In Silico Discovery of a Novel Natural Product Targeting PI3Kα for the Treatment of Head and Neck Squamous Cell Carcinoma
Abstract
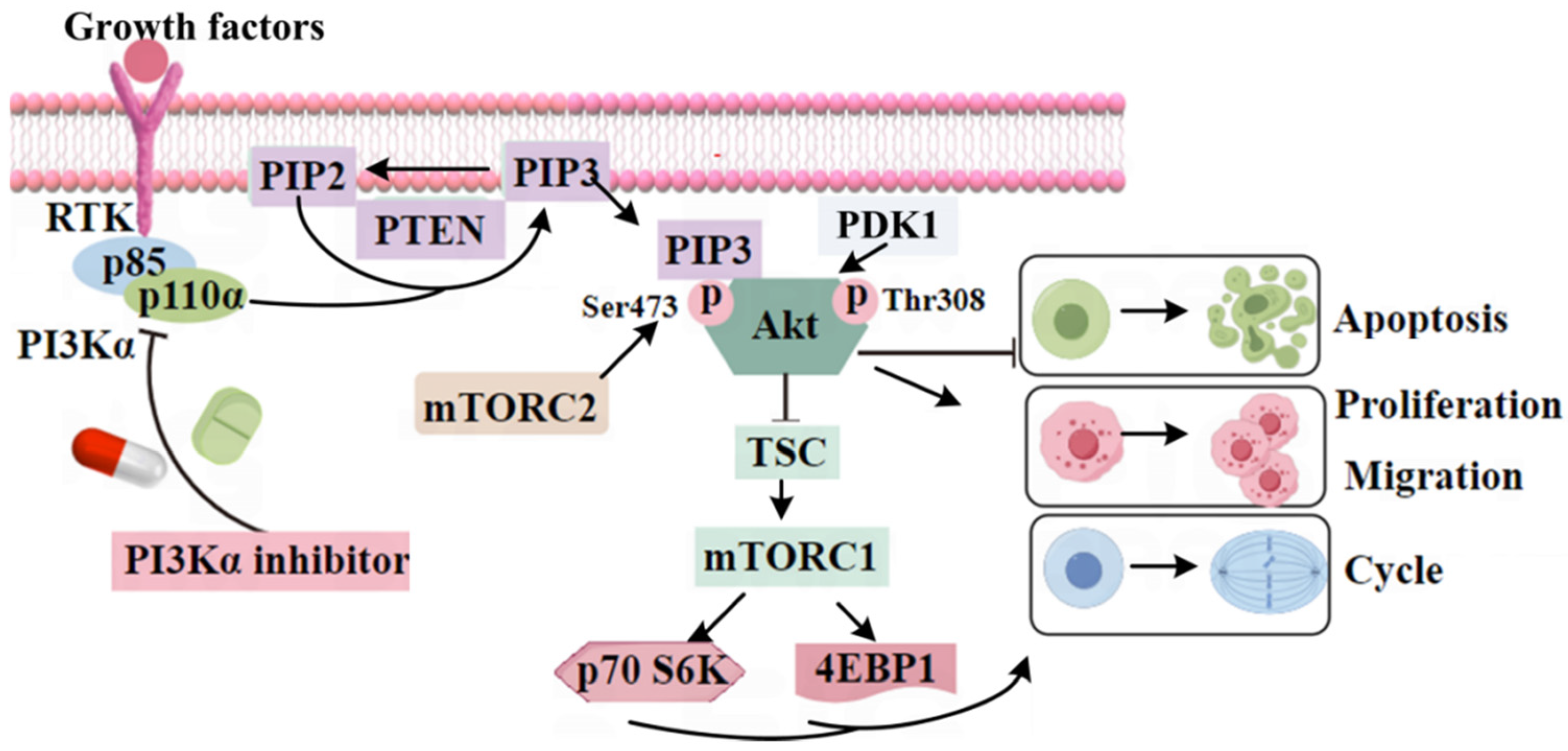
1. Introduction
2. Results
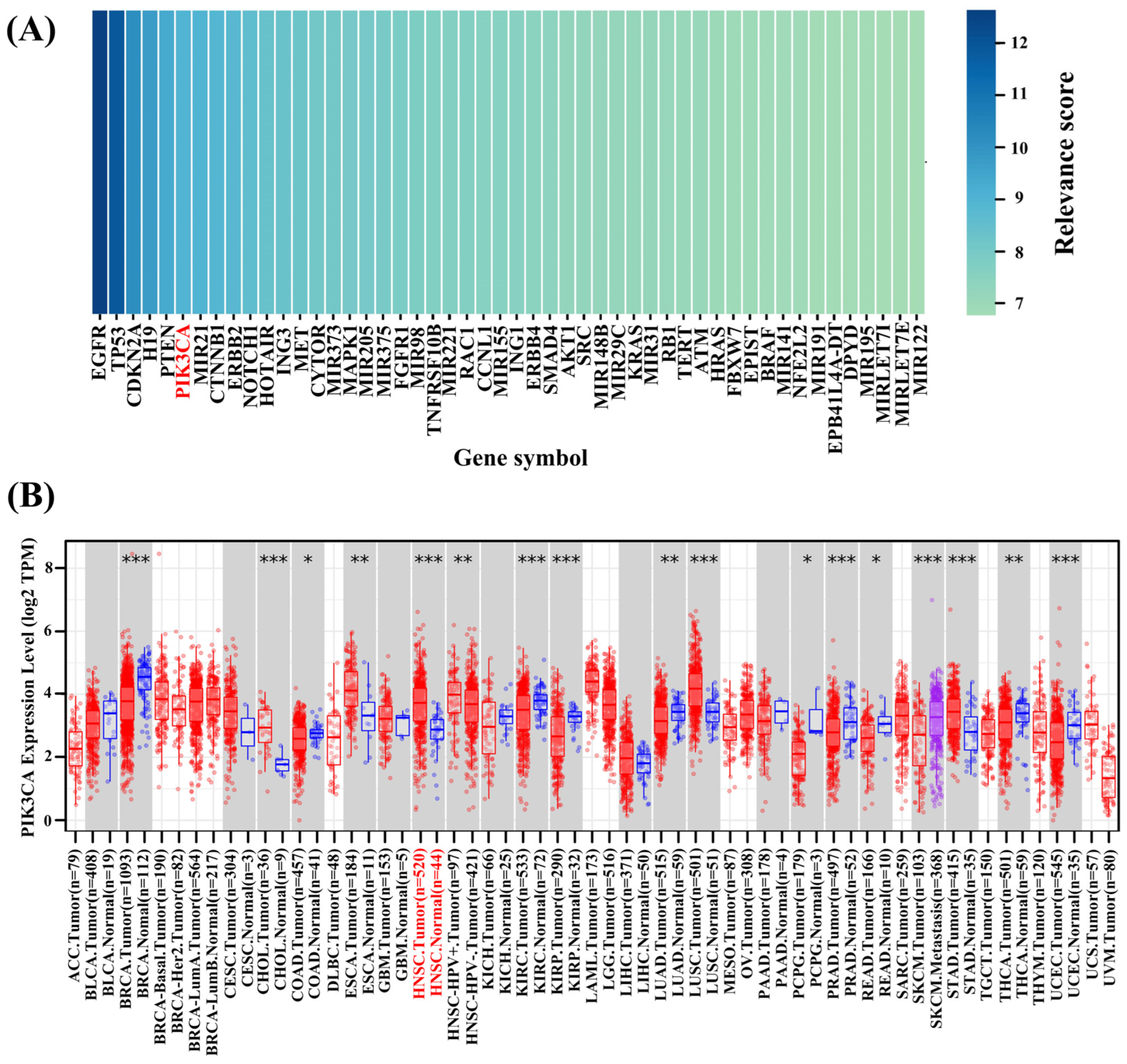
2.1. Correlation Analysis Between Genes and HNSCC
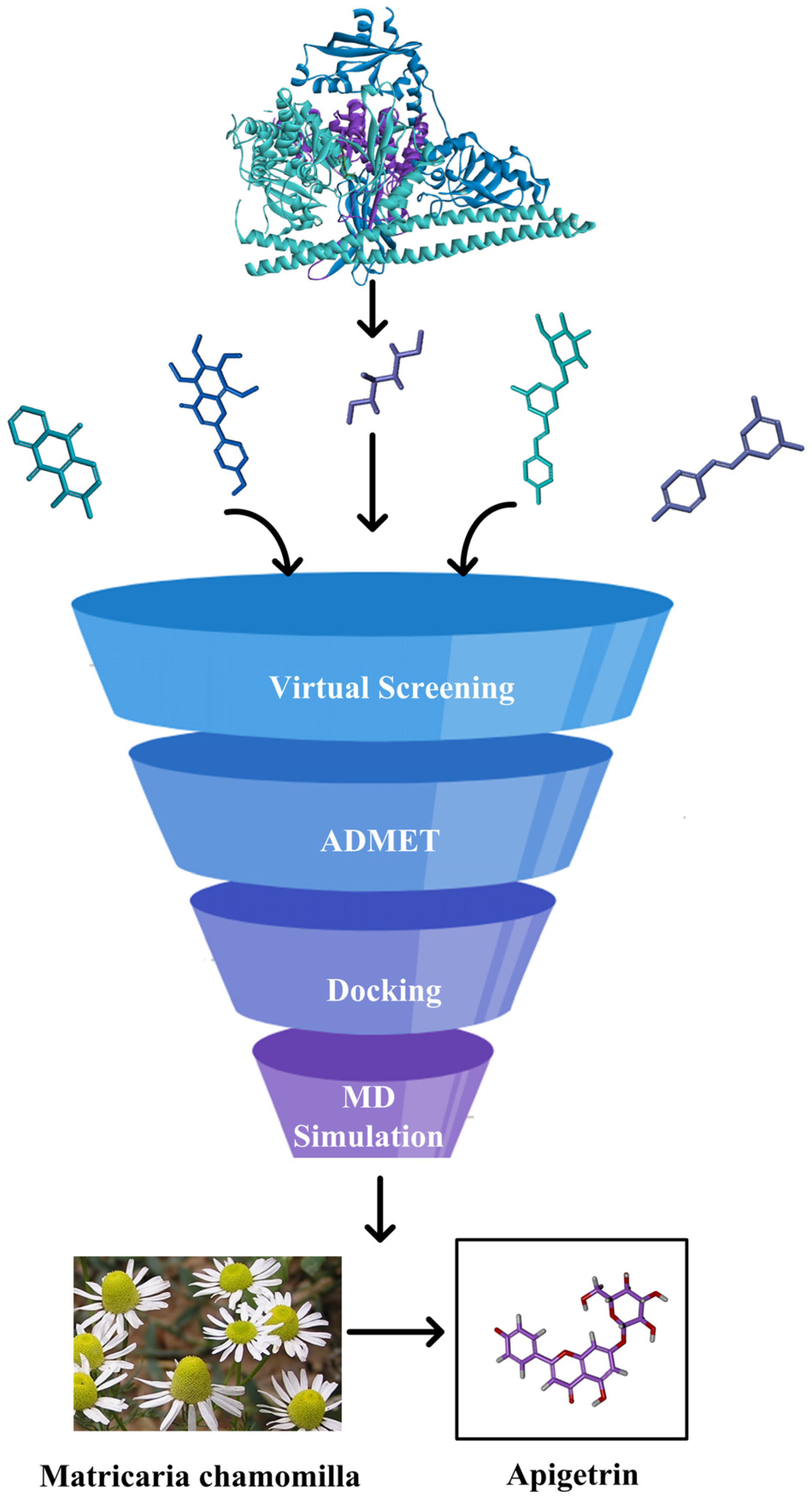
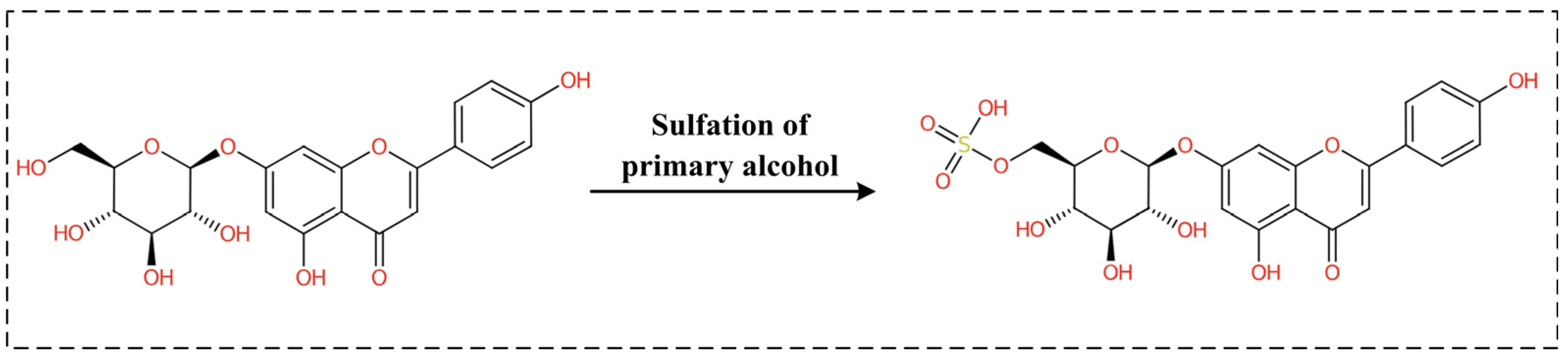
2.2. Virtual Database Screening
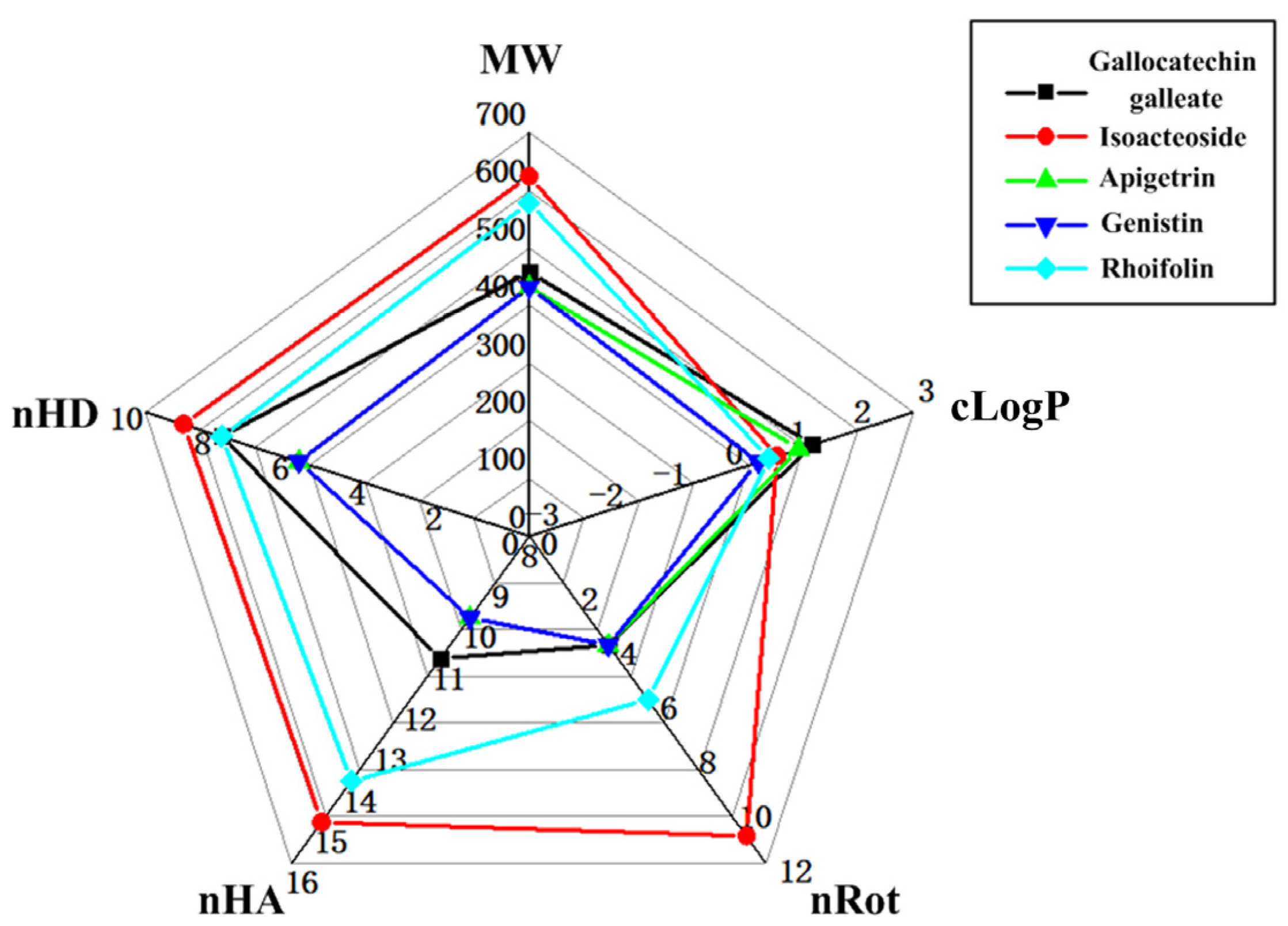
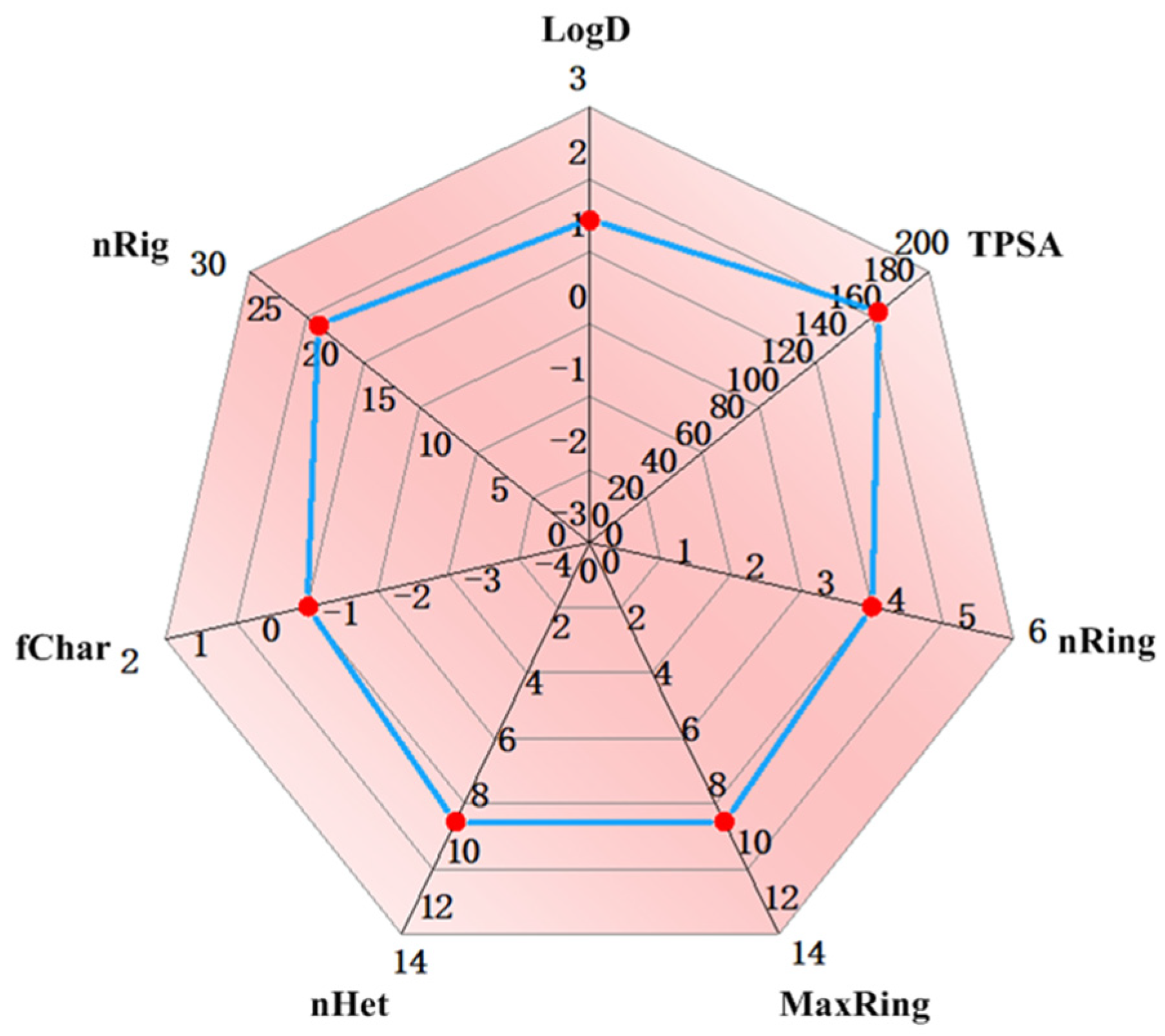
2.3. The Evaluation of the Druggability
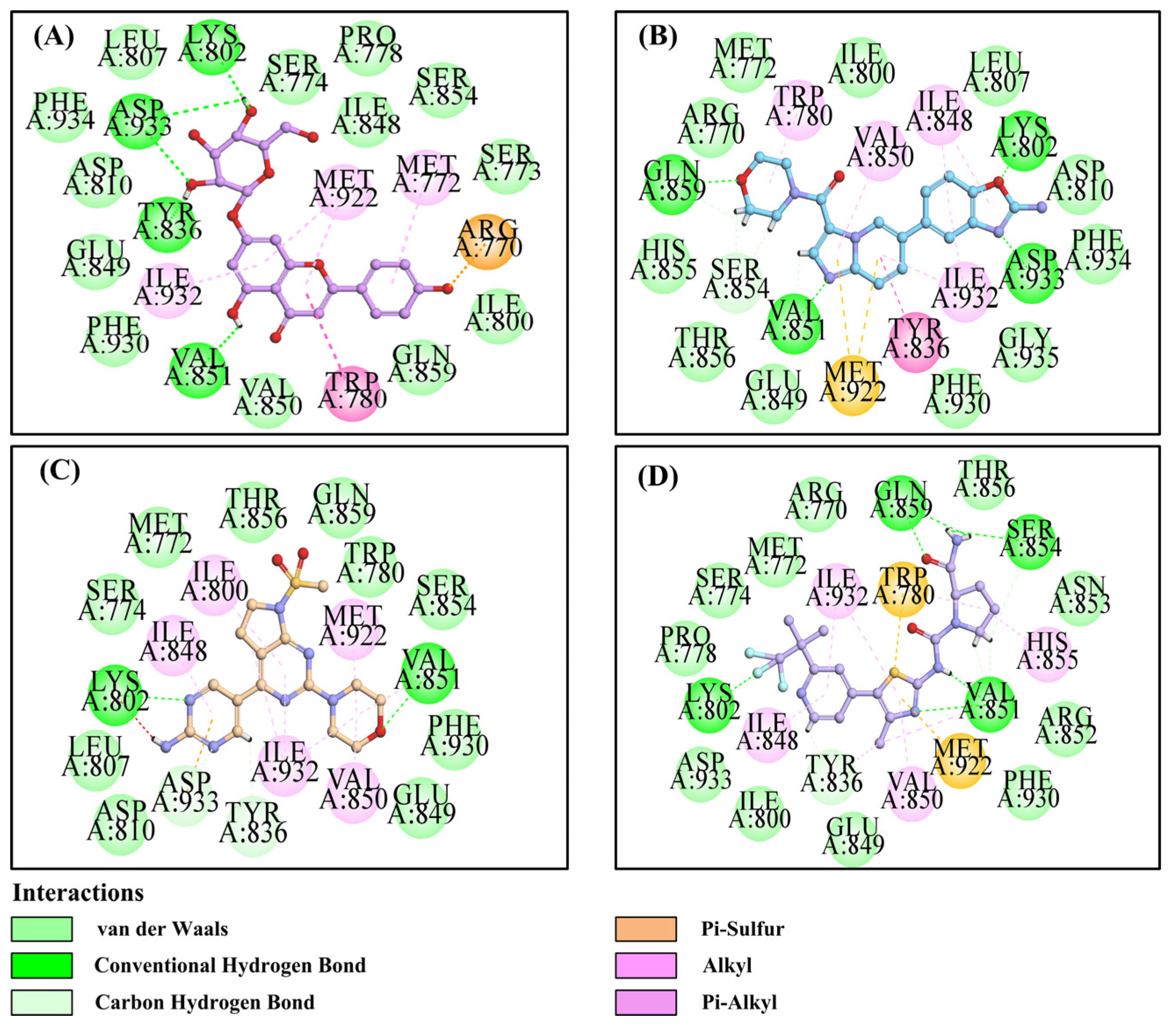
2.4. The Mode of Apigetrin Binding to PI3Kα
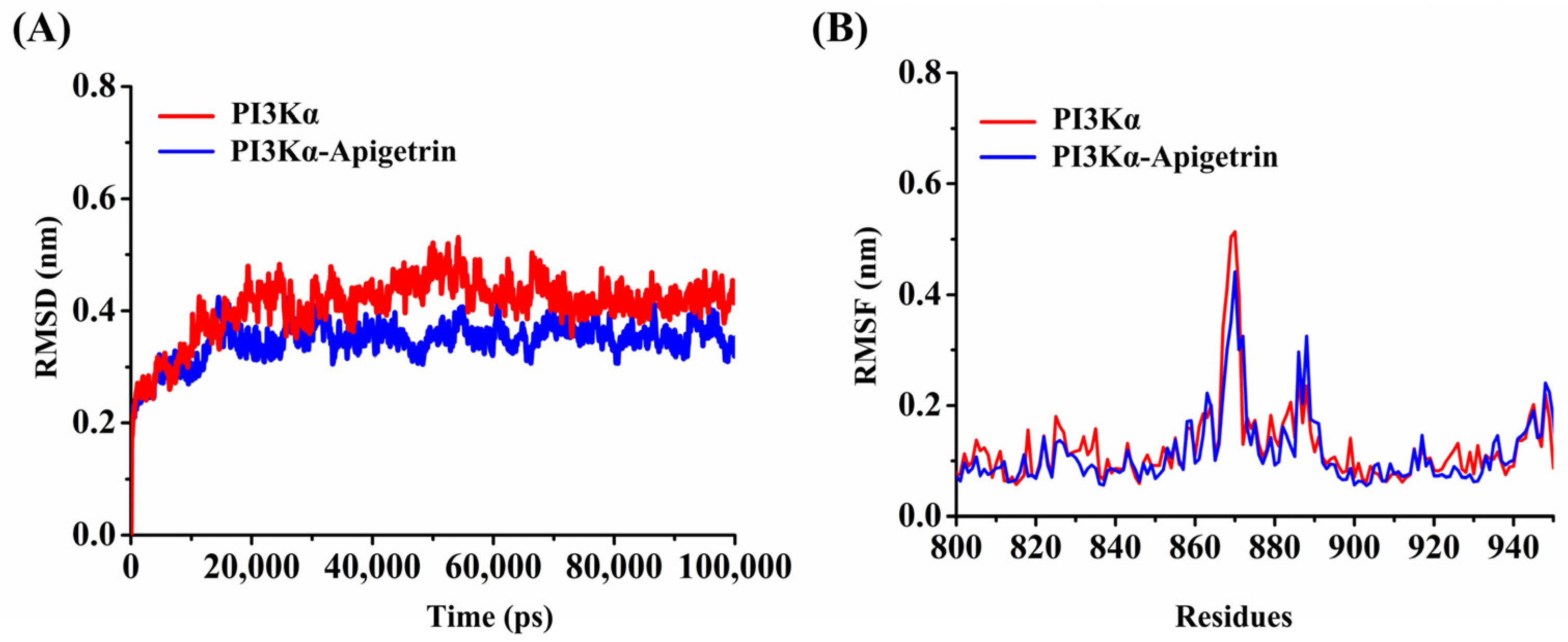
2.5. Molecular Dynamics Simulation of the PI3Kα–Apigetrin System
3. Discussion
4. Materials and Methods
4.1. Correlation Analysis Between PIK3CA and HNSCC
4.2. Virtual Screening Study
4.3. Lipinski’s Filter and ADMET Study
4.4. Molecular Dynamics Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boussios, S.; Sheriff, M.; Ovsepian, S.V. Molecular biology of cancer-interplay of malignant cells with emerging therapies. Int. J. Mol. Sci. 2024, 25, 13090. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, A.T.; Li, H.; Vujanovic, L.; Zandberg, D.P.; Ferris, R.L.; Bruno, T.C. Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment. Nat. Rev. Cancer 2023, 23, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Zhu, Y.; Huang, N.; Qu, N. New advances in the therapeutic strategy of head and neck squamous cell carcinoma: A review of latest therapies and cutting-edge research. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189230. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, G.; Cheng, X.; Zhang, R.; Ma, Y. In silico discovery of a novel potential allosteric PI3Kα inhibitor incorporating 2-oxopropyl urea targeting head and neck squamous cell carcinoma. BMC Chem. 2025, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Lee, S.H.; Cotton, S.; Kennedy, C. Therapeutic exercises for affecting post-treatment swallowing in people treated for advanced-stage head and neck cancers. Cochrane Database Syst. Rev. 2016, 2016, Cd011112. [Google Scholar] [CrossRef]
- Su, Y.C.; Lee, W.C.; Wang, C.C.; Yeh, S.A.; Chen, W.H.; Chen, P.J. Targeting PI3K/AKT/mTOR signaling pathway as a radiosensitization in head and neck squamous cell carcinomas. Int. J. Mol. Sci. 2022, 23, 15749. [Google Scholar] [CrossRef]
- Ahn, S.H.; Hong, H.J.; Kwon, S.Y.; Kwon, K.H.; Roh, J.L.; Ryu, J.; Park, J.H.; Baek, S.K.; Lee, G.H.; Lee, S.Y.; et al. Guidelines for the surgical management of laryngeal cancer: Korean society of thyroid-head and neck surgery. Clin. Exp. Otorhinolaryngol. 2017, 10, 1–43. [Google Scholar]
- Elkabets, M.; Pazarentzos, E.; Juric, D.; Sheng, Q.; Pelossof, R.A.; Brook, S.; Benzaken, A.O.; Rodon, J.; Morse, N.; Yan, J.J.; et al. AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 2015, 27, 533–546. [Google Scholar] [CrossRef]
- Vitale, S.R.; Martorana, F.; Stella, S.; Motta, G.; Inzerilli, N.; Massimino, M.; Tirrò, E.; Manzella, L.; Vigneri, P. PI3K inhibition in breast cancer: Identifying and overcoming different flavors of resistance. Crit. Rev. Oncol. Hematol. 2021, 162, 103334. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Marshall, J.D.S.; Whitecross, D.E.; Mellor, P.; Anderson, D.H. Impact of p85α alterations in cancer. Biomolecules 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule phosphatidylinositol 3-kinase inhibitors prescribed for the treatment of malignancies. Pharmacol. Res. 2021, 168, 105579. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin. Cancer Biol. 2019, 59, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A. Targeting the RAS upstream and downstream signaling pathway for cancer treatment. Eur. J. Pharmacol. 2024, 979, 176727. [Google Scholar] [CrossRef]
- Miled, N.; Yan, Y.; Hon, W.C.; Perisic, O.; Zvelebil, M.; Inbar, Y.; Schneidman-Duhovny, D.; Wolfson, H.J.; Backer, J.M.; Williams, R.L. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science 2007, 317, 239–242. [Google Scholar] [CrossRef]
- Burke, J.E.; Perisic, O.; Masson, G.R.; Vadas, O.; Williams, R.L. Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110α (PIK3CA). Proc. Natl. Acad. Sci. USA 2012, 109, 15259–15264. [Google Scholar] [CrossRef]
- Burke, J.E.; Williams, R.L. Synergy in activating class I PI3Ks. Trends Biochem. Sci. 2015, 40, 88–100. [Google Scholar] [CrossRef]
- Loyo, M.; Li, R.J.; Bettegowda, C.; Pickering, C.R.; Frederick, M.J.; Myers, J.N.; Agrawal, N. Lessons learned from next-generation sequencing in head and neck cancer. Head Neck 2013, 35, 454–463. [Google Scholar] [CrossRef]
- Simpson, D.R.; Mell, L.K.; Cohen, E.E. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral. Oncol. 2015, 51, 291–298. [Google Scholar] [CrossRef]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Rocco, E.G.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN alterations and their role in cancer management: Are we making headway on precision medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef]
