Enrichment of RedoxifibromiR miR-21-5p in Plasma Exosomes of Hypertensive Patients with Renal Injury
Abstract
1. Introduction
2. Results
2.1. Study Cohorts
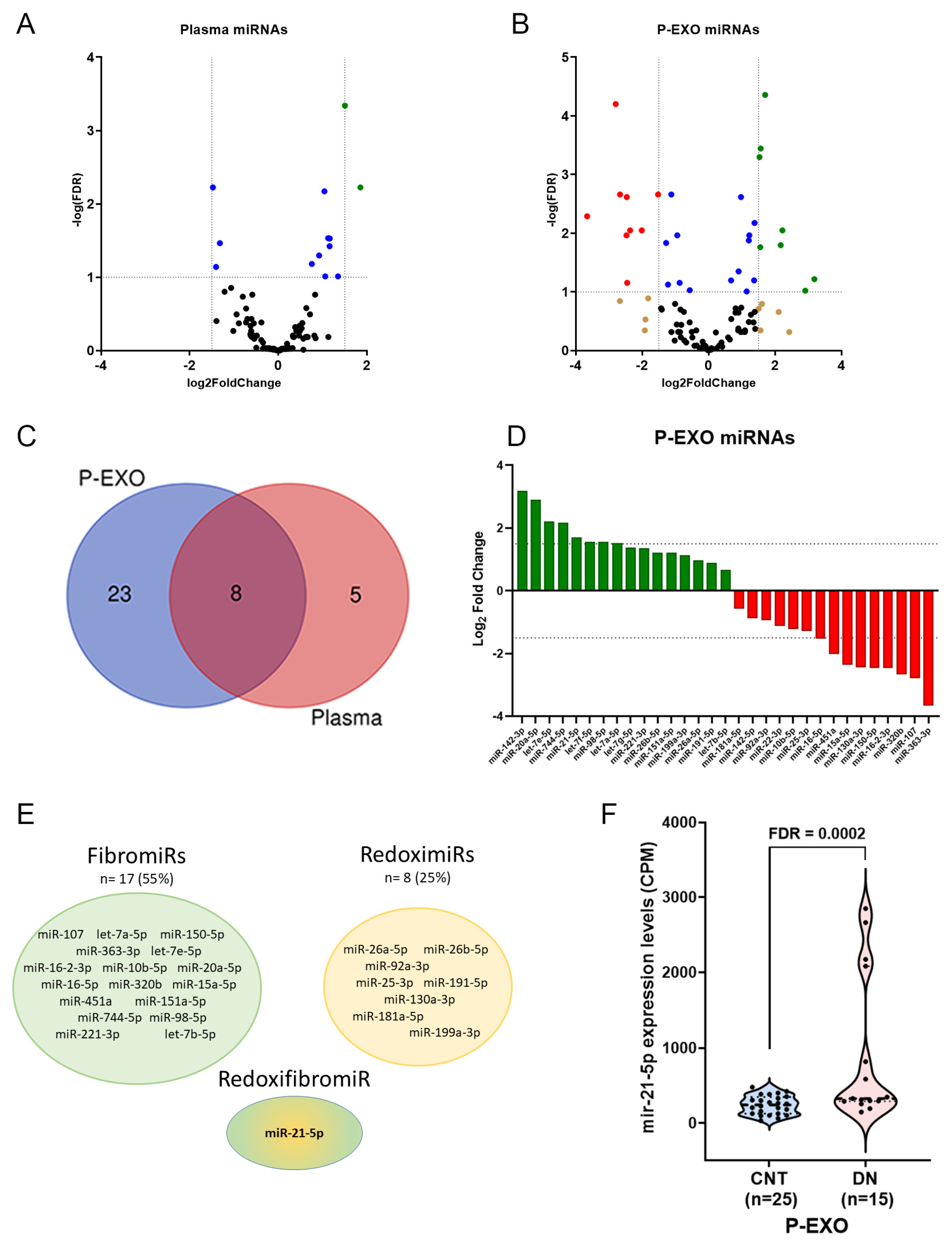
2.2. Differential Expression of miRNAs in Plasma and P-EXO
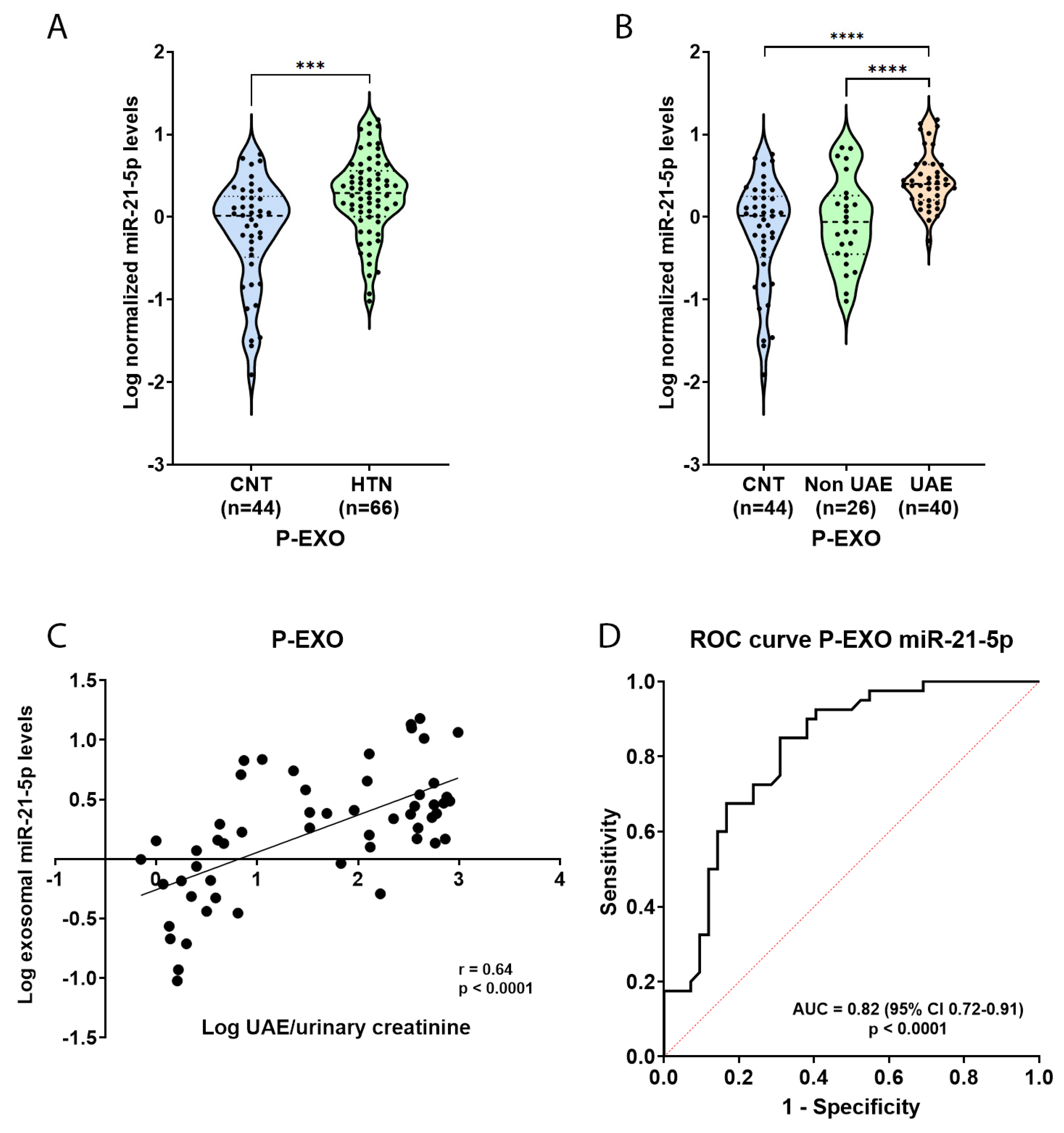
2.3. Elevated miR-21-5p Levels in P-EXO of Hypertensive and Albuminuric Patients
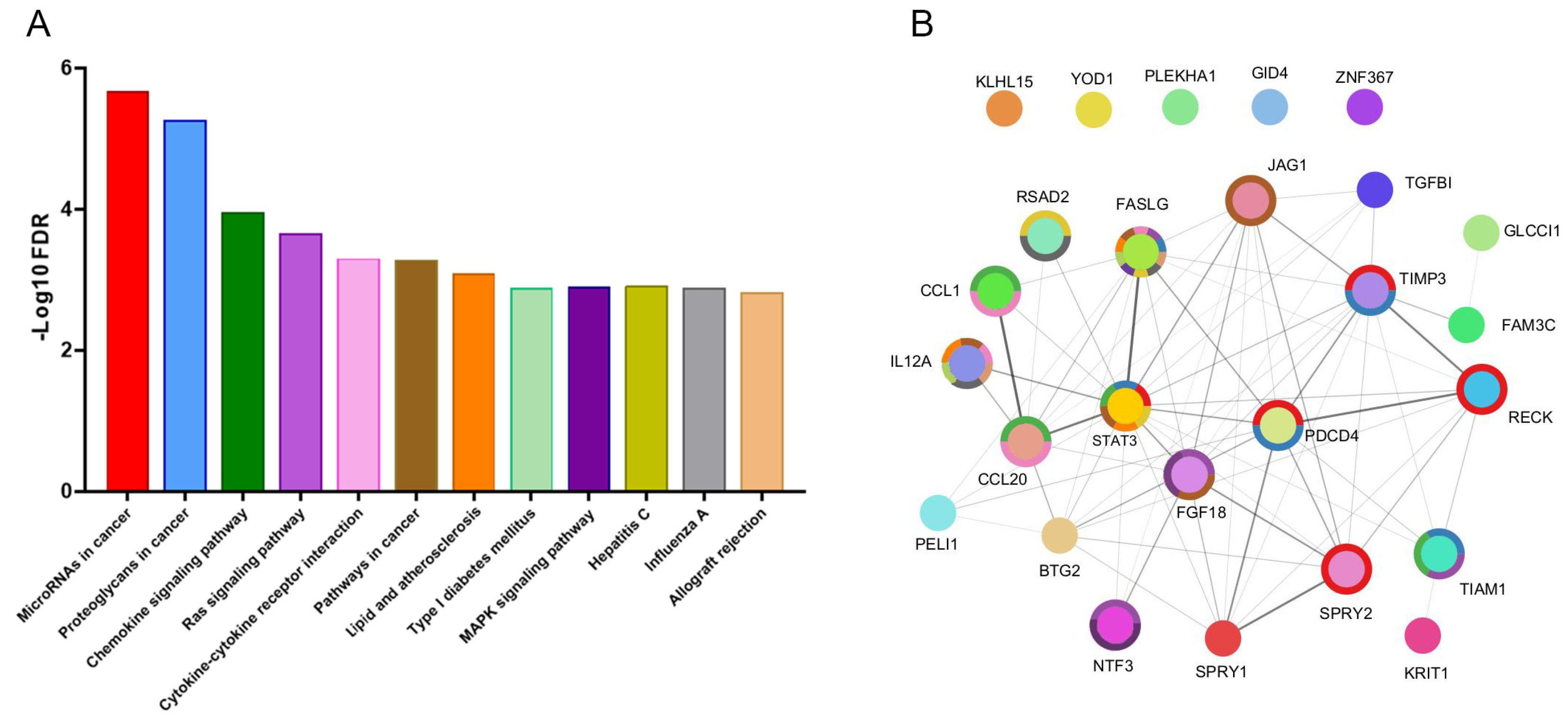
2.4. Identification of miR-21-5p Targets and Functional Enrichment Analysis
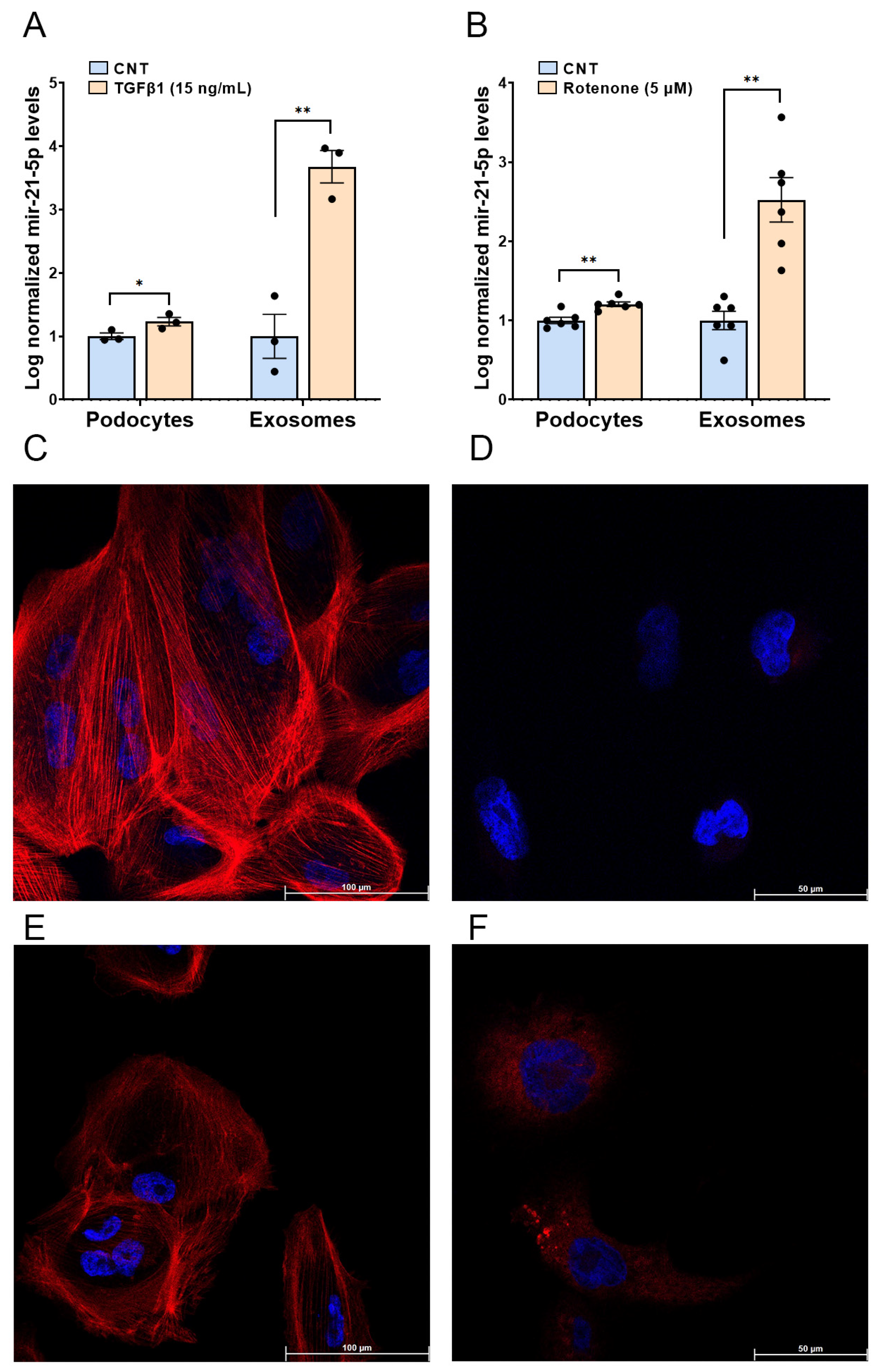
2.5. Functional In Vitro Experiments
3. Discussion
4. Materials and Methods
4.1. Patients Cohort
4.2. Extracellular Vesicle (EV) Isolation from Plasma Samples and Podocyte Cell Cultures
4.3. RNA Extraction, Small RNA Library Preparation, and Next-Generation Sequencing
4.4. miRNA Levels Quantification by RT-qPCR
4.5. Molecular Pathways Analyses
4.6. Podocyte Cell Culture and Stimulation with TGF-β1 and Rotenone
4.7. Immunofluorescence Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohishi, M. Hypertension with Diabetes Mellitus: Physiology and Pathology. Hypertens. Res. 2018, 41, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; Chirinos, J.A.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.-P.; Hering, D.; et al. A Call to Action and a Lifecourse Strategy to Address the Global Burden of Raised Blood Pressure on Current and Future Generations: The Lancet Commission on Hypertension. Lancet 2016, 388, 2665–2712. [Google Scholar] [CrossRef]
