Improved Synthesis of Effective 3-(Indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1H-pyrrolo[2,3-b]pyridine-Based Inhibitors of NADPH Oxidase 2
Abstract
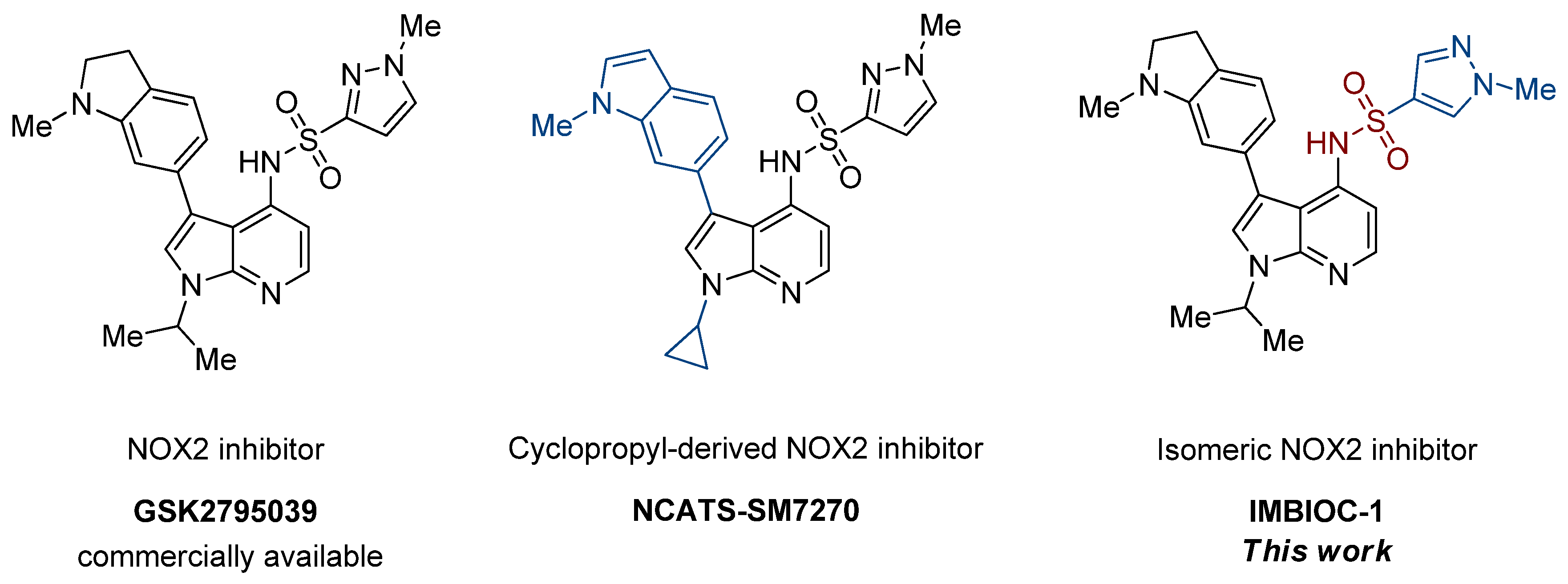
1. Introduction
2. Results and Discussion
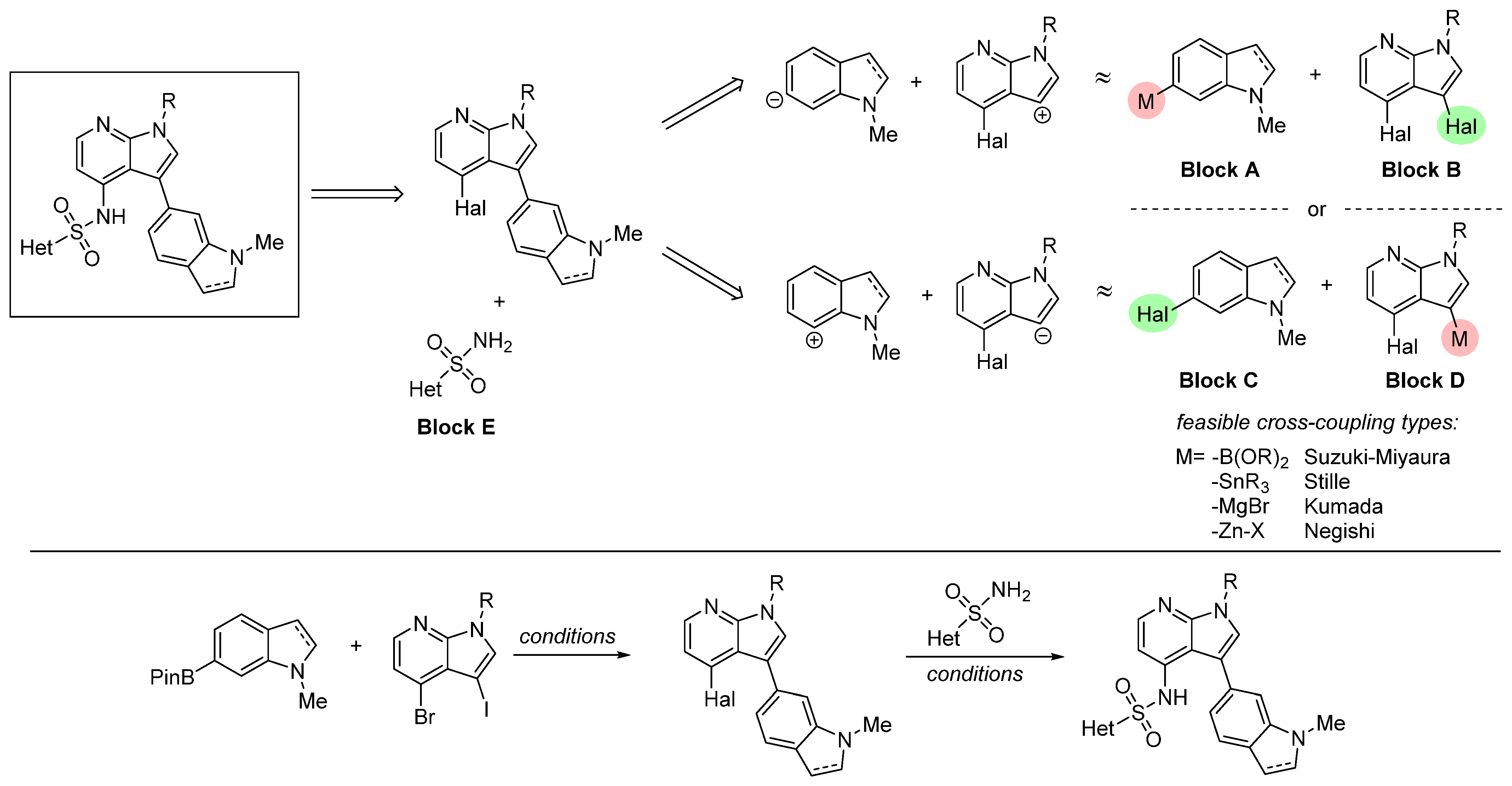
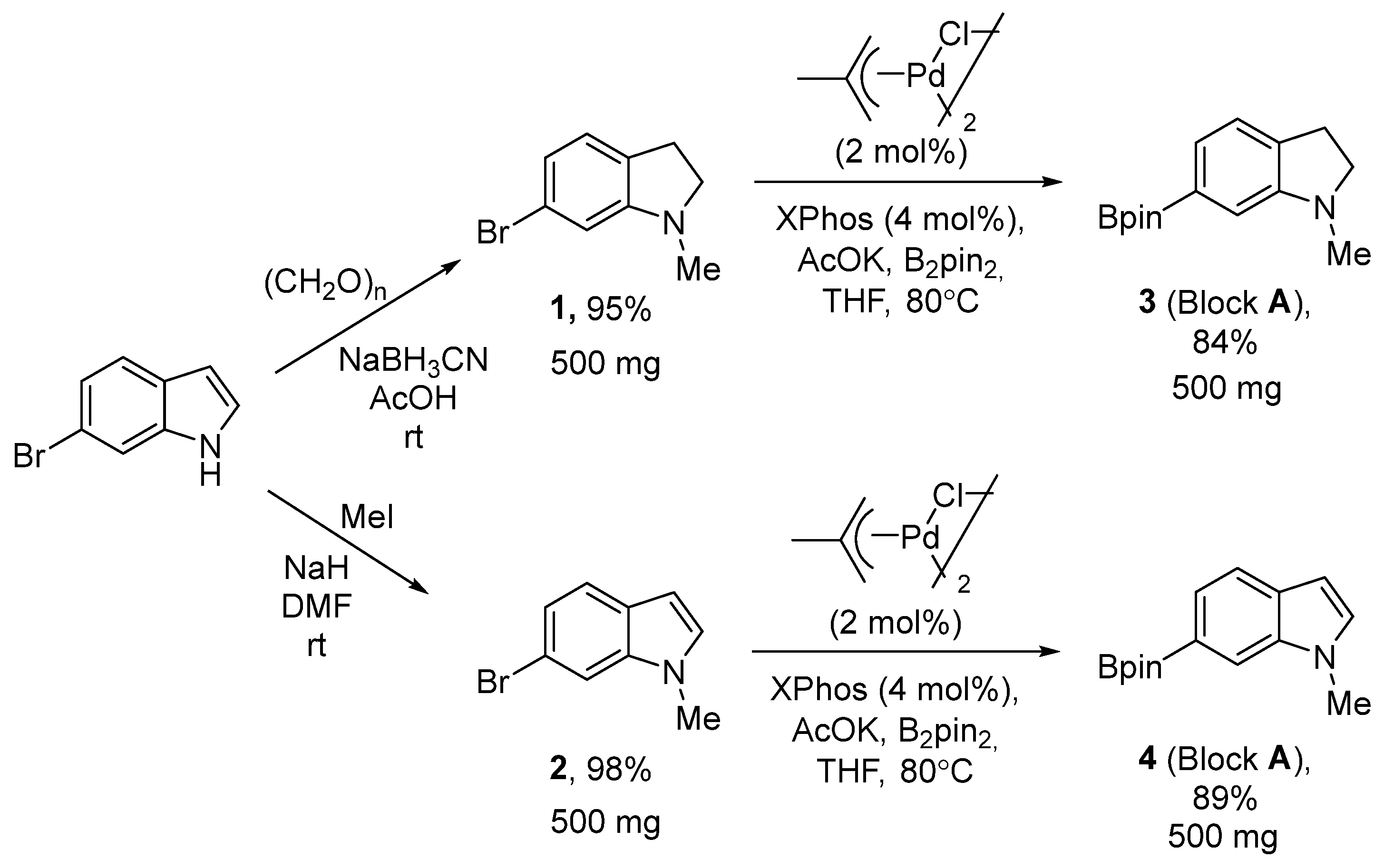
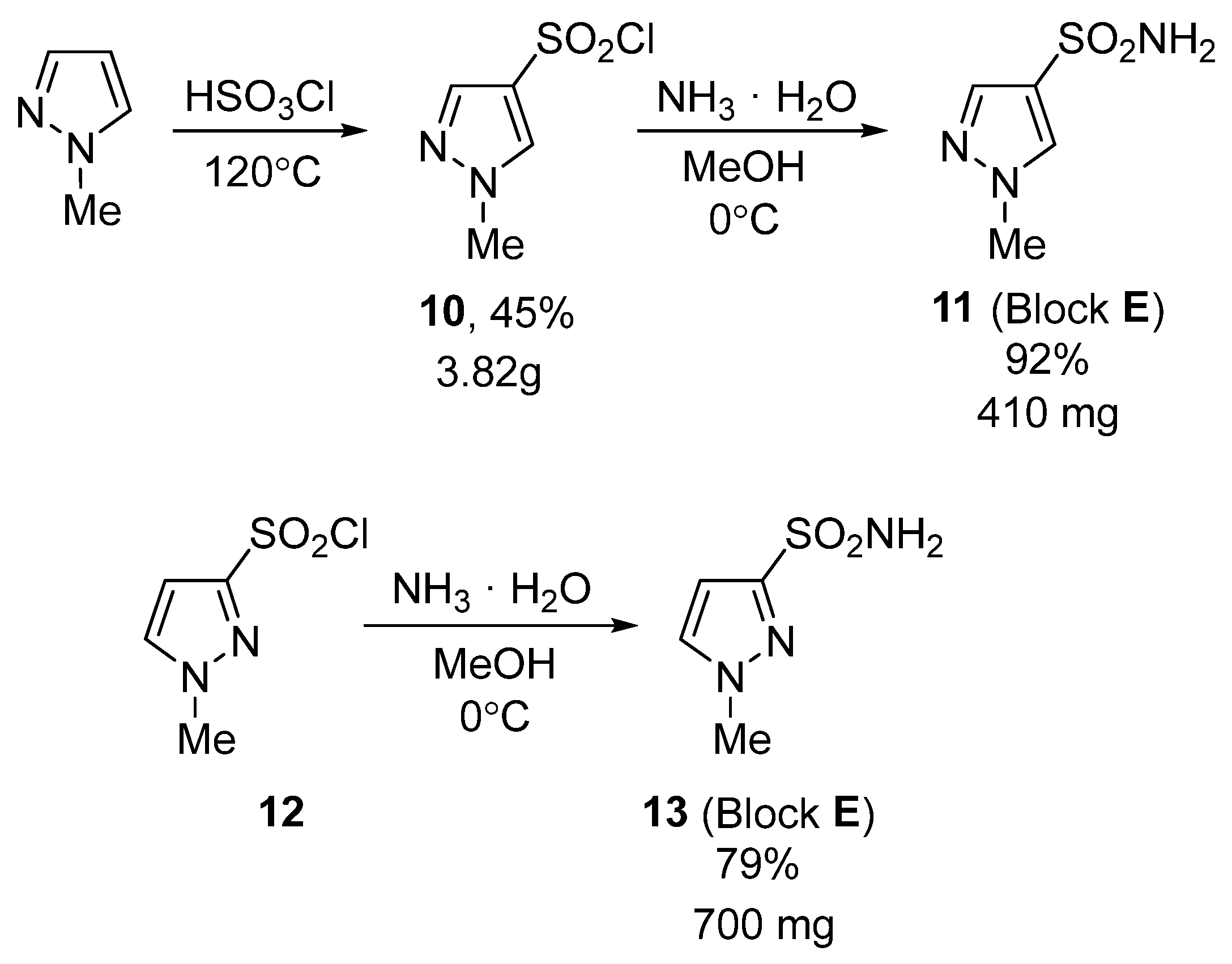
2.1. Chemical Synthesis
2.2. Modeling of NOX2 Complexes with Inhibitors
2.3. Effect on ROS Production and Cytotoxic Properties of NOX2 Inhibitors
3. Materials and Methods
3.1. Human Microglia Cell (HMC3) Culture
3.2. Treatment with Aβ and NOX2 Inhibitors
3.3. Flow Cytometry Analysis
3.4. Statistical Analysis
3.5. Synthesis
3.6. Molecular Docking Analysis
3.7. MTT Cytotoxicity Assay
3.8. Determination of Half Maximal Inhibitory Concentration (IC50)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McBean, G.J.; López, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Wang, J.; Zhang, Q. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 1–28. [Google Scholar]
