A Chloroform Fraction Derived from Vitis vinifera Root Ethanol Extract Attenuates Lipopolysaccharide-Induced Inflammatory Responses and Cognitive Dysfunction in BV-2 Microglia Cells and C57BL/6J Mouse Model
Abstract
1. Introduction
2. Results
2.1. In Vitro Study
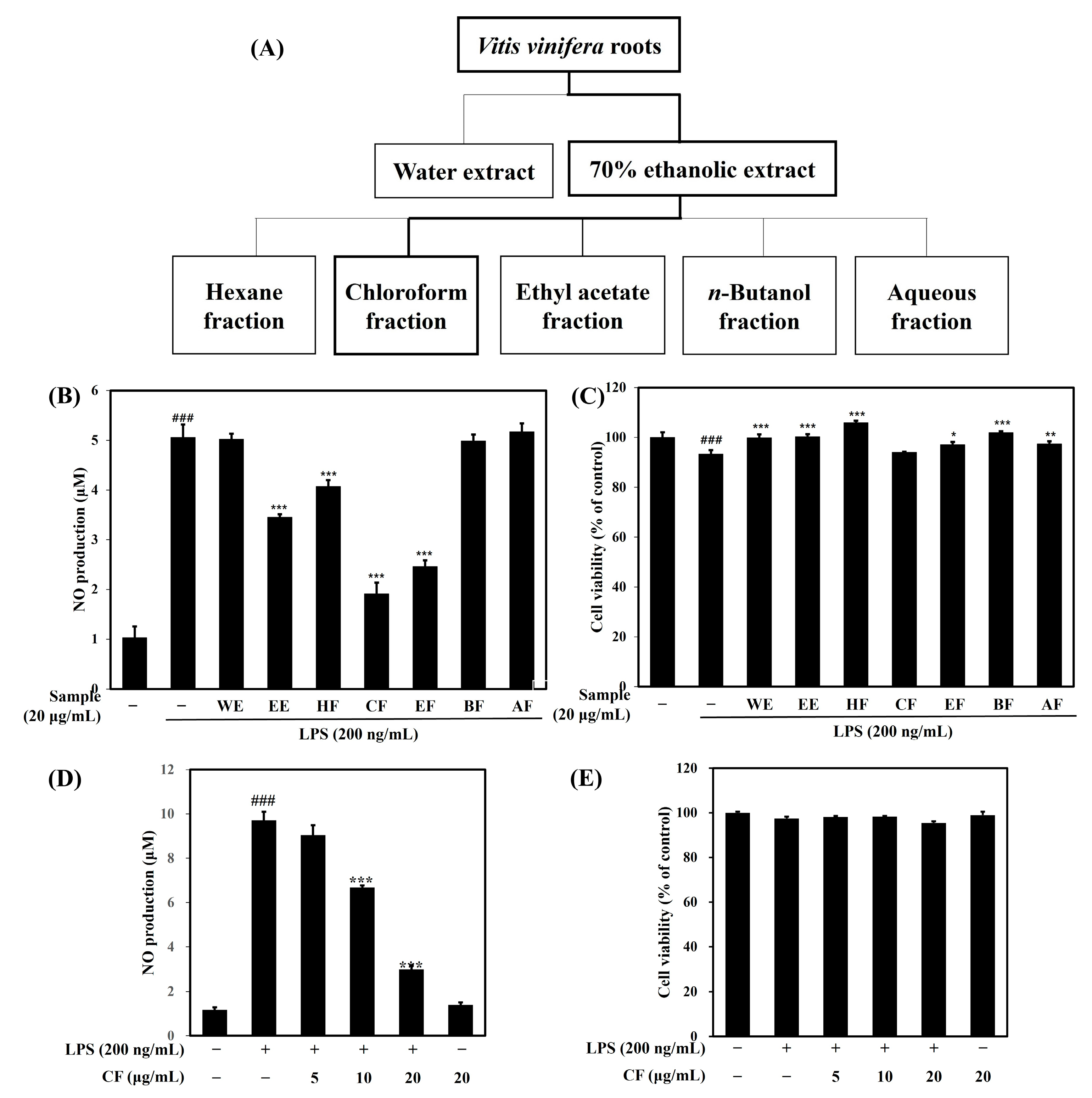
2.1.1. Effects of Vitis vinifera Extracts and Fractions on NO Production and Cell Viability
2.1.2. Effects of the CF on iNOS and COX-2 Protein Expression
2.1.3. Effects of the CF on Inflammatory Cytokines
2.1.4. Effects of the CF on MAPK Protein Expression
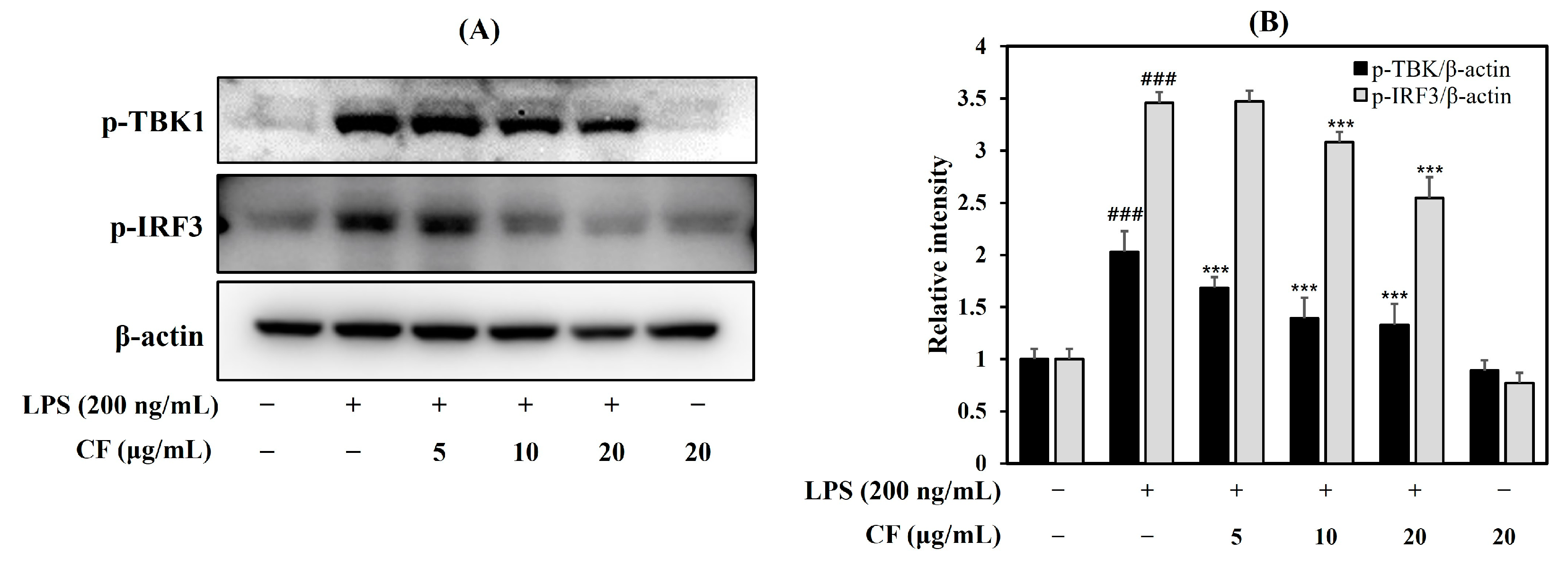
2.1.5. Effects of the CF on TBK1 and IRF3 Protein Expression
2.1.6. LC-MS and HPLC Analysis of CF
2.1.7. Anti-Neuro Inflammatory Effects of Ethyl Caffeate
2.2. In Vivo Study
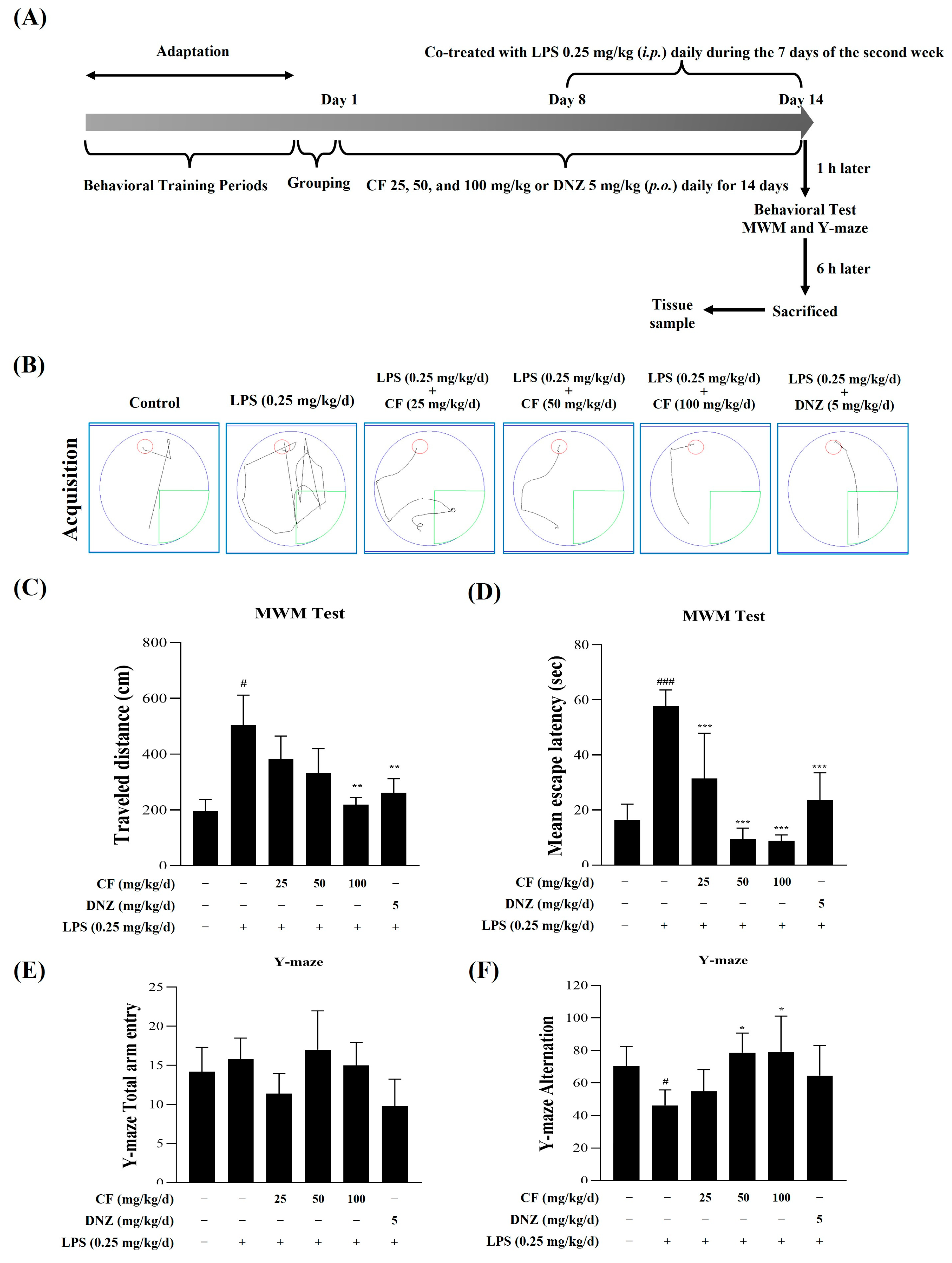
2.2.1. The Effects of CF and LPS on MWM Test and Y-Maze Test
2.2.2. The Effects of CF Against LPS on Serum IL-6 and TNF-α Levels
2.2.3. The Effects of CF Against LPS on Whole Brain iNOS and COX-2 Levels
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Materials
4.1.2. Preparation of the Extract and Its Fractions
4.1.3. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
4.1.4. High-Performance Liquid Chromatography (HPLC) Analysis
4.2. In Vitro Study
4.2.1. Cell Culture and Cell Viability Assays
4.2.2. NO Assay
4.2.3. Measurement of the Levels of TNF-α and IL-6 by ELISA
4.2.4. Western Blotting
4.2.5. RT-PCR Analysis
4.2.6. Immunocytochemical Staining
4.3. In Vivo Study
4.3.1. Animals Handling and Treatment
4.3.2. Morris Water Maze (MWM) Test
4.3.3. Y-Maze Test
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, G.; Pan, C.; Liu, H.; Dong, C.; Chang, X.; Zhou, W.; Wang, S.; Du, Z. Oxyresveratrol Improves Cognitive Impairments and Episodic-like Memory through Modulating Neuroinflammation and PI3K-Akt Signaling Pathway in LPS-Induced Mice. Molecules 2024, 29, 1272. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-Derived Microparticles Mediate Neuroinflammation after Traumatic Brain Injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Tohidpour, A.; Morgun, A.V.; Boitsova, E.B.; Malinovskaya, N.A.; Martynova, G.P.; Khilazheva, E.D.; Kopylevich, N.V.; Gertsog, G.E.; Salmina, A.B. Neuroinflammation and Infection: Molecular Mechanisms Associated with Dysfunction of Neurovascular Unit. Front. Cell. Infect. Microbiol. 2017, 7, 276. [Google Scholar] [CrossRef]
- Hensley, K. Neuroinflammation in Alzheimer’s Disease: Mechanisms, Pathologic Consequences, and Potential for Therapeutic Manipulation. JAD J. Alzheimer’s Dis. 2010, 21, 1–14. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Sheppard, O.; Coleman, M.P.; Durrant, C.S. Lipopolysaccharide-Induced Neuroinflammation Induces Presynaptic Disruption through a Direct Action on Brain Tissue Involving Microglia-Derived Interleukin 1 Beta. J. Neuroinflamm. 2019, 16, 106. [Google Scholar] [CrossRef]
- Walker, A.K.; Wing, E.E.; Banks, W.A.; Dantzer, R. Leucine Competes with Kynurenine for Blood-to-Brain Transport and Prevents Lipopolysaccharide-Induced Depression-like Behavior in Mice. Mol. Psychiatry 2019, 24, 1523–1532. [Google Scholar] [CrossRef]
- Schedlowski, M.; Engler, H.; Grigoleit, J.-S. Endotoxin-Induced Experimental Systemic Inflammation in Humans: A Model to Disentangle Immune-to-Brain Communication. Brain Behav. Immun. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Kraft, A.D.; Harry, G.J. Features of Microglia and Neuroinflammation Relevant to Environmental Exposure and Neurotoxicity. Int. J. Environ. Res. Public Health IJERPH 2011, 8, 2980–3018. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; De Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef]
- Carniglia, L.; Ramírez, D.; Durand, D.; Saba, J.; Turati, J.; Caruso, C.; Scimonelli, T.N.; Lasaga, M. Neuropeptides and Microglial Activation in Inflammation, Pain, and Neurodegenerative Diseases. Mediat. Inflamm. 2017, 2017, 5048616. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Kim, E.-K.; Tang, Y.; Cha, K.-S.; Choi, H.; Lee, C.B.; Yoon, J.-H.; Kim, S.B.; Kim, J.