High Glucose Reduces Influenza and Parainfluenza Virus Productivity by Altering Glycolytic Pattern in A549 Cells
Abstract
1. Introduction
2. Results
2.1. Determination of Influenza and Sendai Virus Replication in Normal, +10 mM, and +25 mM Glucose Concentrations
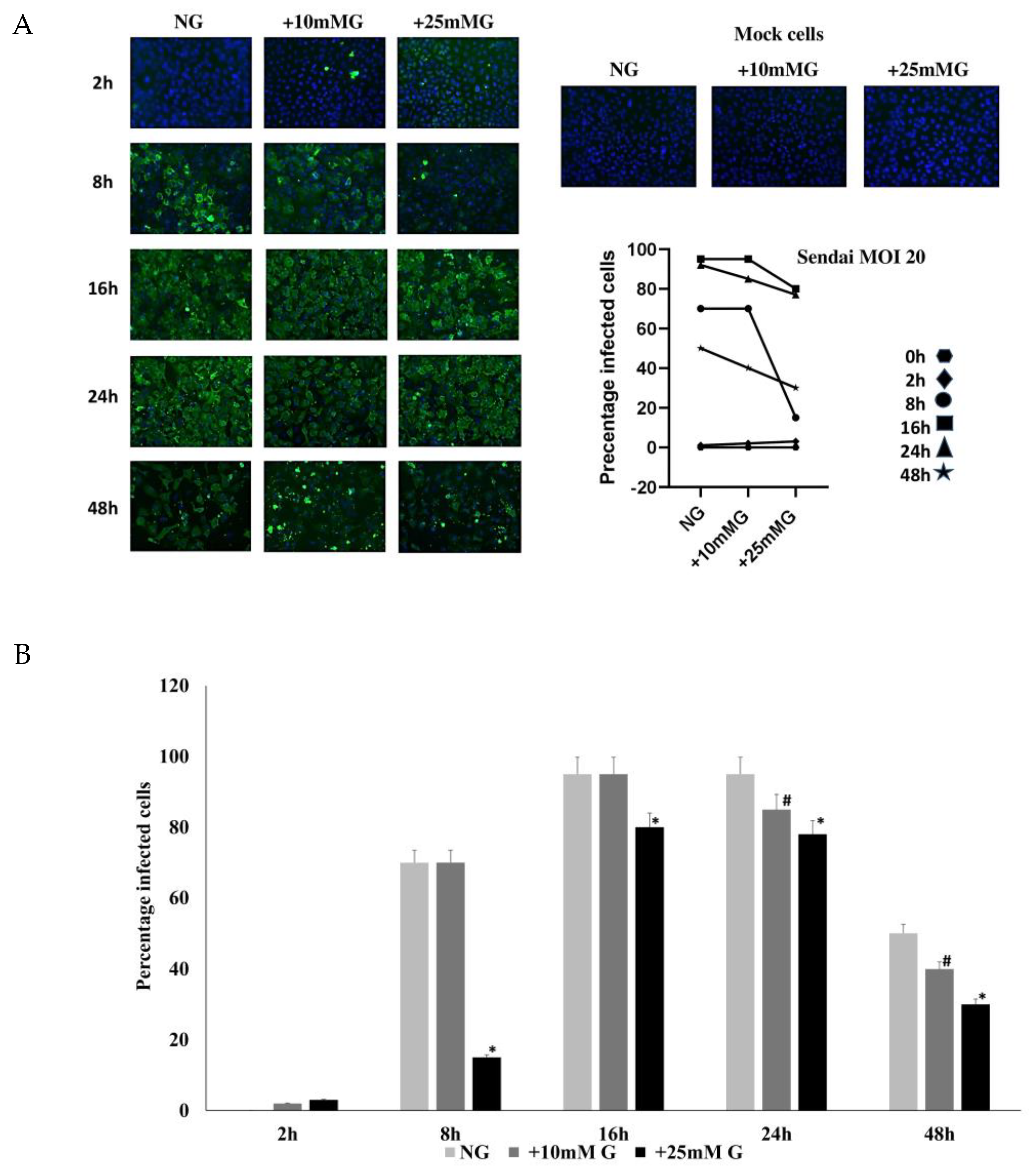
2.2. Immunofluorescence Staining of H1N1 or Sendai Virus NP in A549 Cells Control and Infected with H1N1 (MOI 20) for 2–48 h PI in NG, +10 mM G, and +25 mM G
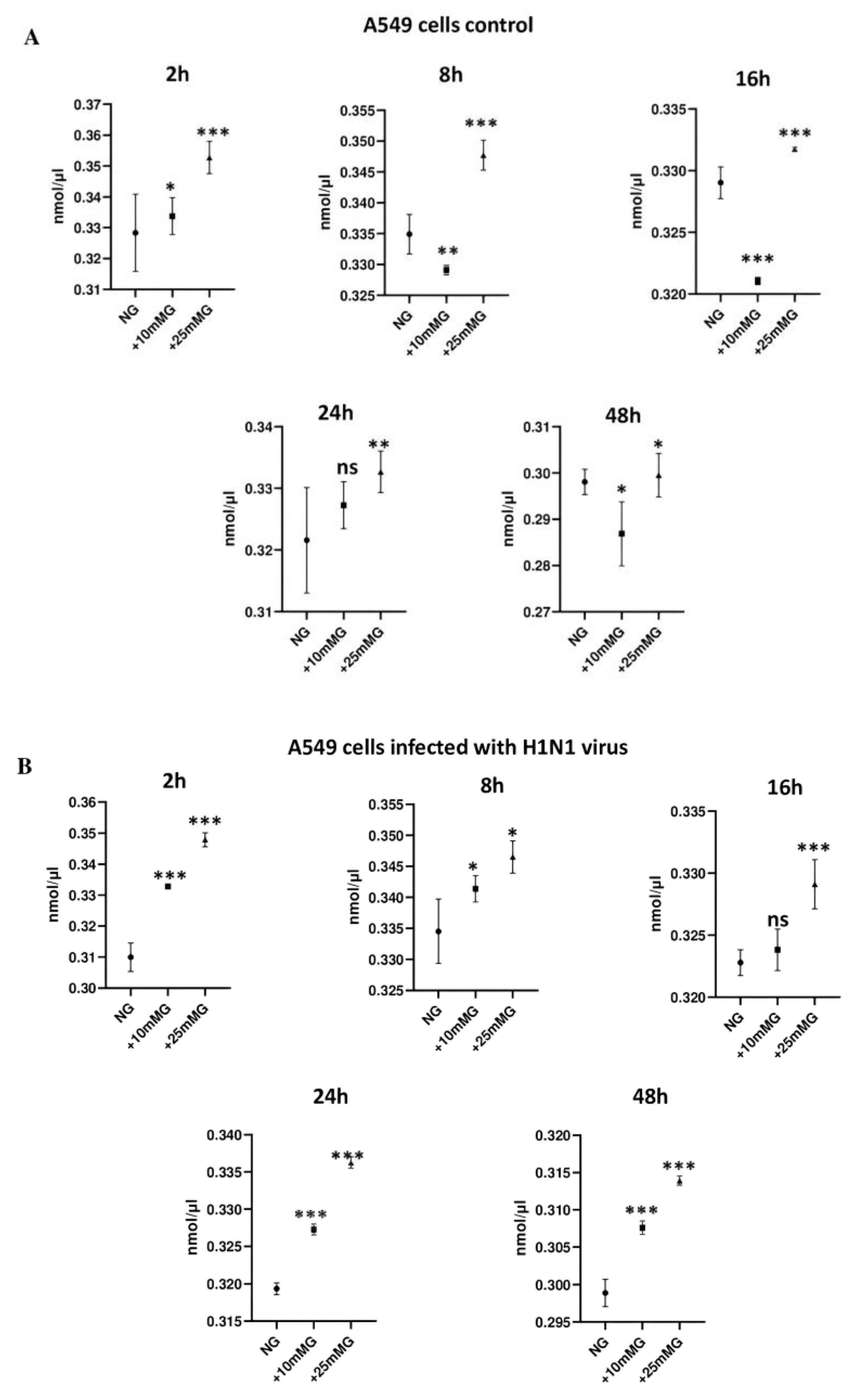
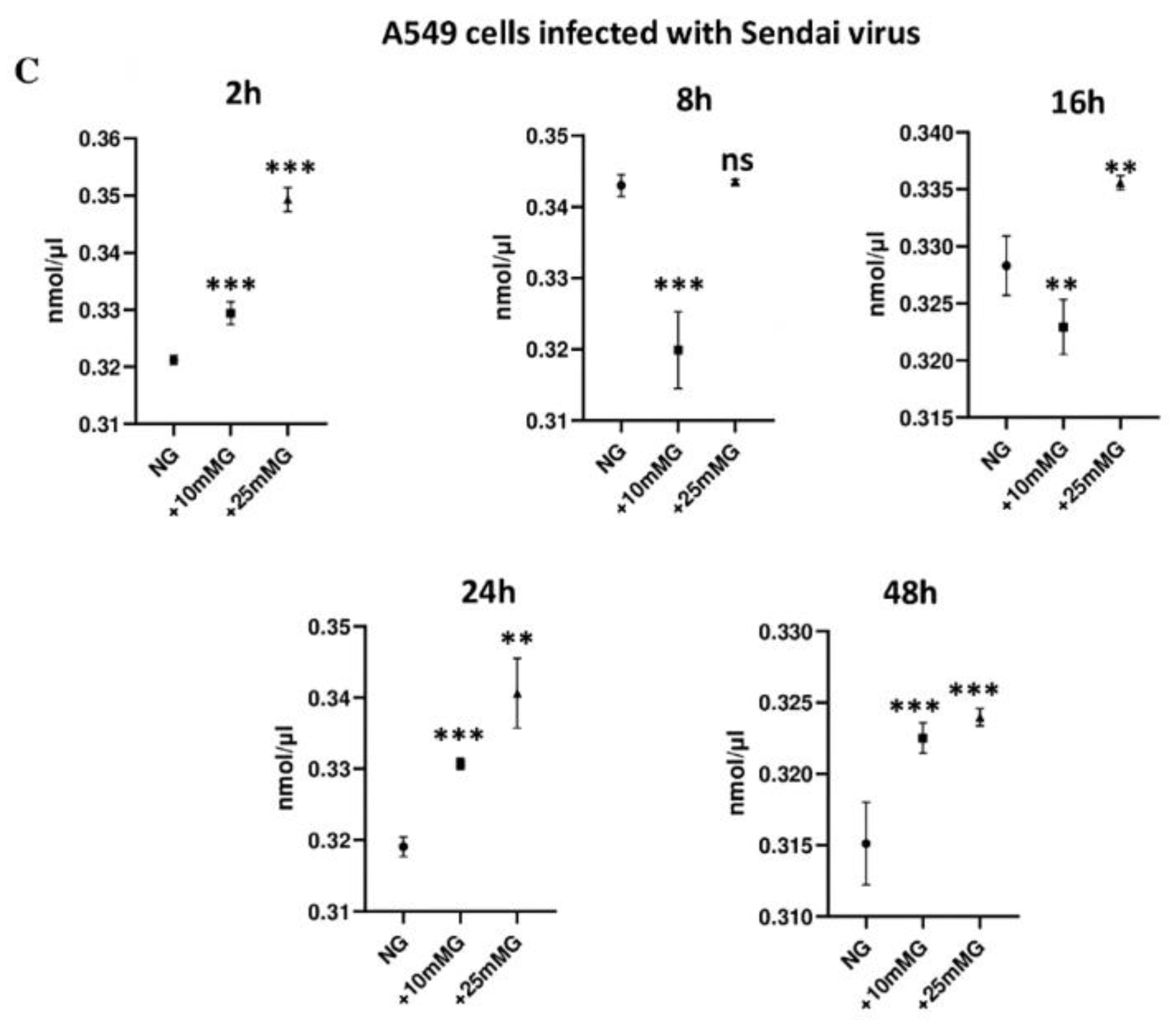
2.3. Lactate Concentration (nmol/µL) in Culture Media of A549 Cells Control and Infected with H1N1 or Sendai Virus (MOI 10) at 2–48 h PI in NG, +10 mM G, and +25 mM G
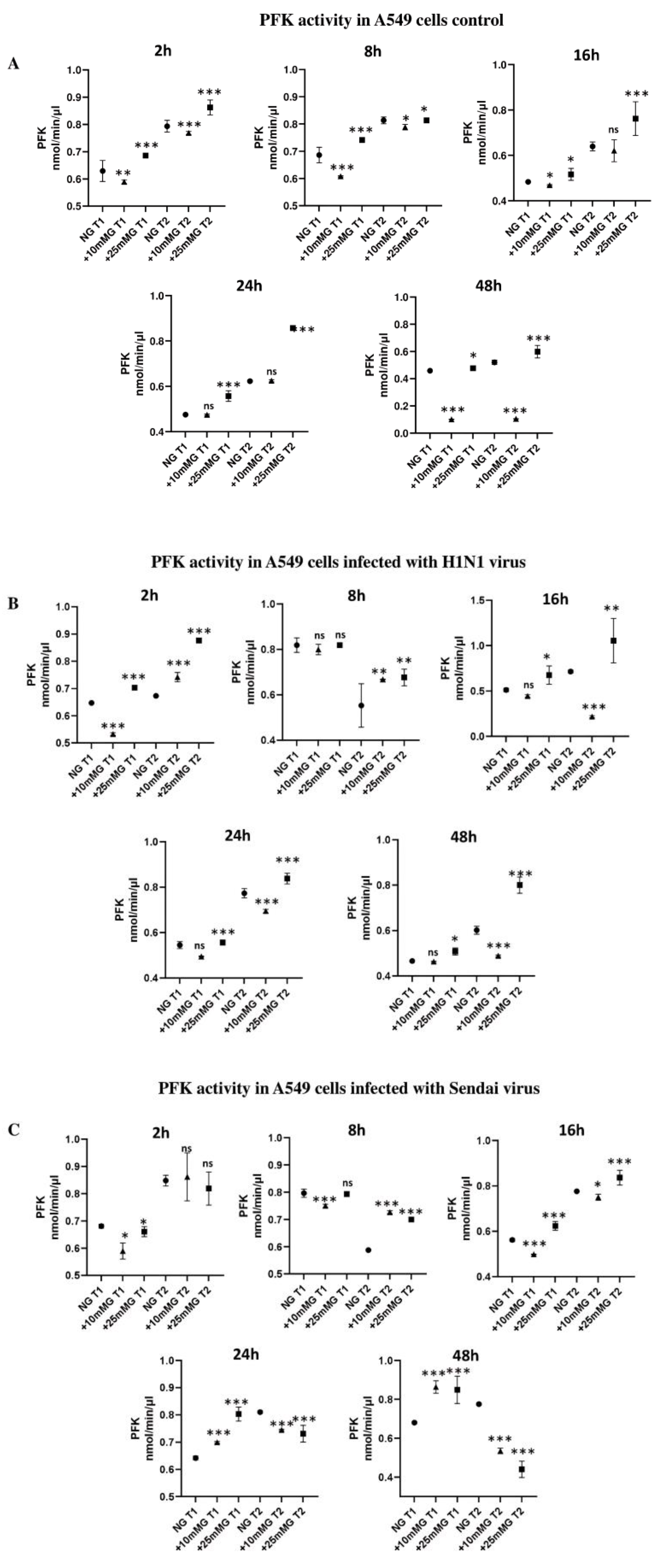
2.4. Phosphofructokinase (PFK) Enzyme Activity (nmol/min/µL) in Culture Media of A549 Cells Control and Infected with H1N1 or Sendai Virus (MOI 10) at 2–48 h PI in NG, +10 mM G, and +25 mM G
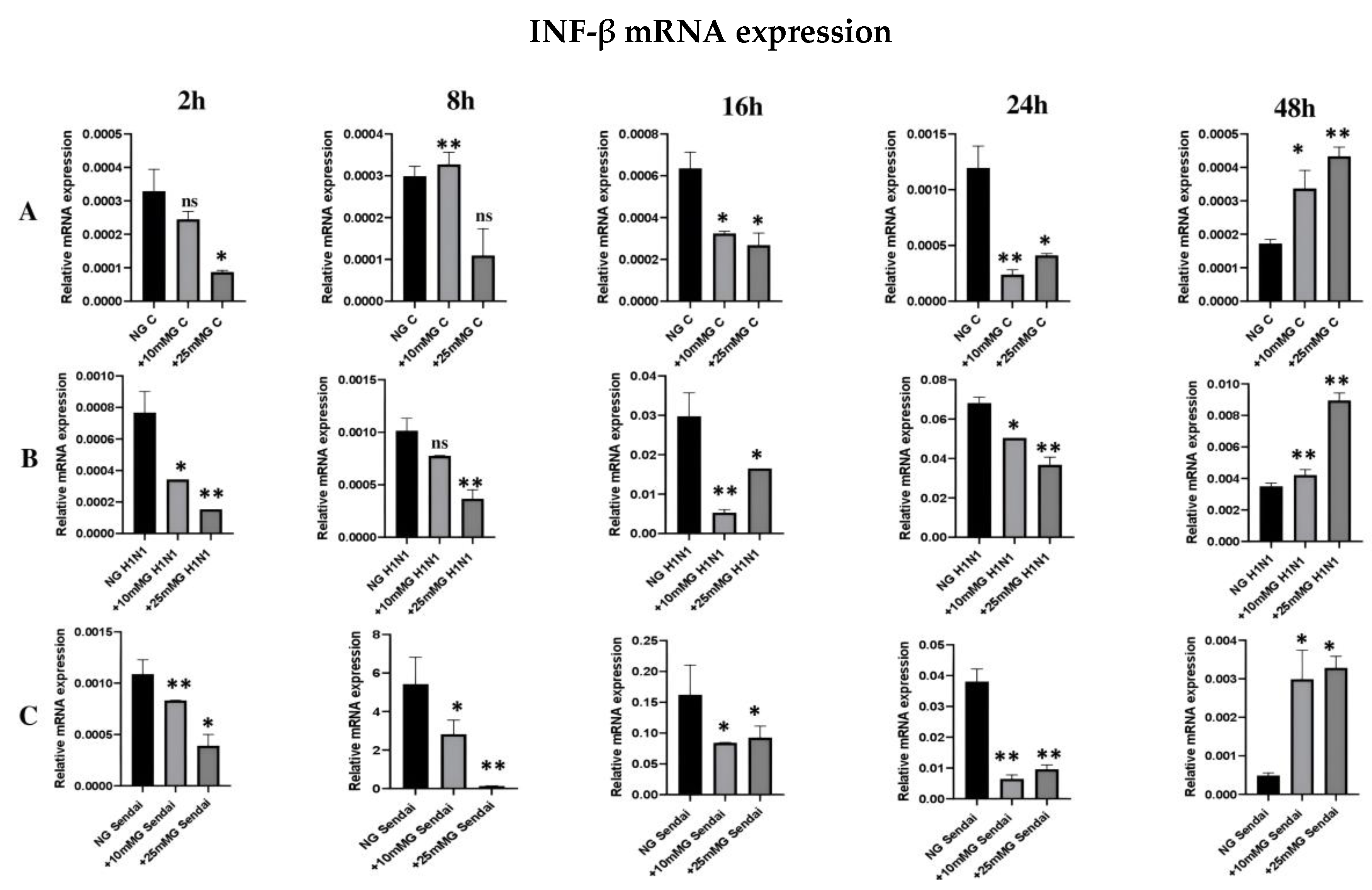
2.5. qRT-PCR Expression of IFN-β mRNA in the Lysates of Uninfected or H1N1 or Sendai Virus-Infected A549 Cells in Normal, +10 mM Glucose, or +25 mM Glucose Concentration
2.6. qRT-PCR Analysis of TGF-β1 mRNA Expression in Total Cellular RNA of Uninfected or H1N1 or Sendai Virus-Infected A549 Cells in Normal, +10 mM Glucose, or +25 mM Glucose Concentration
3. Discussion
4. Methods
4.1. Cell Culture and Viruses
4.2. Determination of Influenza and Sendai Viruses Productivity in Normal, +10 mM, and +25 mM Glucose Concentrations by Immunofluorescence Staining (IF)
