Markers of Kidney Injury: Proenkephalin A and Uromodulin, but Not Dickkopf-3, Are Elevated in Patients After Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, E.D.; Lochte, H.L., Jr.; Lu, W.C.; Ferrebee, J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R. Hematopoietic-cell transplantation at 50. N. Engl. J. Med. 2007, 357, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Haematopoietic Stem Cell Transplantation HSCtx; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Bazinet, A.; Popradi, G. A general practitioner’s guide to hematopoietic stem-cell transplantation. Curr. Oncol. 2019, 26, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.D.; Buckner, C.D.; Banaji, M.; Clift, R.A.; Fefer, A.; Flournoy, N.; Goodell, B.W.; Hickman, R.O.; Lerner, K.G.; Neiman, P.E.; et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 1977, 49, 511–533. [Google Scholar] [CrossRef]
- Gertz, M.A. Current status of stem cell mobilization. Br. J. Haematol. 2010, 150, 647–662. [Google Scholar] [CrossRef]
- Stem Cell Trialists’ Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: An individual patient data meta-analysis of nine randomized trials. J. Clin. Oncol. 2005, 23, 5074–5087. [Google Scholar] [CrossRef]
- Renaghan, A.D.; Jaimes, E.A.; Malyszko, J.; Perazella, M.A.; Sprangers, B.; Rosner, M.H. Acute Kidney Injury and CKD Associated with Hematopoietic Stem Cell Transplantation. Clin. J. Am. Soc. Nephrol. 2020, 15, 289–297. [Google Scholar] [CrossRef]
- Kępska-Dzilińska, M.; Zhymaila, A.; Malyszko, J. Kidney damage in patients after allogeneic stem cell transplantation. Wiad Lek 2022, 75 Pt 1, 877–880. [Google Scholar] [CrossRef]
- Saddadi, F.; Hakemi, M.; Najafi, I.; Moghadam, K.; Ghavamzadeh, A.; Jahani, M.; Ganji, M.; Amini, M.; Soleimanian, T. Chronic kidney disease after hematopoietic cell transplantation: Frequency, risk factors, and outcomes. Transpl. Proc. 2009, 41, 2895–2897. [Google Scholar] [CrossRef]
- Bennett, M.; Dent, C.L.; Ma, Q.; Dastrala, S.; Grenier, F.; Workman, R.; Syed, H.; Ali, S.; Barasch, J.; Devarajan, P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin. J. Am. Soc. Nephrol. 2008, 3, 665–673. [Google Scholar] [CrossRef]