-S.; Kim, J.M.; Han, W.C.; et al. Artemisia Asiatica Nakai Attenuates the Expression of Proinflammatory Mediators in Stimulated Macrophages Through Modulation of Nuclear Factor-κB and Mitogen-Activated Protein Kinase Pathways. J. Med. Food 2015, 18, 921–928. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwaśniak, B.; Sikora, E.; Szopa, A. Vitis vinifera (Vine Grape) as a Valuable Cosmetic Raw Material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef]
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Inês, A.; Oliveira, A.A.; Falco, V. Chitosan Upregulates the Genes of the ROS Pathway and Enhances the Antioxidant Potential of Grape (Vitis vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) Tissues. Antioxidants 2019, 8, 525. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Bioactive Chemicals and Health Benefits of Grapevine Products. In Bioactive Foods in Promoting Health; Elsevier: Amsterdam, The Netherlands, 2010; pp. 581–620. ISBN 978-0-12-374628-3. [Google Scholar]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Falchi, M.; Bertelli, A.; Lo Scalzo, R.; Morassut, M.; Morelli, R.; Das, S.; Cui, J.; Das, D.K. Comparison of Cardioprotective Abilities between the Flesh and Skin of Grapes. J. Agric. Food Chem. 2006, 54, 6613–6622. [Google Scholar] [CrossRef]
- Hudson, T.S.; Hartle, D.K.; Hursting, S.D.; Nunez, N.P.; Wang, T.T.Y.; Young, H.A.; Arany, P.; Green, J.E. Inhibition of Prostate Cancer Growth by Muscadine Grape Skin Extract and Resveratrol through Distinct Mechanisms. Cancer Res. 2007, 67, 8396–8405. [Google Scholar] [CrossRef]
- Bralley, E.E.; Hargrove, J.L.; Greenspan, P.; Hartle, D.K. Topical Anti-Inflammatory Activities of Vitis rotundifolia (Muscadine Grape) Extracts in the Tetradecanoylphorbol Acetate Model of Ear Inflammation. J. Med. Food 2007, 10, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, J.G.; Akila, S.; Narendhirakannan, R.T.; Chatterjee, S. Vitis Vinifera Peel Polyphenols Stabilized Gold Nanoparticles Induce Cytotoxicity and Apoptotic Cell Death in A431 Skin Cancer Cell Lines. Adv. Powder Technol. 2017, 28, 1170–1184. [Google Scholar] [CrossRef]