4.3. Lactate Assay
4.4. Phosphofructokinase (PFK) Assay
4.5. Quantitative Real-Time Reverse Transcriptase PCR (qRT-PCR) of H1N1 and Sendai Viruse Matrix Protein Genes (M1 and M, respectively), IFN-β, or TGF-β1 mRNA
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Høiby, N. Pandemics: Past, present, future: That is like choosing between cholera and plague. APMIS 2021, 129, 352–371. [Google Scholar] [CrossRef] [PubMed]
- Castro, Í.A.; Yang, Y.; Gnazzo, V.; Kim, D.H.; Van Dyken, S.J.; López, C.B. Murine parainfluenza virus persists in lung innate immune cells sustaining chronic lung pathology. Nat. Microbiol. 2024, 9, 2803–2816. [Google Scholar] [CrossRef]
- Matikainen, S.; Sirén, J.; Tissari, J.; Veckman, V.; Pirhonen, J.; Severa, M.; Sun, Q.; Lin, R.; Meri, S.; Uzé, G.; et al. Tumor necrosis factor alpha enhances influenza A virus-induced expression of antiviral cytokines by activating RIG-I gene expression. J. Virol. 2006, 80, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, R.; Evans, E.A. The metabolism of virus-infected animal cells. Prog. Med. Virol. 1959, 2, 73–105. [Google Scholar]
- Awad, K.; Maghraby, A.S.; Abd-Elshafy, D.N.; Bahgat, M.M. Carbohydrate metabolic signatures in immune cells: Response to infection. Front. Immunol. 2022, 13, 912899. [Google Scholar] [CrossRef]