- Wen, Y.; Parikh, C.R. Current concepts and advances in biomarkers of acute kidney injury. Crit. Rev. Clin. Lab. Sci. 2021, 58, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury—Pathophysiological basis and clinical performance. Acta Physiol. 2017, 219, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Endre, Z.H. Assessing renal recovery after acute kidney injury: Can biomarkers help? Nephron 2018, 140, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Denning, G.M.; Ackermann, L.W.; Barna, T.J.; Armstrong, J.G.; Stoll, L.L.; Weintraub, N.L.; Dickson, E.W. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides 2008, 29, 83–92. [Google Scholar] [CrossRef]
- Shah, K.S.; Taub, P.; Patel, M.; Rehfeldt, M.; Struck, J.; Clopton, P.; Mehta, R.L.; Maisel, A.S. Proenkephalin predicts acute kidney injury in cardiac surgery patients. Clin. Nephrol. 2015, 83, 29–35. [Google Scholar] [CrossRef]
- Marino, R.; Struck, J.; Hartmann, O.; Maisel, A.S.; Rehfeldt, M.; Magrini, L.; Melander, O.; Bergmann, A.; Di Somma, S. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J. Nephrol. 2015, 28, 717–724. [Google Scholar] [CrossRef]
- Schulz, C.-A.; Christensson, A.; Ericson, U.; Almgren, P.; Hindy, G.; Nilsson, P.M.; Struck, J.; Bergmann, A.; Melander, O.; Orho-Melander, M. High level of fasting plasma proenkephalin-A predicts deterioration of kidney function and incidence of CKD. J. Am. Soc. Nephrol. 2017, 28, 291–303. [Google Scholar] [CrossRef]
- Zewinger, S.; Rauen, T.; Rudnicki, M.; Federico, G.; Wagner, M.; Triem, S.; Schunk, S.J.; Petrakis, I.; Schmit, D.; Wagenpfeil, S.; et al. Dickkopf-3 (DKK3) in Urine Identifies Patients with Short-Term Risk of eGFR Loss. J. Am. Soc. Nephrol. 2018, 29, 2722–2733. [Google Scholar] [CrossRef]
- Schunk, S.J.; Speer, T.; Petrakis, I.; Fliser, D. Dickkopf 3-a novel biomarker of the ‘kidney injury continuum’. Nephrol. Dial. Transpl. 2021, 36, 761–767. [Google Scholar] [CrossRef]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT–β-catenin signaling—A versatile player in kidney injury and repair. Nature 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Dawnay, A.B.; Cattell, W.R. Serum Tamm-Horsfall Glycoprotein Levels in Health and in Renal Disease. Clin. Nephrol. 1981, 15, 5–8. [Google Scholar] [PubMed]
