PDLIM3 Regulates Migration and Invasion of Head and Neck Squamous Cell Carcinoma via YAP–Mediated Epithelial–Mesenchymal Transition
Abstract
:1. Introduction
2. Results
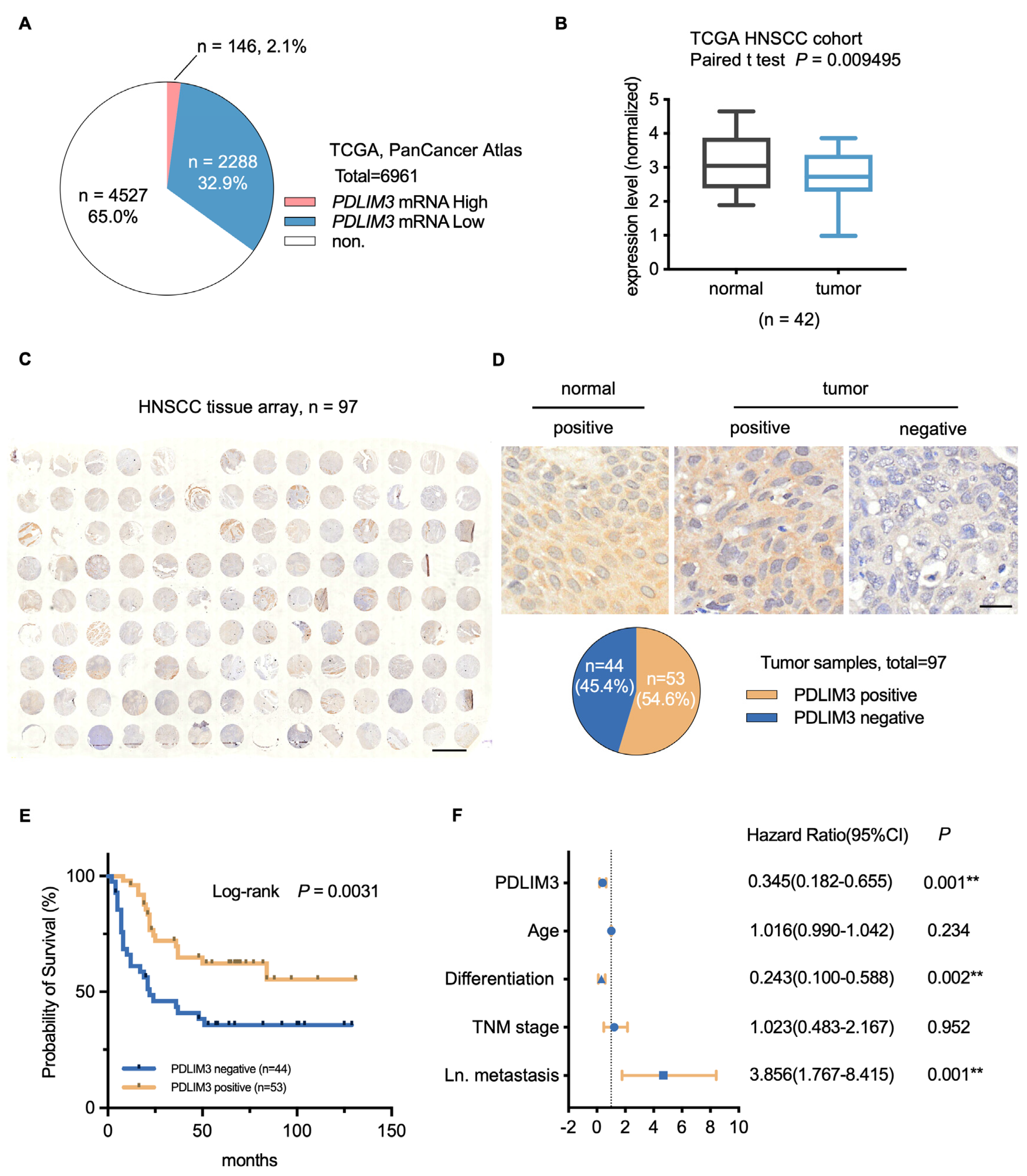
2.1. PDLIM3 Is Downregulated in HNSCC and Correlates with Poor Patient Prognosis
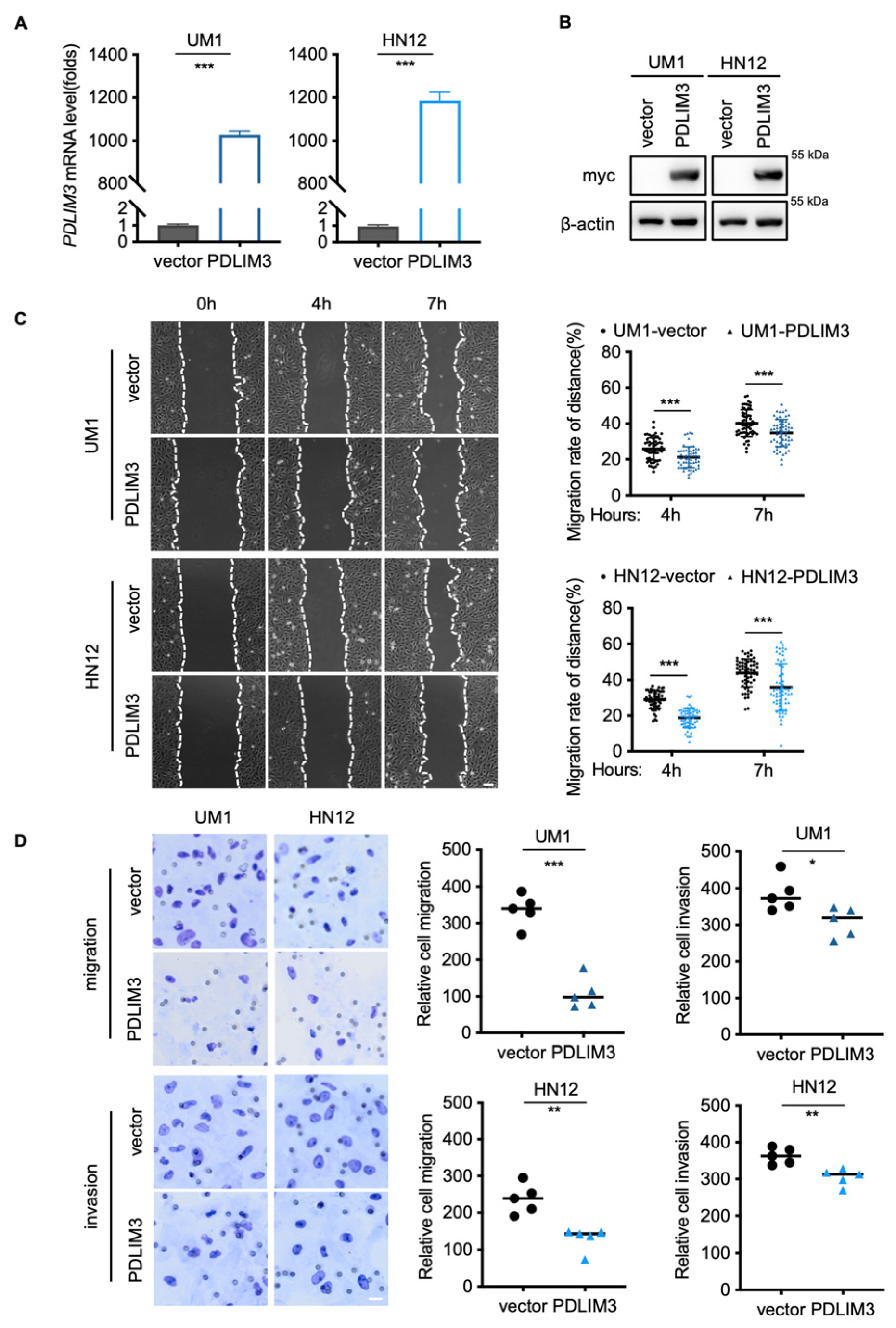
2.2. PDLIM3 Inhibits the Migration and Invasion of HNSCC
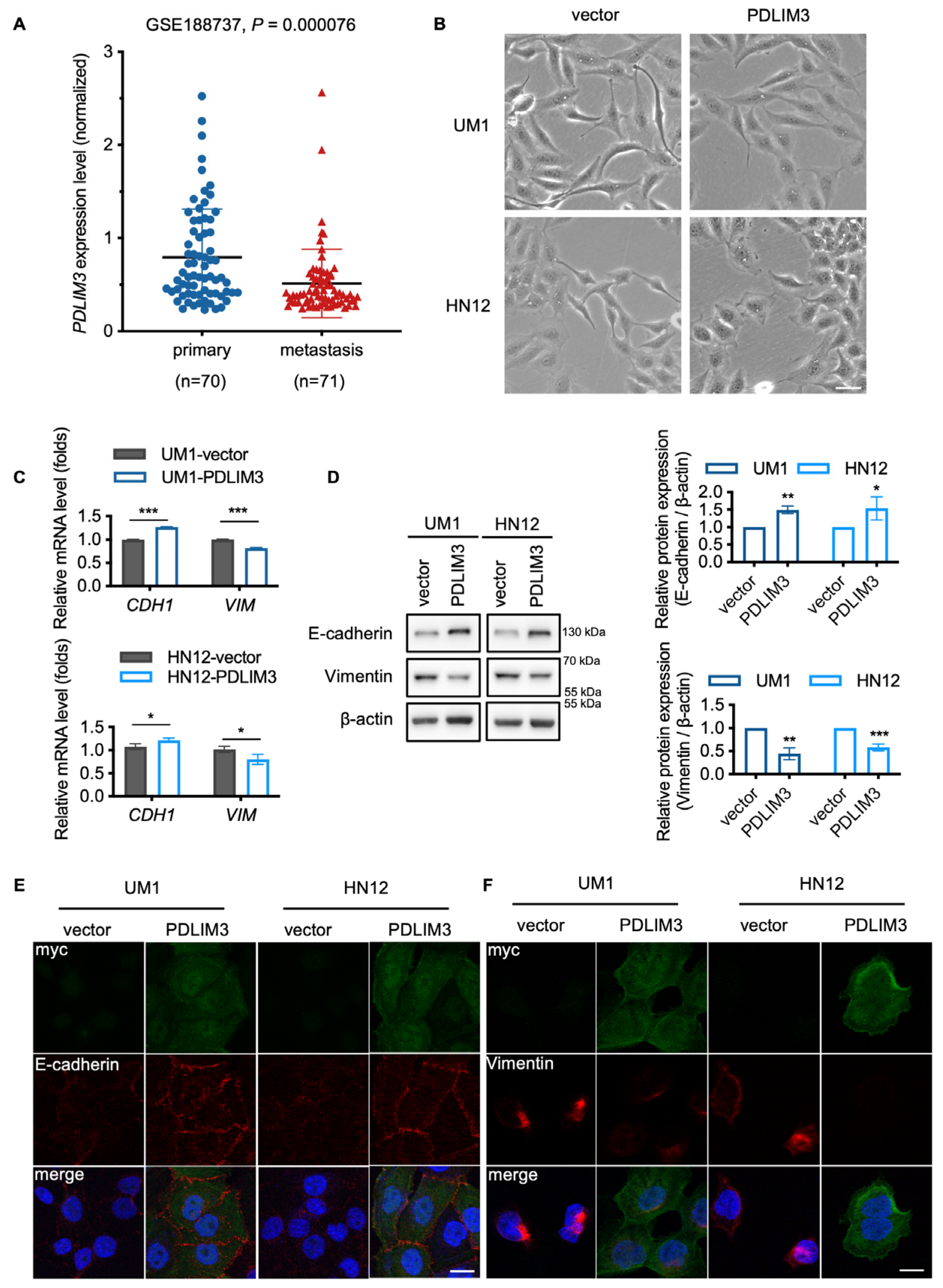
2.3. PDLIM3 Partially Impeding the Process of EMT in HNSCC
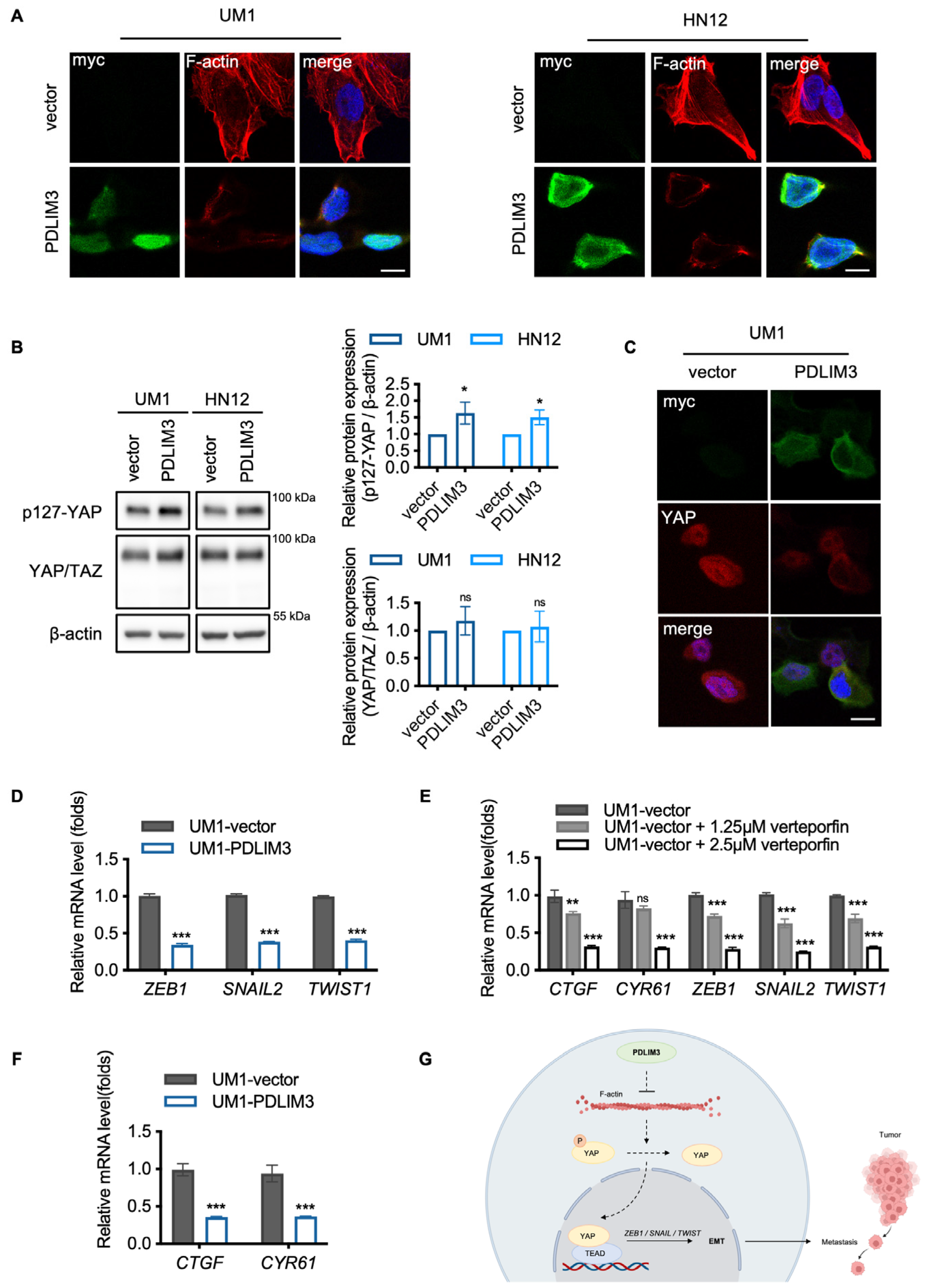
2.4. The Potential Role of Yes–Associated Protein (YAP) in PDLIM3–Mediated Suppression of EMT in HNSCC
3. Discussion
4. Materials and Methods
4.1. Tissue Microarray Analysis
4.2. Cell Line and Cell Culture
4.3. Plasmid Construction, Lentivirus Production, and Stable Cell Line Construction
4.4. Quantitative Real–Time PCR (qPCR) Analysis
4.5. Western Blotting
4.6. In Vitro Cell Proliferation Assay
4.7. 5–Ethynyl–2′–Deoxyuridine (EdU) Assay
4.8. Wound Healing Assay
4.9. Transwell Assay
4.10. Immunofluorescence
4.11. Immunohistochemistry
4.12. Third–Party mRNA Sequencing Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bizzoca, M.E.; Caponio, V.C.A.; Lo Muzio, L.; Claudio, P.P.; Cortese, A. Methods for Overcoming Chemoresistance in Head and Neck Squamous Cell Carcinoma: Keeping the Focus on Cancer Stem Cells, a Systematic Review. Cancers 2024, 16, 3004. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, W.; Wang, W.; Wu, Y.; Fang, M.; Huang, X.; Han, P.; Zhang, Q.; Dong, P.; Zhou, X.; et al. Finotonlimab with chemotherapy in recurrent or metastatic head and neck cancer: A randomized phase 3 trial. Nat. Med. 2024, 30, 2568–2575. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Kurten, C.H.L.; Kulkarni, A.; Cillo, A.R.; Santos, P.M.; Roble, A.K.; Onkar, S.; Reeder, C.; Lang, S.; Chen, X.; Duvvuri, U.; et al. Investigating immune and non–immune cell interactions in head and neck tumors by single–cell RNA sequencing. Nat. Commun. 