Pre-Existing Allergic Inflammation Alters Both Innate and Adaptive Immune Responses in Mice Co-Infected with Influenza Virus
Abstract
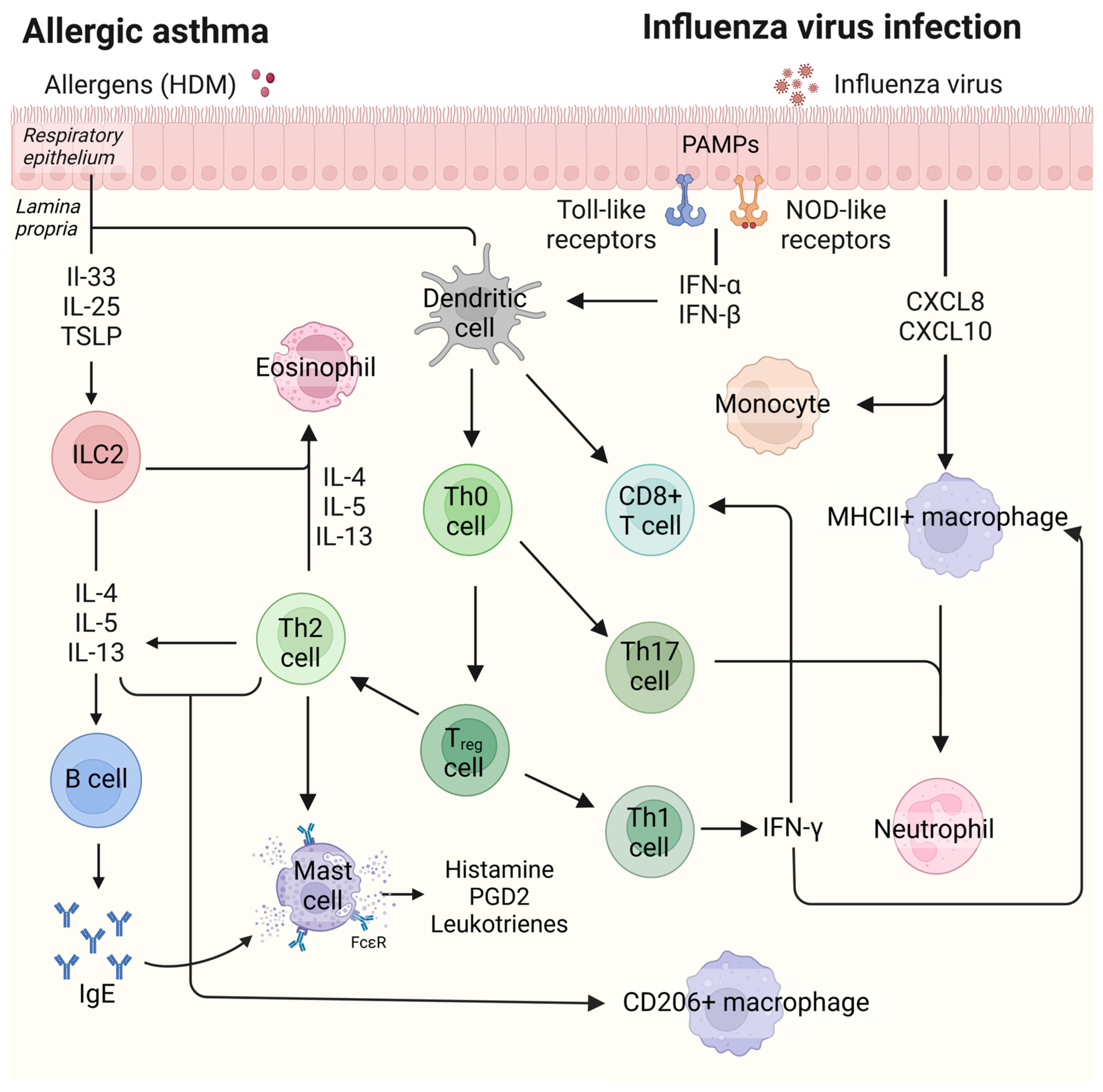
1. Introduction
2. Results
2.1. Both Influenza Infection and HDM Exposure Result in Weight Loss, and Animals Exposed to Both Lose the Most Weight
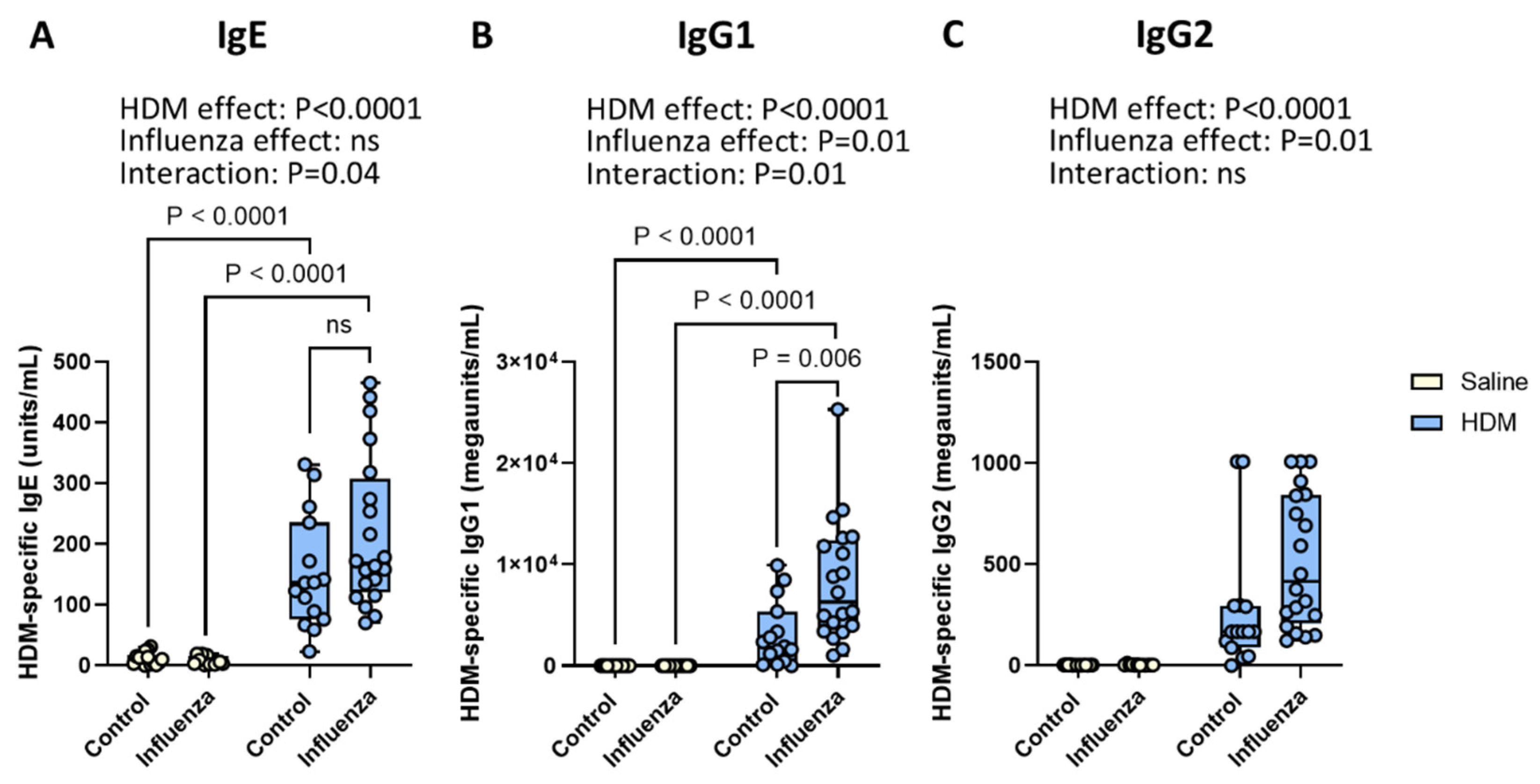
2.2. Exposure to HDM and Influenza Results in Higher Serum Levels of HDM-Specific IgE and IgG1 than Either Exposure Alone
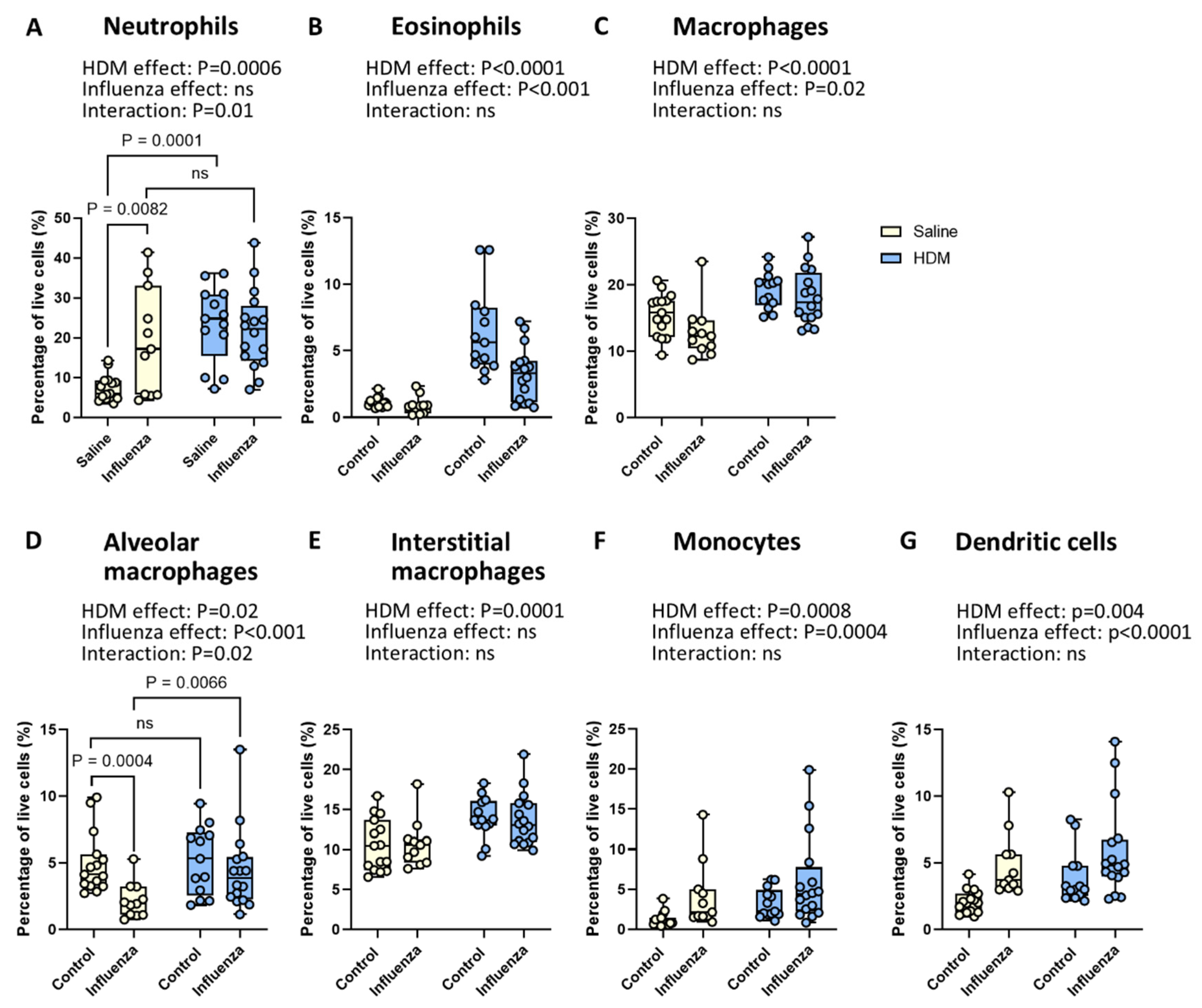
2.3. Exposure to HDM and Influenza Results in Fewer Neutrophils and More Alveolar Macrophages than Expected
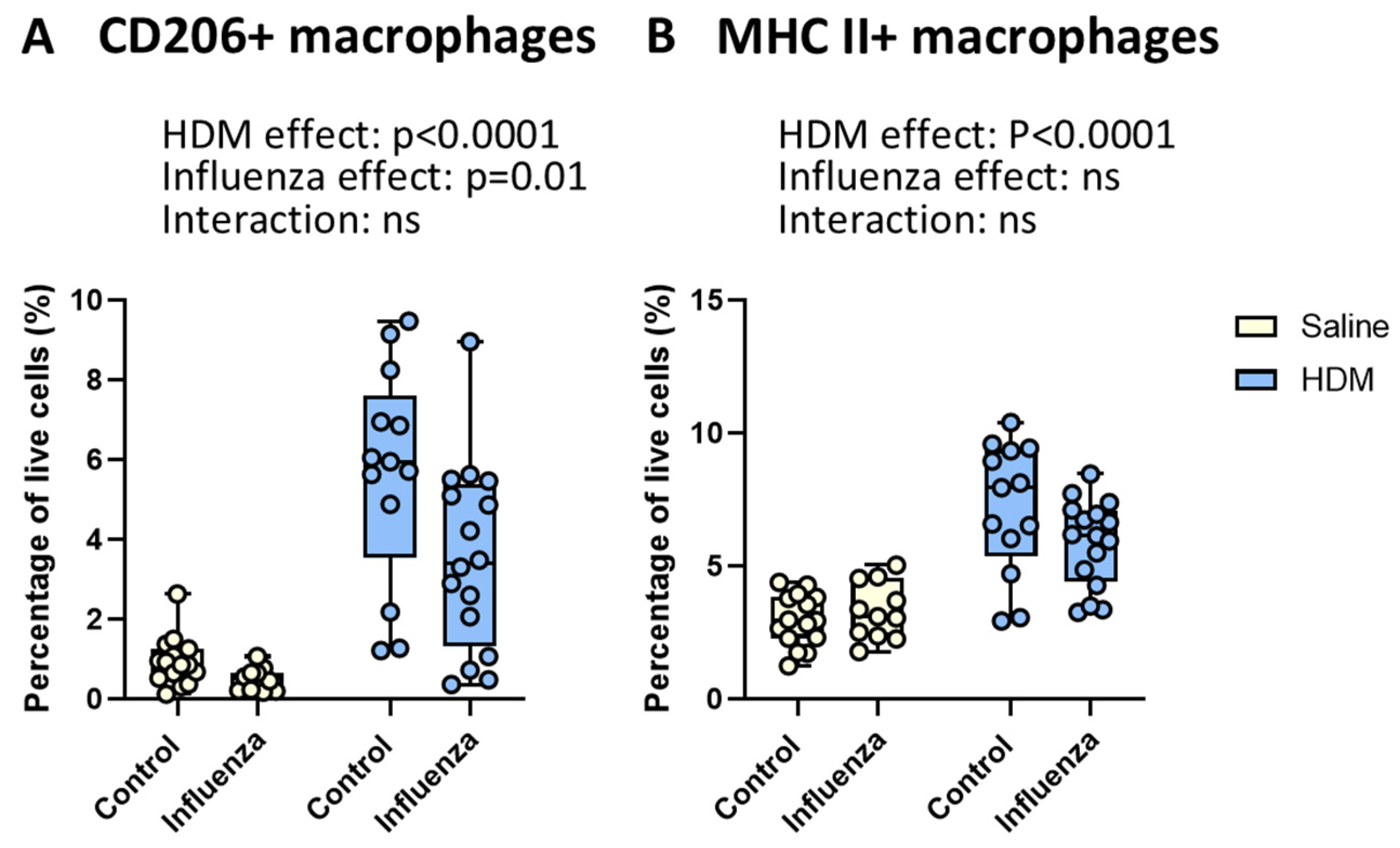
2.4. HDM Exposure Results in More, Whereas Influenza Infection Results in Fewer CD206+ Macrophages
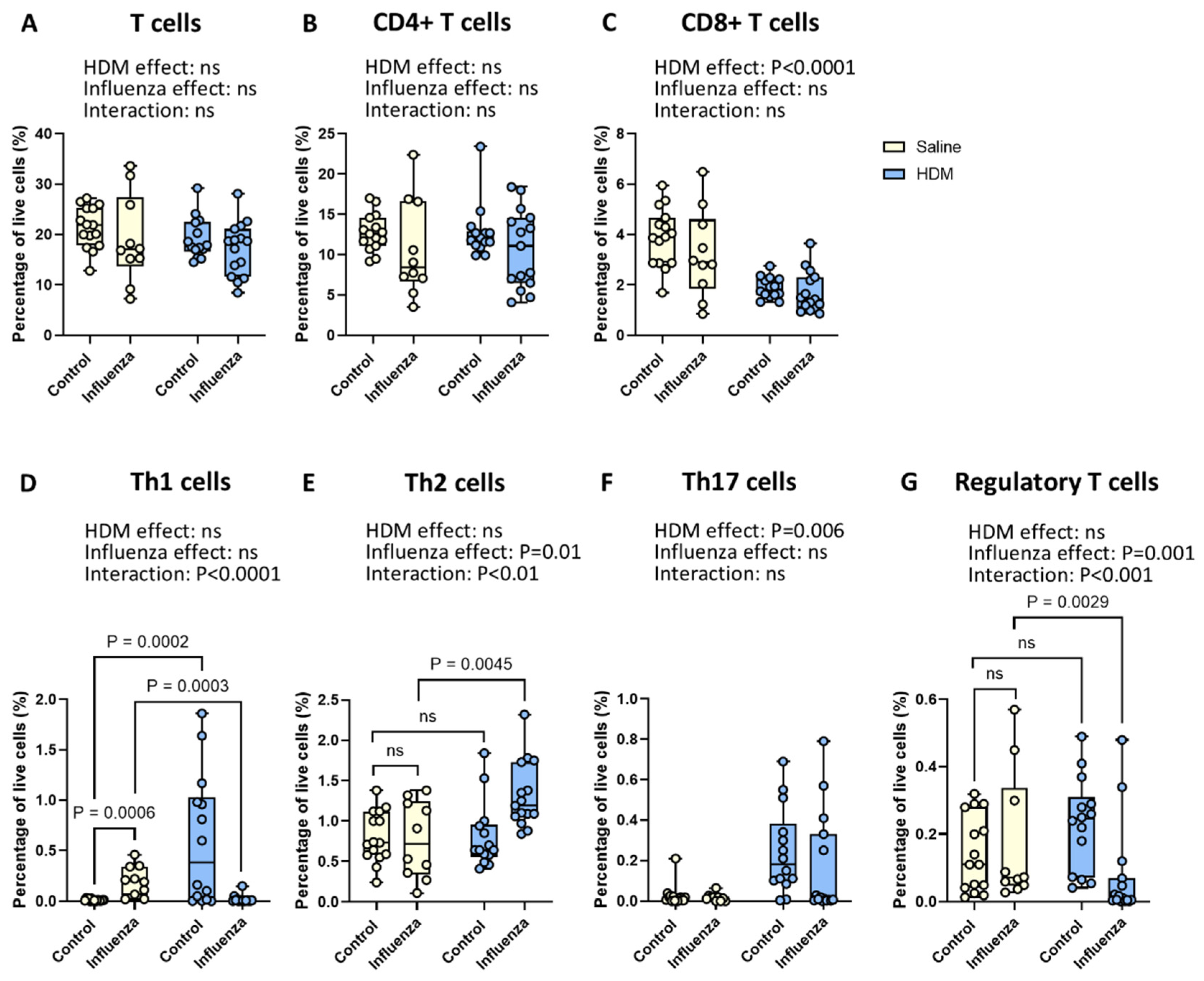
2.5. Exposure to HDM Plus Influenza Results in Fewer Pulmonary Th1 and Regulatory T Cells and More Th2 Cells than Either Exposure Alone
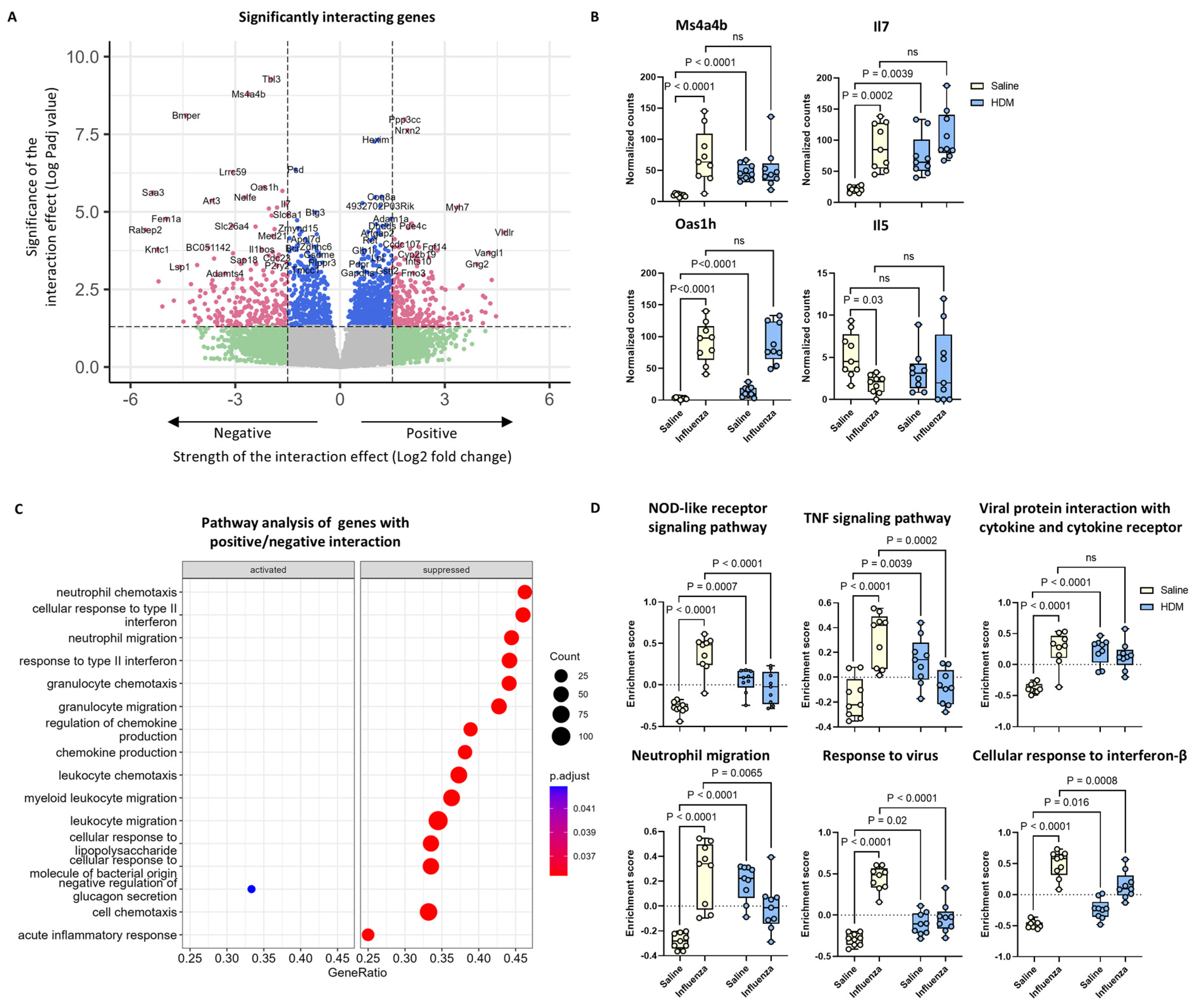
2.6. Th1 and Regulatory T Cell-Related Gene Expression Was Altered by the Interacting Effects of HDM and Influenza Virus Infection
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Flow Cytometry
4.4. Immunoglobulins Measurement
4.5. Bulk RNA Sequencing Analysis
4.6. Lung Virus Titration
4.7. Statistical Analysis
4.8. AI-Assisted Writing Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDM | House dust mite |
| HK | Hong Kong |
| RNA-Seq | RNA sequencing |
| IgE | Immunoglobulin E |
| IgG1 | Immunoglobulin G1 |
| IgG2 | Immunoglobulin G2 |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| Th17 | T helper 17 |
| IL-4 | Interleukin 4 |
| IL-5 | Interleukin 5 |
| IL-13 | Interleukin 13 |
| PCA | Principal component analysis |
| GSEA | Gene set enrichment analysis |
| GSVA | Gene set variation analysis |
| GEO | Gene Expression Omnibus |
| LoD | Limit of detection |
| Q–Q plot | Quantile–quantile plot |
| ANOVA | Analysis of variance |
| MHC II | Major histocompatibility complex class II |
| IFN-γ | Interferon gamma |
References
- Global Asthma Network. The Global Asthma Report 2022. Available online: www.globalasthmareport.org (accessed on 13 February 2025).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2023. Updated July 2023. Available online: www.ginasthma.org (accessed on 13 February 2025).