- Ricardo Da Silva, J.M.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin Dimers and Trimers from Grape Seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Esfahanian, Z.; Behbahani, M.; Shanehsaz, M.; Hessami, M.J.; Nejatian, M.A. Evaluation of Anticancer Activity of Fruit and Leave Extracts from Virus Infected and Healthy Cultivars of Vitis vinifera. Cell J. 2013, 15, 116–123. [Google Scholar]
- Esatbeyoglu, T.; Ewald, P.; Yasui, Y.; Yokokawa, H.; Wagner, A.E.; Matsugo, S.; Winterhalter, P.; Rimbach, G. Chemical characterization, free radical scavenging, and cellular antioxidant and anti-inflammatory properties of a stilbenoid-rich root extract of Vitis vinifera. Oxid. Med. Cell. Longev. 2016, 1, 8591286. [Google Scholar] [CrossRef]
- Ardid-Ruiz, A.; Harazin, A.; Barna, L.; Walter, F.R.; Bladé, C.; Suárez, M.; Deli, M.A.; Aragonès, G. The effects of Vitis vinifera L. phenolic compounds on a blood-brain barrier culture model: Expression of leptin receptors and protection against cytokine-induced damage. J. Ethnopharmacol. 2020, 247, 112253. [Google Scholar] [CrossRef]
- Habashy, N.H.; Kodous, A.S.; Abu-Serie, M.M. Targeting ROS/NF-κB signaling pathway by the seedless black Vitis vinifera polyphenols in CCl4-intoxicated kidney, lung, brain, and spleen in rats. Sci. Rep. 2021, 11, 16575. [Google Scholar] [CrossRef]
- Lakshmi, B.V.S.; Sudhakar, M.; Anisha, M. Neuroprotective role of hydroalcoholic extract of Vitis vinifera against aluminium-induced oxidative stress in rat brain. Neurotoxicology 2014, 41, 73–79. [Google Scholar]
- Hong, Y.; Choi, Y.H.; Han, Y.E.; Oh, S.J.; Lee, A.; Lee, B.; Magnan, R.; Ryu, S.Y.; Choi, C.W.; Kim, M.S. Central administration of ampelopsin A isolated from Vitis vinifera ameliorates cognitive and memory function in a scopolamine-induced dementia model. Antioxidants 2021, 10, 835. [Google Scholar] [CrossRef]
- Calapai, G.; Bonina, F.; Bonina, A.; Rizza, L.; Mannucci, C.; Arcoraci, V.; Laganà, G.; Alibrandi, A.; Pollicino, C.; Inferrera, S.; et al. A randomized, double-blinded, clinical trial on effects of a Vitis vinifera extract on cognitive function in healthy older adults. Front. Pharmacol. 2017, 8, 776. [Google Scholar] [CrossRef]
