Molecular Insights into the Positive Role of Soybean Nodulation by GmWRKY17
Abstract
:1. Introduction
2. Results
2.1. Multiple Sequence Alignment and Phylogenetic Analysis of GmWRKY17
2.2. Expression Pattern of GmWRKY17 in Response to Rhizobium Inoculation
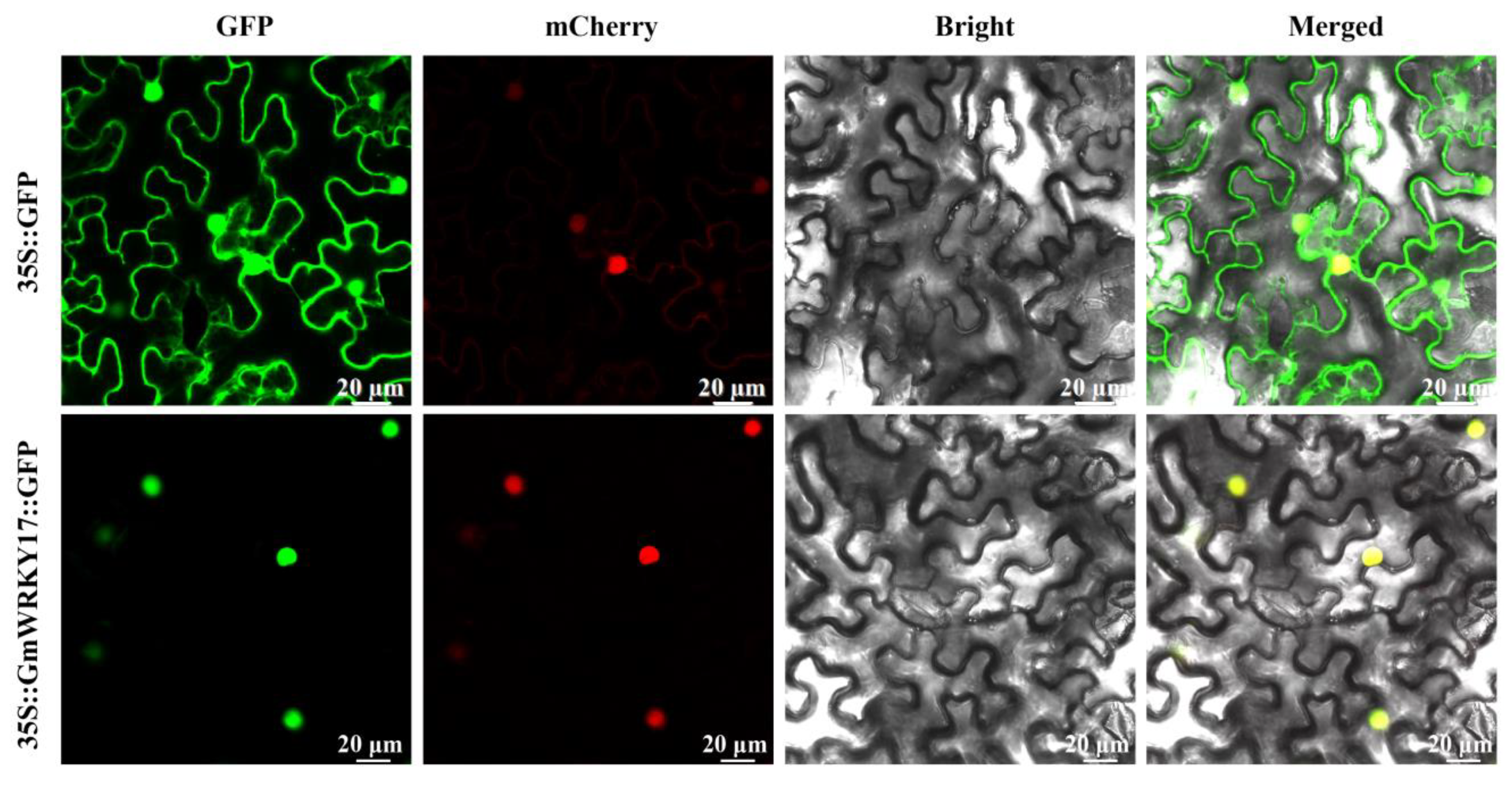
2.3. GmWRKY17 Was Localized in the Nucleus
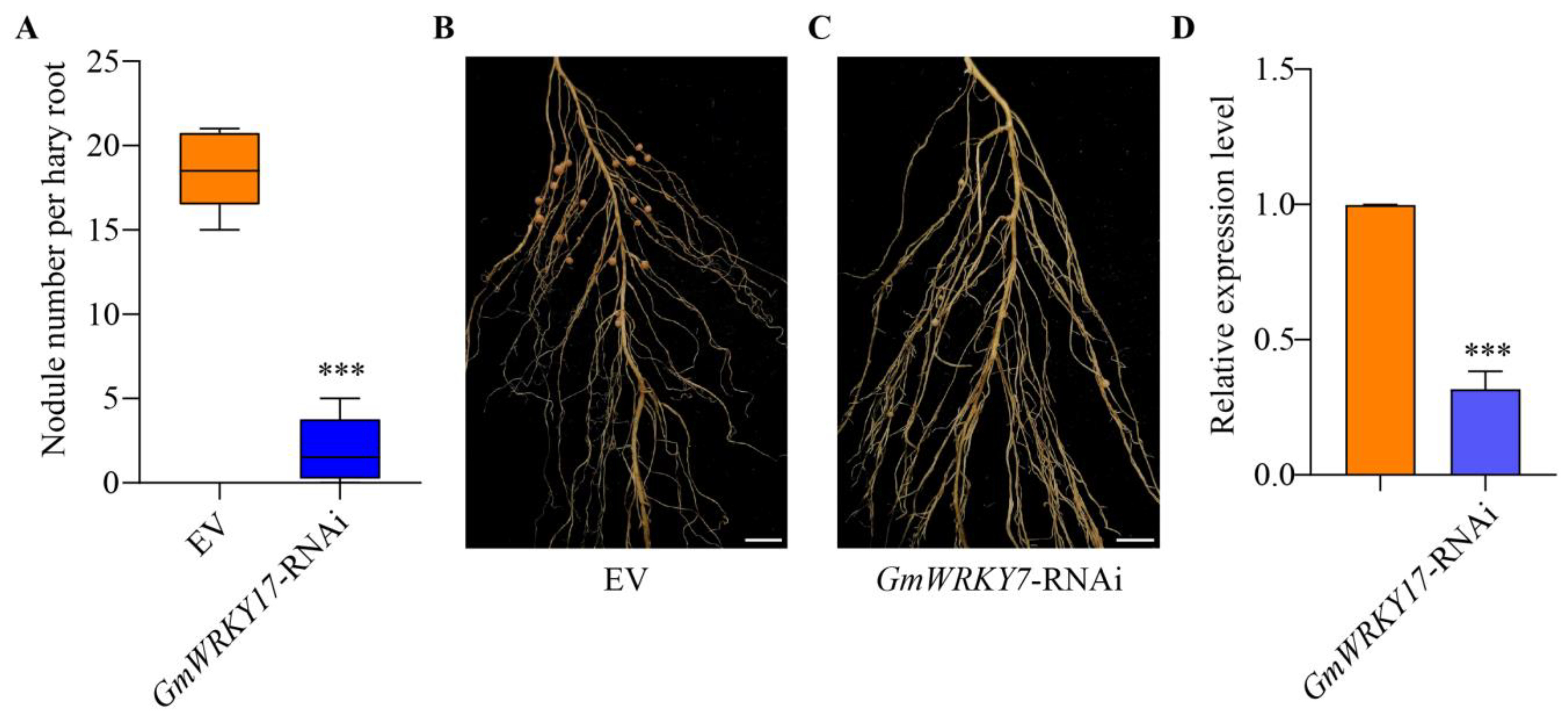
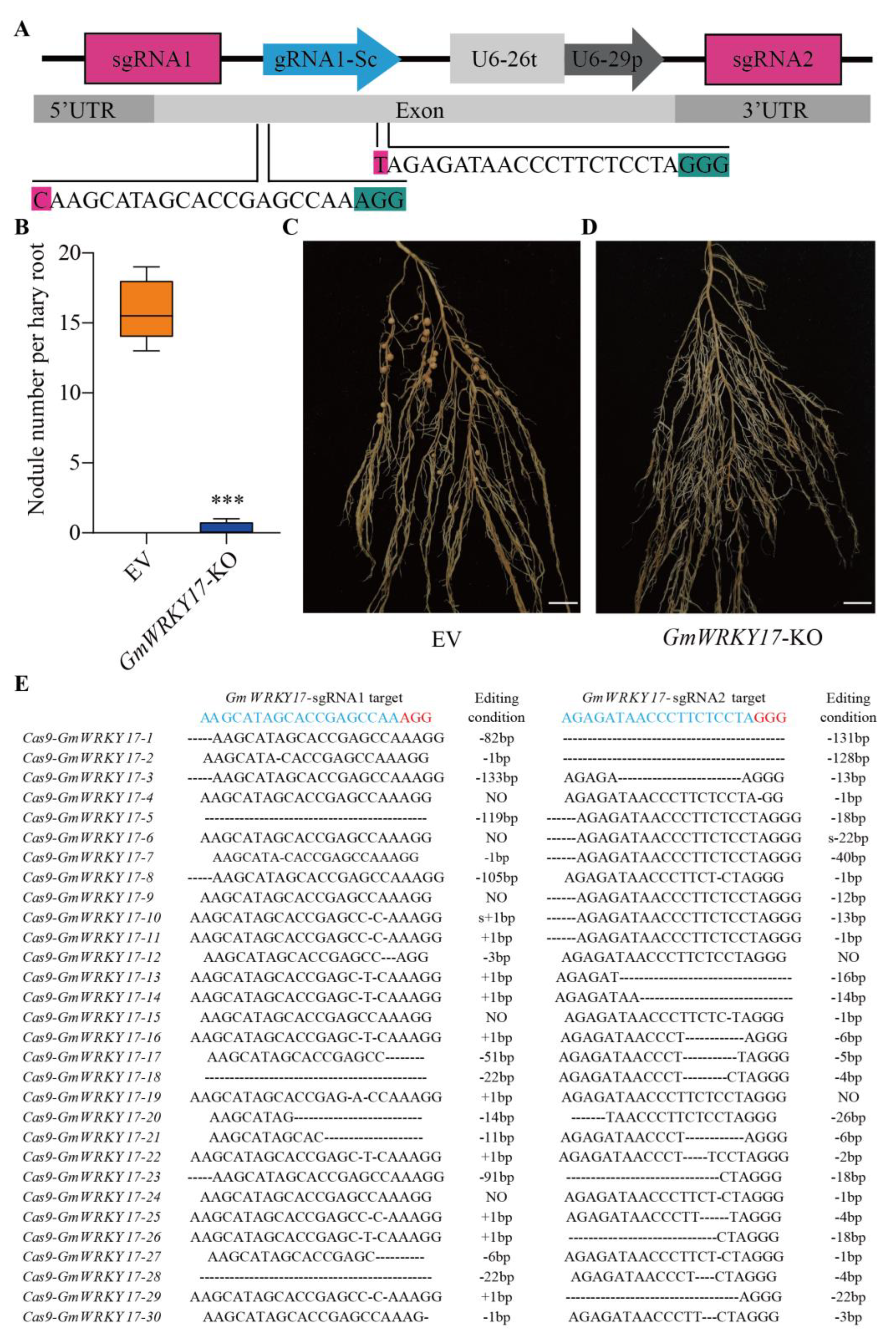
2.4. GmWRKY17 Is an Important Regulator in Regulating Soybean Nodulation
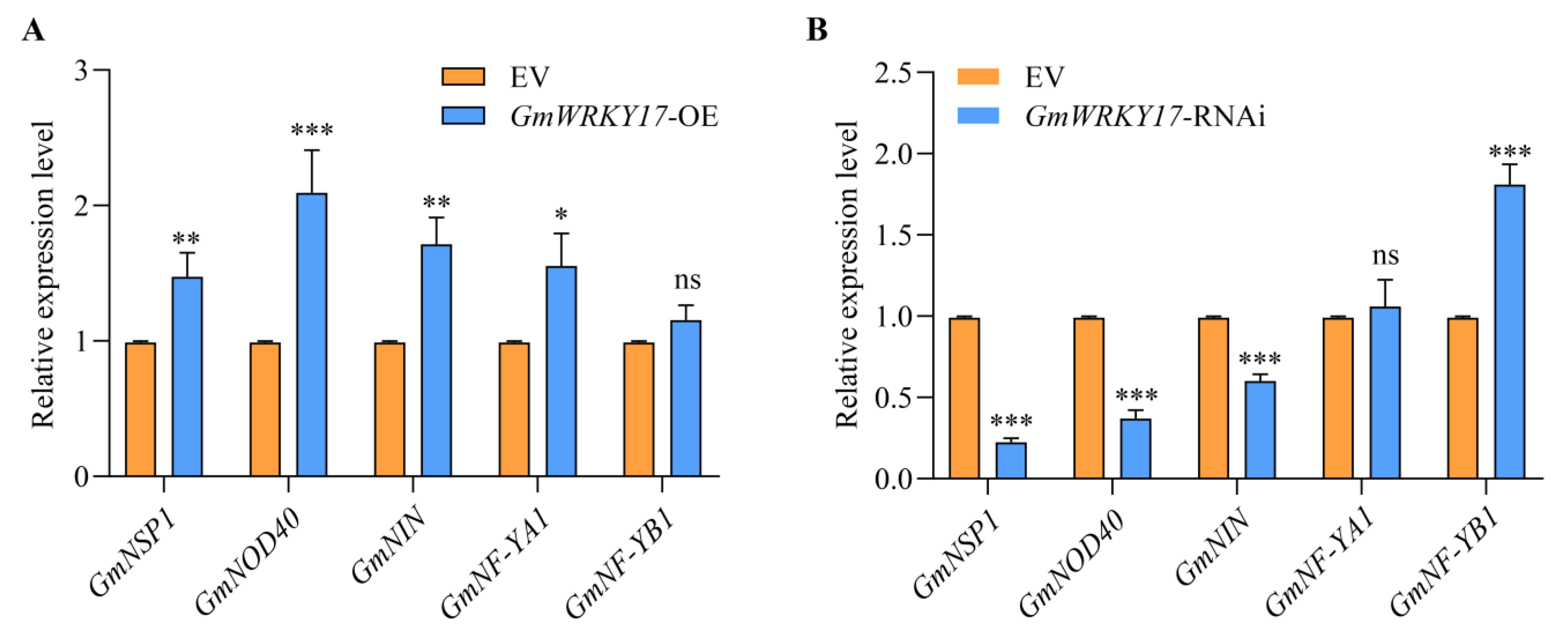
2.5. GmWRKY17 Regulates the Expression of Critical Genes Associated with the NF Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Bioinformatics and Phylogenetic Analysis of GmWRKY17
4.2. Rhizobial Strain, Plant Materials and Growth Conditions
4.3. RNA Extraction and Real-Time RT-qPCR
4.4. Cloning of the GmWRKY17 Gene and Vector Construction
4.5. Genetic Transformation of Soybean Hairy Roots and Subcellular Localization
4.6. Histochemical Analysis of GmWRKY17 Expression
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global Inputs of Biological Nitrogen Fixation in Agricultural Systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Zhao, B.; Jia, X.; Yu, N.; Murray, J.D.; Yi, K.; Wang, E. Microbe-Dependent and Independent Nitrogen and Phosphate Acquisition and Regulation in Plants. New Phytol. 2024, 242, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, T.; Yoro, E.; Kawaguchi, M. Chapter Three—Leguminous Plants: Inventors of Root Nodules to Accommodate Symbiotic Bacteria. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 316, pp. 111–158. [Google Scholar]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms Underlying Legume-Rhizobium Symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, J.J.; Hui, C.; Keet, J.-H.; Ellis, A.G. Co-Introduction vs Ecological Fitting as Pathways to the Establishment of Effective Mutualisms during Biological Invasions. New Phytol. 2017, 215, 1354–1360. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume Nodulation: The Host Controls the Party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Stacey, G.; McAlvin, C.B.; Kim, S.