Knockdown of BAP31 Suppresses Tumorigenesis and Stemness in Breast Cancer Cells via the Hippo Pathway
Abstract
1. Introduction
2. Results
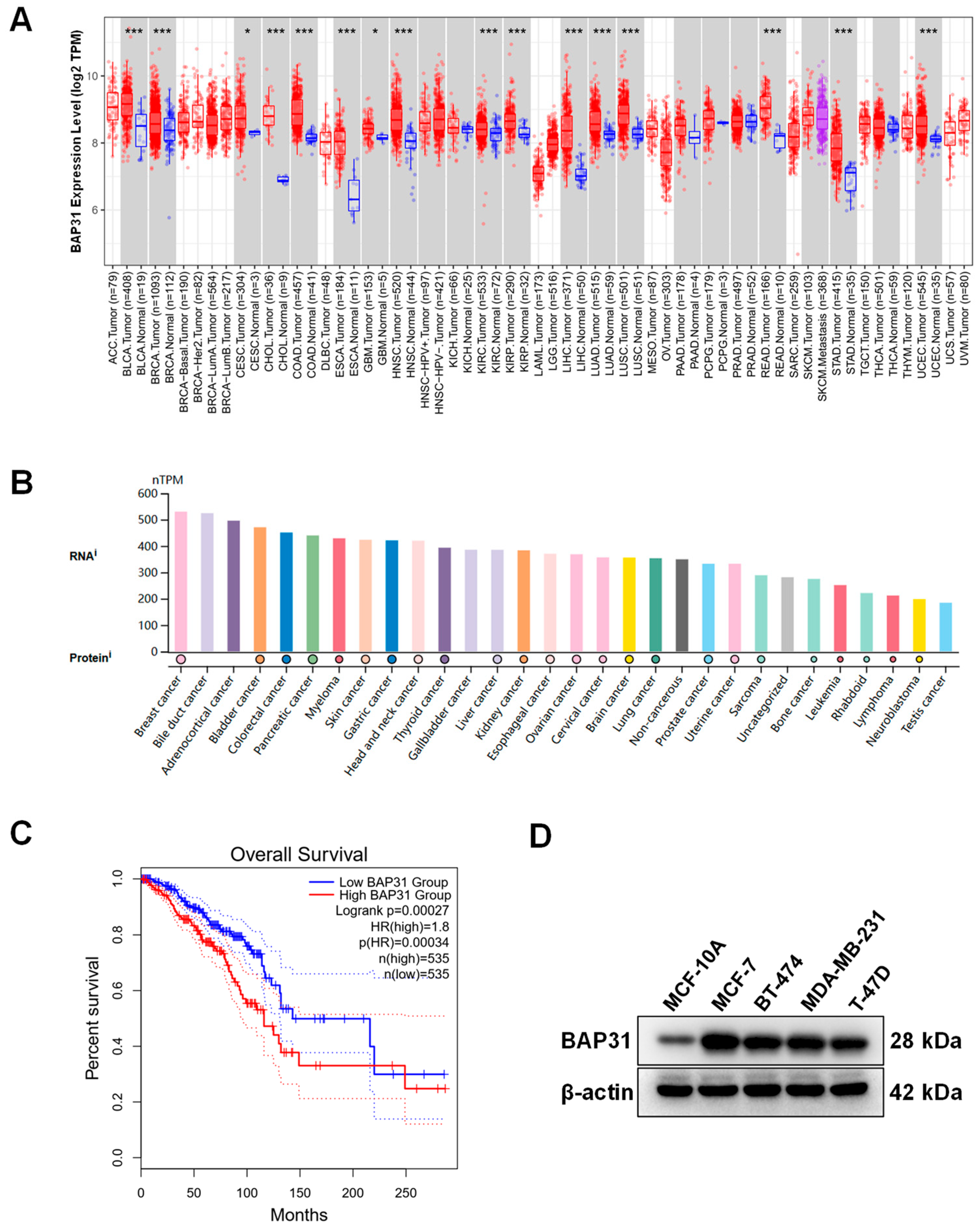
2.1. High Expression of BAP31 Associated with Poor Prognosis in Breast Cancer
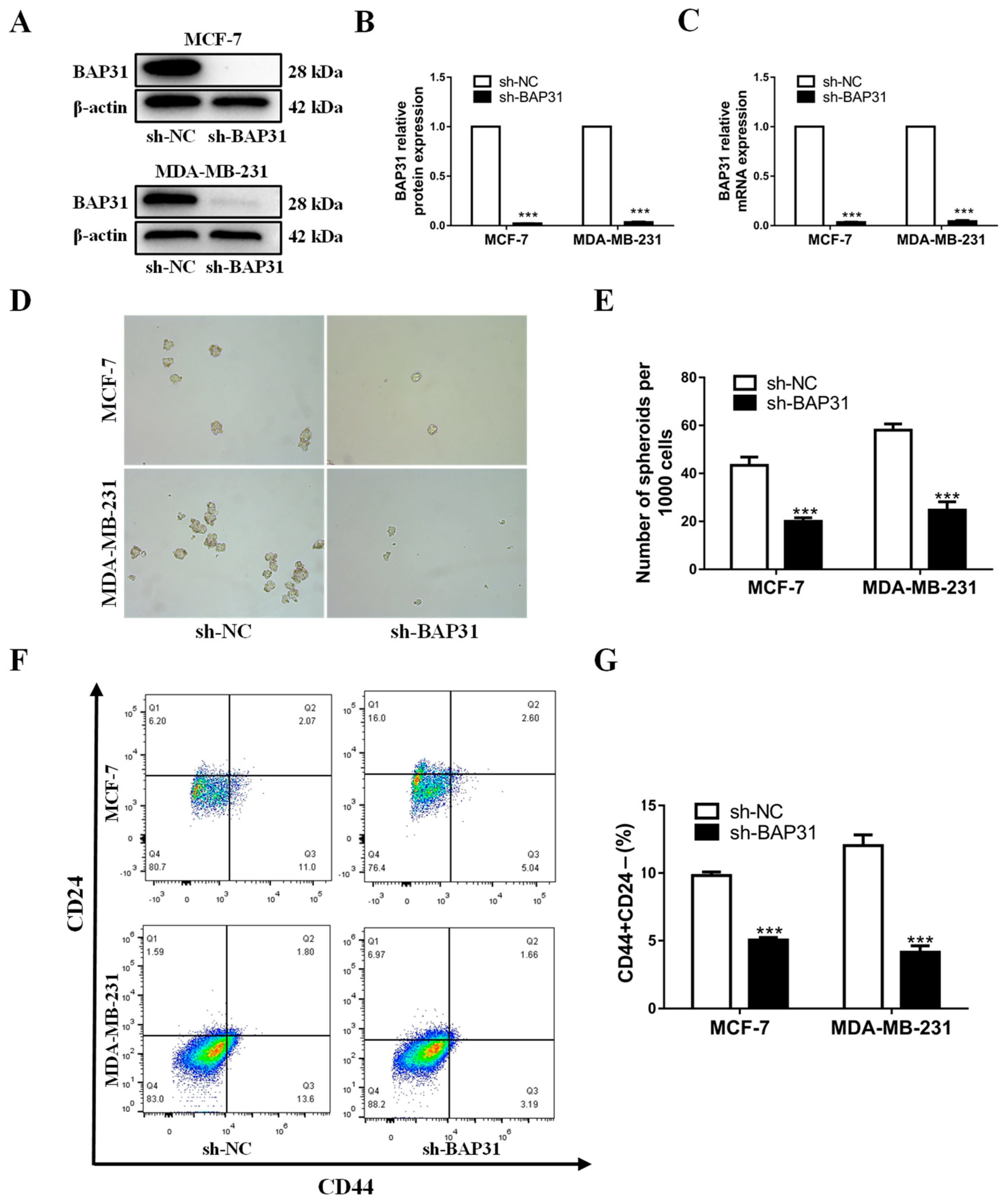
2.2. BAP31 Plays an Important Role in Breast Cancer Tumorigenesis and Stemness
2.3. The Relationship of BAP31 with Core Stemness Factors
2.4. Knockdown of BAP31 Suppresses Tumorigenesis and Stemness of Breast Cancer Cells In Vivo
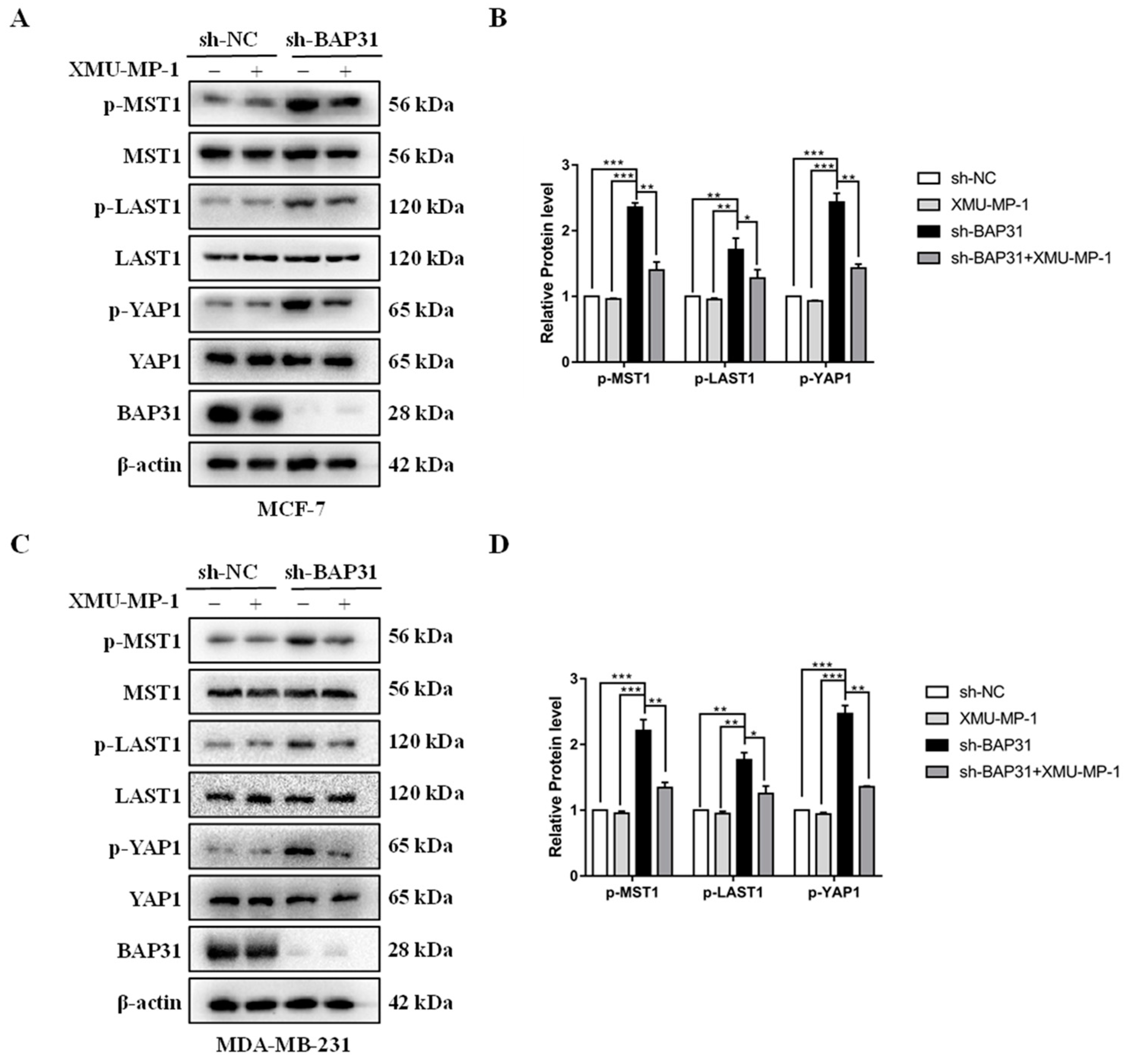
2.5. BAP31 Regulates the Hippo Pathway
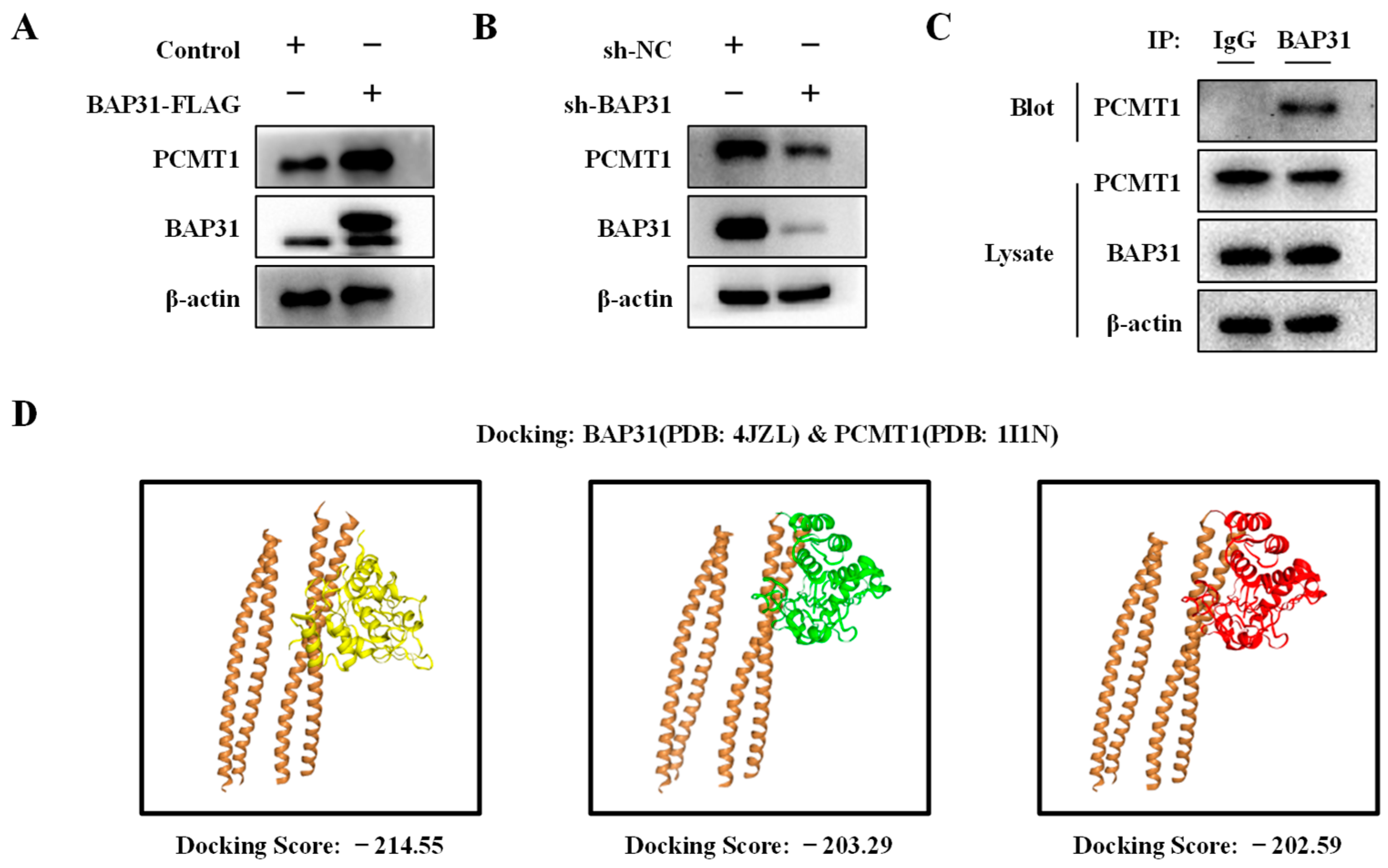
2.6. BAP31 Interacts with PCMT1 and Regulates Its Expression
2.7. Restoration of BAP31 Protein Increased the Expression of PCMT1 and Decreased p-MST1 Protein
3. Discussion
4. Materials and Methods
4.1. Analysis of the Expression Level of BAP31 in the Pan-Cancer Datasets and Prognosis Analysis in Breast Cancer
4.2. Cell Culture and Chemicals
4.3. Construction of Stable Cell Lines
4.4. Western Blot Analysis
4.5. Quantitative Real-Time PCR
4.6. Tumorsphere Formation Assay
4.7. Flow Cytometry Analysis
4.8. Immunoprecipitation (IP) Assay
4.9. Protein–Protein Docking
4.10. Xenograft Tumors in Nude Mice
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qin, Y.; Liu, S. Cytokines, breast cancer stem cells (BCSCs) and chemoresistance. Clin. Transl. Med. 2018, 7, 27. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.L.; Shin, Y.; Yang, G.; Furmanski, P.; Suh, N. Breast cancer stem cells: A review of their characteristics and the agents that affect them. Mol. Carcinog. 2021, 60, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Adachi, T.; Nielsen, P.J.; Terashima, M.; Lamers, M.C.; Kohler, G.; Reth, M. Two new proteins preferentially associated with membrane immunoglobulin D. EMBO J. 1994, 13, 3793–3800. [Google Scholar] [CrossRef]
