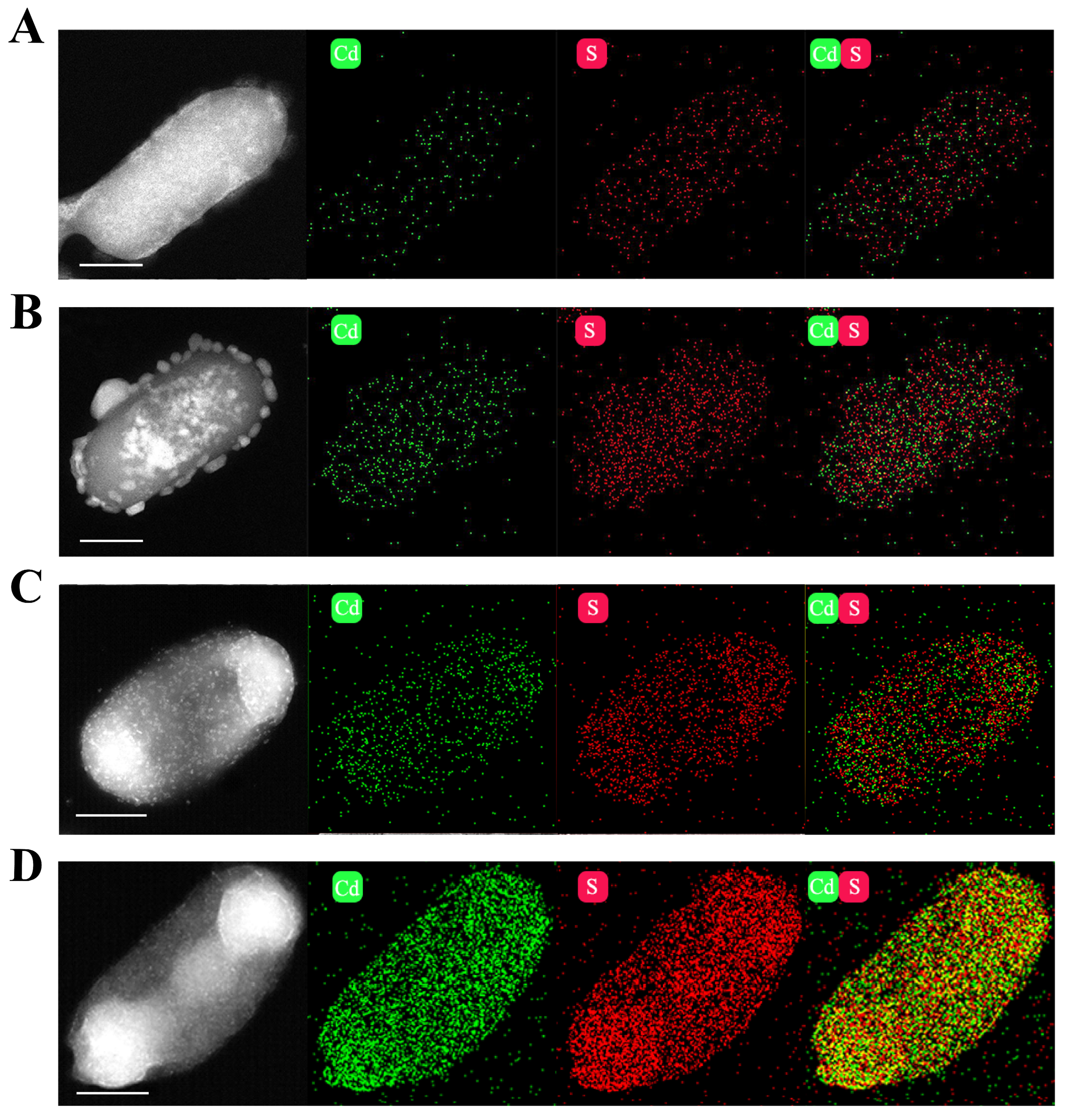
2.4. Characterization of CdS Quantum Dots in E. coli
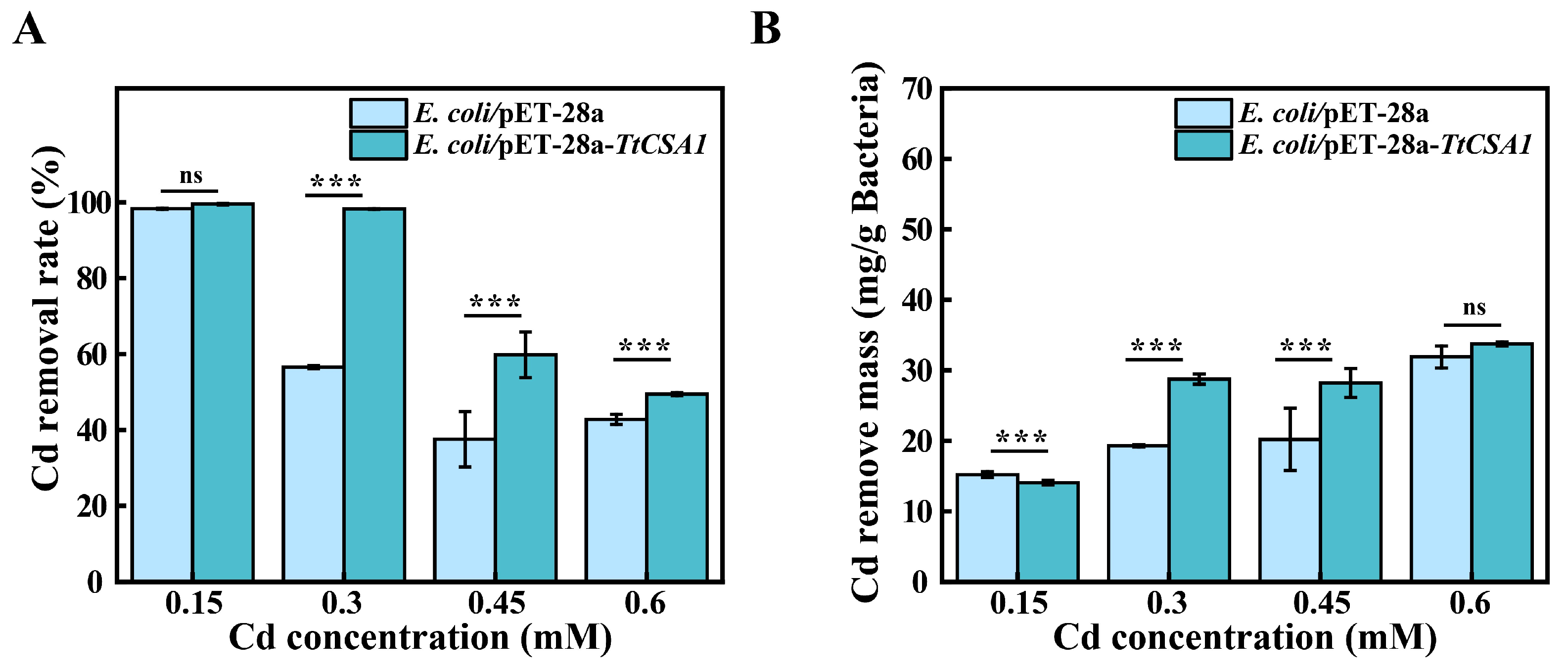
2.5. Removal of Cadmium Ions from Rich Medium
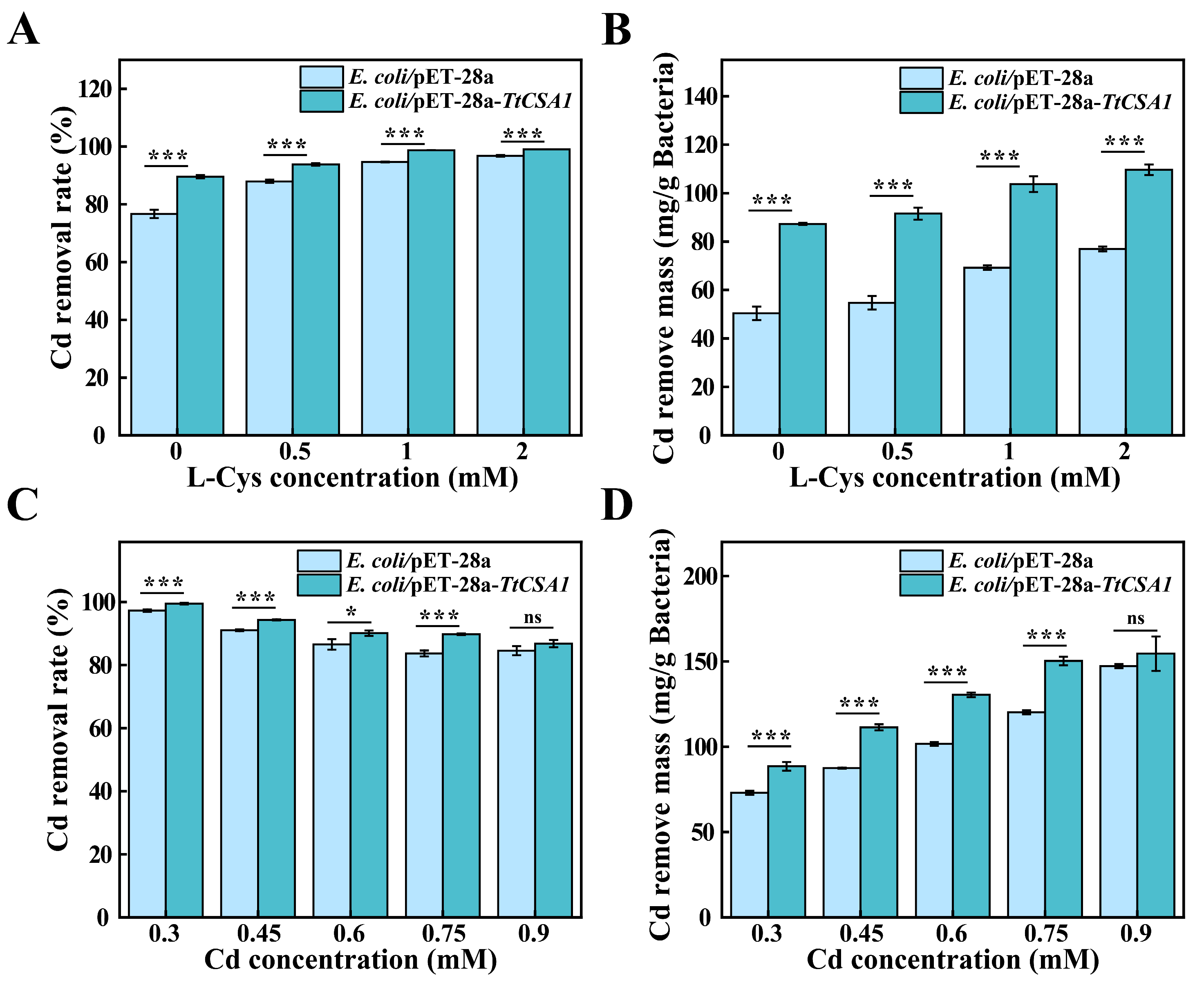
2.6. Removal of Cadmium Ions from Simulated Wastewater
3. Discussion
4. Materials and Methods
4.1. Strains, Media, and Reagents
4.2. Determination of Growth Curves and Minimum Inhibitory Concentration of Cadmium Ions
4.3. Preparation of E. coli/CdS and Fluorescence Intensity Analysis
4.4. Production and Detection of H2S
4.5. The Absorption and Emission Spectrum of CdS QDs
4.6. Characterization of CdS QDs by Transmission Electron Microscope and Energy Dispersive X-Ray Spectroscopy (EDX)
4.7. X-Ray Diffraction of E. coli/pET-28a-TtCSA1-CdS
4.8. Cadmium Removal in LB Medium and Simulated Wastewater
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Cheng, J.; Wang, J.; Zhang, F.; Tian, Y.; Liu, C.; Cao, L.; Zhou, Y. Efficient Remediation of Cadmium Contamination in Soil by Functionalized Biochar: Recent Advances, Challenges, and Future Prospects. Processes 2022, 10, 1627. [Google Scholar] [CrossRef]
- Cristani, M.; Naccari, C.; Nostro, A.; Pizzimenti, A.; Trombetta, D.; Pizzimenti, F. Possible use of Serratia marcescens in toxic metal biosorption (removal). Environ. Sci. Pollut. Res. 2012, 19, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Q.; Li, Y.; Wan, S.; Yin, Z.; Zhao, S.; Dai, X.; Wang, R.; Wang, S.; Zhai, Y.; et al. Stress Response of Aphid Population Under Combined Stress of Cadmium and Lead and Its Effects on Development of Harmonia axyridis. Int. J. Mol. Sci. 2024, 25, 20. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Ko, D.; Kim, H.; Lee, H.; Yavuz, C.T.; Andersen, H.R.; Hwang, Y. Applicability of disulfide-polymer particles surface embedded on alginate beads for cadmium removal from airport derived stormwater. J. Environ. Chem. Eng. 2018, 6, 4124–4129. [Google Scholar] [CrossRef]
- Qiu, M.; Bi, X.; Liu, Y.; Li, H.; Li, D.; Chen, G. Toxicology Effects of Cadmium in Pomacea canaliculate: Accumulation, Oxidative Stress, Microbial Community, and Transcriptome Analysis. Int. J. Mol. Sci. 2025, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.W.; Li, T.; Gao, Z.H.; Xiong, J.; Miao, W. Construction of CdS-Tetrahymena thermophila hybrid system by efficient cadmium adsorption for dye removal under light irradiation. J. Hazard. Mater. 2022, 439, 129683. [Google Scholar] [CrossRef]
