Myocardial Revascularization in Patients with Diabetes and Heart Failure—A Narrative Review
Abstract
:1. Heart Failure: Definition, Epidemiology, and Types
2. Pathophysiological and Molecular Mechanisms of Ischemic Heart Failure
3. Diabetes Mellitus: Definition, Types, and Epidemiology
4. Heart Failure and Diabetes Mellitus
5. Diabetes Mellitus, Atherosclerosis, and Revascularization Failure
5.1. Endothelial Dysfunction and Inflammation
5.2. Platelet Activation and Advanced Glycation End Products
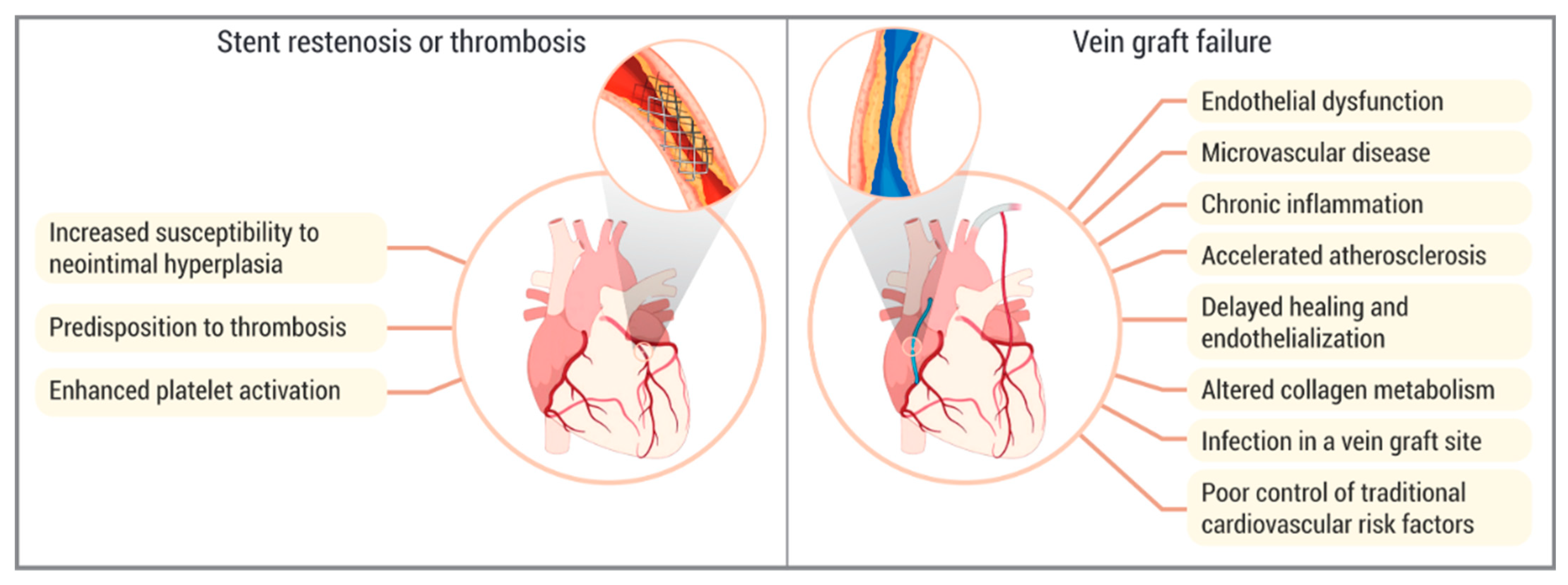
5.3. Neointimal Hyperplasia and In-Stent Restenosis
5.4. Stent Thrombosis
5.5. Vein Graft Failure
6. The Aim
7. Key Clinical Trials
| Study Name | STICH/STICHES | REVIVED-BCIS2 | FREEDOM | CARDia | BARI | SYNTAX | COURAGE | ISCHEMIA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of first published results | 2011 [49]/2016 [51] | 2022 [53] | 2012 [55] | 2010 [62] | 1996 [64] | 2009 [68] | 2007 [73] | 2020 [74] | ||||||||
| Primary outcome | Death from any cause | Death from any cause or hospitalization for HF | MACCE * | MACCE ** | All-cause mortality (1); Q-wave-myocardial-infarction-free survival rate (2) | MACCE *** | Death from any cause or nonfatal myocardial infarction | Death from cardiovascular causes, myocardial infarction, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest | ||||||||
| Number of participants | 1212 | 700 | 1900 | 600 | 1829 | 1800 | 2287 | 5179 | ||||||||
| Population | CAD + HF | CAD + HF | MVCAD + DM | CAD + DM | MVCAD | Three-vessel and/or left main CAD | Stable CAD | Stable CAD | ||||||||
| Treatment modality | CABG + OMT | OMT | PCI + OMT | OMT | CABG | PCI | CABG | PCI | CABG | PCI/POBA | CABG | PCI | PCI + OMT | OMT | CABG/PCI + OMT | OMT |
| Severe LV dysfunction (%) | 100 | 100 | 100 | 100 | 1.7 | 3.3 | 16.4 | 20.3 | Unknown | Unknown | 1.3 | 2.5 | Exclusion criterion | Exclusion criterion | ||
| DM (%) | 39 | 40 | 39 | 43 | 100 | 100 | 100 | 100 | 25/20 on treatment | 24/19 on treatment | 24.6 | 25.6 | 32 | 35 | 41.4 | 42.2 |
| Primary outcome occurrence (%) | 58.9 | 66.1 | 37.2 | 38.0 | 18.7 | 26.6 | 10.5 | 13.0 | 10.7 (1); 80.4 (2) | 13.7 (1); 78.7 (2) | 26.9 | 37.3 | 19.0 | 18.5 | 12.3 | 13.6 |
| p value | 0.12 (5-yYear FU) [49]/0.02 (10-yYear FU) [51] | 0.96 (2-yYear FU) [53] | 0.005 (5-yYear FU) [55] | 0.393 (1-yYear FU) [62] | 0.19 (1); 0.84 (2) (5-yYear FU) [64] | <0.0001 (5-yYear FU) [70] | 0.62 (4.6-yYear FU) [73] | 0.34 (3.2-yYear FU) [74] | ||||||||
8. Special Populations
8.1. Advanced Age
8.2. Left Main Coronary Artery Disease
8.3. Chronic Kidney Disease
8.4. Peripheral Artery Disease
8.5. Chronic Obstructive Pulmonary Disease