2021, 12, 7338. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, Y.; Omar, M.; Broner, E.C.; Agrawal, N.; Pearson, A.T.; Rosenberg, A.J.; Mishra, V.; Singh, A.; El–Naaj, I.A.; Savage, P.A.; et al. A robust and interpretable gene signature for predicting the lymph node status of primary T1/T2 oral cavity squamous cell carcinoma. Int. J. Cancer 2022, 150, 450–460. [Google Scholar] [CrossRef]
- Jagadeeshan, S.; Prasad, M.; Badarni, M.; Ben–Lulu, T.; Liju, V.B.; Mathukkada, S.; Saunders, C.; Shnerb, A.B.; Zorea, J.; Yegodayev, K.M.; et al. Mutated HRAS Activates YAP1–AXL Signaling to Drive Metastasis of Head and Neck Cancer. Cancer Res. 2023, 83, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous–cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Solomon, B.; Young, R.J.; Rischin, D. Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin. Cancer Biol. 2018, 52, 228–240. [Google Scholar] [CrossRef]
- Kitamura, N.; Sento, S.; Yoshizawa, Y.; Sasabe, E.; Kudo, Y.; Yamamoto, T. Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 22, 240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, Z.; Jiang, S.; Wang, H.; Xiao, M.; Shi, Y.; Wang, K. PDZ and LIM Domain–Encoding Genes: Their Role in Cancer Development. Cancers 2023, 15, 5042. [Google Scholar] [CrossRef] [PubMed]
- te Velthuis, A.J.; Bagowski, C.P. PDZ and LIM domain–encoding genes: Molecular interactions and their role in development. Sci. World J. 2007, 7, 1470–1492. [Google Scholar] [CrossRef]
- Krcmery, J.; Camarata, T.; Kulisz, A.; Simon, H.G. Nucleocytoplasmic functions of the PDZ–LIM protein family: New insights into organ development. Bioessays 2010, 32, 100–108. [Google Scholar] [CrossRef]