- Castillo, J.R.; Peters, S.P.; Busse, W.W. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J. Allergy Clin. Immunol. Pract. 2017, 5, 918–927. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Christodoulou, I.; Rohde, G.; Agache, I.; Almqvist, C.; Bruno, A.; Bonini, S.; Bont, L.; Bossios, A.; Bousquet, J.; et al. Viruses and Bacteria in Acute Asthma Exacerbations—A GA2 LEN-DARE Systematic Review. Allergy 2011, 66, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Bakakos, A.; Sotiropoulou, Z.; Vontetsianos, A.; Zaneli, S.; Papaioannou, A.I.; Bakakos, P. Epidemiology and Immunopathogenesis of Virus-Associated Asthma Exacerbations. J. Asthma Allergy 2023, 16, 1025–1040. [Google Scholar] [CrossRef]
- Whetstone, C.E.; Ranjbar, M.; Omer, H.; Cusack, R.P.; Gauvreau, G.M. The Role of Airway Epithelial Cell Alarmins in Asthma. Cells 2022, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Wallrapp, A.; Riesenfeld, S.J.; Burkett, P.R.; Kuchroo, V.K. Type 2 Innate Lymphoid Cells in the Induction and Resolution of Tissue Inflammation. Immunol. Rev. 2018, 286, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Harker, J.A.; Lloyd, C.M. T Helper 2 Cells in Asthma. J. Exp. Med. 2023, 220, e20221094. [Google Scholar] [CrossRef]
- Draijer, C.; Robbe, P.; Boorsma, C.; Hylkema, M.; Melgert, B. Characterization of Macrophage Phenotypes in Three Murine Models of House Dust Mite-Induced Asthma. Mediat. Inflamm. 2013, 2013, 632049. [Google Scholar] [CrossRef]
- Robbe, P.; Draijer, C.; Borg, T.; Luinge, M.; Timens, W.; Wouters, I.; Melgert, B.; Hylkema, M. Distinct Macrophage Phenotypes in Allergic and Nonallergic Lung Inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L358–L367. [Google Scholar] [CrossRef]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef]
- Thomas, R.; Qiao, S.; Yang, X. Th17/Treg Imbalance: Implications in Lung Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 4865. [Google Scholar] [CrossRef]
- Gambadauro, A.; Galletta, F.; Li Pomi, A.; Manti, S.; Piedimonte, G. Immune Response to Respiratory Viral Infections. Int. J. Mol. Sci. 2024, 25, 6178. [Google Scholar] [CrossRef]