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baman, J.R.; Ahmad, F.S. Heart Failure. JAMA 2020, 324, 1015. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287, Erratum in: Cardiovasc. Res. 2023, 119, 1453. https://doi.org/10.1093/cvr/cvad026. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischemia/reperfusion: Translational pathophysiology of ischemic heart disease. Med 2024, 5, 10–31. [Google Scholar] [CrossRef] [PubMed]
- Canty, J.M., Jr. Myocardial injury, troponin release, and cardiomyocyte death in brief ischemia, failure, and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1–H15. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E. Hypoxic Stress-Dependent Regulation of Na,K-ATPase in Ischemic Heart Disease. Int. J. Mol. Sci. 2023, 24, 7855. [Google Scholar] [CrossRef]
- Stanley, W.C. Myocardial energy metabolism during ischemia and the mechanisms of metabolic therapies. J. Cardiovasc. Pharmacol. Ther. 2004, 9 (Suppl. S1), S31–S45. [Google Scholar] [CrossRef] [PubMed]
- Humeres, C.; Venugopal, H.; Frangogiannis, N.G. Smad-dependent pathways in the infarcted and failing heart. Curr. Opin. Pharmacol. 2022, 64, 102207. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Dong, Z.; Lu, X.; Hong, W.; Liu, J.; Zou, X.; Gao, J.; Jiang, H.; Sun, X.; et al. An ischemic area-targeting, peroxynitrite-responsive, biomimetic carbon monoxide nanogenerator for preventing myocardial ischemia-reperfusion injury. Bioact. Mater. 2023, 28, 480–494. [Google Scholar] [CrossRef]
- An, Y.; Wang, X.; Guan, X.; Yuan, P.; Liu, Y.; Wei, L.; Wang, F.; Qi, X. Endoplasmic reticulum stress-mediated cell death in cardiovascular disease. Cell Stress Chaperones 2024, 29, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nishida, K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc. Res. 2009, 81, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khanbabapour Sasi, A.; Hussen, B.M.; Shoorei, H.; Siddiq, A.; Taheri, M.; Ayatollahi, S.A. Interplay between PI3K/AKT pathway and heart disorders. Mol. Biol. Rep. 2022, 49, 9767–9781. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Du, T.; Long, T.; Liao, X.; Dong, Y.; Huang, Z.P. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct. Target Ther. 2022, 7, 134. [Google Scholar] [CrossRef]
- Maryam, P.D.; Varghese, T.P.; Tazneem, B. Unraveling the complex pathophysiology of heart failure: Insights into the role of renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS). Curr. ProblCardiol. 2024, 49, 102411. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69, Erratum in: Diabetes Care 2010, 33, e57. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 26 November 2024).