- Usui, R.; Ogawa, T.; Takahashi, H.; Iwasaki, C.; Koike, M.; Morito, T.; Hatano, M.; Nitta, K. Serum Uromodulin Is a Novel Renal Function Marker in the Japanese Population. Clin. Exp. Nephrol. 2021, 25, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Block, M.; Herbst, V.; Nockher, W.A.; Schlumberger, W.; Satanovskij, R.; Angermann, S.; Hasenau, A.-L.; Stecher, L.; Heemann, U.; et al. Plasma Uromodulin Correlates with Kidney Function and Identifies Early Stages in Chronic Kidney Disease Patients. Medicine 2016, 95, e3011. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.V.; Khasun, M.; Kayukov, I.G.; Galkina, O.V.; Sipovski, V.G.; Parastaeva, M.M.; Bogdanova, E.O. Serum Uromodulin as an Early Biomarker of Tubular Atrophy and Interstitial Fibrosis in Patients with Glomerulopathies. Ter. Arkhiv 2018, 90, 41–47. [Google Scholar] [CrossRef]
- Forni, L.G.; Darmon, M.; Ostermann, M.; Oudemans-van Straaten, H.M.; Pettilä, V.; Prowle, J.R.; Schetz, M.; Joannidis, M. Renal recovery after acute kidney injury. Intensive Care Med. 2017, 43, 855–866. [Google Scholar] [CrossRef]
- Kellum, J.A.; Sileanu, F.E.; Bihorac, A.; Hoste, E.A.; Chawla, L.S. Recovery after acute kidney injury. Am. J. Respir. Crit. Care Med. 2017, 195, 784–791. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Hansrivijit, P.; Kovvuru, K.; Kanduri, S.R.; Torres-Ortiz, A.; Acharya, P.; Gonzalez-Suarez, M.L.; Kaewput, W.; Bathini, T.; Cheungpasitporn, W. Diagnostics, risk factors, treatment and outcomes of acute kidney injury in a new paradigm. J. Clin. Med. 2020, 9, 1104. [Google Scholar] [CrossRef]
- Coca, S.G.; King, J.T., Jr.; Rosenthal, R.A.; Perkal, M.F.; Parikh, C.R. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010, 78, 926–933. [Google Scholar] [CrossRef]
- Cerdá, J.; Liu, K.D.; Cruz, D.N.; Jaber, B.L.; Koyner, J.L.; Heung, M.; Okusa, M.D.; Faubel, S. Promoting kidney function recovery in patients with AKI requiring RRT. Clin. J. Am. Soc. Nephrol. 2015, 10, 1859–1867. [Google Scholar] [CrossRef]
- Kanduri, S.R.; Cheungpasitporn, W.; Thongprayoon, C.; Bathini, T.; Kovvuru, K.; Garla, V.; Medaura, J.; Vaitla, P.; Kashani, K.B. Incidence and mortality of acute kidney injury in patients undergoing hematopoietic stem cell transplantation: A systematic review and meta-analysis. QJM Int. J. Med. 2020, 113, 621–632. [Google Scholar] [CrossRef]