- Lukhele, S.; Boukhaled, G.M.; Brooks, D.G. Type I Interferon Signaling, Regulation and Gene Stimulation in Chronic Virus Infection. Semin. Immunol. 2019, 43, 101277. [Google Scholar] [CrossRef]
- Tavares, L.P.; Teixeira, M.M.; Garcia, C.C. The Inflammatory Response Triggered by Influenza Virus: A Two-Edged Sword. Inflamm. Res. 2017, 66, 283–302. [Google Scholar] [CrossRef]
- Draijer, C.; Boorsma, C.E.; Robbe, P.; Timens, W.; Hylkema, M.N.; Ten Hacken, N.H.; van den Berge, M.; Postma, D.S.; Melgert, B.N. Human Asthma Is Characterized by More IRF5+ M1 and CD206+ M2 Macrophages and Less IL-10+ M2-like Macrophages Around Airways Compared with Healthy Airways. J. Allergy Clin. Immunol. 2017, 140, 280–283.e3. [Google Scholar] [CrossRef] [PubMed]
- Waithman, J.; Mintern, J.D. Dendritic Cells and Influenza A Virus Infection. Virulence 2012, 3, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Ravanetti, L.; Dijkhuis, A.; Sabogal Pineros, Y.S.; Bal, S.M.; Dierdorp, B.S.; Dekker, T.; Logiantara, A.; Adcock, I.M.; Rao, N.L.; Boon, L.; et al. An Early Innate Response Underlies Severe Influenza-Induced Exacerbations of Asthma in a Novel Steroid-Insensitive and Anti-IL-5-Responsive Mouse Model. Allergy 2017, 72, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Li, B.W.S.; de Bruijn, M.J.W.; Lukkes, M.; van Nimwegen, M.; Bergen, I.M.; KleinJan, A.; GeurtsvanKessel, C.H.; Andeweg, A.; Rimmelzwaan, G.F.; Hendriks, R.W. T Cells and ILC2s Are Major Effector Cells in Influenza-Induced Exacerbation of Allergic Airway Inflammation in Mice. Eur. J. Immunol. 2019, 49, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Nouri, H.R.; Schaunaman, N.; Kraft, M.; Li, L.; Numata, M.; Chu, H.W. Tollip Deficiency Exaggerates Airway Type 2 Inflammation in Mice Exposed to Allergen and Influenza A Virus: Role of the ATP/IL-33 Signaling Axis. Front. Immunol. 2023, 14, 1304758. [Google Scholar] [CrossRef] [PubMed]
- Shahangian, K.; Ngan, D.A.; Chen, H.H.R.; Oh, Y.; Tam, A.; Wen, J.; Cheung, C.; Knight, D.A.; Dorscheid, D.R.; Hackett, T.L.; et al. IL-4Rα Blockade Reduces Influenza-Associated Morbidity in a Murine Model of Allergic Asthma. Respir. Res. 2021, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Aloe, C.; McQualter, J.; Papanicolaou, A.; Vlahos, R.; Wilson, N.; Bozinovski, S. G-CSFR Antagonism Reduces Mucosal Injury and Airways Fibrosis in a Virus-Dependent Model of Severe Asthma. Br. J. Pharmacol. 2021, 178, 1869–1885. [Google Scholar] [CrossRef]