- Annaert, W.G.; Becker, B.; Kistner, U.; Reth, M.; Jahn, R. Export of cellubrevin from the endoplasmic reticulum is controlled by BAP31. J. Cell Biol. 1997, 139, 1397–1410. [Google Scholar] [CrossRef]
- Breckenridge, D.G.; Stojanovic, M.; Marcellus, R.C.; Shore, G.C. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochromec release to the cytosol. J. Cell Biol. 2003, 160, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Namba, T. BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites. Sci. Adv. 2019, 5, eaaw1386. [Google Scholar] [CrossRef]
- Machihara, K.; Namba, T. BAP31 Inhibits Cell Adaptation to ER Stress Conditions, Negatively Regulating Autophagy Induction by Interaction with STX17. Cells 2019, 8, 1350. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, B.; Cao, Y.H.; Yan, J.C.; Sun, L.J.; Liu, X.; Xu, Y.; Wang, X.Y.; Wang, B. BCR-Associated Protein 31 Regulates Macrophages Polarization and Wound Healing Function via Early Growth Response 2/C/EBPbeta and IL-4Ralpha/C/EBPbeta Pathways. J. Immunol. 2022, 209, 1059–1070. [Google Scholar] [CrossRef]
- Cacciagli, P.; Sutera-Sardo, J.; Borges-Correia, A.; Roux, J.; Dorboz, I.; Desvignes, J.; Badens, C.; Delepine, M.; Lathrop, M.; Cau, P.; et al. Mutations in BCAP31 Cause a Severe X-Linked Phenotype with Deafness, Dystonia, and Central Hypomyelination and Disorganize the Golgi Apparatus. Am. J. Hum. Genet. 