- Arcos, M.; Goodla, L.; Kim, H.; Desai, S.P.; Liu, R.; Yin, K.; Liu, Z.; Martin, D.R.; Xue, X. PINK1-deficiency facilitates mitochondrial iron accumulation and colon tumorigenesis. Autophagy 2025, 21, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Chen, H.Y.; Hsu, S.Y.; Pang, J.H.; Wang, S.Y.; Hsu, J.T.; Yeh, T.S.; Chen, L.W.; Kuo, S.F.; Sun, C.C.; et al. PTEN insufficiency modulates ER+ breast cancer cell cycle progression and increases cell growth in vitro and in vivo. Drug Des. Dev. Ther. 2015, 9, 4631–4638. [Google Scholar] [CrossRef] [PubMed]
- Carver, B.S.; Tran, J.; Gopalan, A.; Chen, Z.; Shaikh, S.; Carracedo, A.; Alimonti, A.; Nardella, C.; Varmeh, S.; Scardino, P.T.; et al. Author Correction: Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat. Genet 2020, 52, 984. [Google Scholar] [CrossRef] [PubMed]
- Keam, B.; Kim, S.; Ahn, Y.O.; Kim, T.M.; Lee, S.H.; Kim, D.W.; Heo, D.S. In vitro anticancer activity of PI3K alpha selective inhibitor BYL719 in head and neck cancer. Anticancer Res. 2015, 35, 175–182. [Google Scholar]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT pathway as a target for cancer treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Stephens, L.; Hawkins, P. PI3K signalling: The path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 2012, 13, 195–203. [Google Scholar] [CrossRef]
- Ranek, M.J.; Kokkonen-Simon, K.M.; Chen, A.; Dunkerly-Eyring, B.L.; Vera, M.P.; Oeing, C.U.; Patel, C.H.; Nakamura, T.; Zhu, G.; Bedja, D.; et al. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature 2019, 566, 264–269. [Google Scholar] [CrossRef]