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arroyo, O.; Ortega, A.; Redon, J.; Cortes, R. Therapeutic Potential of Extracellular Vesicles in Hypertension-Associated Kidney Disease. Hypertension 2021, 77, 28–38. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Z.-J.; Song, J.-W.; Liang, L.-R.; Sun, L.-L.; Liu, X.-Y.; Miao, R.; Xu, Y.-L.; Li, X.-T.; Zhang, M.-W.; et al. MicroRNA-122-5p Promotes Renal Fibrosis and Injury in Spontaneously Hypertensive Rats by Targeting FOXO3. Exp. Cell Res. 2022, 411, 113017. [Google Scholar] [CrossRef] [PubMed]
- Fierro-Fernández, M.; Miguel, V.; Lamas, S. Role of redoximiRs in Fibrogenesis. Redox Biol. 2016, 7, 58–67. [Google Scholar] [CrossRef]
- Pérez-Carrillo, L.; Giménez-Escamilla, I.; García-Manzanares, M.; Triviño, J.C.; Feijóo-Bandín, S.; Aragón-Herrera, A.; Lago, F.; Martínez-Dolz, L.; Portolés, M.; Tarazón, E.; et al. Altered MicroRNA Maturation in Ischemic Hearts: Implication of Hypoxia on XPO5 and DICER1 Dysregulation and RedoximiR State. Antioxidants 2023, 12, 1337. [Google Scholar] [CrossRef]
- Hennino, M.-F.; Buob, D.; Van der Hauwaert, C.; Gnemmi, V.; Jomaa, Z.; Pottier, N.; Savary, G.; Drumez, E.; Noël, C.; Cauffiez, C.; et al. miR-21-5p Renal Expression Is Associated with Fibrosis and Renal Survival in Patients with IgA Nephropathy. Sci. Rep. 2016, 6, 27209. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Riffo-Campos, A.L.; Ortega, A.; Martinez-Arroyo, O.; Perez-Gil, D.; Olivares, D.; Solaz, E.; Martinez, F.; Martínez-Hervás, S.; Chaves, F.J.; et al. Urinary- and Plasma-Derived Exosomes Reveal a Distinct MicroRNA Signature Associated with Albuminuria in Hypertension. Hypertension 2021, 77, 960–971. [Google Scholar] [CrossRef]