- Hingorani, S. Renal complications of hematopoietic-cell transplantation. N. Engl. J. Med. 2016, 374, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Gorab, D.L.; Fernandes, C.R.; Macedo, E. Role of proenkephalin in the diagnosis of severe and subclinical acute kidney injury during the perioperative period of liver transplantation. Pract. Lab Med. 2022, 31, e00278. [Google Scholar] [CrossRef] [PubMed]
- Beunders, R.; van Groenendael, R.; Leijte, G.P.; Kox, M.; Pickkers, P. Proenkephalin Compared to Conventional Methods to Assess Kidney Function in Critically Ill Sepsis Patients. Shock 2020, 54, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Hartmann, O.; Struck, J.; Bergmann, A.; Gansevoort, R.T.; Joosten, M.M.; van den Berg, E.; de Boer, R.A.; Bakker, S.J. Plasma Proenkephalin and Poor Long-Term Outcome in Renal Transplant Recipients. Transpl. Direct 2017, 3, e190. [Google Scholar] [CrossRef]
- Beunders, R.; Donato, L.J.; van Groenendael, R.; Arlt, B.; Carvalho-Wodarz, C.; Schulte, J.; Coolen, A.C.; Lieske, J.C.; Meeusen, J.W.; Jaffe, A.S.; et al. Assessing GFR with Proenkephalin. Kidney Int. Rep. 2023, 8, 2345–2355. [Google Scholar] [CrossRef]
- Nilsson, C.; Christensson, A.; Nilsson, P.M.; Melander, O.; Bennet, L. Pro-Enkephalin and its association with renal function in Middle Eastern immigrants and native Swedes. Scand. J. Clin. Lab. Investig. 2021, 81, 573–578. [Google Scholar] [CrossRef]
- Khorashadi, M.; Beunders, R.; Pickkers, P.; Legrand, M. Proenkephalin: A New Biomarker for Glomerular Filtration Rate and Acute Kidney Injury. Nephron 2020, 144, 655–661. [Google Scholar] [CrossRef]
- Smeets, N.J.L.; Hartmann, O.; Schulte, J.; Schreuder, M.F.; de Wildt, S.N. Proenkephalin A as a marker for glomerular filtration rate in critically ill children: Validation against gold standard iohexol GFR measurements. Clin. Chem. Lab. Med. 2022, 61, 104–111. [Google Scholar] [CrossRef]
- Caironi, P.; Latini, R.; Struck, J.; Hartmann, O.; Bergmann, A.; Bellato, V.; Ferraris, S.; Tognoni, G.; Pesenti, A.; Gattinoni, L.; et al. Circulating Proenkephalin, Acute Kidney Injury, and Its Improvement in Patients with Severe Sepsis or Shock. Clin. Chem. 2018, 64, 1361–1369. [Google Scholar] [CrossRef]