- Lee, D.K.; Lee, K.P.; Kim, H.; Choi, B.J.; Chang, H.R.; Park, W.H. Effect of the Ethanol Extract of Vitis labrusca Root on Apoptosis in Hep G2 Cells. Korean J. Orient. Physiol. Pathol. 2008, 22, 377–384. [Google Scholar]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Xu, Y.; Jin, M.-Z.; Yang, Z.-Y.; Jin, W.-L. Microglia in Neurodegenerative Diseases. Neural Regen. Res. 2021, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial Activation and Chronic Neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Youssef, M.; Ibrahim, A.; Akashi, K.; Hossain, M.S. PUFA-Plasmalogens Attenuate the LPS-Induced Nitric Oxide Production by Inhibiting the NF-kB, P38 MAPK and JNK Pathways in Microglial Cells. Neuroscience 2019, 397, 18–30. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Wang, W.; Bai, Y.; Jia, H.; Yuan, Z.; Yang, Z. Role and Mechanisms of the NF-ĸB Signaling Pathway in Various Developmental Processes. Biomed. Pharmacother. 2022, 153, 113513. [Google Scholar] [CrossRef]
- Xie, Q.; Wu, G.-Z.; Yang, N.; Shen, Y.-H.; Tang, J.; Zhang, W.-D. Delavatine A, an Unusual Isoquinoline Alkaloid Exerts Anti-Inflammation on LPS-Induced Proinflammatory Cytokines Production by Suppressing NF-κB Activation in BV-2 Microglia. Biochem. Biophys. Res. Commun. 2018, 502, 202–208. [Google Scholar] [CrossRef]
- Mukherjee, S.; Karmakar, S.; Babu, S.P.S. TLR2 and TLR4 Mediated Host Immune Responses in Major Infectious Diseases: A Review. Braz. J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jain, A.; Gao, Y.; Dozmorov, I.M.; Mandraju, R.; Wakeland, E.K.; Pasare, C. Differential Outcome of TRIF-Mediated Signaling in TLR4 and TLR3 Induced DC Maturation. Proc. Natl. Acad. Sci. USA 2015, 112, 13994–13999. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.-L.; Gioia, R.; Gatot, J.-S.; Patrascu, F.; Carpentier, I.; Chapelle, J.-P.; O’Neill, L.; Beyaert, R.; Piette, J.; Chariot, A. Are the IKKs and IKK-Related Kinases TBK1 and IKK-ɛ Similarly Activated? Trends Biochem. Sci. 2008, 33, 171–180. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.E.; Carpenter, S.; Pålsson-McDermott, E.M.; Gearing, L.J.; O’Neill, L.A.J. Glucocorticoids Inhibit IRF3 Phosphorylation in Response to Toll-like Receptor-3 and -4 by Targeting TBK1 Activation. J. Biol. Chem. 2008, 283, 14277–14285. [Google Scholar] [CrossRef]
- Yanai, H.; Chiba, S.; Hangai, S.; Kometani, K.; Inoue, A.; Kimura, Y.; Abe, T.; Kiyonari, H.; Nishio, J.; Taguchi-Atarashi, N.; et al. Revisiting the Role of IRF3 in Inflammation and Immunity by Conditional and Specifically Targeted Gene Ablation in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 5253–5258. [Google Scholar] [CrossRef]
- Sung, M.J.; Davaatseren, M.; Kim, W.; Park, S.K.; Kim, S.H.; Hur, H.J.; Kim, M.S.; Kim, Y.S.; Kwon, D.Y. Vitisin A suppresses LPS-induced NO production by inhibiting ERK, p38, and NF-kappaB activation in RAW 264.7 cells. Int. Immunopharmacol. 2009, 9, 319–323. [Google Scholar] [CrossRef]
- Wang, H.; Song, D.; Wei, L.; Huang, L.; Wei, D.; Su, Y.; Liang, J.; Lian, H.; Zhao, J. Ethyl caffeate inhibits macrophage polarization via SIRT1/NF-κB to attenuate traumatic heterotopic ossification in mice. Biomed. Pharmacother. 2023, 161, 114508. [Google Scholar] [CrossRef]
- Choi, D.Y.; Lee, J.W.; Lin, G.; Lee, Y.K.; Lee, Y.H.; Choi, I.S.; Han, S.B.; Jung, J.K.; Kim, Y.H.; Kim, K.H.; et al. Obovatol attenuates LPS-induced memory impairments in mice via inhibition of NF-κB signaling pathway. Neurochem. Int. 2012, 60, 68–77. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, B.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Rapaka, D.; Bitra, V.R.; Vishala, T.C.; Akula, A. Vitis vinifera acts as anti-Alzheimer’s agent by modulating biochemical parameters implicated in cognition and memory. J. Ayurveda Integr. Med. 2019, 10, 241–247. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Kim, Y.S.; Kim, G.W.; Kim, W.J.; Hong, S.M.; Kim, C.G.; Choi, D.K. Standardized extract of Glehnia Littoralis abrogates memory impairment and neuroinflammation by regulation of CREB/BDNF and NF-κB/MAPK signaling in scopolamine-induced amnesic mice model. Biomed. Pharmacother. 2023, 165, 115106. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]