- Aronson, D.; Edelman, E.R. Revascularization for coronary artery disease in diabetes mellitus: Angioplasty, stents and coronary artery bypass grafting. Rev. Endocr. Metab. Disord. 2010, 11, 75–86. [Google Scholar] [CrossRef]
- Schramm, T.K.; Gislason, G.H.; Køber, L.; Rasmussen, S.; Rasmussen, J.N.; Abildstrøm, S.Z.; Hansen, M.L.; Folke, F.; Buch, P.; Madsen, M.; et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: A population study of 3.3 million people. Circulation 2008, 117, 1945–1954. [Google Scholar] [CrossRef]
- Bangalore, S.; Kumar, S.; Fusaro, M.; Amoroso, N.; Kirtane, A.J.; Byrne, R.A.; Williams, D.O.; Slater, J.; Cutlip, D.E.; Feit, F. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: Mixed treatment comparison analysis of 22, 844 patient years of follow-up from randomized trials. BMJ 2012, 345, e5170. [Google Scholar] [CrossRef]
- Li, X.; Zhou, X.; Gao, L. Diabetes and Heart Failure: A Literature Review, Reflection and Outlook. Biomedicines 2024, 12, 1572. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Iacoviello, M. Diabetes leading to heart failure and heart failure leading to diabetes: Epidemiological and clinical evidence. Heart Fail. Rev. 2023, 28, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Al Madhoun, A. Teneligliptin: A potential therapeutic approach for diabetic cardiomyopathy. World J. Diabetes 2024, 15, 1654–1658. [Google Scholar] [CrossRef]
- Rosano, G.M.; Vitale, C.; Seferovic, P. Heart Failure in Patients with Diabetes Mellitus. Card. Fail. Rev. 2017, 3, 52–55. [Google Scholar] [CrossRef]
- Nagoshi, T.; Yoshimura, M.; Rosano, G.M.; Lopaschuk, G.D.; Mochizuki, S. Optimization of cardiac metabolism in heart failure. Curr. Pharm. Des. 2011, 17, 3846–3853. [Google Scholar] [CrossRef]
- Rosano, G.M.; Fini, M.; Caminiti, G.; Barbaro, G. Cardiac metabolism in myocardial ischemia. Curr. Pharm. Des. 2008, 14, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Yokoyama, M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arter. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Verbeuren, T.J.; Van de Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Hartge, M.M.; Unger, T.; Kintscher, U. The endothelium and vascular inflammation in diabetes. Diab Vasc. Dis. Res. 2007, 4, 84–88. [Google Scholar] [CrossRef]
- Vinik, A.I.; Erbas, T.; Park, T.S.; Nolan, R.; Pittenger, G.L. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001, 24, 1476–1485. [Google Scholar] [CrossRef]
- Bierhaus, A.; Nawroth, P.P. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 2009, 52, 2251–2263. [Google Scholar] [CrossRef]
- Kornowski, R.; Mintz, G.S.; Kent, K.M.; Pichard, A.D.; Satler, L.F.; Bucher, T.A.; Hong, M.K.; Popma, J.J.; Leon, M.B. Increased restenosis in diabetes mellitus after coronary interventions is due to exaggerated intimal hyperplasia. A serial intravascular ultrasound study. Circulation 1997, 95, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Terashima, M.; Rathore, S.; Itoh, T.; Habara, M.; Nasu, K.; Kimura, M.; Itoh, T.; Kinoshita, Y.; Ehara, M.; et al. Different patterns of vascular response between patients with or without diabetes mellitus after drug-eluting stent implantation: Optical coherence tomographic analysis. JACC Cardiovasc. Interv. 2010, 3, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Colwell, J.A.; Nesto, R.W. The platelet in diabetes: Focus on prevention of ischemic events. Diabetes Care 2003, 26, 2181–2188. [Google Scholar] [CrossRef]
- De Vries, M.R.; Simons, K.H.; Jukema, J.W.; Braun, J.; Quax, P.H. Vein graft failure: From pathophysiology to clinical outcomes. Nat. Rev. Cardiol. 2016, 13, 451–470. [Google Scholar] [CrossRef]