- Donato, L.J.; Meeusen, J.W.; Lieske, J.C.; Bergmann, D.; Sparwaßer, A.; Jaffe, A.S. Analytical performance of an immunoassay to measure proenkephalin. Clin. Biochem. 2018, 58, 72–77. [Google Scholar] [CrossRef]
- Tichy, J.; Hausmann, A.; Lanzerstorfer, J.; Ryz, S.; Wagner, L.; Lassnigg, A.; Bernardi, M.H. Prediction of Successful Liberation from Continuous Renal Replacement Therapy Using a Novel Biomarker in Patients with Acute Kidney Injury after Cardiac Surgery-An Observational Trial. Int. J. Mol. Sci. 2024, 25, 10873. [Google Scholar] [CrossRef] [PubMed]
- Von Groote, T.; Albert, F.; Meersch, M.; Koch, R.; Gerss, J.; Arlt, B.; Sadjadi, M.; Porschen, C.; Pickkers, P.; Zarbock, A.; et al. Evaluation of Proenkephalin A 119–159 for liberation from renal replacement therapy: An external, multicenter pilot study in critically ill patients with acute kidney injury. Crit. Care 2023, 27, 276. [Google Scholar] [CrossRef] [PubMed]
- Dépret, F.; Hollinger, A.; Cariou, A.; Deye, N.; Vieillard-Baron, A.; Fournier, M.C.; Jaber, S.; Damoisel, C.; Lu, Q.; Monnet, X.; et al. Incidence and Outcome of Subclinical Acute Kidney Injury Using penKid in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2020, 202, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Bullen, A.L.; Katz, R.; Poursadrolah, S.; Short, S.A.P.; Long, D.L.; Cheung, K.L.; Sharma, S.; Al-Rousan, T.; Fregoso, A.; Schulte, J.; et al. Plasma proenkephalin A and incident chronic kidney disease and albuminuria in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. BMC Nephrol. 2024, 25, 16. [Google Scholar] [CrossRef]
- Walczak-Wieteska, P.; Zuzda, K.; Małyszko, J.; Andruszkiewicz, P. Proenkephalin A 119–159 in Perioperative and Intensive Care-A Promising Biomarker or Merely Another Option? Diagnostics 2024, 14, 2364. [Google Scholar] [CrossRef]
- Grycuk, W.; Jakubowska, Z.; Malyszko, J. Proenkephalin (PENK): A functional biomarker in chronic kidney diseases—Hope or just a new bystander? J. Nephrol. 2025. accepted. [Google Scholar]
- Shi, K.; Jiang, W.; Song, L.; Li, X.; Zhang, C.; Li, L.; Feng, Y.; Yang, J.; Wang, T.; Wang, H.; et al. Persistent acute kidney injury biomarkers: A systematic review and meta-analysis. Clin. Chim. Acta 2025, 564, 119907. [Google Scholar] [CrossRef]