- Ritter, J.B.; Wahl, A.S.; Freund, S.; Genzel, Y.; Reichl, U. Metabolic effects of influenza virus infection in cultured animal cells: Intra- and extracellular metabolite profiling. BMC Syst. Biol. 2010, 4, 61. [Google Scholar]
- Motawi, T.K.; Shahin, N.N.; Maghraby, A.S.; Kirschfink, M.; Abd-Elshafy, D.N.; Awad, K.; Bahgat, M.M. H1N1 Infection Reduces Glucose Level in Human U937 Monocytes Culture. Viral Immunol. 2020, 33, 384–390. [Google Scholar] [CrossRef]
- Motawi, T.K.; Shahin, N.N.; Awad, K.; Maghraby, A.S.; Abd-Elshafy, D.N.; Bahgat, M.M. Glycolytic and immunological alterations in human U937 monocytes in response to H1N1 infection. IUBMB Life 2020, 72, 2481–2498. [Google Scholar] [CrossRef]
- Thyrsted, J.; Storgaard, J.; Blay-Cadanet, J.; Heinz, A.; Thielke, A.L.; Crotta, S.; de Paoli, F.; Olagnier, D.; Wack, A.; Hiller, K.; et al. Influenza A induces lactate formation to inhibit type I IFN in primary human airway epithelium. iScience 2021, 24, 103300. [Google Scholar] [CrossRef] [PubMed]
- Veckman, V.; Osterlund, P.; Fagerlund, R.; Melén, K.; Matikainen, S.; Julkunen, I. TNF-alpha and IFN-alpha enhance influenza-A-virus-induced chemokine gene expression in human A549 lung epithelial cells. Virology 2006, 345, 96–104. [Google Scholar] [CrossRef]
- Jakovcevski, I.; Andjus, P.R.; Förster, E. Editorial: Extracellular matrix in development and disorders of the nervous system. Front. Cell Dev. Biol. 2023, 11, 1153484. [Google Scholar]