- Mason, H.; Rai, G.; Kozyr, A.; De Jonge, N.; Gliniewicz, E.; Berg, L.J.; Wald, G.; Dorrier, C.; Henderson, M.J.; Zakharov, A.; et al. Development of an improved and specific inhibitor of NADPH oxidase 2 to treat traumatic brain injury. Redox Biol. 2023, 60, 102611. [Google Scholar] [CrossRef] [PubMed]
- Dohi, K.; Ohtaki, H.; Nakamachi, T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflamm. 2010, 7, 41. [Google Scholar] [CrossRef]
- Surace, M.J.; Block, M.L. Targeting microglia-mediated neurotoxicity: The potential of NOX2 inhibitors. Cell. Mol. Life Sci. 2012, 69, 2409–2427. [Google Scholar] [CrossRef]
- Park, L.; Zhou, P.; Pitstick, R.; Capone, C.; Anrather, J.; Norris, E.H.; Younkin, L.; Younkin, S.; Carlson, G.; McEwen, B.S.; et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2008, 105, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Cuvelier, E.; Destée, A. NADPH oxidases in Parkinson’s disease: A systematic review. Mol. Neurodegener. 2017, 12, 84. [Google Scholar] [CrossRef]
- Reis, J.; Massari, M.; Marchese, S.; Ceccon, M.; Aalbers, F.S.; Corana, F.; Valente, S.; Mai, A.; Magnani, F.; Mattevi, A. A closer look into NADPH oxidase inhibitors: Validation and insight into their mechanism of action. Redox Biol. 2020, 32, 101466. [Google Scholar] [CrossRef]
- Liu, F.-C.; Yu, H.-P.; Chen, P.-J.; Yang, H.-W.; Chang, S.-H.; Tzeng, C.-C.; Cheng, W.-J.; Chen, Y.-R.; Chen, Y.-L.; Hwang, T.-L. A novel NOX2 inhibitor attenuates human neutrophil oxidative stress and ameliorates inflammatory arthritis in mice. Redox Biol. 2019, 26, 101273. [Google Scholar] [CrossRef]
- Juric, M.; Rawat, V.; Amaradhi, R.; Zielonka, J.; Ganesh, T. Novel NADPH Oxidase-2 Inhibitors as Potential Anti-Inflammatory and Neuroprotective Agents. Antioxidants 2023, 12, 1660. [Google Scholar] [CrossRef]
- Sorce, S.; Stocker, R.; Seredenina, T.; Holmdahl, R.; Aguzzi, A.; Chio, A.; Depaulis, A.; Heitz, F.; Olofsson, P.; Olsson, T.; et al. NADPH oxidases as drug targets and biomarkers in neurodegenerative diseases: What is the evidence? Free