- Riffo-Campos, A.L.; Perez-Hernandez, J.; Ortega, A.; Martinez-Arroyo, O.; Flores-Chova, A.; Redon, J.; Cortes, R. Exosomal and Plasma Non-Coding RNA Signature Associated with Urinary Albumin Excretion in Hypertension. Int. J. Mol. Sci. 2022, 23, 823. [Google Scholar] [CrossRef] [PubMed]
- Flores-Chova, A.; Martinez-Arroyo, O.; Riffo-Campos, A.L.; Ortega, A.; Forner, M.J.; Cortes, R. Plasma Exosomal Non-Coding RNA Profile Associated with Renal Damage Reveals Potential Therapeutic Targets in Lupus Nephritis. Int. J. Mol. Sci. 2023, 24, 7088. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, X.; Scicluna, B.J.; Coleman, B.M.; Hill, A.F. Characterization and Deep Sequencing Analysis of Exosomal and Non-Exosomal miRNA in Human Urine. Kidney Int. 2014, 86, 433–444. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Le, T.H. Extracellular Vesicles as a Novel Diagnostic and Research Tool for Patients with HTN and Kidney Disease. Am. J. Physiol. Ren. Physiol. 2019, 317, F641–F647. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Tian, N.; Zou, D.; Shi, Y.; Zhang, N. Astragaloside IV Improves Renal Function and Fibrosis via Inhibition of miR-21-Induced Podocyte Dedifferentiation and Mesangial Cell Activation in Diabetic Mice. Drug Des. Devel Ther. 2018, 12, 2431–2442. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Liu, X.-S.; Huang, X.-R.; Yu, X.-Q.; Lan, H.-Y. Diverse Role of TGF-β in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Nasci, V.L.; Chuppa, S.; Griswold, L.; Goodreau, K.A.; Dash, R.K.; Kriegel, A.J. miR-21-5p Regulates Mitochondrial Respiration and Lipid Content in H9C2 Cells. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H710–H721. [Google Scholar] [CrossRef]