- Kharel, H.L.; Jha, L.; Tan, M.; Selvaratnam, T. Removal of Cadmium (II) from Aqueous Solution Using Galdieria sulphuraria CCMEE 5587.1. BioTech 2024, 13, 28. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Palanivelu, J.; Hemavathy, R.V. Sustainable approaches for removing toxic heavy metal from contaminated water: A comprehensive review of bioremediation and biosorption techniques. Chemosphere 2024, 357, 141933. [Google Scholar] [CrossRef]
- Lopes, C.S.C.; Teixeira, D.B.; Braz, B.F.; Santelli, R.E.; de Castilho, L.V.A.; Gomez, J.G.C.; Castro, R.P.V.; Seldin, L.; Freire, D.M.G. Application of rhamnolipid surfactant for remediation of toxic metals of long- and short-term contamination sites. Int. J. Environ. Sci. Technol. 2020, 18, 575–588. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- Peng, W.; Li, X.; Song, J.; Jiang, W.; Liu, Y.; Fan, W. Bioremediation of cadmium- and zinc-contaminated soil using Rhodobacter sphaeroides. Chemosphere 2018, 197, 33–41. [Google Scholar] [CrossRef]
- Sreedevi, P.R.; Suresh, K.; Jiang, G. Bacterial bioremediation of heavy metals in wastewater: A review of processes and applications. J. Water Process Eng. 2022, 48, 102884. [Google Scholar] [CrossRef]
- Srivastava, S.; Agrawal, S.B.; Mondal, M.K. A review on progress of heavy metal removal using adsorbents of microbial and plant origin. Environ. Sci. Pollut. Res. 2015, 22, 15386–15415. [Google Scholar] [CrossRef]
- Chen, G.; Yi, B.; Zeng, G.; Niu, Q.; Yan, M.; Chen, A.; Du, J.; Huang, J.; Zhang, Q. Facile green extracellular biosynthesis of CdS quantum dots by white rot fungus Phanerochaete chrysosporium. Colloids Surf. B. 2014, 117, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Cai, R.; Sun, C. Threonine dehydratase enhances bacterial cadmium resistance via driving cysteine desulfuration and biomineralization of cadmium sulfide nanocrystals. J. Hazard. Mater. Adv. 2021, 417, 126102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, M.; Zhang, J.; Wang, H. Cysteine and thiosulfate promoted cadmium immobilization in strain G303 by the formation of extracellular CdS. Sci. Total Environ. 2024, 923, 171457. [Google Scholar] [CrossRef]