2013, 93, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, Y.; Jiang, D.; Wang, J.; Dang, E.; Li, Z.; Zhou, J.; Lu, Y.; Shi, J.; Tao, L.; et al. MiR-362 suppresses cervical cancer progression via directly targeting BAP31 and activating TGFβ/Smad pathway. Cancer Med. 2021, 10, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, D.; Yang, S.; Sun, Y.; Liu, Y.; Shi, J.; Hu, C.; Pan, J.; Liu, T.; Jin, B.; et al. BAP31 Promotes Tumor Cell Proliferation by Stabilizing SERPINE2 in Hepatocellular Carcinoma. Front. Cell. Dev. Biol. 2020, 8, 607906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C.; Wu, Y.; Liu, J.; Wang, T.; Wang, B. BAP31-Mediated miR-206/133b Cluster Promotes Transendothelial Migration and Metastasis of Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 16740. [Google Scholar] [CrossRef]
- Chen, J.; Guo, H.; Jiang, H.; Namusamba, M.; Wang, C.; Lan, T.; Wang, T.; Wang, B. A BAP31 intrabody induces gastric cancer cell death by inhibiting p27kip1 proteasome degradation. Int. J. Cancer 2019, 144, 2051–2062. [Google Scholar] [CrossRef]
- Song, K.; Farzaneh, M. Signaling pathways governing breast cancer stem cells behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef]
- Wang, X.; Jung, Y.; Jun, S.; Lee, S.; Wang, W.; Schneider, A.; Sun Oh, Y.; Lin, S.H.; Park, B.; Chen, J.; et al. PAF-Wnt signaling-induced cell plasticity is required for maintenance of breast cancer cell stemness. Nat. Commun. 2016, 7, 10633. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, D.; Chi, F.; Zhao, B. The Hippo pathway regulates stem cell proliferation, self-renewal, and differentiation. Protein Cell 2012, 3, 291–304. [Google Scholar] [CrossRef]
- Wang, L.; Baker, N.E. Salvador-Warts-Hippo Pathway in a Developmental Checkpoint Monitoring Helix-Loop-Helix Proteins. Dev. Cell 2015, 32, 191–202. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Y.; He, Y.; Li, H.; Li, W. Activation of the RhoA-YAP-β-catenin signaling axis promotes the expansion of inner ear progenitor cells in 3D culture. Stem Cells 2020, 38, 860–874. [Google Scholar] [CrossRef]