- Ulyanova, V.; Mahmud, R.S.; Laikov, A.; Dudkina, E.; Markelova, M.; Mostafa, A.; Pleschka, S.; Ilinskaya, O. Anti-Influenza Activity of the Ribonuclease Binase: Cellular Targets Detected by Quantitative Proteomics. Int. J. Mol. Sci. 2020, 21, 8294. [Google Scholar] [CrossRef]
- Levin, S.; Hahn, T. Interferon deficiency syndrome. Clin. Exp. Immunol. 1985, 60, 267–273. [Google Scholar] [PubMed]
- Jarosz, A.; Wrona, J.; Balcerzyk-Matić, A.; Szyluk, K.; Nowak, T.; Iwanicki, T.; Iwanicka, J.; Kalita, M.; Kania, W.; Gawron, K.; et al. Association of the TGFB1 gene polymorphisms with pain symptoms and the effectiveness of platelet-rich plasma in the treatment of lateral elbow tendinopathy: A prospective cohort study. Int. J. Mol. Sci. 2025, 26, 2431. [Google Scholar] [CrossRef]
- Kawata, M.; Koinuma, D.; Ogami, T.; Umezawa, K.; Iwata, C.; Watabe, T.; Miyazono, K. TGF-β-induced epithelial-mesenchymal transition of A549 lung adenocarcinoma cells is enhanced by pro-inflammatory cytokines derived from RAW 264.7 macrophage cells. J. Biochem. 2012, 151, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Awad, K.; Kakkola, L.; Julkunen, I. High Glucose increases lactate and induces the transforming growth factor beta-Smad 1/5 atherogenic pathway in primary human macrophages. Biomedicines 2024, 12, 1575. [Google Scholar] [CrossRef]
- Ward, R.; Ergul, A. Relationship of endothelin-1 and NLRP3 inflammasome activation in HT22 hippocampal cells in diabetes. Life Sci. 2016, 159, 97–103. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, F.; Liu, Y.; Yi, Z.; Wang, X.; Wu, Y.; Gong, P. 1α,25 Dihydroxyvitamin D3 promotes angiogenesis by alleviating AGEs-induced autophagy. Arch. Biochem. Biophys. 2021, 712, 109041. [Google Scholar] [CrossRef]