- Xu, S.; Luo, X.; Huang, Q.; Chen, W. Calcium-crosslinked alginate-encapsulated bacteria for remediating of cadmium-polluted water and production of CdS nanoparticles. Appl. Microbiol. Biotechnol. 2021, 105, 2171–2179. [Google Scholar] [CrossRef]
- Arif, M.S.; Yasmeen, T.; Abbas, Z.; Ali, S.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; Abdel-Daim, M.M. Role of Exogenous and Endogenous Hydrogen Sulfide (H2S) on Functional Traits of Plants Under Heavy Metal Stresses: A Recent Perspective. Front. Plant Sci. 2021, 11, 545453. [Google Scholar] [CrossRef]
- Venegas, F.A.; Saona, L.A.; Monrás, J.P.; Órdenes-Aenishanslins, N.; Giordana, M.F.; Ulloa, G.; Collao, B.; Bravo, D.; Pérez-Donoso, J.M. Biological phosphorylated molecules participate in the biomimetic and biological synthesis of cadmium sulphide quantum dots by promoting H2S release from cellular thiols. RSC Adv. 2017, 7, 40270–40278. [Google Scholar] [CrossRef]
- Tan, B.H.; Wong, P.T.H.; Bian, J.-S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Du, Q.-Q.; Qian, J.; Wan, D.-Y.; Wu, S.-M. Eco-friendly intracellular biosynthesis of CdS quantum dots without changing Escherichia coli’s antibiotic resistance. Enzyme Microb. Technol. 2017, 96, 96–102. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Shen, W. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. BioMetals 2012, 25, 617–631. [Google Scholar] [CrossRef]
- Ma, N.; Sha, Z.; Sun, C. Formation of cadmium sulfide nanoparticles mediates cadmium resistance and light utilization of the deep-sea bacterium Idiomarina sp. OT37-5b. Appl. Environ. Microbiol. 2021, 23, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Monras, J.P.; Plaza, D.O.; Collao, B.; Saona, L.A.; Duran-Toro, V.; Venegas, F.A.; Soto, C.; Ulloa, G.; Vasquez, C.C.; et al. Low-temperature biosynthesis of fluorescent semiconductor nanoparticles (CdS) by oxidative stress resistant Antarctic bacteria. J. Biotechnol. 2014, 187, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.F.; Tian, H.F.; Yan, Z.; Song, C.; Wang, S.G. Stability and biomineralization of cadmium sulfide nanoparticles biosynthesized by the bacterium Rhodopseudomonas palustris under light. J. Hazard. Mater. Adv. 2023, 458, 131937. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Chang, S. The construction of an engineered bacterium to remove cadmium from wastewater. Water Sci. Technol. 2014, 70, 2015–2021. [Google Scholar] [CrossRef]