- Canki, E.; Kho, E.; Hoenderop, J.G.J. Urinary biomarkers in kidney disease. Clin. Chim. Acta 2024, 555, 117798. [Google Scholar] [CrossRef]
- Dziamałek-Macioszczyk, P.; Winiarska, A.; Pawłowska, A.; Wojtacha, P.; Stompór, T. Patterns of Dickkopf-3 Serum and Urine Levels at Different Stages of Chronic Kidney Disease. J. Clin. Med. 2023, 12, 4705. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, Z.; Yang, S.; Hao, C.; Zhao, H.; An, Y. Advances and insights for DKK3 in non-cancerous diseases: A systematic review. PeerJ 2025, 13, e18935. [Google Scholar] [CrossRef]
- Schunk, S.J.; Zarbock, A.; Meersch, M.; Küllmar, M.; Kellum, J.A.; Schmit, D.; Wagner, M.; Triem, S.; Wagenpfeil, S.; Gröne, H.J.; et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: An observational cohort study. Lancet 2019, 394, 488–496. [Google Scholar] [CrossRef]
- González, J.; Jatem, E.; Roig, J.; Valtierra, N.; Ostos, E.; Abó, A.; Santacana, M.; García, A.; Segarra, A. Usefulness of urinary biomarkers to estimate the interstitial fibrosis surface in diabetic nephropathy with normal kidney function. Nephrol. Dial Transpl. 2022, 37, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Watchorn, J.C.; Zarbock, A.; Forni, L.G. Prognostic Biomarkers and AKI: Potential to Enhance the Identification of Post-Operative Patients at Risk of Loss of Renal Function. Res. Rep. Urol. 2024, 16, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Jiang, Z.; Wu, Y.; Ou, S.; Qin, J.; Xue, L.; Wu, W. The role of urinary Dickkopf-3 in the prediction of acute kidney injury: A systematic review meta-analysis. Int. Urol. Nephrol. 2023, 55, 3175–3188. [Google Scholar] [CrossRef] [PubMed]
- Seibert, F.S.; Heringhaus, A.; Pagonas, N.; Rohn, B.; Bauer, F.; Trappe, H.J.; Landmesser, U.; Babel, N.; Westhoff, T.H. Dickkopf-3 in the prediction of contrast media induced acute kidney injury. J. Nephrol. 2021, 34, 821–828. [Google Scholar] [CrossRef]
- Roscigno, G.; Quintavalle, C.; Biondi-Zoccai, G.; De Micco, F.; Frati, G.; Affinito, A.; Nuzzo, S.; Condorelli, G.; Briguori, C. Urinary Dickkopf-3 and Contrast-Associated Kidney Damage. J. Am. Coll. Cardiol. 2021, 77, 2667–2676. [Google Scholar] [CrossRef]