- Zhao, S.; Li, W.; Yu, W.; Rao, T.; Li, H.; Ruan, Y.; Yuan, R.; Li, C.; Ning, J.; Li, S.; et al. Exosomal miR-21 from Tubular Cells Contributes to Renal Fibrosis by Activating Fibroblasts via Targeting PTEN in Obstructed Kidneys. Theranostics 2021, 11, 8660–8673. [Google Scholar] [CrossRef] [PubMed]
- Larrue, R.; Fellah, S.; Van der Hauwaert, C.; Hennino, M.-F.; Perrais, M.; Lionet, A.; Glowacki, F.; Pottier, N.; Cauffiez, C. The Versatile Role of miR-21 in Renal Homeostasis and Diseases. Cells 2022, 11, 3525. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, X.; Kang, Z.; Wang, Y.; Xia, T.; Ding, N.; Yin, Y. Elevation of miR-21, through Targeting MKK3, May Be Involved in Ischemia Pretreatment Protection from Ischemia-Reperfusion Induced Kidney Injury. J. Nephrol. 2016, 29, 27–36. [Google Scholar] [CrossRef]
- Lu, X.; Yu, Y.; Tan, S. The Role of the miR-21-5p-Mediated Inflammatory Pathway in Ulcerative Colitis. Exp. Ther. Med. 2020, 19, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Mehrtabar, S.; Jabraeilipour, A.; Doustar, N.; Rahmani Youshanlouei, H.; Tahavvori, A.; Fattahi, P.; Alavi, S.M.A.; Taha, S.R.; Fazlollahpour-Naghibi, A.; et al. Inhibitory Effect of microRNA-21 on Pathways and Mechanisms Involved in Cardiac Fibrosis Development. Ther. Adv. Cardiovasc. Dis. 2024, 18, 17539447241253134. [Google Scholar] [CrossRef]