- Shen, J.J.; Qiao, Z.J.; Xing, T.J.; Zhang, L.P.; Liang, Y.L.; Jin, Z.P.; Yang, G.D.; Wang, R.; Pei, Y.X. Cadmium toxicity is alleviated by AtLCD and AtDCD inEscherichia coli. J. Appl. Microbiol. 2012, 113, 1130–1138. [Google Scholar] [CrossRef]
- Jafarian, V.; Ghaffari, F. A unique metallothionein-engineered in Escherichia coli for biosorption of lead, zinc, and cadmium; absorption or adsorption? Microbiology 2017, 86, 73–81. [Google Scholar] [CrossRef]
- Li, Y.; Cui, R.; Zhang, P. Mechanism-Oriented Controllability of Intracellular Quantum Dots Formation: The Role of Glutathione Metabolic Pathway. ACS Nano 2012, 7, 2240–2248. [Google Scholar] [CrossRef]
- Kawashima, C.G.; Noji, M.; Nakamura, M.; Ogra, Y. Heavy metal tolerance of transgenic tobacco plants over-expressing cysteine synthase. Biotechnol. Lett. 2004, 26, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, K.; Jing, Y.; Zuo, J.; Hu, J.; Lv, X.; Chen, Z.; Mi, R.; Huang, Y.; Yu, S.; et al. LsrB-based and temperature-dependent identification of bacterial AI-2 receptor. AMB Express 2017, 7, 188. [Google Scholar] [CrossRef]
- Liu, Y.; Pei, R.; Huang, Z.; Xiao, J.; Yao, A.; Xu, K.; Li, Y.; Ullah, S.; Yu, Z.; Wang, Y.; et al. Green immobilization of CdS-Pt nanoparticles on recombinant Escherichia coli boosted by overexpressing cysteine desulfurase for photocatalysis application. Bioresour. Technol. Rep. 2021, 16, 100823. [Google Scholar] [CrossRef]
- Lv, H.; Xu, J.; Bo, T.; Wang, W. Characterization of Cystathionine β-Synthase TtCbs1 and Cysteine Synthase TtCsa1 Involved in Cysteine Biosynthesis in Tetrahymena thermophila. J. Eukaryotic Microbiol. 2021, 68, 12834. [Google Scholar] [CrossRef]
- Lv, H.; Xu, J.; Bo, T.; Wang, W. Comparative transcriptome analysis uncovers roles of hydrogen sulfide for alleviating cadmium toxicity in Tetrahymena thermophila. BMC Genom. 2021, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Liu, J.; Zhang, W.; Xu, J.; Bo, T.; Wang, Z.; Wang, W. Photocatalytic degradation of methylene blue by CdS quantum dots biosynthesized by cysteine synthetase TtCsa1 from Tetrahymena thermophila. Int. J. Biol. Macromol. 2025, 305, 141166. [Google Scholar] [CrossRef]