- Enko, D.; Meinitzer, A.; Scherberich, J.E.; März, W.; Herrmann, M.; Artinger, K.; Rosenkranz, A.R.; Zitta, S. Individual Uromodulin Serum Concentration Is Independent of Glomerular Filtration Rate in Healthy Kidney Donors. Clin. Chem. Lab. Med. CCLM 2021, 59, 563–570. [Google Scholar] [CrossRef]
- Garimella, P.S.; Biggs, M.L.; Katz, R.; Ix, J.H.; Bennett, M.R.; Devarajan, P.; Kestenbaum, B.R.; Siscovick, D.S.; Jensen, M.K.; Shlipak, M.G.; et al. Urinary Uromodulin, Kidney Function, and Cardiovascular Disease in Elderly Adults. Kidney Int. 2015, 88, 1126–1134. [Google Scholar] [CrossRef]
- Steubl, D.; Block, M.; Herbst, V.; Nockher, W.A.; Schlumberger, W.; Kemmner, S.; Bachmann, Q.; Angermann, S.; Wen, M.; Heemann, U.; et al. Urinary Uromodulin Independently Predicts End-Stage Renal Disease and Rapid Kidney Function Decline in a Cohort of Chronic Kidney Disease Patients. Medicine 2019, 98, e15808. [Google Scholar] [CrossRef]
- Vonbrunn, E.; Ebert, N.; Cordasic, N.; Amann, K.; Büttner, A.; Büttner-Herold, M.; Scherberich, J.E.; Daniel, C. Serum Uromodulin as early marker for ischemic acute kidney injury and nephron loss: Association with kidney tissue distribution pattern. J. Transl. Med. 2025, 23, 323. [Google Scholar] [CrossRef]
- Kaszynska, A.; Kepska-Dzilinska, M.; Karakulska-Prystupiuk, E.; Tomaszewska, A.; Basak, G.; Zorawski, M.; Drozak, I.; Jakubowska, Z.; Malyszko, J. 226.9: Proenkephalin and DKK-3 levels and kidney function in patients after hematopoietic stem cell transplantation. Transplantation 2024, 108. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef]
| Control Group | HSCT | |
|---|---|---|
| Age (years) | 49 ± 10 | 48 ± 14 |
| Time after transplantation (months) | NA | 103.3 ± 50.5 |
| Hemoglobin (g/dL) | 13.87 ± 0.89 | 12.87 ± 1.99 |
| Acute myeloid leukemia (%) | NA | 43 |
| serum creatinine (mg/dL) | 0.86 ± 0.35 | 1.1 ± 0.37 * |
| eGFR by CKD-EPI (mL/min/1.72 m2) | 96.9 ± 13.0 | 73.5 ± 23.5 ** |
| Erythrocyturia (%) | 0 | 11 |
| Leukocyturia (%) | 0 | 6.25 |
| HSCT with eGFR > 60 (mL/min/1.72 m2) | HSCT with eGFR < 60 (mL/min/1.72 m2) | |
|---|---|---|
| Age (years) | 46 ± 10 | 50 ± 17 |
| Time after transplantation (months) | 87 ± 43 | 119 ± 52 * |