-Y.; Olivares, J.; Soto, M.J. Effects of Endogenous Salicylic Acid on Nodulation in the Model Legumes Lotus japonicus and Medicago truncatula. Plant Physiol. 2006, 141, 1473–1481. [Google Scholar] [CrossRef]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Felle, H.H.; Umehara, Y.; Grønlund, M.; Sato, S.; Nakamura, Y.; Tabata, S.; Sandal, N.; et al. Plant Recognition of Symbiotic Bacteria Requires Two LysM Receptor-like Kinases. Nature 2003, 425, 585–592. [Google Scholar] [CrossRef]
- Madsen, E.B.; Madsen, L.H.; Radutoiu, S.; Olbryt, M.; Rakwalska, M.; Szczyglowski, K.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N.; et al. A Receptor Kinase Gene of the LysM Type Is Involved in Legume Perception of Rhizobial Signals. Nature 2003, 425, 637–640. [Google Scholar] [CrossRef]
- Limpens, E.; Franken, C.; Smit, P.; Willemse, J.; Bisseling, T.; Geurts, R. LysM Domain Receptor Kinases Regulating Rhizobial Nod Factor-Induced Infection. Science 2003, 302, 630–633. [Google Scholar] [CrossRef]
- Arrighi, J.-F.; Barre, A.; Ben Amor, B.; Bersoult, A.; Soriano, L.C.; Mirabella, R.; de Carvalho-Niebel, F.; Journet, E.-P.; Ghérardi, M.; Huguet, T.; et al. The Medicago Truncatula Lysin Motif-Receptor-like Kinase Gene Family Includes NFP and New Nodule-Expressed Genes. Plant Physiol. 2006, 142, 265–279. [Google Scholar] [CrossRef]
- Charpentier, M.; Oldroyd, G.E.D. Nuclear Calcium Signaling in Plants. Plant Physiol. 2013, 163, 496–503. [Google Scholar] [PubMed]
- Lévy, J.; Bres, C.; Geurts, R.; Chalhoub, B.; Kulikova, O.; Duc, G.; Journet, E.-P.; Ané, J.-M.; Lauber, E.; Bisseling, T.; et al. A Putative Ca2+ and Calmodulin-Dependent Protein Kinase Required for Bacterial and Fungal Symbioses. Science 2004, 303, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Kim, J.; Muñoz, A.; Heckmann, A.B.; Downie, J.A.; Oldroyd, G.E.D. GRAS Proteins Form a DNA Binding Complex to Induce Gene Expression during Nodulation Signaling in Medicago truncatula. Plant Cell 2009, 21, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, H.; Luo, D.; Yu, N.; Dong, W.; Wang, C.; Zhang, X.; Dai, H.; Yang, J.; Wang, E. DELLA Proteins Are Common Components of Symbiotic Rhizobial and Mycorrhizal Signalling Pathways. Nat. Commun. 2016, 7, 12433. [Google Scholar]