- Juric, D.; Rodon, J.; Tabernero, J.; Janku, F.; Burris, H.A.; Schellens, J.H.M.; Middleton, M.R.; Berlin, J.; Schuler, M.; Gil-Martin, M.; et al. Phosphatidylinositol 3-kinase α-selective inhibition with Alpelisib (BYL719) in PIK3CA-altered solid tumors: Results from the first-in-human study. J. Clin. Oncol. 2018, 36, 1291–1299. [Google Scholar] [CrossRef]
- Kearney, A.L.; Vasan, N. A New Wave of PI3Kα Inhibitors. Cancer Discov. 2023, 13, 2313–2315. [Google Scholar] [CrossRef]
- Turner, N.C.; Im, S.A.; Saura, C.; Juric, D.; Loibl, S.; Kalinsky, K.; Schmid, P.; Loi, S.; Sunpaweravong, P.; Musolino, A.; et al. Inavolisib-based therapy in PIK3CA-mutated advanced breast cancer. N. Engl. J. Med. 2024, 391, 1584–1596. [Google Scholar] [CrossRef]
- Wei, X.L.; Liu, F.R.; Liu, J.H.; Zhao, H.Y.; Zhang, Y.; Wang, Z.Q.; Qiu, M.Z.; Xu, F.; Yu, Q.Q.; Du, Y.W.; et al. First-in-human phase Ia study of the PI3Kα inhibitor CYH33 in patients with solid tumors. Nat. Commun. 2022, 13, 7012. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, X.; Wang, R.J.; Ma, Q.Y.; Xu, L.; Wang, Y.; Liao, H.P.; Wang, H.L.; Hu, L.D.; Kong, X.; et al. PI3Kα inhibitor CYH33 triggers antitumor immunity in murine breast cancer by activating CD8(+)T cells and promoting fatty acid metabolism. J. Immunother. Cancer 2021, 9, e003093. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25 (Suppl. S2), 41–59. [Google Scholar] [CrossRef] [PubMed]