- Zhan, L.; Mu, Z.; Jiang, H.; Zhang, S.; Pang, Y.; Jin, H.; Chen, J.; Jia, C.; Guo, H. MiR-21-5p Protects against Ischemic Stroke by Targeting IL-6R. Ann. Transl. Med. 2023, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Lorz, C.; Egido, J. The Fas Ligand/Fas System in Renal Injury. Nephrol. Dial. Transplant. 1999, 14, 1831–1834. [Google Scholar] [CrossRef][Green Version]
- Wang, G.; Yu, Y.; Sun, C.; Liu, T.; Liang, T.; Zhan, L.; Lin, X.; Feng, X.-H. STAT3 Selectively Interacts with Smad3 to Antagonize TGF-β Signalling. Oncogene 2016, 35, 4388–4398. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Agrawal, V.; Kamthan, S.; Prasad, N.; Agarwal, V. Blood TGF-Β1 and miRNA-21-5p Levels Predict Renal Fibrosis and Outcome in IgA Nephropathy. Int. Urol. Nephrol. 2023, 55, 1557–1564. [Google Scholar] [CrossRef]
- Loboda, A.; Sobczak, M.; Jozkowicz, A.; Dulak, J. TGF-Β1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediat. Inflamm. 2016, 2016, 8319283. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Gao, Y.-B.; Zhang, N.; Zou, D.-W.; Wang, P.; Zhu, Z.-Y.; Li, J.-Y.; Zhou, S.-N.; Wang, S.-C.; Wang, Y.-Y.; et al. miR-21 Overexpression Enhances TGF-Β1-Induced Epithelial-to-Mesenchymal Transition by Target Smad7 and Aggravates Renal Damage in Diabetic Nephropathy. Mol. Cell. Endocrinol. 2014, 392, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, L.; Liu, R.; Liu, J.; Cheng, Q. Exosomal miR-21-5p Derived from Multiple Myeloma Cells Promote Renal Epithelial-Mesenchymal Transition through Targeting TGF-β/SMAD7 Signalling Pathway. Clin. Exp. Pharmacol. Physiol. 2023, 50, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.; Viggiani, G.; Chen, Y.