- Wu, C.; Deng, C.F.; Zhang, J.X.; Pan, W.; Yang, L.Y.; Pan, K.; Tan, Q.G.; Yue, T.T.; Miao, A.J. Silica nanoparticles inhibit cadmium uptake by the protozoan Tetrahymena thermophila without the need for adsorption. J. Hazard. Mater. 2024, 466, 133569. [Google Scholar] [CrossRef] [PubMed]
- Meena, O.P. Heavy Metals Toxicity and the Environment Protection. Hilaris SRL 2021, 8, 326. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Wang, B.; Wang, T.; Wang, Y.; Meng, D. Synthesis and photocatalytic property of cubic phase CdS. Solid State Sci. 2019, 92, 31–35. [Google Scholar] [CrossRef]
- Cui, Y.H.; Wu, J.H.; Wei, W.; Zhang, F.; Li, L.L.; Tian, L.J.; Li, W.W.; Lam, P.K.S.; Yu, H.Q. Intracellular Hybrid Biosystem in a Protozoan to Trigger Visible-Light-Driven Photocatalysis. ACS Appl. Mater. Interfaces. 2021, 13, 19846–19854. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Dou, Y.; Liu, D.; Si, W.; Fang, H.; Zhao, C.; Chen, S.; Xi, J.; Li, J. Hydrogen sulfide—Cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 2016, 6, 39702. [Google Scholar] [CrossRef]
- Cao, H.; Liang, Y.; Zhang, L.; Liu, Z.; Liu, D.; Cao, X.; Deng, X.; Jin, Z.; Pei, Y. AtPRMT5-mediated AtLCD methylation improves Cd2+ tolerance via increased H2S production in Arabidopsis. Plant Physiol. 2022, 190, 2637–2650. [Google Scholar] [CrossRef]
- Wang, C.L.; Lum, A.M.; Ozuna, S.C.; Clark, D.S.; Keasling, J.D. Aerobic sulfide production and cadmium precipitation by Escherichia coli expressing the Treponema denticola cysteine desulfhydrase gene. Appl. Microbiol. Biotechnol. 2001, 56, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, J.-H.; Meng, B.; Mao, K.; Zheng, M.; Tan, A.; Yang, G.; Feng, X. LmGSTF3 Overexpression Enhances Cadmium Tolerance in Lemna minor. Environ. Sci. Technol. 2024, 10, 1021. [Google Scholar] [CrossRef]
- Valenzuela-Ibaceta, F.; Torres-Olea, N.; Ramos-Zúñiga, J.; Dietz-Vargas, C.; Navarro, C.A.; Pérez-Donoso, J.M. Minicells as an Escherichia colimechanism for the accumulation and disposal of fluorescent cadmium sulphide nanoparticles. J. Nanobiotechnol. 2024, 22, 1. [Google Scholar] [CrossRef]