- Mori, H.; Parker, N.S.; Rodrigues, D.; Hulland, K.; Chappell, D.; Hincks, J.S.; Bright, H.; Evans, S.M.; Lamb, D.J. Differences in Respiratory Syncytial Virus and Influenza Infection in a House-Dust-Mite-Induced Asthma Mouse Model: Consequences for Steroid Sensitivity. Clin. Sci. 2013, 125, 565–574. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, C.; Lange, T.; Kolahian, S. Myeloid-Derived Suppressor Cells in Influenza Virus-Induced Asthma Exacerbation. Front. Immunol. 2024, 15, 1342497. [Google Scholar] [CrossRef] [PubMed]
- Martucci, C.; Allen, A.D.; Moretto, N.; Bagnacani, V.; Fioni, A.; Patacchini, R.; Civelli, M.; Villetti, G.; Facchinetti, F. CHF6297: A Novel Potent and Selective p38 MAPK Inhibitor with Robust Anti-Inflammatory Activity and Suitable for Inhaled Pulmonary Administration as Dry Powder. Front. Pharmacol. 2024, 15, 1343941, Erratum in Front. Pharmacol. 2024, 15, 1497520. https://doi.org/10.3389/fphar.2024.1497520. [Google Scholar] [CrossRef]
- Williams, J.W.; Tjota, M.Y.; Sperling, A.I. The Contribution of Allergen-Specific IgG to the Development of Th2-Mediated Airway Inflammation. J. Allergy 2012, 2012, 236075. [Google Scholar] [CrossRef]
- Draijer, C.; Robbe, P.; Boorsma, C.E.; Hylkema, M.N.; Melgert, B.N. Dual Role of YM1+ M2 Macrophages in Allergic Lung Inflammation. Sci. Rep. 2018, 8, 5105. [Google Scholar] [CrossRef]
- Vlasma, J.R.; van der Veen, T.A.; de Jager, M.H.; Nawijn, M.C.; Brandsma, C.A.; Melgert, B.N. Cigarette Smoking Prolongs Inflammation Associated with Influenza Infection and Delays Its Clearance in Mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 327, L634–L645. [Google Scholar] [CrossRef]
- Kalil, A.C.; Thomas, P.G. Influenza Virus-Related Critical Illness: Pathophysiology and Epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 Inflammation in Asthma—Present in Most, Absent in Many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The Basic Immunology of Asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Iikura, M.; Hojo, M.; Koketsu, R.; Watanabe, S.; Sato, A.; Chino, H.; Ro, S.; Masaki, H.; Hirashima, J.; Ishii, S.; et al. The Importance of Bacterial and Viral Infections Associated with Adult Asthma Exacerbations in Clinical Practice. PLoS ONE 2015, 10, e0123584. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, J.; Openshaw, P.; Jha, A.; Del Giacco, S.R.; Firinu, D.; Tsilochristou, O.; Roberts, G.; Selby, A.; Akdis, C.; Agache, I.; et al. Influenza Burden, Prevention, and Treatment in Asthma—A Scoping Review by the EAACI Influenza in Asthma Task Force. Allergy 2018, 73, 1151–1181. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Parisini, A.; Calzi, A.; Pallavicini, F.M.; Cassola, G.; Artioli, S.; Anselmo, M.; Pagano, G.; Rezza, G.; Viscoli, C.; et al. Risk Factors for Severe Complications of the Novel Influenza A (H1N1): Analysis of Patients Hospitalized in Italy. Clin. Microbiol. Infect. 2011, 17, 247–250. [Google Scholar] [CrossRef]
- Coverstone, A.M.; Wang, L.; Sumino, K. Beyond Respiratory Syncytial Virus and Rhinovirus in the Pathogenesis and Exacerbation of Asthma: The Role of Metapneumovirus, Bocavirus, and Influenza Virus. Immunol. Allergy Clin. N. Am. 2019, 39, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kamimoto, L.; Bramley, A.M.; Schmitz, A.M.; Benoit, S.R.; Louie, J.; Sugerman, D.E.; Druckenmiller, J.K.; Ritger, K.A.; Chugh, R.; et al. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April–June 2009. N. Engl. J. Med. 2009, 361, 1935–1944. [Google Scholar] [CrossRef]
- Feldman, L.Y.; Zhu, J.; To, T. Estimating Age-Specific Influenza-Associated Asthma Morbidity in Ontario, Canada. Respir. Med. 2019, 155, 104–112. [Google Scholar] [CrossRef]
- Jacquet, A. Innate Immune Responses in House Dust Mite Allergy. ISRN Allergy 2013, 2013, 735031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Betts, R.J.; Kemeny, D.M. CD8+ T Cells in Asthma: Friend or Foe? Pharmacol. Ther. 2009, 121, 123–131. [Google Scholar] [CrossRef]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Types of Influenza Viruses. 18 September 2024. Available online: https://www.cdc.gov/flu/about/viruses-types.html?CDC_AAref_Val=https://www.cdc.gov/flu/about/viruses/types.htm (accessed on 11 March 2025).