- Cunningham, R.; Hansen, C.G. The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clin. Sci. 2022, 136, 197–222. [Google Scholar] [CrossRef]
- Guo, C.; Tommasi, S.; Liu, L.; Yee, J.; Dammann, R.; Pfeifer, G.P. RASSF1A Is Part of a Complex Similar to the Drosophila Hippo/Salvador/Lats Tumor-Suppressor Network. Curr. Biol. 2007, 17, 700–705. [Google Scholar] [CrossRef]
- Zeng, Q.; Hong, W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 2008, 13, 188–192. [Google Scholar] [CrossRef]
- Qiao, M.; Wang, Y.; Xu, X.; Lu, J.; Dong, Y.; Tao, W.; Stein, J.; Stein, G.S.; Iglehart, J.D.; Shi, Q.; et al. Mst1 is an interacting protein that mediates PHLPPs’ induced apoptosis. Mol. Cell 2010, 38, 512–523. [Google Scholar] [CrossRef]
- Ren, A.; Yan, G.; You, B.; Sun, J. Down-regulation of Mammalian Sterile 20–Like Kinase 1 by Heat Shock Protein 70 Mediates Cisplatin Resistance in Prostate Cancer Cells. Cancer Res. 2008, 68, 2266–2274. [Google Scholar] [CrossRef]
- Yan, G.; Qin, Q.; Yi, B.; Chuprun, K.; Sun, H.; Huang, S.; Sun, J. Protein-L-isoaspartate (D-aspartate) O-methyltransferase protects cardiomyocytes against hypoxia induced apoptosis through inhibiting proapoptotic kinase Mst1. Int. J. Cardiol. 2013, 168, 3291–3299. [Google Scholar] [CrossRef]
- Zhao, P.; Xia, G.; Dong, S.; Jiang, Z.X.; Chen, M. An iTEP-salinomycin nanoparticle that specifically and effectively inhibits metastases of 4T1 orthotopic breast tumors. Biomaterials 2016, 93, 10633. [Google Scholar] [CrossRef]