- Pirhonen, J.; Sareneva, T.; Kurimoto, M.; Julkunen, I.; Matikainen, S. Virus infection activates IL-1β and IL-18 production in human macrophages by a caspase-1-dependent pathway. J. Immunol. 1999, 162, 7322–7373. [Google Scholar]
- Kohio, H.P.; Adamson, A.L. Glycolytic control of vacuolar-type ATPase activity: A mechanism to regulate influenza viral infection. Virology 2013, 444, 301–309. [Google Scholar] [CrossRef]
- Racker, E.; Krimsky, I. Inhibition of phosphorylation of glucose in mouse brains by viruses and its prevention by preparations of diphosphopyridine nucleotide. J. Exp. Med. 1946, 84, 191–203. [Google Scholar] [PubMed]
- Hulme, K.D.; Yan, L.; Marshall, R.J.; Bloxham, C.J.; Upton, K.R.; Hasnain, S.Z.; Bielefeldt-Ohmann, H.; Loh, Z.; Ronacher, K.; Chew, K.Y.; et al. High glucose levels increase influenza-associated damage to the pulmonary epithelial-endothelial barrier. Elife 2020, 9, e56907. [Google Scholar] [CrossRef] [PubMed]
- Rivelli, J.F.; Amaiden, M.R.; Monesterolo, N.E.; Previtali, G.; Santander, V.S.; Fernandez, A.; Arce, C.A.; Casale, C.H. High glucose levels induce inhibition of Na,K-ATPase via stimulation of Aldose Reductase, formation of microtubules and formation of an acetylated tubulin/Na,K-ATPase complex. Int. J. Biochem. Cell Biol. 2012, 44, 1203–1213. [Google Scholar] [CrossRef]