- Rodriguez-Ramirez, H.G.; Salinas-Carmona, M.C.; Barboza-Quintana, O.; Melo-de la Garza, A.; Ceceñas-Falcon, L.A.; Rangel-Martinez, L.M.; Rosas-Taraco, A.G. CD206+ Cell Number Differentiates Influenza A (H1N1)pdm09 from Seasonal Influenza A Virus in Fatal Cases. Mediat. Inflamm. 2014, 2014, 921054. [Google Scholar] [CrossRef]
- Ghoneim, H.E.; Thomas, P.G.; McCullers, J.A. Depletion of Alveolar Macrophages During Influenza Infection Facilitates Bacterial Superinfections. J. Immunol. 2013, 191, 1250–1259. [Google Scholar] [CrossRef]
- Duan, M.; Li, W.C.; Vlahos, R.; Maxwell, M.J.; Anderson, G.P.; Hibbs, M.L. Distinct Macrophage Subpopulations Characterize Acute Infection and Chronic Inflammatory Lung Disease. J. Immunol. 2012, 189, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.P.; Samarasinghe, A.E. The Role of Innate Leukocytes During Influenza Virus Infection. J. Immunol. Res. 2019, 2019, 8028725. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, A.E.; Melo, R.C.; Duan, S.; LeMessurier, K.S.; Liedmann, S.; Surman, S.L.; Lee, J.J.; Hurwitz, J.L.; Thomas, P.G.; McCullers, J.A. Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. J. Immunol. 2017, 198, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Sabogal Piñeros, Y.S.; Bal, S.M.; Dijkhuis, A.; Majoor, C.J.; Dierdorp, B.S.; Dekker, T.; Hoefsmit, E.P.; Bonta, P.I.; Picavet, D.; van der Wel, N.N.; et al. Eosinophils Capture Viruses, a Capacity That Is Defective in Asthma. Allergy 2019, 74, 1898–1909. [Google Scholar] [CrossRef]
- Giurgea, L.T.; Cervantes-Medina, A.; Walters, K.A.; Scherler, K.; Han, A.; Czajkowski, L.M.; Baus, H.A.; Hunsberger, S.; Klein, S.L.; Kash, J.C.; et al. Sex Differences in Influenza: The Challenge Study Experience. J. Infect. Dis. 2022, 225, 715–722. [Google Scholar] [CrossRef]
- Robinson, D.P.; Lorenzo, M.E.; Jian, W.; Klein, S.L. Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses. PLoS Pathog. 2011, 7, e1002149. [Google Scholar] [CrossRef]
- Lorenzo, M.E.; Hodgson, A.; Robinson, D.P.; Kaplan, J.B.; Pekosz, A.; Klein, S.L. Antibody Responses and Cross Protection Against Lethal Influenza A Viruses Differ Between the Sexes in C57BL/6 Mice. Vaccine 2011, 29, 9246–9255. [Google Scholar] [CrossRef]
- Vermillion, M.S.; Ursin, R.L.; Kuok, D.I.T.; Vom Steeg, L.G.; Wohlgemuth, N.; Hall, O.J.; Fink, A.L.; Sasse, E.; Nelson, A.; Ndeh, R.; et al. Production of Amphiregulin and Recovery from Influenza Is Greater in Males Than Females. Biol. Sex Differ. 2018, 9, 24. [Google Scholar] [CrossRef]