- Folgar, S.; Torres, E.; Pérez-Rama, M.; Cid, A.; Herrero, C.; Abalde, J. Dunaliella salina as marine microalga highly tolerant to but a poor remover of cadmium. J. Hazard. Mater. 2009, 165, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Wakeman, K.D.; Erving, L.; Riekkola-Vanhanen, M.L.; Puhakka, J.A. Silage supports sulfate reduction in the treatment of metals- and sulfate-containing waste waters. Water Res. 2010, 44, 4932–4939. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cheng, L.; Tao, M.; Tong, S.; Yao, X.; Liu, Y. Using modified quartz sand for phosphate pollution control in cemented phosphogypsum (PG) backfill. J. Clean. Prod. 2021, 283, 124652. [Google Scholar] [CrossRef]
- Ren, W.; Wan, C.; Li, Z.; Liu, X.; Zhang, R.; Yang, X.; Lee, D.J. Functional CdS nanocomposites recovered from biomineralization treatment ofsulfate wastewater and its applications in the perspective of photocatalysis and electrochemistry. Sci. Total Environ. 2020, 742, 140646. [Google Scholar] [CrossRef]
- Yagi, T.; Ogata, M. Catalytic properties of adenylylsulfate reductase from Desulfovibrio vulgaris Miyazaki. Biochimie 1996, 78, 838–846. [Google Scholar] [CrossRef]
- Dostal Webster, A.; Staley, C.; Hamilton, M.J.; Huang, M.; Fryxell, K.; Erickson, R.; Kabage, A.J.; Sadowsky, M.J.; Khoruts, A. Influence of short-term changes in dietary sulfur on the relative abundances of intestinal sulfate-reducing bacteria. Gut Microbes. 2019, 10, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.; Lien, T.; Birkeland, N. A novel organization of the dissimilatory sulfite reductase operon of Thermodesulforhabdus norvegica verified by RT-PCR. FEMS Microbiol. Lett. 2001, 203, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Li, L.; Young, E.; Carey, M.; Li, G.; Zhu, Y.-G.; Banwart, S.; Meharg, A.A.; Meharg, C. Fertilization Enhances Grain Inorganic Arsenic Assimilation in Rice. Expos. Health 2023, 16, 417–430. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Hu, Q.; Wei, T.; Wang, J.; Chen, H.; Wu, C. Temperature-Driven Metalloprotein-Based Hybrid Hydrogels for Selective and Reversible Removal of Cadmium(II) from Water. ACS Appl. Mater. Interfaces 2019, 12, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lu, C.; Hou, N.; Xin, Y.; Liu, J.; Liu, H.; Xun, L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef]