-W.; Coulis, G.; Castaldi, A. MicroRNA and ROS Crosstalk in Cardiac and Pulmonary Diseases. Int. J. Mol. Sci. 2020, 21, 4370. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.-O.; Divya, S.P.; Turcios, L.; Roy, R.V.; Hitron, J.A.; Wang, L.; Kim, D.; Dai, J.; Asha, P.; et al. Hexavalent Chromium Induces Malignant Transformation of Human Lung Bronchial Epithelial Cells via ROS-Dependent Activation of miR-21-PDCD4 Signaling. Oncotarget 2016, 7, 51193–51210. [Google Scholar] [CrossRef] [PubMed]
- Dhas, Y.; Arshad, N.; Biswas, N.; Jones, L.D.; Ashili, S. MicroRNA-21 Silencing in Diabetic Nephropathy: Insights on Therapeutic Strategies. Biomedicines 2023, 11, 2583. [Google Scholar] [CrossRef]
- Yasen, A.; Feng, J.; Xie, X.-M.; Li, K.; Cai, Y.-H.; Liao, Z.-H.; Liang, R.-B.; Dai, T.-X.; Wang, G.-Y. Exosomes Derived from TGF-Β1-Pretreated Mesenchymal Stem Cells Alleviate Biliary Ischemia-Reperfusion Injury through Jagged1/Notch1/SOX9 Pathway. Int. Immunopharmacol. 2023, 119, 110253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.-H.; Sun, Y.-H. TGF-Β1 Stimulated Mesenchymal Stem Cells-Generated Exosomal miR-29a Promotes the Proliferation, Migration and Fibrogenesis of Tenocytes by Targeting FABP3. Cytokine 2023, 162, 156090. [Google Scholar] [CrossRef]
- Ilg, M.M.; Bustin, S.A.; Ralph, D.J.; Cellek, S. TGF-Β1 Induces Formation of TSG-6-Enriched Extracellular Vesicles in Fibroblasts Which Can Prevent Myofibroblast Transformation by Modulating Erk1/2 Phosphorylation. Sci. Rep. 2024, 14, 12389. [Google Scholar] [CrossRef]