- Shauly-Aharonov, M.; Shafrir, A.; Paltiel, O.; Calderon-Margalit, R.; Safadi, R.; Bicher, R.; Barenholz-Goultschin, O.; Stokar, J. Both high and low pre-infection glucose levels associated with increased risk for severe COVID-19, New insights from a population-based study. PLoS ONE 2021, 16, e0254847. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Atkin, S.; Kesharwani, P.; Jamialahmadi, T.; Sahebkar, A. Anti-inflammatory effects of sodium-glucose cotransporter-2 inhibitors in COVID-19. IUBMB Life 2023, 75, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Kasper, J.; van der Aa, S.; Andeweg, A.C.; Zaaraoui-Boutahar, F.; Goeijenbier, M.; Richard, M.; Herold, S.; Becker, C.; Scott, D.P.; et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur. Respir. J. 2016, 47, 954–966. [Google Scholar] [CrossRef]
- Hegelund, M.H.; Glenthøj, A.; Ryrsø, C.K.; Ritz, C.; Dungu, A.M.; Sejdic, A.; List, K.C.K.; Krogh-Madsen, R.; Lindegaard, B.; Kurtzhals, J.A.L.; et al. Biomarkers for iron metabolism among patients hospitalized with community-acquired pneumonia caused by infection with SARS-CoV-2, bacteria, and influenza. APMIS 2022, 130, 590–596. [Google Scholar] [CrossRef]
- Denney, L.; Branchett, W.; Gregory, L.G.; Oliver, R.A.; Lloyd, C.M. Epithelial-derived TGF-β1 acts as a pro-viral factor in the lung during influenza A infection. Mucosal Immunol. 2018, 11, 523–535. [Google Scholar] [CrossRef]