- Namusamba, M.; Li, Z.; Zhang, Q.; Wang, C.; Wang, T.; Wang, B. Biological roles of the B cell receptor-associated protein 31: Functional Implication in Cancer. Mol. Biol. Rep. 2021, 48, 773–786. [Google Scholar] [CrossRef]
- Quistgaard, E.M. BAP31: Physiological functions and roles in disease. Biochimie 2021, 186, 105–129. [Google Scholar] [CrossRef]
- Manley, H.A.; Lennon, V.A. Endoplasmic reticulum membrane-sorting protein of lymphocytes (BAP31) is highly expressed in neurons and discrete endocrine cells. J. Histochem. Cytochem. 2001, 49, 1235–1243. [Google Scholar] [CrossRef]
- Namusamba, M.; Wu, Y.; Yang, J.; Zhang, Q.; Wang, C.; Wang, T.; Wang, B. BAP31 Promotes Angiogenesis via Galectin-3 Upregulation in Neuroblastoma. Int. J. Mol. Sci. 2024, 25, 2946. [Google Scholar] [CrossRef]
- Gao, H.; Chakraborty, G.; Zhang, Z.; Akalay, I.; Gadiya, M.; Gao, Y.; Sinha, S.; Hu, J.; Jiang, C.; Akram, M.; et al. Multi-organ Site Metastatic Reactivation Mediated by Non-canonical Discoidin Domain Receptor 1 Signaling. Cell 2016, 166, 47–62. [Google Scholar] [CrossRef]
- Adorno-Cruz, V.; Kibria, G.; Liu, X.; Doherty, M.; Junk, D.J.; Guan, D.; Hubert, C.; Venere, M.; Mulkearns-Hubert, E.; Sinyuk, M.; et al. Cancer stem cells: Targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015, 75, 924–929. [Google Scholar] [CrossRef]
- Gao, L.; Guo, Q.; Li, X.; Yang, X.; Ni, H.; Wang, T.; Zhao, Q.; Liu, H.; Xing, Y.; Xi, T.; et al. MiR-873/PD-L1 axis regulates the stemness of breast cancer cells. eBioMedicine 2019, 41, 395–407. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, P.; Wang, J.; Shu, Y.; Zhong, X.; Gao, Z.; Yang, J.; Jiang, Y.; Zhou, X.; et al. Long noncoding RNA HOTAIR regulates the stemness of breast cancer cells via activation of the NF-κB signaling pathway. J. Biol. Chem. 2022, 298, 102630. [Google Scholar] [CrossRef]