- Heckmann, A.B.; Sandal, N.; Bek, A.S.; Madsen, L.H.; Jurkiewicz, A.; Nielsen, M.W.; Tirichine, L.; Stougaard, J. Cytokinin Induction of Root Nodule Primordia in Lotus japonicus Is Regulated by a Mechanism Operating in the Root Cortex. Mol. Plant-Microbe Interact. 2011, 24, 1385–1395. [Google Scholar]
- Kosuta, S.; Held, M.; Hossain, M.S.; Morieri, G.; Macgillivary, A.; Johansen, C.; Antolín-Llovera, M.; Parniske, M.; Oldroyd, G.E.D.; Downie, A.J.; et al. Lotus japonicus symRK-14 Uncouples the Cortical and Epidermal Symbiotic Program. Plant J. 2011, 67, 929–940. [Google Scholar]
- Popp, C.; Ott, T. Regulation of Signal Transduction and Bacterial Infection during Root Nodule Symbiosis. Curr. Opin. Plant Biol. 2011, 14, 458–467. [Google Scholar]
- Suzaki, T.; Ito, M.; Yoro, E.; Sato, S.; Hirakawa, H.; Takeda, N.; Kawaguchi, M. Endoreduplication-Mediated Initiation of Symbiotic Organ Development in Lotus japonicus. Development 2014, 141, 2441–2445. [Google Scholar]
- Liu, H.; Zhang, C.; Yang, J.; Yu, N.; Wang, E. Hormone Modulation of Legume-Rhizobial Symbiosis. J. Integr. Plant Biol. 2018, 60, 632–648. [Google Scholar] [CrossRef]
- Guinel, F.C. Ethylene, a Hormone at the Center-Stage of Nodulation. Front. Plant Sci. 2015, 6, 1121. [Google Scholar]
- Chen, J.; Wang, Z.; Wang, L.; Hu, Y.; Yan, Q.; Lu, J.; Ren, Z.; Hong, Y.; Ji, H.; Wang, H.; et al. The B-Type Response Regulator GmRR11d Mediates Systemic Inhibition of Symbiotic Nodulation. Nat. Commun. 2022, 13, 7661. [Google Scholar] [PubMed]
- Cerri, M.R.; Frances, L.; Laloum, T.; Auriac, M.-C.; Niebel, A.; Oldroyd, G.E.D.; Barker, D.G.; Fournier, J.; de Carvalho-Niebel, F. Medicago truncatula ERN Transcription Factors: Regulatory Interplay with NSP1/NSP2 GRAS Factors and Expression Dynamics throughout Rhizobial Infection. Plant Physiol. 2012, 160, 2155–2172. [Google Scholar] [PubMed]
- Diédhiou, I.; Diouf, D. Transcription Factors Network in Root Endosymbiosis Establishment and Development. World J. Microbiol. Biotechnol. 2018, 34, 37. [Google Scholar]