Radic. Biol. Med. 2017, 112, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Chen, W.S.; Chueng, A.L.; Dunne, A.A.; Seredenina, T.; Filippova, A.; Ramachandran, S.; Bridges, A.; Chaudry, L.; Pettman, G.; et al. Discovery of GSK2795039, a Novel Small Molecule NADPH Oxidase 2 Inhibitor. Antioxid. Redox Signal. 2015, 23, 358–374. [Google Scholar] [CrossRef]
- Padilha, E.C.; Shah, P.; Rai, G.; Xu, X. NOX2 inhibitor GSK2795039 metabolite identification towards drug optimization. J. Pharm. Biomed. Anal. 2021, 201, 114102. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhang, Y.; Li, G.-G.; Yu, H.-H.; Bai, S.; Guo, G.-Y.; Guo, W.-L.; Ma, Y.; Wang, J.-H.; Liu, N.; et al. P2 × 7 receptor activation aggravates NADPH oxidase 2-induced oxidative stress after intracerebral hemorrhage. Neural Regener. Res. 2021, 16, 1582–1591. [Google Scholar]
- Wang, M.; Luo, L. An Effective NADPH Oxidase 2 Inhibitor Provides Neuroprotection and Improves Functional Outcomes in Animal Model of Traumatic Brain Injury. Neurochem. Res. 2020, 45, 1097–1106. [Google Scholar] [CrossRef]