- Shi, L.; Tang, X.; Qian, M.; Liu, Z.; Meng, F.; Fu, L.; Wang, Z.; Zhu, W.G.; Huang, J.D.; Zhou, Z.; et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene 2018, 37, 6299–6315. [Google Scholar] [CrossRef]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef]
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.; Abujarour, R.; et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010, 24, 1106–1118. [Google Scholar] [CrossRef]
- Zanconato, F.; Forcato, M.; Battilana, G.; Azzolin, L.; Quaranta, E.; Bodega, B.; Rosato, A.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 2015, 17, 1218–1227. [Google Scholar] [CrossRef]
- Yimlamai, D.; Fowl, B.H.; Camargo, F.D. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J. Hepatol. 2015, 63, 1491–1501. [Google Scholar] [CrossRef]
- Nishina, H. Physiological and pathological roles of the Hippo-YAP/TAZ signaling pathway in liver formation, homeostasis, and tumorigenesis. Cancer Sci. 2022, 113, 1900–1908. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Yang, Y.; Ji, W.; Yu, Y.; Niu, X.; Zeng, Q.; Xia, W.; Lu, S. The Hippo/YAP1 pathway interacts with FGFR1 signaling to maintain stemness in lung cancer. Cancer Lett. 2018, 423, 36–46. [Google Scholar] [CrossRef]
- Escoll, M.; Lastra, D.; Pajares, M.; Robledinos-Anton, N.; Rojo, A.I.; Fernandez-Gines, R.; Mendiola, M.; Martinez-Marin, V.; Esteban, I.; Lopez-Larrubia, P.; et al. Transcription factor NRF2 uses the Hippo pathway effector TAZ to induce tumorigenesis in glioblastomas. Redox Biol. 2020, 30, 101425. [Google Scholar] [CrossRef]
- Kang, J.; Wang, J.; Yao, Z.; Hu, Y.; Ma, S.; Fan, Q.; Gao, F.; Sun, Y.; Sun, J. Fascin induces melanoma tumorigenesis and stemness through regulating the Hippo pathway. Cell Commun. Signal. 2018, 16, 37. [Google Scholar] [CrossRef]