- Liu, W.; Kohlen, W.; Lillo, A.; Op den Camp, R.; Ivanov, S.; Hartog, M.; Limpens, E.; Jamil, M.; Smaczniak, C.; Kaufmann, K.; et al. Strigolactone Biosynthesis in Medicago truncatula and Rice Requires the Symbiotic GRAS-Type Transcription Factors NSP1 and NSP2. Plant Cell 2011, 23, 3853–3865. [Google Scholar]
- Fu, M.; Sun, J.; Li, X.; Guan, Y.; Xie, F. Asymmetric Redundancy of Soybean Nodule Inception (NIN) Genes in Root Nodule Symbiosis. Plant Physiol. 2022, 188, 477–489. [Google Scholar]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA Encoding a Novel DNA-Binding Protein, SPF1, That Recognizes SP8 Sequences in the 5’ Upstream Regions of Genes Coding for Sporamin and Beta-Amylase from Sweet Potato. Mol. Gen. Genet. 1994, 244, 563–5716. [Google Scholar]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY Superfamily of Plant Transcription Factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar]
- Ciolkowski, I.; Wanke, D.; Birkenbihl, R.P.; Somssich, I.E. Studies on DNA-Binding Selectivity of WRKY Transcription Factors Lend Structural Clues into WRKY-Domain Function. Plant Mol. Biol. 2008, 68, 81–92. [Google Scholar]
- Ma, Z.; Hu, L. WRKY Transcription Factor Responses and Tolerance to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2024, 25, 6845. [Google Scholar] [CrossRef]
- Song, H.; Wang, P.; Hou, L.; Zhao, S.; Zhao, C.; Xia, H.; Li, P.; Zhang, Y.; Bian, X.; Wang, X. Global Analysis of WRKY Genes and Their Response to Dehydration and Salt Stress in Soybean. Front. Plant Sci. 2016, 7, 9. [Google Scholar]
- Zhou, Q.-Y.; Tian, A.-G.; Zou, H.-F.; Xie, Z.-M.; Lei, G.; Huang, J.; Wang, C.-M.; Wang, H.-W.; Zhang, J.-S.; Chen, S.-Y. Soybean WRKY-Type Transcription Factor Genes, GmWRKY13, GmWRKY21, and GmWRKY54, Confer Differential Tolerance to Abiotic Stresses in Transgenic Arabidopsis Plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, H.-W.; Li, Q.-T.; Wei, W.; Li, W.; Zhang, W.-K.; Ma, B.; Bi, Y.-D.; Lai, Y.-C.; Liu, X.