- Arola, A.M.; Sanchez, X.; Murphy, R.T.; Hasle, A.; Li, H.; Elliott, P.M.; McKenna, W.J.; Towbin, J.A.; Bowles, N.E. Mutations in PDLIM3 and MYOZ1 encoding myocyte Z line proteins are infrequently found in idiopathic dilated cardiomyopathy. Mol. Genet. Metab. 2007, 90, 435–440. [Google Scholar] [CrossRef]
- Ohsawa, N.; Koebis, M.; Suo, S.; Nishino, I.; Ishiura, S. Alternative splicing of PDLIM3/ALP, for alpha–actinin–associated LIM protein 3, is aberrant in persons with myotonic dystrophy. Biochem. Biophys. Res. Commun. 2011, 409, 64–69. [Google Scholar] [CrossRef]
- Zheng, M.; Cheng, H.; Banerjee, I.; Chen, J. ALP/Enigma PDZ–LIM domain proteins in the heart. J. Mol. Cell Biol. 2010, 2, 96–102. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Li, X.; Li, G.; Mizukami, T.; Liu, Y.; Wang, Y.; Xu, G.; Roder, H.; Zhang, L.; et al. PDLIM3 supports hedgehog signaling in medulloblastoma by facilitating cilia formation. Cell Death Differ. 2023, 30, 1198–1210. [Google Scholar] [CrossRef]
- Hu, X.; Chen, M.; Ruan, Q.; Shi, C.; Pan, J.; Luo, L. Comprehensive Analysis of PDLIM3 Expression Profile, Prognostic Value, and Correlations with Immune Infiltrates in Gastric Cancer. J. Immunol. Res. 2022, 2022, 2039447. [Google Scholar] [CrossRef]
- Piao, S.; Zheng, L.; Zheng, H.; Zhou, M.; Feng, Q.; Zhou, S.; Ke, M.; Yang, H.; Wang, X. High Expression of PDLIM2 Predicts a Poor Prognosis in Prostate Cancer and Is Correlated with Epithelial–Mesenchymal Transition and Immune Cell Infiltration. J. Immunol. Res. 2022, 2022, 2922832. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Kang, Y. Context–dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Lambert, A.W.; Weinberg, R.A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 2021, 21, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Feng, X.; Degese, M.S.; Iglesias–Bartolome, R.; Vaque, J.P.; Molinolo, A.A.; Rodrigues, M.; Zaidi, M.R.; Ksander, B.R.; Merlino, G.; Sodhi, A.; et al. Hippo–independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio–regulated rho GTPase signaling circuitry. Cancer Cell 2014, 25, 831–845. [Google Scholar] [CrossRef]