- Ward, A.O.; Caputo, M.; Angelini, G.D.; George, S.J.; Zakkar, M. Activation and inflammation of the venous endothelium in vein graft disease. Atherosclerosis 2017, 265, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Gasper, W.J.; Rahman, A.S.; Conte, M.S. Vein graft failure. J. Vasc. Surg. 2015, 61, 203–216. [Google Scholar] [CrossRef]
- De Vries, M.R.; Quax, P.H.A. Inflammation in Vein Graft Disease. Front. Cardiovasc. Med. 2018, 5, 3. [Google Scholar] [CrossRef]
- Sarjeant, J.M.; Rabinovitch, M. Understanding and treating vein graft atherosclerosis. Cardiovasc. Pathol. 2002, 11, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Xenogiannis, I.; Zenati, M.; Bhatt, D.L.; Rao, S.V.; Rodés-Cabau, J.; Goldman, S.; Shunk, K.A.; Mavromatis, K.; Banerjee, S.; Alaswad, K.; et al. Saphenous Vein Graft Failure: From Pathophysiology to Prevention and Treatment Strategies. Circulation 2021, 144, 728–745. [Google Scholar] [CrossRef]
- Parang, P.; Arora, R. Coronary vein graft disease: Pathogenesis and prevention. Can. J. Cardiol. 2009, 25, e57–e62. [Google Scholar] [CrossRef]
- Lee, R.; Margaritis, M.; Channon, K.M.; Antoniades, C. Evaluating oxidative stress in human cardiovascular disease: Methodological aspects and considerations. Curr. Med. Chem. 2012, 19, 2504–2520. [Google Scholar] [CrossRef]
- Bulkley, B.H.; Hutchins, G.M. Acute postoperative graft phlebitis: A rare cause of saphenous vein-coronary artery bypass failure. Am. Heart J. 1978, 95, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Llerena-Velastegui, J.; Zumbana-Podaneva, K.; Velastegui-Zurita, S.; Mejia-Mora, M.; Perez-Tomassetti, J.; Cabrera-Cruz, A.; Haro-Arteaga, P.; de Jesus, A.C.F.S.; Coelho, P.M.; Sanahuja-Montiel, C. Comparative Efficacy of Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting in the Treatment of Ischemic Heart Disease: A Systematic Review and Meta-Analysis of Recent Randomized Controlled Trials. Cardiol. Res. 2024, 15, 153–168. [Google Scholar] [CrossRef]
- Feng, S.; Li, M.; Fei, J.; Dong, A.; Zhang, W.; Fu, Y.; Zhao, Y. Ten-year outcomes after percutaneous coronary intervention versus coronary artery bypass grafting for multivessel or left main coronary artery disease: A systematic review and meta-analysis. J. Cardiothorac. Surg. 2023, 18, 54. [Google Scholar] [CrossRef]
- Xie, Q.; Huang, J.; Zhu, K.; Chen, Q. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with coronary heart disease and type 2 diabetes mellitus: Cumulative meta-analysis. Clin. Cardiol. 2021, 44, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.X.; Yan, J.; Wang, M.Y.; Chen, R.; Luo, J.Y.; Li, X.M.; Xie, X.; Ma, Y.T. Effects of Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting on Clinical Outcomes in Patients with Reduced Ejection Fraction Heart Failure and Coronary Heart Disease: A Meta-Analysis. Heart Surg. Forum 2023, 26, E062–E073. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Lee, K.L.; O’Connor, C.M.; Oh, J.K.; Bonow, R.O.; Pohost, G.M.; Feldman, A.M.; Mark, D.B.; Panza, J.A.; Sopko, G.; et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J. Thorac. Cardiovasc. Surg. 2007, 134, 1540–1547. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef]
- MacDonald, M.R.; She, L.; Doenst, T.; Binkley, P.F.; Rouleau, J.L.; Tan, R.S.; Lee, K.L.; Miller, A.B.; Sopko, G.; Szalewska, D.; et al. Clinical characteristics and outcomes of patients with and without diabetes in the Surgical Treatment for Ischemic Heart Failure (STICH) trial. Eur. J. Heart Fail. 2015, 17, 725–734. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; Petrie, M.C.; Greenwood, J.P.; O’Kane, P.D.; Evans, R.; Sculpher, M.; Mcdonagh, T.; Gershlick, A.; de Belder, M.; et al. Percutaneous Revascularization for Ischemic Ventricular Dysfunction: Rationale and Design of the REVIVED-BCIS2 Trial: Percutaneous Coronary Intervention for Ischemic Cardiomyopathy. JACC Heart Fail. 2018, 6, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Farkouh, M.E.; Dangas, G.; Leon, M.B.; Smith, C.; Nesto, R.; Buse, J.B.; Cohen, D.J.; Mahoney, E.; Sleeper, L.; King, S., 3rd; et al. Design of the Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) Trial. Am. Heart J. 2008, 155, 215–223. [Google Scholar] [CrossRef]