- Fink, A.L.; Engle, K.; Ursin, R.L.; Tang, W.Y.; Klein, S.L. Biological Sex Affects Vaccine Efficacy and Protection Against Influenza in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12477–12482. [Google Scholar] [CrossRef]
- Vom Steeg, L.G.; Dhakal, S.; Woldetsadik, Y.A.; Park, H.S.; Mulka, K.R.; Reilly, E.C.; Topham, D.J.; Klein, S.L. Androgen Receptor Signaling in the Lungs Mitigates Inflammation and Improves the Outcome of Influenza in Mice. PLoS Pathog. 2020, 16, e1008506. [Google Scholar] [CrossRef] [PubMed]
- Eshima, N.; Tokumaru, O.; Hara, S.; Bacal, K.; Korematsu, S.; Tabata, M.; Karukaya, S.; Yasui, Y.; Okabe, N.; Matsuishi, T. Sex- and Age-Related Differences in Morbidity Rates of 2009 Pandemic Influenza A H1N1 Virus of Swine Origin in Japan. PLoS ONE 2011, 6, e19409. [Google Scholar] [CrossRef]
- Kumar, A.; Zarychanski, R.; Pinto, R.; Cook, D.J.; Marshall, J.; Lacroix, J.; Stelfox, T.; Bagshaw, S.; Choong, K.; Lamontagne, F.; et al. Critically Ill Patients with 2009 Influenza A(H1N1) Infection in Canada. JAMA 2009, 302, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Luscombe, G.M.; Hawke, C. Influenza Infections in Australia 2009–2015: Is There a Combined Effect of Age and Sex on Susceptibility to Virus Subtypes? BMC Infect. Dis. 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Mertz, D.; Lo, C.K.; Lytvyn, L.; Ortiz, J.R.; Loeb, M.; FLURISK-INVESTIGATORS. Pregnancy as a Risk Factor for Severe Influenza Infection: An Individual Participant Data Meta-Analysis. BMC Infect. Dis. 2019, 19, 683. [Google Scholar] [CrossRef]
- World Health Organization. Sex, Gender and Influenza; WHO: Geneva, Switzerland, 2010.
- Kliem, C.V.; Schaub, B. The Role of Regulatory B Cells in Immune Regulation and Childhood Allergic Asthma. Mol. Cell. Pediatr. 2024, 11, 1. [Google Scholar] [CrossRef]
- Bodewes, R.; Rimmelzwaan, G.F.; Osterhaus, A.D. Animal Models for the Preclinical Evaluation of Candidate Influenza Vaccines. Expert Rev. Vaccines 2010, 9, 59–72. [Google Scholar] [CrossRef]