- Akrida, I.; Bravou, V.; Papadaki, H. The deadly cross–talk between Hippo pathway and epithelial–mesenchymal transition (EMT) in cancer. Mol. Biol. Rep. 2022, 49, 10065–10076. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef]
- Piccolo, S.; Panciera, T.; Contessotto, P.; Cordenonsi, M. YAP/TAZ as master regulators in cancer: Modulation, function and therapeutic approaches. Nat. Cancer 2023, 4, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, X.; Liu, J.; Hao, J. The Hippo/YAP signaling pathway: The driver of cancer metastasis. Cancer Biol. Med. 2023, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Du, S.; Gao, P.; Zheng, J. Verteporfin suppresses the proliferation, epithelial–mesenchymal transition and stemness of head and neck squamous carcinoma cells via inhibiting YAP1. J. Cancer 2019, 10, 4196–4207. [Google Scholar] [CrossRef]
- Liu–Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Akter, R.; Shahriar, M.F.; Uddin, M.N. Cancer–Associated Stromal Fibroblast–Derived Transcriptomes Predict Poor Clinical Outcomes and Immunosuppression in Colon Cancer. Pathol. Oncol. Res. 2022, 28, 1610350. [Google Scholar] [CrossRef]
- Kolluru, V.; Tyagi, A.; Chandrasekaran, B.; Damodaran, C. Profiling of differentially expressed genes in cadmium–induced prostate carcinogenesis. Toxicol. Appl. Pharmacol. 2019, 375, 57–63. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, H.; Cao, Y.; Feng, W.; Li, M.; Li, X. PDZ and LIM domain protein 4 suppresses the growth and invasion of ovarian cancer cells via inactivation of STAT3 signaling. Life Sci. 2019, 233, 116715. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, C.; Zhou, S.; Lau, W.B.; Lau, B.; Luo, Z.; Lin, Q.; Yang, H.; Xuan, Y.; Yi, T.; et al. Epigenetic repression of PDZ–LIM domain–containing protein 2 promotes ovarian cancer via NOS2–derived nitric oxide signaling. Oncotarget 2016, 7, 1408–1420. [Google Scholar] [CrossRef]