- Zang, J.; Maxwell, A.P.; Simpson, D.A.; McKay, G.J. Differential Expression of Urinary Exosomal MicroRNAs miR-21-5p and miR-30b-5p in Individuals with Diabetic Kidney Disease. Sci. Rep. 2019, 9, 10900. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arroyo, O.; Flores-Chova, A.; Sanchez-Garcia, B.; Redon, J.; Cortes, R.; Ortega, A. Rab3A/Rab27A System Silencing Ameliorates High Glucose-Induced Injury in Podocytes. Biology 2023, 12, 690. [Google Scholar] [CrossRef]
| Variables | HTN UAE (n = 40) | HTN NON-UAE (n = 26) | CNT (n = 44) |
|---|---|---|---|
| Age (years) | 58 ± 11 *** | 55 ± 6 | 41 ± 10 |
| Gender (male) | 74% | 64% | 57.0% |
| SBP (mmHg) | 138± 19 *** | 136 ± 24 | 117 ± 13 |
| DBP (mmHg) | 83 ± 11 *** | 87 ± 14 | 73 ± 7 |
| PP (mmHg) | 54 ± 15 ** | 48 ± 17 | 43 ± 9 |
| Glucose (mg/dL) | 130 ± 50 ** | 119 ± 41 | 98 ± 12 |
| Glycated hemoglobin (%) | 6.9 ± 1.2 * | 6.0 ± 0.9 | 5.6 ± 0.4 |
| Total Cholesterol (mg/dL) | 188 ± 35 | 173 ± 29 | 193 ± 41 |
| LDL (mg/dL) | 117 ± 31 | 108 ± 25 | 123 ± 34 |
| HDL (mg/dL) | 47 ± 13 ** | 50 ± 10 | 59 ± 14 |
| Triglycerides (mg/dL) | 188 ± 154 *** | 127 ± 60 | 99 ± 87 |
| Plasma creatinine (mg/dL) | 1.01 ± 0.40 ** | 0.90 ± 0.22 | 0.76 ± 0.21 |
| GFR (mL/min/1.73 m2) | 86 ± 30 ** | 87 ± 19 | 107 ± 20 |
| Body mass index (kg/m2) | 33 ± 7 *** | 30 ± 5 | 24 ± 4 |
| Obesity grade (%) | |||
| Grade I | 21 ** | 18 | 2 |
| Grade II | 23 ** | 11 | 0 |
| Grade III | 13 ** | 11 | 0 |
| Diabetes (%) | 62 *** | 64 | 2 |
| Dyslipidemia (%) | 90 *** | 86 | 5 |
| Smoking (%) | 36 ** | 46 | 5 |
| UAE/Creatinine (mg/g) | 307 ± 231 *** | 3.1 ± 1.7 | 4.4 ± 2.3 |
| Treatment (%) | |||