- Kleinehr, J.; Schöfbänker, M.; Daniel, K.; Günl, F.; Mohamed, F.F.; Janowski, J.; Brunotte, L.; Boergeling, Y.; Liebmann, M.; Behrens, M.; et al. Glycolytic interference blocks influenza A virus propagation by impairing viral polymerase-driven synthesis of genomic vRNA. PLoS Pathog. 2023, 19, e1010986. [Google Scholar] [CrossRef]
- Awad, A.M.; Hansen, K.; Del Rio, D.; Flores, D.; Barghash, R.F.; Kakkola, L.; Julkunen, I.; Awad, K. Insights into COVID-19, Perspectives on Drug Remedies and Host Cell Responses. Biomolecules 2023, 13, 1452. [Google Scholar] [CrossRef]
- Kolehmainen, P.; Heroum, J.; Jalkanen, P.; Huttunen, M.; Toivonen, L.; Marjomäki, V.; Waris, M.; Smura, T.; Kakkola, L.; Tauriainen, S.; et al. Serological follow-up study indicates high seasonal coronavirus infection and reinfection rates in early childhood. Microbiol. Spectr. 2022, 10, e0196721. [Google Scholar] [CrossRef]
- Keskinen, P.; Nyqvist, M.; Sareneva, T.; Pirhonen, J.; Melen, K.; Julkunen, I. Impaired antiviral response in human hepatoma cells. Virology 1999, 263, 364–375. [Google Scholar] [PubMed]
- Julkunen, I.; Hautanen, A.; Keski-Oja, J. Interaction of viral envelope glycoproteins with fibronectin. Infect. Immun. 1983, 40, 876–881. [Google Scholar] [PubMed]
- Julkunen, I.; Melén, K.; Nyqvist, M.; Pirhonen, J.; Sareneva, T.; Matikainen, S. Inflammatory responses in influenza A virus infection. Vaccine 2000, 19 (Suppl. S1), S32–S37. [Google Scholar] [CrossRef]