- Wang, P.; Li, G.Y.; Zhou, L.; Jiang, H.L.; Yang, Y.; Wu, H.T. Exosomes from M2 macrophages promoted glycolysis in FaDu cells by inhibiting PDLIM2 expression to stabilize PFKL. Neoplasma 2022, 69, 1041–1053. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kang, Y.; Im, N.R.; Kim, B.; Kwon, T.K.; Jung, K.Y.; Baek, S.K. Actin–Associated Gene Expression is Associated with Early Regional Metastasis of Tongue Cancer. Laryngoscope 2021, 131, 813–819. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, J.K.; Wang, K.; Chen, H.; Qin, S.; Liu, J.; Luo, M.; Chen, Y.; Jiang, J.; Zhou, L.; et al. PDLIM1 Inhibits Tumor Metastasis Through Activating Hippo Signaling in Hepatocellular Carcinoma. Hepatology 2020, 71, 1643–1659. [Google Scholar] [CrossRef] [PubMed]
- Healy, N.C.; O’Connor, R. Sequestration of PDLIM2 in the cytoplasm of monocytic/macrophage cells is associated with adhesion and increased nuclear activity of NF–kappaB. J. Leukoc. Biol. 2009, 85, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, T.; Wu, Y.; Xu, H.; Xie, C.; Dong, Y.; Zhong, L.; Wang, Z.; Zhao, H.; Zhou, Y.; et al. GPR39 Overexpression in OSCC Promotes YAP–Sustained Malignant Progression. J. Dent. Res. 2020, 99, 949–958. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K.L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Deng, L.; Zou, H.; Guo, Y.; Tong, T.; Huang, M.; Ling, G.; Li, P. New insights into the ambivalent role of YAP/TAZ in human cancers. J. Exp. Clin. Cancer Res. 2023, 42, 130. [Google Scholar] [CrossRef] [PubMed]
- Imodoye, S.O.; Adedokun, K.A. EMT–induced immune evasion: Connecting the dots from mechanisms to therapy. Clin. Exp. Med. 2023, 23, 4265–4287. [Google Scholar] [CrossRef]