- Draijer, C.; Florez-Sampedro, L.; Reker-Smit, C.; Post, E.; van Dijk, F.; Melgert, B.N. Explaining the Polarized Macrophage Pool During Murine Allergic Lung Inflammation. Front. Immunol. 2022, 13, 1056477. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhu, A.; Ibrahim, J.G.; Love, M.I. Heavy-Tailed Prior Distributions for Sequence Count Data: Removing the Noise and Preserving Large Differences. Bioinformatics 2019, 35, 2084–2092. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene Set Variation Analysis for Microarray and RNA-Seq Data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Audouy, S.A.; van der Schaaf, G.; Hinrichs, W.L.; Frijlink, H.W.; Wilschut, J.; Huckriede, A. Development of a Dried Influenza Whole Inactivated Virus Vaccine for Pulmonary Immunization. Vaccine 2011, 29, 4345–4352. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; van der Veen, T.A.; de Groot, L.E.S.; de Jager, M.H.; Lan, A.; Baarsma, H.A.; Lutter, R.; van der Graaf, K.; Gosens, R.; Schmidt, M.; et al. Pre-Existing Allergic Inflammation Alters Both Innate and Adaptive Immune Responses in Mice Co-Infected with Influenza Virus. Int. J. Mol. Sci. 2025, 26, 3483. https://doi.org/10.3390/ijms26083483
Li D, van der Veen TA, de Groot LES, de Jager MH, Lan A, Baarsma HA, Lutter R, van der Graaf K, Gosens R, Schmidt M, et al. Pre-Existing Allergic Inflammation Alters Both Innate and Adaptive Immune Responses in Mice Co-Infected with Influenza Virus. International Journal of Molecular Sciences. 2025; 26(8):3483. https://doi.org/10.3390/ijms26083483
Chicago/Turabian StyleLi, Dan, T. Anienke van der Veen, Linsey E. S. de Groot, Marina H. de Jager, Andy Lan, Hoeke A. Baarsma, René Lutter, Kees van der Graaf, Reinoud Gosens, Martina Schmidt, and et al. 2025. "Pre-Existing Allergic Inflammation Alters Both Innate and Adaptive Immune Responses in Mice Co-Infected with Influenza Virus" International Journal of Molecular Sciences 26, no. 8: 3483. https://doi.org/10.3390/ijms26083483
APA StyleLi, D., van der Veen, T. A., de Groot, L. E. S., de Jager, M. H., Lan, A., Baarsma, H. A., Lutter, R., van der Graaf, K., Gosens, R., Schmidt, M., & Melgert, B. N. (2025). Pre-Existing Allergic Inflammation Alters Both Innate and Adaptive Immune Responses in Mice Co-Infected with Influenza Virus. International Journal of Molecular Sciences, 26(8), 3483. https://doi.org/10.3390/ijms26083483