- Farkouh, M.E.; Domanski, M.; Sleeper, L.A.; Siami, F.S.; Dangas, G.; Mack, M.; Yang, M.; Cohen, D.J.; Rosenberg, Y.; Solomon, D.; et al. Strategies for multivessel revascularization in patients with diabetes. N. Engl. J. Med. 2012, 367, 2375–2384. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165, Erratum in: Eur. Heart J. 2019, 40, 3096. https://doi.org/10.1093/eurheartj/ehz507. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17, Erratum in: Circulation 2022, 145, e771. https://doi.org/10.1161/CIR.0000000000001061. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119, Erratum in: Circulation 2023, 148, e148. https://doi.org/10.1161/CIR.0000000000001183. Erratum in: Circulation 2023, 148, e186. https://doi.org/10.1161/CIR.0000000000001195. [Google Scholar] [CrossRef]
- Ramanathan, K.; Abel, J.G.; Park, J.E.; Fung, A.; Mathew, V.; Taylor, C.M.; Mancini, G.B.J.; Gao, M.; Ding, L.; Verma, S.; et al. Surgical Versus Percutaneous Coronary Revascularization in Patients with Diabetes and Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2017, 70, 2995–3006. [Google Scholar] [CrossRef]
- Kapur, A.; Malik, I.S.; Bagger, J.P.; Anderson, J.R.; Kooner, J.S.; Thomas, M.; Punjabi, P.; Mayet, J.; Millane, T.; Goedicke, J.; et al. The Coronary Artery Revascularisation in Diabetes (CARDia) trial: Background, aims, and design. Am. Heart J. 2005, 149, 13–19. [Google Scholar] [CrossRef]
- Kapur, A.; Hall, R.J.; Malik, I.S.; Qureshi, A.C.; Butts, J.; de Belder, M.; Baumbach, A.; Angelini, G.; de Belder, A.; Oldroyd, K.G.; et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J. Am. Coll. Cardiol. 2010, 55, 432–440. [Google Scholar] [CrossRef]
- Frye, R.L.; Sopko, G.; Detre, K.M. The BARI trial: Baseline observations. The BARI Investigators. Trans. Am. Clin. Climatol. Assoc. 1993, 104, 26–30. [Google Scholar] [PubMed]
- Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N. Engl. J. Med. 1996, 335, 217–225. [Google Scholar] [CrossRef]
- BARI Investigators. The final 10-year follow-up results from the BARI randomized trial. J. Am. Coll. Cardiol. 2007, 49, 1600–1606. [Google Scholar] [CrossRef]
- Ong, A.T.; Serruys, P.W.; Mohr, F.W.; Morice, M.C.; Kappetein, A.P.; Holmes, D.R., Jr.; Mack, M.J.; van den Brand, M.; Morel, M.A.; van Es, G.A.; et al. The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: Design, rationale, and run-in phase. Am. Heart J. 2006, 151, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Sianos, G.; Morel, M.A.; Kappetein, A.P.; Morice, M.C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005, 1, 219–227. [Google Scholar]
- Serruys, P.W.; Morice, M.C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; van den Brand, M.; Bass, E.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972, Erratum in: N. Engl. J. Med. 2013, 368, 584. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Feldman, T.E.; Mack, M.J.; Morice, M.C.; Holmes, D.R.; Ståhle, E.; Dawkins, K.D.; Mohr, F.W.; Serruys, P.W.; Colombo, A. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur. Heart J. 2011, 32, 2125–2134. [Google Scholar] [CrossRef]
- Mohr, F.W.; Morice, M.C.; Kappetein, A.P.; Feldman, T.E.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Morel, M.A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Thuijs, D.J.F.M.; Kappetein, A.P.; Serruys, P.W.; Mohr, F.W.; Morice, M.C.; Mack, M.J.; Holmes, D.R., Jr.; Curzen, N.; Davierwala, P.; Noack, T.; et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019, 394, 1325–1334, Erratum in: Lancet 2020, 395, 870. https://doi.org/10.1016/S0140-6736(20)30249-X. [Google Scholar] [CrossRef]