-L.; et al. GmWRKY27 Interacts with GmMYB174 to Reduce Expression of GmNAC29 for Stress Tolerance in Soybean Plants. Plant J. 2015, 83, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-Y.; Du, Y.-T.; Ma, J.; Min, D.-H.; Jin, L.-G.; Chen, J.; Chen, M.; Zhou, Y.-B.; Ma, Y.-Z.; Xu, Z.-S.; et al. The WRKY Transcription Factor GmWRKY12 Confers Drought and Salt Tolerance in Soybean. Int. J. Mol. Sci. 2018, 19, 4087. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y. GmWRKY17-Mediated Transcriptional Regulation of GmDREB1D and GmABA2 Controls Drought Tolerance in Soybean. Plant Mol. Biol. 2023, 113, 157–170. [Google Scholar] [CrossRef]
- Ma, Q.; Xia, Z.; Cai, Z.; Li, L.; Cheng, Y.; Liu, J.; Nian, H. GmWRKY16 Enhances Drought and Salt Tolerance Through an ABA-Mediated Pathway in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1979. [Google Scholar] [CrossRef]
- Qiu, J.-L.; Fiil, B.K.; Petersen, K.; Nielsen, H.B.; Botanga, C.J.; Thorgrimsen, S.; Palma, K.; Suarez-Rodriguez, M.C.; Sandbech-Clausen, S.; Lichota, J.; et al. Arabidopsis MAP Kinase 4 Regulates Gene Expression through Transcription Factor Release in the Nucleus. EMBO J. 2008, 27, 2214–2221. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol 2016, 16, 86. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Li, D.; Hong, B.; Gao, J.; Zhang, Z. Rose WRKY13 Promotes Disease Protection to Botrytis by Enhancing Cytokinin Content and Reducing Abscisic Acid Signaling. Plant Physiol. 2023, 191, 679–693. [Google Scholar] [CrossRef]
- Penmetsa, R.V.; Uribe, P.; Anderson, J.; Lichtenzveig, J.; Gish, J.-C.; Nam, Y.W.; Engstrom, E.; Xu, K.; Sckisel, G.; Pereira, M.; et al. The Medicago Truncatula Ortholog of Arabidopsis EIN2, Sickle, Is a Negative Regulator of Symbiotic and Pathogenic Microbial Associations. Plant J. 2008, 55, 580–595. [Google Scholar] [CrossRef]
- Ding, Y.; Kalo, P.; Yendrek, C.; Sun, J.; Liang, Y.; Marsh, J.F.; Harris, J.M.; Oldroyd, G.E.D. Abscisic Acid Coordinates Nod Factor and Cytokinin Signaling during the Regulation of Nodulation in Medicago truncatula. Plant Cell 2008, 20, 2681–2695. [Google Scholar] [CrossRef]