| RT (min) | m/z ([M + H]+) | Formula ([M + H]+) | Compound |
|---|---|---|---|
| 7.71 | 453.1317 | C28H21O6 | Viniferifuran |
| 8.49 | 267.1558 | C11H13O4 | Ethyl caffeate |
| 9.38 | 249.1113 | C14H17O4 | Prenyl caffeate |
| 11.18 | 455.1469 | C28H23O6 | Maximol A or Pallidol |
| 12.90 | 907.2713 | C56H43O12 | H-ampelopsin or viticanol B or viticanol C or Vitisin A or Vitisin C or Hopeaphenol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Lee, S.-B.; Kang, S.-I.; Kim, W.-J.; Choi, D.-K. A Chloroform Fraction Derived from Vitis vinifera Root Ethanol Extract Attenuates Lipopolysaccharide-Induced Inflammatory Responses and Cognitive Dysfunction in BV-2 Microglia Cells and C57BL/6J Mouse Model. Int. J. Mol. Sci. 2025, 26, 3126. https://doi.org/10.3390/ijms26073126
Kim Y-S, Lee S-B, Kang S-I, Kim W-J, Choi D-K. A Chloroform Fraction Derived from Vitis vinifera Root Ethanol Extract Attenuates Lipopolysaccharide-Induced Inflammatory Responses and Cognitive Dysfunction in BV-2 Microglia Cells and C57BL/6J Mouse Model. International Journal of Molecular Sciences. 2025; 26(7):3126. https://doi.org/10.3390/ijms26073126
Chicago/Turabian StyleKim, Yon-Suk, Sang-Bong Lee, Shin-Il Kang, Woo-Jung Kim, and Dong-Kug Choi. 2025. "A Chloroform Fraction Derived from Vitis vinifera Root Ethanol Extract Attenuates Lipopolysaccharide-Induced Inflammatory Responses and Cognitive Dysfunction in BV-2 Microglia Cells and C57BL/6J Mouse Model" International Journal of Molecular Sciences 26, no. 7: 3126. https://doi.org/10.3390/ijms26073126
APA StyleKim, Y.-S., Lee, S.-B., Kang, S.-I., Kim, W.-J., & Choi, D.-K. (2025). A Chloroform Fraction Derived from Vitis vinifera Root Ethanol Extract Attenuates Lipopolysaccharide-Induced Inflammatory Responses and Cognitive Dysfunction in BV-2 Microglia Cells and C57BL/6J Mouse Model. International Journal of Molecular Sciences, 26(7), 3126. https://doi.org/10.3390/ijms26073126