- Wang, R.; Serruys, P.W.; Gao, C.; Hara, H.; Takahashi, K.; Ono, M.; Kawashima, H.; O’leary, N.; Holmes, D.R.; Witkowski, A.; et al. Ten-year all-cause death after percutaneous or surgical revascularization in diabetic patients with complex coronary artery disease. Eur. Heart J. 2021, 43, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Varghese, K.S.; Fusco, P.J.; Mathew, D.M.; Mathew, S.M.; Ahmed, S.; Rogando, D.O.; Salazar, S.A.; Pandey, R.; Awad, A.K.; et al. Coronary Revascularization in Patients with Diabetes: A Meta-Analysis of Randomized Controlled Trials and Propensity-Matched Studies. Innovations 2023, 18, 29–40. [Google Scholar] [CrossRef]
- Al-Sadawi, M.; Tao, M.; Dhaliwal, S.; Radakrishnan, A.; Liu, Y.; Gier, C.; Masson, R.; Rahman, T.; Tam, E.; Mann, N. Utility of coronary revascularization in patients with ischemic left ventricular dysfunction. Cardiovasc. Revasc Med. 2024, 65, 88–97. [Google Scholar] [CrossRef]
- Pei, J.; Wang, X.; Xing, Z.; Zheng, K.; Hu, X. Short-term and long-term outcomes of revascularization interventions for patients with severely reduced left ventricular ejection fraction: A meta-analysis. ESC Heart Fail. 2021, 8, 634–643. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.; Hua, K.; Liu, T.S.; Zhou, S.Y.; Zhou, N.; Zhang, H.J. Outcomes of Coronary Artery Bypass Graft Surgery Versus Percutaneous Coronary Intervention in Patients Aged 18-45 Years with Diabetes Mellitus. Chin. Med. J. 2017, 130, 2906–2915. [Google Scholar] [CrossRef]
- Tam, D.Y.; Dharma, C.; Rocha, R.; Farkouh, M.E.; Abdel-Qadir, H.; Sun, L.Y.; Wijeysundera, H.C.; Austin, P.C.; Udell, J.A.; Gaudino, M.; et al. Long-Term Survival After Surgical or Percutaneous Revascularization in Patients with Diabetes and Multivessel Coronary Disease. J. Am. Coll. Cardiol. 2020, 76, 1153–1164. [Google Scholar] [CrossRef]
- Bianco, V.; Kilic, A.; Mulukutla, S.R.; Gleason, T.G.; Kliner, D.; Aranda-Michel, E.; Brown, J.A.; Wang, Y.; Allen, C.C.; Habertheuer, A.; et al. Coronary Artery Bypass Grafting vs. Percutaneous Coronary Intervention in Patients with Diabetes. Semin. Thorac. Cardiovasc. Surg. 2021, 33, 368–377. [Google Scholar] [CrossRef]
- Nagendran, J.; Norris, C.M.; Graham, M.M.; Ross, D.B.; Macarthur, R.G.; Kieser, T.M.; Maitland, A.M.; Southern, D.; Meyer, S.R. Coronary revascularization for patients with severe left ventricular dysfunction. Ann. Thorac. Surg. 2013, 96, 2038–2044. [Google Scholar] [CrossRef]
- Iribarne, A.; DiScipio, A.W.; Leavitt, B.J.; Baribeau, Y.R.; McCullough, J.N.; Weldner, P.W.; Huang, Y.L.; Robich, M.P.; Clough, R.A.; Sardella, G.L.; et al. Comparative effectiveness of coronary artery bypass grafting versus percutaneous coronary intervention in a real-world Surgical Treatment for Ischemic Heart Failure trial population. J. Thorac. Cardiovasc. Surg 2018, 156, 1410–1421.e2. [Google Scholar] [CrossRef]
- Sun, L.Y.; Gaudino, M.; Chen, R.J.; Bader Eddeen, A.; Ruel, M. Long-term Outcomes in Patients with Severely Reduced Left Ventricular Ejection Fraction Undergoing Percutaneous Coronary Intervention vs. Coronary Artery Bypass Grafting. JAMA Cardiol. 2020, 5, 631–641, Erratum in: JAMA Cardiol. 2020, 5, 732. https://doi.org/10.1001/jamacardio.2020.1655. [Google Scholar] [CrossRef]
- Nagendran, J.; Bozso, S.J.; Norris, C.M.; McAlister, F.A.; Appoo, J.J.; Moon, M.C.; Freed, D.H.; Nagendran, J. Coronary Artery Bypass Surgery Improves Outcomes in Patients with Diabetes and Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.Y.; Climie, R.E.; Shu, M.; Grieve, S.M.; Kozor, R.; Figtree, G.A. Vascular aging and cardiovascular disease: Pathophysiology and measurement in the coronary arteries. Front. Cardiovasc. Med. 2023, 28, 1206156. [Google Scholar] [CrossRef]