- Reid, D.E.; Heckmann, A.B.; Novák, O.; Kelly, S.; Stougaard, J. CYTOKININ OXIDASE/DEHYDROGENASE3 Maintains Cytokinin Homeostasis during Root and Nodule Development in Lotus Japonicus. Plant Physiol. 2016, 170, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.-H.; Lin, Y.-H.; Reid, D.E.; Gresshoff, P.M. Molecular Analysis of Legume Nodule Development and Autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Smit, P.; Raedts, J.; Portyanko, V.; Debellé, F.; Gough, C.; Bisseling, T.; Geurts, R. NSP1 of the GRAS Protein Family Is Essential for Rhizobial Nod Factor-Induced Transcription. Science 2005, 308, 1789–1791. [Google Scholar] [CrossRef] [PubMed]
- Charon, C.; Johansson, C.; Kondorosi, E.; Kondorosi, A.; Crespi, M. Enod40 Induces Dedifferentiation and Division of Root Cortical Cells in Legumes. Proc. Natl. Acad. Sci. USA 1997, 94, 8901–8906. [Google Scholar] [CrossRef]
- Feng, J.; Lee, T.; Schiessl, K.; Oldroyd, G.E.D. Processing of NODULE INCEPTION Controls the Transition to Nitrogen Fixation in Root Nodules. Science 2021, 374, 629–632. [Google Scholar] [CrossRef]
- Soyano, T.; Kouchi, H.; Hirota, A.; Hayashi, M. Nodule Inception Directly Targets NF-Y Subunit Genes to Regulate Essential Processes of Root Nodule Development in Lotus japonicus. PLoS Genet. 2013, 9, e1003352. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Amyot, L.; Tian, L.; Xu, Z.; Gruber, M.Y.; Hannoufa, A. Ectopic Expression of miR156 Represses Nodulation and Causes Morphological and Developmental Changes in Lotus japonicus. Mol. Genet. Genom. 2015, 290, 471–484. [Google Scholar] [CrossRef]
- Huo, X.; Schnabel, E.; Hughes, K.; Frugoli, J. RNAi Phenotypes and the Localization of a Protein::GUS Fusion Imply a Role for Medicago truncatula PIN Genes in Nodulation. J. Plant Growth Regul. 2006, 25, 156–165. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Y.; Zhu, L.; Tian, Y.; Chen, L.; Sun, Z.; Ullah, I.; Li, X. GmTIR1/GmAFB3-Based Auxin Perception Regulated by miR393 Modulates Soybean Nodulation. New Phytol. 2017, 215, 672–686. [Google Scholar] [CrossRef]