- Farouk, H.M.; Attia, E.Z.; Shaban, G.M.; Abdelmohsen, U.R.; El-Katatny, M.H. Antimicrobial secondary metabolites and antioxidant activities of fungal endophytes associated with Ziziphus spina-christi (L.) Desf. (Nabq) leaves. Nat. Prod. Res. 2024, 17, 1–5. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Zoi, V.; Kyritsis, A.P.; Galani, V.; Lazari, D.; Sioka, C.; Voulgaris, S.; Alexiou, G.A. The role of curcumin in cancer: A focus on the PI3K/Akt pathway. Cancers 2024, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C.; Lai, C.S.; Chung, M.C.; Kalyanam, N.; Majeed, M.; Ho, C.T.; Ho, Y.S.; Pan, M.H. Potent anti-cancer effect of 3′-hydroxypterostilbene in human colon xenograft tumors. PLoS ONE 2014, 9, e111814. [Google Scholar] [CrossRef]
- Mostefai, N.; Cherif, F.Y.; Hosen, M.N.; Ouici, H.B.; Brahim, H.; Guendouzi, A.; Belkhiri, L.; Guendouzi, A.; Alharbi, H.M.; Jawi, M.; et al. Identification of acetylcholinesterase inhibitors and stability analysis of THC@HP-β-CD inclusion complex: A comprehensive computational study. Talanta 2025, 286, 127370. [Google Scholar] [CrossRef]
- Roney, M.; Wong, K.K.V.; Uddin, M.N.; Rullah, K.; Septama, A.W.; Antika, L.D.; Mohd Aluwi, M.F.F. Design, synthesis, structural characterization, cytotoxicity and computational studies of usnic acid derivative as potential anti-breast cancer agent against MCF7 and T47D cell lines. Comput. Biol. Chem. 2025, 115, 108303. [Google Scholar] [CrossRef]
- Nichols, A.C.; Palma, D.A.; Chow, W.; Tan, S.; Rajakumar, C.; Rizzo, G.; Fung, K.; Kwan, K.; Wehrli, B.; Winquist, E.; et al. High frequency of activating PIK3CA mutations in human papillomavirus-positive oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 617–622. [Google Scholar] [CrossRef]