- Yoon, H.; Dehart, J.P.; Murphy, J.M.; Lim, S.T. Understanding the roles of FAK in cancer: Inhibitors, genetic models, and new insights. J. Histochem. Cytochem. 2015, 63, 114–128. [Google Scholar] [CrossRef]
- Rentala, S.; Chintala, R.; Guda, M.; Chintala, M.; Komarraju, A.L.; Mangamoori, L.N. Atorvastatin inhibited Rho–associated kinase 1 (ROCK1) and focal adhesion kinase (FAK) mediated adhesion and differentiation of CD133+CD44+ prostate cancer stem cells. Biochem. Biophys. Res. Commun. 2013, 441, 586–592. [Google Scholar] [CrossRef]
- Kang, C.G.; Lee, H.J.; Kim, S.H.; Lee, E.O. Zerumbone Suppresses Osteopontin–Induced Cell Invasion Through Inhibiting the FAK/AKT/ROCK Pathway in Human Non–Small Cell Lung Cancer A549 Cells. J. Nat. Prod. 2016, 79, 156–160. [Google Scholar] [CrossRef]
- Huang, F.; Ren, Y.; Hua, Y.; Wang, Y.; Li, R.; Ji, N.; Zeng, X.; Bai, D.; Chen, Q.; Zhou, X.; et al. m6A–dependent mature miR–151–5p accelerates the malignant process of HNSCC by targeting LYPD3. Mol. Biomed. 2024, 5, 27. [Google Scholar] [CrossRef]
- Yang, D.; Yang, D.; He, Y.; Liu, J.; Song, Y.; Zhou, Y.; Jiang, L.; Zeng, X.; Xu, X.; Xu, H.; et al. MRTF–A/SRF signaling suppresses invasion of oral squamous cell carcinoma. Oral. Dis. 2024, 30, 1919–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lv, D.; Wei, W.; Zhou, T.; Tang, S.; Yang, F.; Zhang, J.; Jiang, L.; Xia, X.; Jiang, Y.; et al. Type 17 immune response promotes oral epithelial cell proliferation in periodontitis. Arch. Oral Biol. 2024, 164, 106005. [Google Scholar] [CrossRef]
- Quah, H.S.; Cao, E.Y.; Suteja, L.; Li, C.H.; Leong, H.S.; Chong, F.T.; Gupta, S.; Arcinas, C.; Ouyang, J.F.; Ang, V.; et al. Single cell analysis in head and neck cancer reveals potential immune evasion mechanisms during early metastasis. Nat. Commun. 2023, 14, 1680. [Google Scholar] [CrossRef]
| Variable | Patients | PDLIM3 | χ² | p | |