- Reid, D.; Nadzieja, M.; Novák, O.; Heckmann, A.B.; Sandal, N.; Stougaard, J. Cytokinin Biosynthesis Promotes Cortical Cell Responses during Nodule Development. Plant Physiol. 2017, 175, 361–375. [Google Scholar] [CrossRef]
- Gauthier-Coles, C.; White, R.G.; Mathesius, U. Nodulating Legumes Are Distinguished by a Sensitivity to Cytokinin in the Root Cortex Leading to Pseudonodule Development. Front. Plant Sci. 2018, 9, 1901. [Google Scholar]
- Zhong, X.; Wang, J.; Shi, X.; Bai, M.; Yuan, C.; Cai, C.; Wang, N.; Zhu, X.; Kuang, H.; Wang, X.; et al. Genetically Optimizing Soybean Nodulation Improves Yield and Protein Content. Nat. Plants 2024, 10, 736–742. [Google Scholar] [PubMed]
- Ke, X.; Xiao, H.; Peng, Y.; Wang, J.; Lv, Q.; Wang, X. Phosphoenolpyruvate Reallocation Links Nitrogen Fixation Rates to Root Nodule Energy State. Science 2022, 378, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Yoro, E.; Suzaki, T.; Toyokura, K.; Miyazawa, H.; Fukaki, H.; Kawaguchi, M. A Positive Regulator of Nodule Organogenesis, NODULE INCEPTION, Acts as a Negative Regulator of Rhizobial Infection in Lotus japonicus. Plant Physiol. 2014, 165, 747–758. [Google Scholar]
- Schauser, L.; Roussis, A.; Stiller, J.; Stougaard, J. A Plant Regulator Controlling Development of Symbiotic Root Nodules. Nature 1999, 402, 191–195. [Google Scholar]
- Mitra, R.M.; Gleason, C.A.; Edwards, A.; Hadfield, J.; Downie, J.A.; Oldroyd, G.E.D.; Long, S.R. A Ca2+/Calmodulin-Dependent Protein Kinase Required for Symbiotic Nodule Development: Gene Identification by Transcript-Based Cloning. Proc. Natl. Acad. Sci. USA 2004, 101, 4701–4705. [Google Scholar]
- Oldroyd, G.E.D.; Long, S.R. Identification and Characterization of Nodulation-Signaling Pathway 2, a Gene of Medicago truncatula Involved in Nod Actor Signaling. Plant Physiol. 2003, 131, 1027–1032. [Google Scholar]
- Kaló, P.; Gleason, C.; Edwards, A.; Marsh, J.; Mitra, R.M.; Hirsch, S.; Jakab, J.; Sims, S.; Long, S.R.; Rogers, J.; et al. Nodulation Signaling in Legumes Requires NSP2, a Member of the GRAS Family of Transcriptional Regulators. Science 2005, 308, 1786–1789. [Google Scholar]