- Cochicho, D.; Esteves, S.; Rito, M.; Silva, F.; Martins, L.; Montalvão, P.; Cunha, M.; Magalhães, M.; Gil da Costa, R.M.; Felix, A. PIK3CA gene mutations in HNSCC: Systematic review and correlations with HPV status and patient survival. Cancers 2022, 14, 1286. [Google Scholar] [CrossRef]
- Kotzampasi, D.M.; Papadourakis, M.; Burke, J.E.; Cournia, Z. Free energy landscape of the PI3Kα C-terminal activation. Comput. Struct. Biotechnol. J. 2024, 23, 3118–3131. [Google Scholar] [CrossRef]
- Liang, H.; Fang, C.; Qiu, M. The multi-target mechanism of action of selaginella doederleinii hieron in the treatment of nasopharyngeal carcinoma: A network pharmacology and multi-omics analysis. Sci. Rep. 2025, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [PubMed]
- Firdous, S.; Bhat, S.H.; Aziz, S.; Jehangir, M.; Syeed, S.; Iqra, Z.; Ahmad, M.A.; Rasool, S.; Khursheed, A.; Shalla, A.H.; et al. Antibacterial potential of Thymus linearis essential oil collected from Wasturwan mountain: A combination of experimental and theoretical studies involving in silico molecular docking simulation of the major compounds against Novobiocin-resistant mutant of DNA Gyrase-B. Microb. Pathog. 2023, 183, 106280. [Google Scholar] [PubMed]
- Gao, K.; Zhou, T.; Yin, Y.; Sun, X.; Jiang, H.; Li, T. Atorvastatin inhibits glioma glycolysis and immune escape by modulating the miR-125a-5p/TXLNA axis. Hereditas 2024, 161, 54. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar]
| Apigetrin | Alpelisib | |
|---|---|---|
| PPB 1 (%) | 83.8% | 97.8% |
| BBB 2 (%) | No | No |
| CLplasma 3 (mL/min/kg) | 3.214 | 4.278 |
| T1/2 4 (h) | 3.454 | 0.815 |
| CYP2D6 inhibitor 5 | No | No |
| Bioavailability score 6 | 0.55 | 0.55 |
| Log Kp 7 (cm/s) | −7.65 | −6.71 |