- Malkov, A.; Popova, I.; Ivanov, A.; Jang, S.S.; Yoon, S.Y.; Osypov, A.; Huang, Y.; Zilberter, Y.; Zilberter, M.A. β initiates brain hypometabolism, network dysfunction and behavioral abnormalities via NOX2-induced oxidative stress in mice. Commun. Biol. 2021, 4, 1054. [Google Scholar] [CrossRef]
- Chen, D.W.; Duncan, S.; King, N.P.; Lee, K.C.; Mak, S.Y.; Rivers, D.A. Novel compounds. WO2012170752A1, 13 December 2012. [Google Scholar]
- Gribble, G.W.; Nutaitis, C.F. Reactions of Sodium Borohydride in Acidic Media; XVI.1 N-Methylation of Amines with Paraformaldehyde/Trifluoroacetic Acid. Synthesis 1987, 8, 709–711. [Google Scholar] [CrossRef]
- Poot, A.J.; van Ameijde, J.; Slijper, M.; van den Berg, A.; Hilhorst, R.; Ruijtenbeek, R.; Rijkers, D.T.S.; Liskamp, R.M.J. Development of Selective Bisubstrate-Based Inhibitors Against Protein Kinase C (PKC) Isozymes By Using Dynamic Peptide Microarrays. ChemBioChem 2009, 10, 2042–2051. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Huang, W.; Tan, W.; Zhang, A. Design. Synthesis and pharmacological evaluation of new acyl sulfonamides as potent and selective Bcl-2 inhibitors. Bioorg. Med. Chem. 2018, 26, 443–454. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Liu, R.; Song, K.; Chen, L. Structure of human phagocyte NADPH oxidase in the activated state. Nature 2024, 627, 189–195. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Bhattacharya, N.; Mishra, S.; Bhattacharya, A.; Banerjee, P.; Senapati, S.; Mishra, R. Microglia Specific Drug Targeting Using Natural Products for the Regulation of Redox Imbalance in Neurodegeneration. Front. Pharmacol. 2021, 12, 654489. [Google Scholar] [CrossRef] [PubMed]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef] [PubMed]


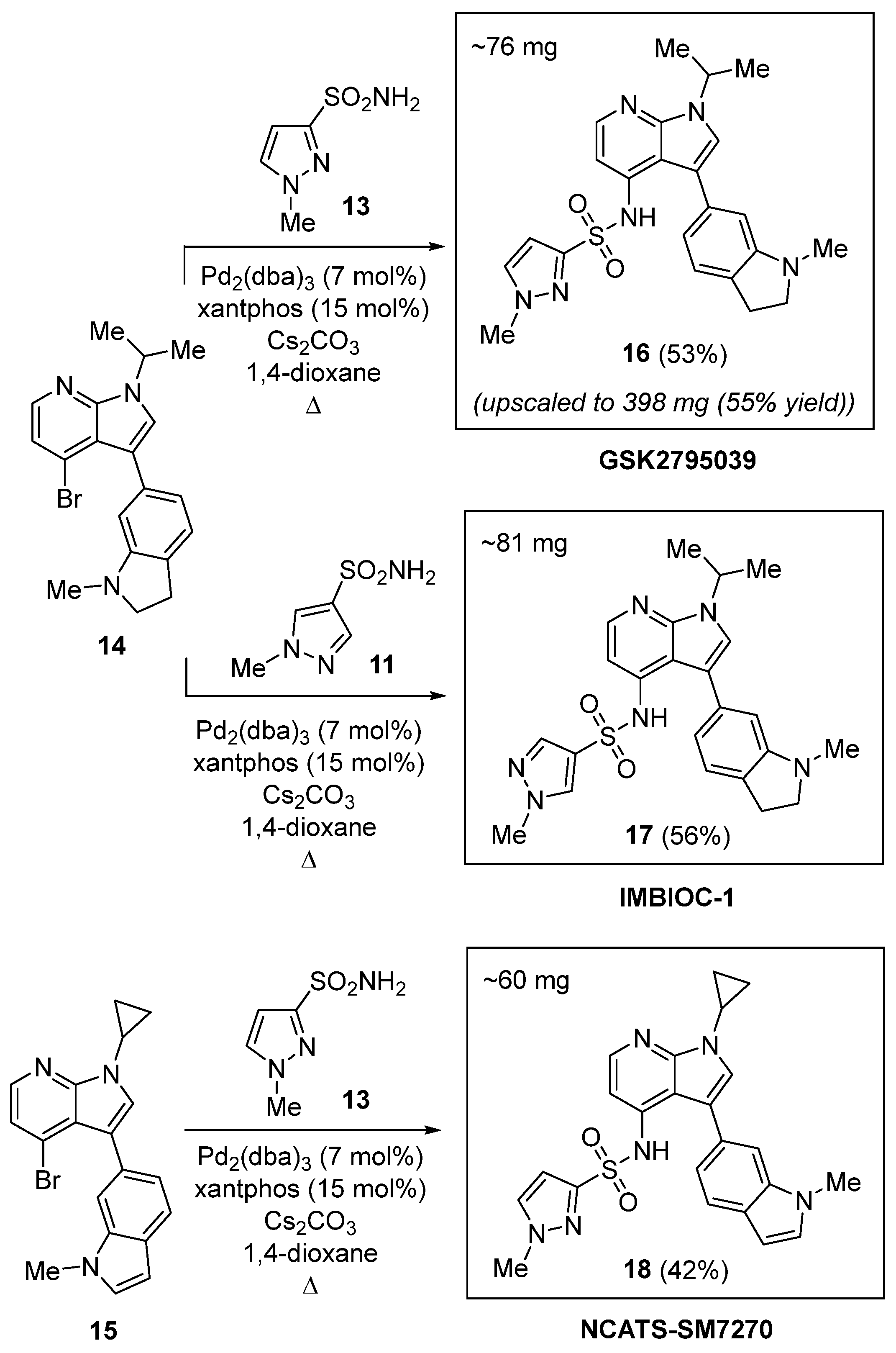
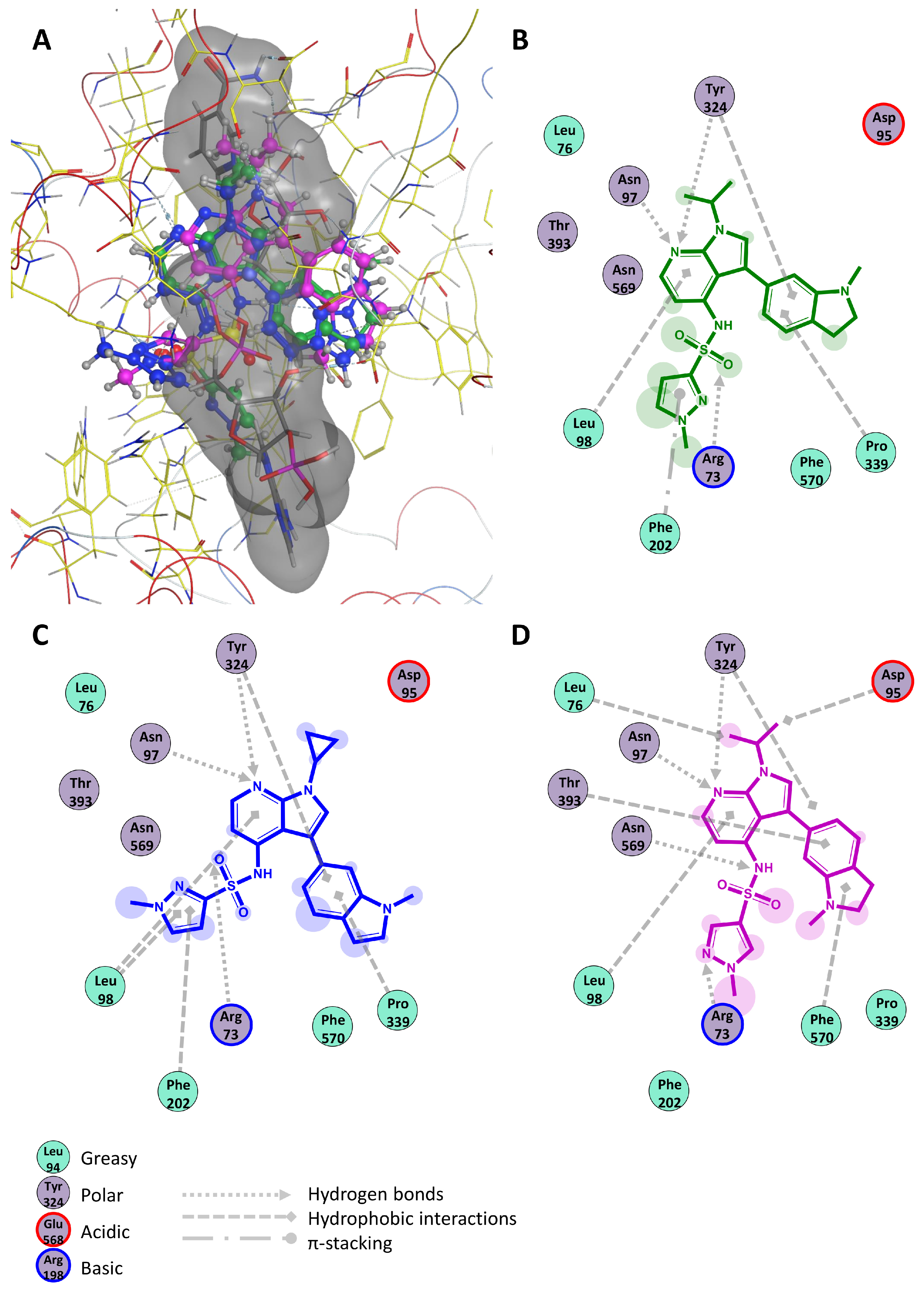
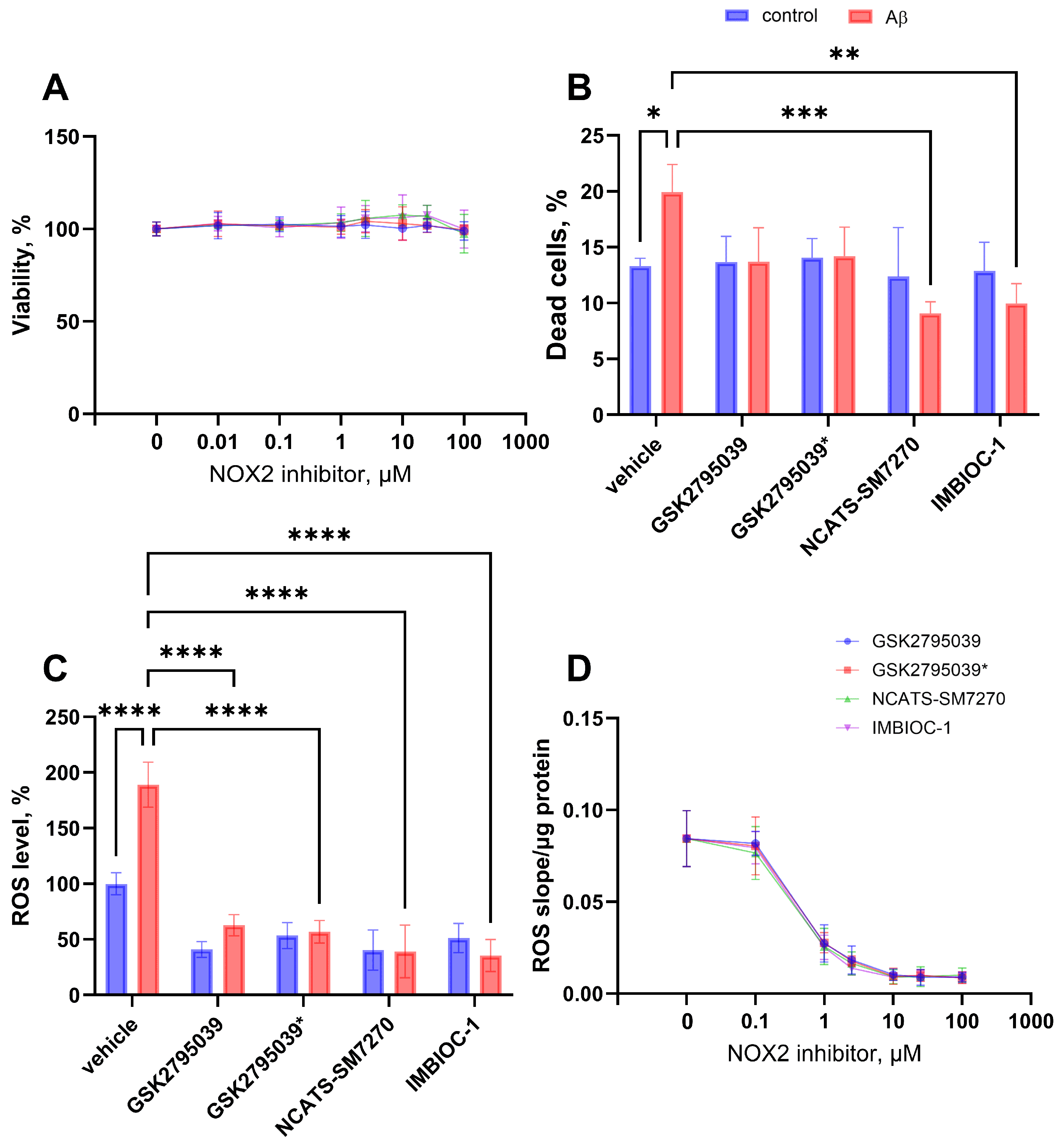
| Compound | ΔG, kcal/mol | Hydrophobic Interactions | Hydrogen Bonds | π-Stacking | Salt Bridges |
|---|---|---|---|---|---|