- Dunleavy, R.; Lu, L.; Kiely, C.J.; McIntosh, S.; Berger, B.W. Single-enzyme biomineralization of cadmium sulfide nanocrystals with controlled optical properties. Proc. Natl. Acad. Sci. USA 2016, 113, 5275–5280. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, N.; Jin, W.; Wu, S.; Sun, C. Genomic and Transcriptomic Insights into Calcium Carbonate Biomineralization by Marine Actinobacterium Brevibacterium linens BS258. Front. Microbiol. 2017, 8, 602. [Google Scholar] [CrossRef]
- Khan, I.; Aftab, M.; Shakir, S.; Ali, M.; Qayyum, S.; Rehman, M.U.; Haleem, K.S.; Touseef, I. Mycoremediation of heavy metal (Cd and Cr)–polluted soil through indigenous metallotolerant fungal isolates. Environ. Monit. Assess. 2019, 191, 9. [Google Scholar] [CrossRef]
- He, X.; Chen, W.; Huang, Q. Surface display of monkey metallothionein α tandem repeats and EGFP fusion protein on Pseudomonas putida X4 for biosorption and detection of cadmium. Appl. Microbiol. Biotechnol. 2011, 95, 1605–1613. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, W.; Liu, J.; Liu, Y.; Xu, J.; Wang, W. Overexpression of Tetrahymena Cysteine Synthetase 1 Promotes Cadmium Removal by Biosynthesizing Cadmium Sulfide Quantum Dots in Escherichia coli. Int. J. Mol. Sci. 2025, 26, 3685. https://doi.org/10.3390/ijms26083685
Lei W, Liu J, Liu Y, Xu J, Wang W. Overexpression of Tetrahymena Cysteine Synthetase 1 Promotes Cadmium Removal by Biosynthesizing Cadmium Sulfide Quantum Dots in Escherichia coli. International Journal of Molecular Sciences. 2025; 26(8):3685. https://doi.org/10.3390/ijms26083685
Chicago/Turabian StyleLei, Wenliang, Juan Liu, Yiwei Liu, Jing Xu, and Wei Wang. 2025. "Overexpression of Tetrahymena Cysteine Synthetase 1 Promotes Cadmium Removal by Biosynthesizing Cadmium Sulfide Quantum Dots in Escherichia coli" International Journal of Molecular Sciences 26, no. 8: 3685. https://doi.org/10.3390/ijms26083685
APA StyleLei, W., Liu, J., Liu, Y., Xu, J., & Wang, W. (2025). Overexpression of Tetrahymena Cysteine Synthetase 1 Promotes Cadmium Removal by Biosynthesizing Cadmium Sulfide Quantum Dots in Escherichia coli. International Journal of Molecular Sciences, 26(8), 3685. https://doi.org/10.3390/ijms26083685