- Wang, T.; Qin, Z.Y.; Wen, L.Z.; Guo, Y.; Liu, Q.; Lei, Z.J.; Pan, W.; Liu, K.J.; Wang, X.W.; Lai, S.J.; et al. Epigenetic restriction of Hippo signaling by MORC2 underlies stemness of hepatocellular carcinoma cells. Cell Death Differ. 2018, 25, 2086–2100. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, J.; Yang, M.J.; Hong, S.P.; Lee, C.K.; Kubota, Y.; Lim, D.S.; Koh, G.Y. A MST1-FOXO1 cascade establishes endothelial tip cell polarity and facilitates sprouting angiogenesis. Nat. Commun. 2019, 10, 838. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Zhao, B.; An, F.; Zhang, W.; Zhu, X.; Meng, S.; Wang, B. Knockdown of BAP31 Suppresses Tumorigenesis and Stemness in Breast Cancer Cells via the Hippo Pathway. Int. J. Mol. Sci. 2025, 26, 3576. https://doi.org/10.3390/ijms26083576
Hao Z, Zhao B, An F, Zhang W, Zhu X, Meng S, Wang B. Knockdown of BAP31 Suppresses Tumorigenesis and Stemness in Breast Cancer Cells via the Hippo Pathway. International Journal of Molecular Sciences. 2025; 26(8):3576. https://doi.org/10.3390/ijms26083576
Chicago/Turabian StyleHao, Zhenzhen, Bo Zhao, Fei An, Wanting Zhang, Xiaoshuang Zhu, Shihao Meng, and Bing Wang. 2025. "Knockdown of BAP31 Suppresses Tumorigenesis and Stemness in Breast Cancer Cells via the Hippo Pathway" International Journal of Molecular Sciences 26, no. 8: 3576. https://doi.org/10.3390/ijms26083576
APA StyleHao, Z., Zhao, B., An, F., Zhang, W., Zhu, X., Meng, S., & Wang, B. (2025). Knockdown of BAP31 Suppresses Tumorigenesis and Stemness in Breast Cancer Cells via the Hippo Pathway. International Journal of Molecular Sciences, 26(8), 3576. https://doi.org/10.3390/ijms26083576