| GSK2795039 | −8.75 | 98Leu 324Tyr 339Pro | 73Arg 97Asn 324Tyr | 202Phe | – |
| NCATS-SM7270 | −8.35 | 98Leu 202Phe 324Tyr 339Pro | 73Arg 97Asn 324Tyr | – | – |
| IMBIOC-1 | −8.85 | 76Leu 95Asp 98Leu 324Tyr 393Thr 570Phe | 73Arg 97Asn 324Tyr 569Asn | – | – |
| NADPH | −11.89 | – | 203Glu 341Thr 354His 358Val | 570Phe | 338His |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapov, K.V.; Platonov, D.N.; Belyy, A.Y.; Novikov, M.A.; Tomilov, Y.V.; Anashkina, A.A.; Mukhina, K.A.; Kechko, O.I.; Solyev, P.N.; Novikov, R.A.; et al. Improved Synthesis of Effective 3-(Indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1H-pyrrolo[2,3-b]pyridine-Based Inhibitors of NADPH Oxidase 2. Int. J. Mol. Sci. 2025, 26, 3647. https://doi.org/10.3390/ijms26083647
Potapov KV, Platonov DN, Belyy AY, Novikov MA, Tomilov YV, Anashkina AA, Mukhina KA, Kechko OI, Solyev PN, Novikov RA, et al. Improved Synthesis of Effective 3-(Indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1H-pyrrolo[2,3-b]pyridine-Based Inhibitors of NADPH Oxidase 2. International Journal of Molecular Sciences. 2025; 26(8):3647. https://doi.org/10.3390/ijms26083647
Chicago/Turabian StylePotapov, Konstantin V., Dmitry N. Platonov, Alexander Yu. Belyy, Maxim A. Novikov, Yury V. Tomilov, Anastasia A. Anashkina, Kristina A. Mukhina, Olga I. Kechko, Pavel N. Solyev, Roman A. Novikov, and et al. 2025. "Improved Synthesis of Effective 3-(Indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1H-pyrrolo[2,3-b]pyridine-Based Inhibitors of NADPH Oxidase 2" International Journal of Molecular Sciences 26, no. 8: 3647. https://doi.org/10.3390/ijms26083647
APA StylePotapov, K. V., Platonov, D. N., Belyy, A. Y., Novikov, M. A., Tomilov, Y. V., Anashkina, A. A., Mukhina, K. A., Kechko, O. I., Solyev, P. N., Novikov, R. A., Makarov, A. A., & Mitkevich, V. A. (2025). Improved Synthesis of Effective 3-(Indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1H-pyrrolo[2,3-b]pyridine-Based Inhibitors of NADPH Oxidase 2. International Journal of Molecular Sciences, 26(8), 3647. https://doi.org/10.3390/ijms26083647