| Hemoglobin (g/dL) | 13.01 ± 0.91 | 12.03 ± 1.01 * |
| Acute myeloid leukemia | 27 | 7 ** |
| Acute lymphocytic leukemia | 10 | 8 |
| Lymphoma | 4 | 4 |
| serum creatinine (mg/dL) | 0.95 ± 0.21 | 1.55 ± 0.30 ** |
| eGFR by CKD-EPI (ml/min/1.72 m2) | 85.28 ± 16.42 | 46.10 ± 7.80 ** |
| Erythrocyturia (%) | 0 | 11 |
| Leukocyturia (%) | 0 | 6.25 |
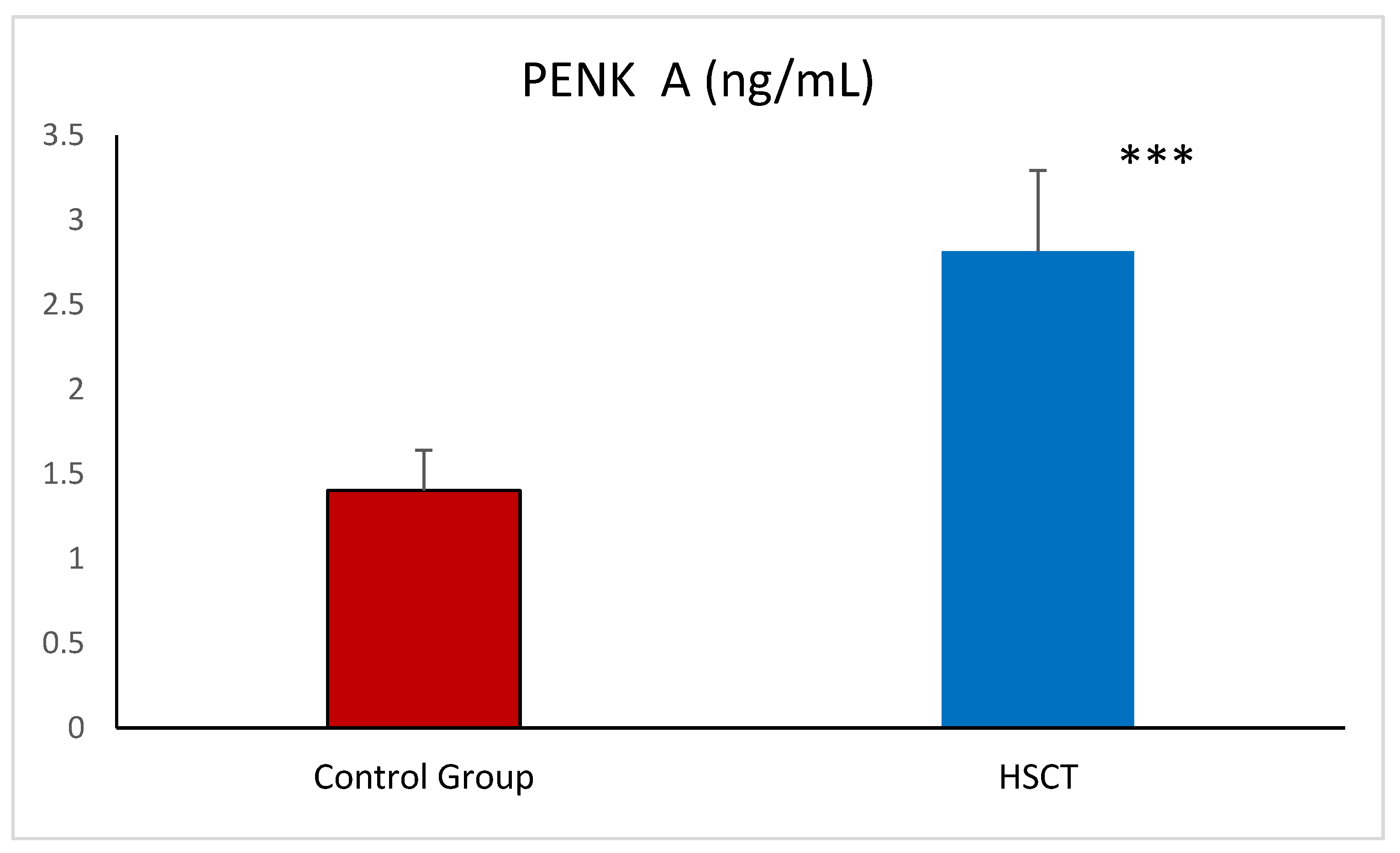
| PENK A (ng/mL) | 2.57 ± 0.43 | 2.90 ± 0.44 * |
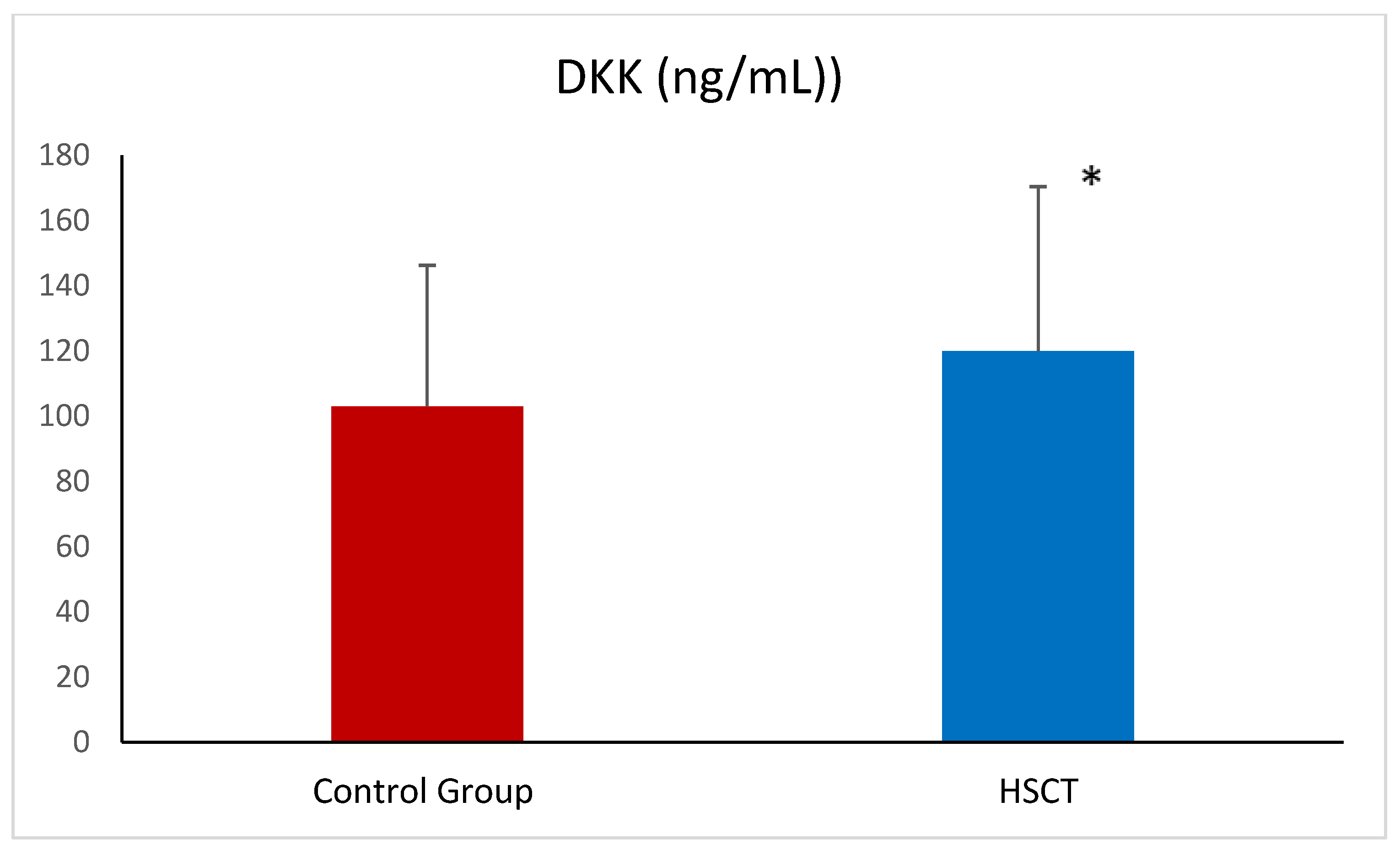
| DKK-3 (ng/mL)) | 47.69 ± 13.25 | 49.15 ± 14.65 |
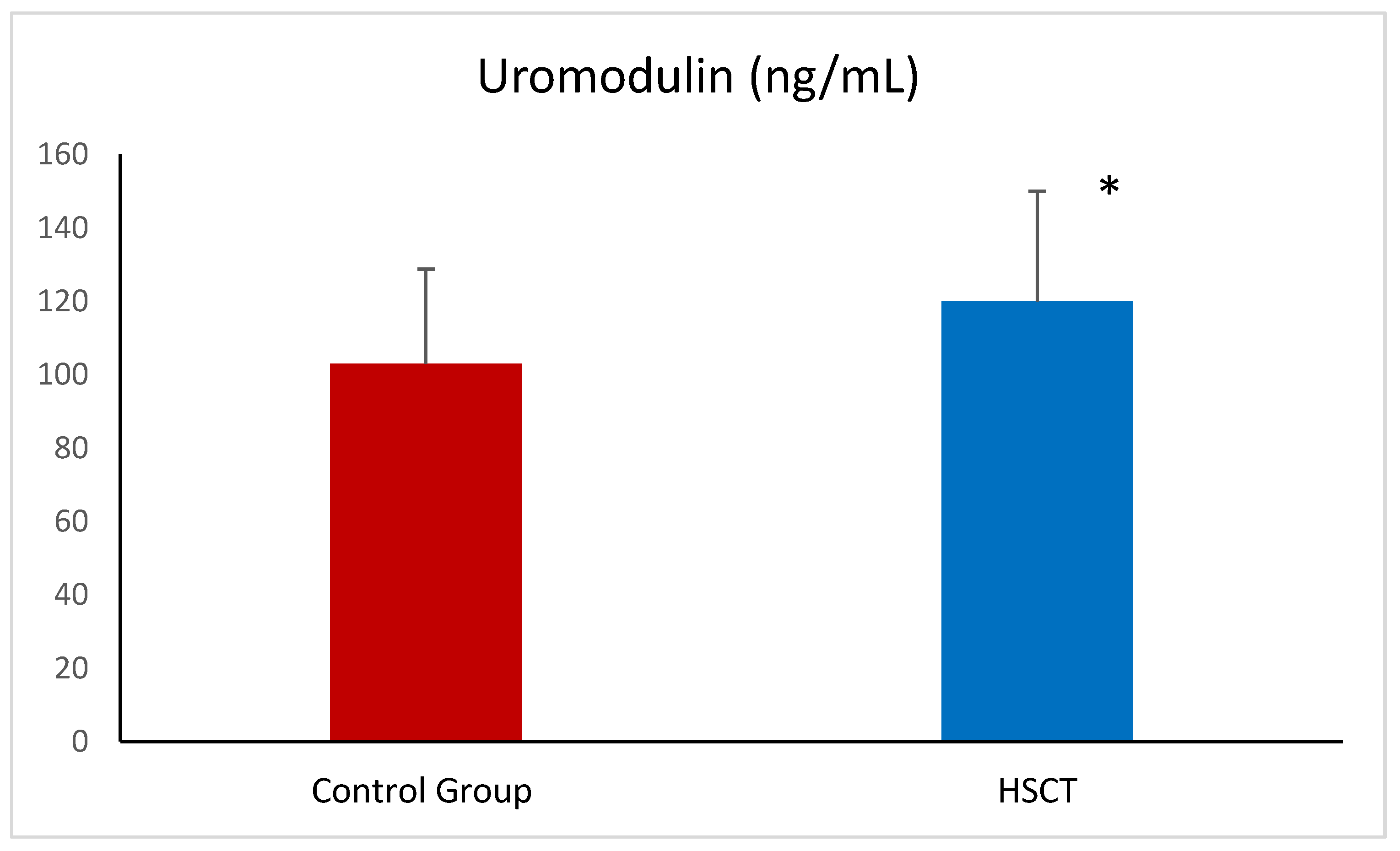
| Uromodulin (ng/mL) | 101.77 ± 28.12 | 119.70 ± 27.73 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaszyńska, A.; Kępska-Dzilińska, M.; Karakulska-Prystupiuk, E.; Tomaszewska, A.; Basak, G.W.; Żórawski, M.; Jakubowska, Z.; Małyszko, J. Markers of Kidney Injury: Proenkephalin A and Uromodulin, but Not Dickkopf-3, Are Elevated in Patients After Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2025, 26, 3581. https://doi.org/10.3390/ijms26083581
Kaszyńska A, Kępska-Dzilińska M, Karakulska-Prystupiuk E, Tomaszewska A, Basak GW, Żórawski M, Jakubowska Z, Małyszko J. Markers of Kidney Injury: Proenkephalin A and Uromodulin, but Not Dickkopf-3, Are Elevated in Patients After Hematopoietic Stem Cell Transplantation. International Journal of Molecular Sciences. 2025; 26(8):3581. https://doi.org/10.3390/ijms26083581
Chicago/Turabian StyleKaszyńska, Aleksandra, Małgorzata Kępska-Dzilińska, Ewa Karakulska-Prystupiuk, Agnieszka Tomaszewska, Grzegorz Władysław Basak, Marcin Żórawski, Zuzanna Jakubowska, and Jolanta Małyszko. 2025. "Markers of Kidney Injury: Proenkephalin A and Uromodulin, but Not Dickkopf-3, Are Elevated in Patients After Hematopoietic Stem Cell Transplantation" International Journal of Molecular Sciences 26, no. 8: 3581. https://doi.org/10.3390/ijms26083581
APA StyleKaszyńska, A., Kępska-Dzilińska, M., Karakulska-Prystupiuk, E., Tomaszewska, A., Basak, G. W., Żórawski, M., Jakubowska, Z., & Małyszko, J. (2025). Markers of Kidney Injury: Proenkephalin A and Uromodulin, but Not Dickkopf-3, Are Elevated in Patients After Hematopoietic Stem Cell Transplantation. International Journal of Molecular Sciences, 26(8), 3581. https://doi.org/10.3390/ijms26083581