- González-Juanatey, C.; Anguita-Sánchez, M.; Barrios, V.; Núñez-Gil, I.; Gómez-Doblas, J.J.; García-Moll, X.; Peral-Disdier, V.; Martínez-Dolz, L.; Rodríguez-Santamarta, M.; Viñolas-Prat, X. Impact of Advanced Age on the Incidence of Major Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus and Stable Coronary Artery Disease in a Real-World Setting in Spain. J. Clin. Med. 2023, 12, 5218. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.K.; Dai, X.Y.; Ren, J.Y.; Liu, T.; Zhang, Y.K.; Hu, S.T.; Wang, P.; Wu, X.; Zhang, J.K.; Tse, G. Prevalence, clinical characteristics, and long-term outcomes of new diabetes diagnosis in elderly patients undergoing percutaneous coronary intervention. Sci. Rep. 2024, 14, 14814. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Rutledge, J.C.; Rogers, J.H. Coronary artery revascularization in patients with diabetes mellitus. Circulation 2013, 128, 1675–1685. [Google Scholar] [CrossRef]
- Ramadan, R.; Boden, W.E.; Kinlay, S. Management of Left Main Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e008151. [Google Scholar] [CrossRef]
- Gaba, P.; Sabik, J.F.; Murphy, S.A.; Bellavia, A.; O’Gara, P.T.; Smith, P.K.; Serruys, P.W.; Kappetein, A.P.; Park, S.J.; Park, D.W.; et al. Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting in Patients with Left Main Disease with and Without Diabetes: Findings from a Pooled Analysis of 4 Randomized Clinical Trials. Circulation 2024, 149, 1328–1338. [Google Scholar] [CrossRef]
- Ahn, J.M.; Roh, J.H.; Kim, Y.H.; Park, D.W.; Yun, S.C.; Lee, P.H.; Chang, M.; Park, H.W.; Lee, S.W.; Lee, C.W.; et al. Randomized Trial of Stents Versus Bypass Surgery for Left Main Coronary Artery Disease: 5-Year Outcomes of the PRECOMBAT Study. J. Am. Coll. Cardiol. 2015, 65, 2198–2206. [Google Scholar] [CrossRef]
- Mäkikallio, T.; Holm, N.R.; Lindsay, M.; Spence, M.S.; Erglis, A.; Menown, I.B.; Trovik, T.; Eskola, M.; Romppanen, H.; Kellerth, T.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): A prospective, randomised, open-label, non-inferiority trial. Lancet 2016, 388, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Serruys, P.W.; Sabik, J.F.; Leon, M.B.; Taggart, D.P.; Morice, M.C.; Gersh, B.J.; Pocock, S.J.; Cohen, D.J.; Wallentin, L.; et al. Design and rationale for a randomised comparison of everolimus-eluting stents and coronary artery bypass graft surgery in selected patients with left main coronary artery disease: The EXCEL trial. EuroIntervention 2016, 12, 861–872. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, O.; Di Franco, A.; Improta, R.; Di Pietro, G.; Leone, A.; Pecoraro, M.; Meynet, P.; Carbone, M.L.; Di Lorenzo, E.; Bruno, F.; et al. Percutaneous coronary intervention versus coronary artery bypass grafting for left main disease according to age: A meta-analysis. J. Thorac. Cardiovasc. Surg. 2024, in press. [Google Scholar] [CrossRef]
- Valdivielso, J.M.; Rodríguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermúdez-López, M.; Sánchez-Niño, M.D.; Pérez-Fernández, M.; Ortiz, A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Czarnik, W.; Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Rysz, J.; Franczyk, B. From Atherosclerotic Plaque to Myocardial Infarction—The Leading Cause of Coronary Artery Occlusion. Int. J. Mol. Sci. 2024, 25, 7295. [Google Scholar] [CrossRef]
- Wheeler, D.C.; James, J.; Patel, D.; Viljoen, A.; Ali, A.; Evans, M.; Fernando, K.; Hicks, D.; Milne, N.; Newland-Jones, P.; et al. SGLT2 Inhibitors: Slowing of Chronic Kidney Disease Progression in Type 2 Diabetes. Diabetes Ther. 2020, 11, 2757–2774. [Google Scholar] [CrossRef]