|---|---|---|---|---|---|
| n (%) | Positive | Negative | |||
| Age | |||||
| ≤60 years | 43 (44) | 20 | 23 | 0.04127 | 0.8390 |
| >60 years | 54 (56) | 24 | 30 | ||
| Gender | |||||
| Male | 63 (65) | 30 | 33 | 0.3698 | 0.5431 |
| Female | 34 (35) | 14 | 20 | ||
| History of smoking | |||||
| No | 59 (61) | 32 | 27 | 0.0098 | 0.9211 |
| Yes | 38 (39) | 21 | 17 | ||
| History of alcohol | |||||
| No | 67 (69) | 36 | 31 | 0.07204 | 0.7884 |
| Yes | 30 (31) | 17 | 13 | ||
| Differentiation | |||||
| High | 68 (70) | 30 | 38 | 2.595 | 0.2732 |
| Moderate | 24 (25) | 10 | 14 | ||
| Low | 5 (5) | 4 | 1 | ||
| TNM stage | |||||
| I/II | 39 (40) | 14 | 25 | 2.357 | 0.1247 |
| III/IV | 58 (60) | 30 | 28 | ||
| Tumor stage | |||||
| T1/T2 | 61 (63) | 26 | 35 | 0.4971 | 0.4808 |
| T3/T4 | 36 (37) | 18 | 18 | ||
| Lymph node metastasis | |||||
| No | 53 (55) | 20 | 33 | 2.741 | 0.0978 |
| Yes | 44 (45) | 24 | 20 | ||
| Radiotherapy | |||||
| No | 82 (85) | 43 | 39 | 1.036 | 0.3088 |
| Yes | 15 (15) | 10 | 5 | ||
| Pre–Chemotherapy | |||||
| No | 66 (68) | 43 | 23 | 9.208 | 0.0024 |
| Yes | 31 (32) | 10 | 21 | ||
| Post–Chemotherapy | |||||
| No | 50 (52) | 25 | 25 | 0.8961 | 0.3438 |
| Yes | 47 (48) | 28 | 19 | ||
| Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p–Value | HR | 95% CI | p–Value | |
| PDLIM3 | 0.419 | 0.229–0.764 | 0.005 | 0.345 | 0.182–0.655 | 0.001 |
| Age | 1.027 | 1.002–1.053 | 0.033 | 1.016 | 0.990–1.042 | 0.234 |
| Gender | 0.640 | 0.329–1.244 | 0.188 | |||
| Differentiation | 0.378 | 0.168–0.850 | 0.019 | 0.243 | 0.100–0.588 | 0.002 |
| TNM stage | 2.377 | 1.279–4.417 | 0.006 | 1.023 | 0.483–2.167 | 0.952 |
| Tumor stage | 1.928 | 0.922–4.034 | 0.081 | |||
| Lymph node metastasis | 3.122 | 1.667–5.849 | <0.001 | 3.856 | 1.767–8.415 | 0.001 |
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
| GAPDH | GAGTCAACGGATTTGGTCGT | TTGATTTTGGAGGGATCTCG |