| Oral antidiabetic | 54 ** | 32 | 2 |
| CCB | 39 ** | 38 | 5 |
| ARA II | 90 *** | 93 | 5 |
| Statins | 32 ** | 8 | 5 |
| BB | 15.4 * | 31 | 0 |
| Diuretics | 44 ** | 29 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Arroyo, O.; Flores-Chova, A.; Mendez-Debaets, M.; Martinez-Hervas, S.; Martinez, F.; Forner, M.J.; Redon, J.; Ortega, A.; Cortes, R. Enrichment of RedoxifibromiR miR-21-5p in Plasma Exosomes of Hypertensive Patients with Renal Injury. Int. J. Mol. Sci. 2025, 26, 590. https://doi.org/10.3390/ijms26020590
Martinez-Arroyo O, Flores-Chova A, Mendez-Debaets M, Martinez-Hervas S, Martinez F, Forner MJ, Redon J, Ortega A, Cortes R. Enrichment of RedoxifibromiR miR-21-5p in Plasma Exosomes of Hypertensive Patients with Renal Injury. International Journal of Molecular Sciences. 2025; 26(2):590. https://doi.org/10.3390/ijms26020590
Chicago/Turabian StyleMartinez-Arroyo, Olga, Ana Flores-Chova, Marta Mendez-Debaets, Sergio Martinez-Hervas, Fernando Martinez, Maria J. Forner, Josep Redon, Ana Ortega, and Raquel Cortes. 2025. "Enrichment of RedoxifibromiR miR-21-5p in Plasma Exosomes of Hypertensive Patients with Renal Injury" International Journal of Molecular Sciences 26, no. 2: 590. https://doi.org/10.3390/ijms26020590
APA StyleMartinez-Arroyo, O., Flores-Chova, A., Mendez-Debaets, M., Martinez-Hervas, S., Martinez, F., Forner, M. J., Redon, J., Ortega, A., & Cortes, R. (2025). Enrichment of RedoxifibromiR miR-21-5p in Plasma Exosomes of Hypertensive Patients with Renal Injury. International Journal of Molecular Sciences, 26(2), 590. https://doi.org/10.3390/ijms26020590