- Bangalore, S.; Maron, D.J.; O’Brien, S.M.; Fleg, J.L.; Kretov, E.I.; Briguori, C.; Kaul, U.; Reynolds, H.R.; Mazurek, T.; Sidhu, M.S.; et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N. Engl. J. Med. 2020, 382, 1608–1618. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, Y.; Chen, S.; Liu, S. Impact of Chronic Kidney Disease on Outcomes of Percutaneous Coronary Intervention in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Tex. Heart Inst. J. 2023, 50, e227873. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Q.; Nong, S.; Chen, Y.; Li, L.; Gui, C. Meta-analysis of clinical adverse events after CABG vs. PCI in patients with chronic kidney disease and coronary artery disease. BMC Cardiovasc. Disord. 2023, 23, 590. [Google Scholar] [CrossRef]
- Shroff, G.R.; Herzog, C.A. Coronary Revascularization in Patients with CKD Stage 5D: Pragmatic Considerations. J. Am. Soc. Nephrol. 2016, 27, 3521–3529. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.G.; Brodmann, M.; Kanoun Schnur, S.S.; Bartus, S.; Vrsalovic, M.; Krestianinov, O.; Kala, P.; Bil, J.; Gil, R.; Kanovsky, J.; et al. Intentional coronary revascularization versus conservative therapy in patients after peripheral artery revascularization due to critical limb ischemia: The INCORPORATE trial. Clin. Res. Cardiol. 2024. Epub ahead of print Erratum in: Clin. Res. Cardiol. 2024. https://doi.org/10.1007/s00392-024-02501-7. [Google Scholar] [CrossRef]
- Chen, H.; Luo, X.; Du, Y.; He, C.; Lu, Y.; Shi, Z.; Zhou, J. Association between chronic obstructive pulmonary disease and cardiovascular disease in adults aged 40 years and above: Data from NHANES 2013–2018. BMC Pulm. Med. 2023, 23, 318. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, D.; Jolicoeur, M.E.; Marquis-Gravel, G.; Fremes, S.E. Is the world ready for the STICH 3.0 trial? Curr. Opin. Cardiol. 2022, 37, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Tannu, M.; Nelson, A.J.; Rymer, J.A.; Jones, W.S. Myocardial Revascularization in Heart Failure: A State-of-the-Art Review. J. Card. Fail. 2024, 30, 1330–1342. [Google Scholar] [CrossRef]
- Fremes, S.E.; Marquis-Gravel, G.; Gaudino, M.F.L.; Jolicoeur, E.M.; Bédard, S.; Masterson Creber, R.; Ruel, M.; Vervoort, D.; Wijeysundera, H.C.; Farkouh, M.E.; et al. STICH3C: Rationale and Study Protocol. Circ. Cardiovasc. Interv. 2023, 16, e012527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivkovic, S.; Mandic, A.; Krupnikovic, K.; Obradovic, A.; Misevic, V.; Farkic, M.; Ilic, I.; Tesic, M.; Aleksandric, S.; Juricic, S.; et al. Myocardial Revascularization in Patients with Diabetes and Heart Failure—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3398. https://doi.org/10.3390/ijms26073398
Zivkovic S, Mandic A, Krupnikovic K, Obradovic A, Misevic V, Farkic M, Ilic I, Tesic M, Aleksandric S, Juricic S, et al. Myocardial Revascularization in Patients with Diabetes and Heart Failure—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(7):3398. https://doi.org/10.3390/ijms26073398
Chicago/Turabian StyleZivkovic, Stefan, Aleksandar Mandic, Kosta Krupnikovic, Aleksa Obradovic, Vojko Misevic, Mihajlo Farkic, Ivan Ilic, Milorad Tesic, Srdjan Aleksandric, Stefan Juricic, and et al. 2025. "Myocardial Revascularization in Patients with Diabetes and Heart Failure—A Narrative Review" International Journal of Molecular Sciences 26, no. 7: 3398. https://doi.org/10.3390/ijms26073398
APA StyleZivkovic, S., Mandic, A., Krupnikovic, K., Obradovic, A., Misevic, V., Farkic, M., Ilic, I., Tesic, M., Aleksandric, S., Juricic, S., Beleslin, B., & Dobric, M. (2025). Myocardial Revascularization in Patients with Diabetes and Heart Failure—A Narrative Review. International Journal of Molecular Sciences, 26(7), 3398. https://doi.org/10.3390/ijms26073398