| PDLIM3 | AACTCGCCAATTGGGCTCTA | CTGGAGCACTCTGAAGGAGC |
| CDH1 | CGAGAGCTACACGTTCACGG | GGGTGTCGAGGGAAAAATAGG |
| VIM | TGCCGTTGAAGCTGCTAACTA | CCAGAGGGAGTGAATCCAGATTA |
| CTGF | GTTTGGCCCAGACCCAACTA | GGCTCTGCTTCTCTAGCCTG |
| CYR61 | CAGGACTGTGAAGATGCGGT | GCCTGTAGAAGGGAAACGCT |
| ZEB1 | GATGATGAATGCGAGTCAGATGC | ACAGCAGTGTCTTGTTGTTGT |
| SNAIL2 | CGAACTGGACACACATACAGTG | CTGAGGATCTCTGGTTGTGGT |
| TWIST1 | GTCCGCAGTCTTACGAGGAG | GCTTGAGGGTCTGAATCTTGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Zhou, Y.; Zhang, Y.; Wei, W.; Huang, F.; Yang, D.; Zhang, Y.; Zhang, R.; Xia, X.; Chen, Q.; et al. PDLIM3 Regulates Migration and Invasion of Head and Neck Squamous Cell Carcinoma via YAP–Mediated Epithelial–Mesenchymal Transition. Int. J. Mol. Sci. 2025, 26, 3147. https://doi.org/10.3390/ijms26073147
Yang F, Zhou Y, Zhang Y, Wei W, Huang F, Yang D, Zhang Y, Zhang R, Xia X, Chen Q, et al. PDLIM3 Regulates Migration and Invasion of Head and Neck Squamous Cell Carcinoma via YAP–Mediated Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences. 2025; 26(7):3147. https://doi.org/10.3390/ijms26073147
Chicago/Turabian StyleYang, Fan, Ying Zhou, You Zhang, Weideng Wei, Fei Huang, Dan Yang, Yixin Zhang, Ruiyang Zhang, Xiaoqiang Xia, Qianming Chen, and et al. 2025. "PDLIM3 Regulates Migration and Invasion of Head and Neck Squamous Cell Carcinoma via YAP–Mediated Epithelial–Mesenchymal Transition" International Journal of Molecular Sciences 26, no. 7: 3147. https://doi.org/10.3390/ijms26073147
APA StyleYang, F., Zhou, Y., Zhang, Y., Wei, W., Huang, F., Yang, D., Zhang, Y., Zhang, R., Xia, X., Chen, Q., Jiang, Y., & Feng, X. (2025). PDLIM3 Regulates Migration and Invasion of Head and Neck Squamous Cell Carcinoma via YAP–Mediated Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences, 26(7), 3147. https://doi.org/10.3390/ijms26073147