| VDss 8 (L/kg) | 0.897 | 1.335 |
| CYP1A2 inhibitor 9 | No | No |
| CYP2C9 inhibitor 10 | No | No |
| CYP3A4 inhibitor 11 | No | Yes |
| Pgp inhibitor (%) 12 | 0–10% | 0–10% |
| Apigetrin | Alpelisib | |
|---|---|---|
| LD50 (mg/kg) | 5000 | 1000 |
| Hepatotoxicity | Inact82% | Inact54% |
| Neurotoxicity | Inact88% | Act74% |
| Carcinogenicity | Inact86% | Inact58% |
| Immunotoxicity | Inact93% | Inact97% |
| Cytotoxicity | Inact69% | Inact68% |
| Apigetrin | Alpelisib | |
|---|---|---|
| Rat oral acute toxicity | 4.7% | 44.4% |
| hERG blockers | 2.8% | 43.5% |
| Drug-induced nephrotoxicity | 34.7% | 88.5% |
| Respiratory toxicity | 3.6% | 68.1% |
| Hematotoxicity | 12.5% | 66.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Cheng, X. In Silico Discovery of a Novel Natural Product Targeting PI3Kα for the Treatment of Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2025, 26, 3565. https://doi.org/10.3390/ijms26083565
Jia W, Cheng X. In Silico Discovery of a Novel Natural Product Targeting PI3Kα for the Treatment of Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2025; 26(8):3565. https://doi.org/10.3390/ijms26083565
Chicago/Turabian StyleJia, Wenqing, and Xianchao Cheng. 2025. "In Silico Discovery of a Novel Natural Product Targeting PI3Kα for the Treatment of Head and Neck Squamous Cell Carcinoma" International Journal of Molecular Sciences 26, no. 8: 3565. https://doi.org/10.3390/ijms26083565
APA StyleJia, W., & Cheng, X. (2025). In Silico Discovery of a Novel Natural Product Targeting PI3Kα for the Treatment of Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences, 26(8), 3565. https://doi.org/10.3390/ijms26083565