- Ronni TSareneva, T.; Pirhonen, J.; Julkunen, I. Activation of IFN-a, IFN-y. MxA, and IFN regulatory factor I genes in influenza A virus-infected human peripheral blood mononuclear cells. J. Immunol. 1995, 154, 2764. [Google Scholar]
- Osterlund, P.; Pirhonen, J.; Ikonen, N.; Rönkkö, E.; Strengell, M.; Mäkelä, S.M.; Broman, M.; Hamming, O.J.; Hartmann, R.; Ziegler, T.; et al. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. J. Virol. 2010, 84, 1414–1422. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, K.; Abdelhadi, M.; Awad, A.M. High Glucose Reduces Influenza and Parainfluenza Virus Productivity by Altering Glycolytic Pattern in A549 Cells. Int. J. Mol. Sci. 2025, 26, 2975. https://doi.org/10.3390/ijms26072975
Awad K, Abdelhadi M, Awad AM. High Glucose Reduces Influenza and Parainfluenza Virus Productivity by Altering Glycolytic Pattern in A549 Cells. International Journal of Molecular Sciences. 2025; 26(7):2975. https://doi.org/10.3390/ijms26072975
Chicago/Turabian StyleAwad, Kareem, Maha Abdelhadi, and Ahmed M. Awad. 2025. "High Glucose Reduces Influenza and Parainfluenza Virus Productivity by Altering Glycolytic Pattern in A549 Cells" International Journal of Molecular Sciences 26, no. 7: 2975. https://doi.org/10.3390/ijms26072975
APA StyleAwad, K., Abdelhadi, M., & Awad, A. M. (2025). High Glucose Reduces Influenza and Parainfluenza Virus Productivity by Altering Glycolytic Pattern in A549 Cells. International Journal of Molecular Sciences, 26(7), 2975. https://doi.org/10.3390/ijms26072975