- Soyano, T.; Hirakawa, H.; Sato, S.; Hayashi, M.; Kawaguchi, M. Nodule Inception Creates a Long-Distance Negative Feedback Loop Involved in Homeostatic Regulation of Nodule Organ Production. Proc. Natl. Acad. Sci. USA 2014, 111, 14607–14612. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Li, M.; Jiang, X.; Jiao, W.; Song, Q. A Telomere-to-Telomere Gap-Free Assembly of Soybean Genome. Molecular Plant 2023, 16, 1711–1714. [Google Scholar]
- Jian, B.; Liu, B.; Bi, Y.; Hou, W.; Wu, C.; Han, T. Validation of Internal Control for Gene Expression Study in Soybean by Quantitative Real-Time PCR. BMC Mol. Biol. 2008, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
- Wang, L.; Sun, Z.; Su, C.; Wang, Y.; Yan, Q.; Chen, J.; Ott, T.; Li, X. A GmNINa-miR172c-NNC1 Regulatory Network Coordinates the Nodulation and Autoregulation of Nodulation Pathways in Soybean. Mol. Plant 2019, 12, 1211–1226. [Google Scholar] [PubMed]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.T.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium Rhizogenes-Mediated Transformation of Soybean to Study Root Biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Mai, C.; Xia, L.; Jia, G.; Li, X.; Lu, Y.; Li, Z.; Yang, H.; Wang, L. Molecular Insights into the Positive Role of Soybean Nodulation by GmWRKY17. Int. J. Mol. Sci. 2025, 26, 2965. https://doi.org/10.3390/ijms26072965
Zhao X, Mai C, Xia L, Jia G, Li X, Lu Y, Li Z, Yang H, Wang L. Molecular Insights into the Positive Role of Soybean Nodulation by GmWRKY17. International Journal of Molecular Sciences. 2025; 26(7):2965. https://doi.org/10.3390/ijms26072965
Chicago/Turabian StyleZhao, Xiaorui, Chunhai Mai, Lintao Xia, Gaiya Jia, Xinhui Li, Yichu Lu, Zhenying Li, Hongbin Yang, and Lixiang Wang. 2025. "Molecular Insights into the Positive Role of Soybean Nodulation by GmWRKY17" International Journal of Molecular Sciences 26, no. 7: 2965. https://doi.org/10.3390/ijms26072965
APA StyleZhao, X., Mai, C., Xia, L., Jia, G., Li, X., Lu, Y., Li, Z., Yang, H., & Wang, L. (2025). Molecular Insights into the Positive Role of Soybean Nodulation by GmWRKY17. International Journal of Molecular Sciences, 26(7), 2965. https://doi.org/10.3390/ijms26072965






