Abstract
Photodynamic therapy (PDT) is an innovative treatment that has recently been approved for clinical use and holds promise for cancer patients. It offers several benefits, such as low systemic toxicity, minimal invasiveness, and the ability to stimulate antitumor immune responses. For certain types of cancer, it has shown positive results with few side effects. However, PDT still faces some challenges, including limited light penetration into deeper tumor tissues, uneven distribution of the photosensitizer (PS) that can also affect healthy cells, and the difficulties posed by the hypoxic tumor microenvironment (TME). In hypoxic conditions, PDT’s effectiveness is reduced due to insufficient production of reactive oxygen species, which limits tumor destruction and can lead to relapse. This review highlights recent advances in photosensitizers and nanotechnologies that are being developed to improve PDT. It focuses on multifunctional nanoplatforms and nanoshuttles that have shown promise in preclinical studies, especially for treating solid tumors. One of the key areas of focus is the development of PSs that specifically target mitochondria to treat deep-seated malignant tumors. New mitochondria-targeting nano-PSs are designed with better water solubility and extended wavelength ranges, allowing them to target tumors more effectively, even in challenging, hypoxic environments. These advancements in PDT are opening new doors for cancer treatment, especially when combined with other therapeutic strategies. Moving forward, research should focus on optimizing PDT, creating more efficient drug delivery systems, and developing smarter PDT platforms. Ultimately, these efforts aim to make PDT a first-choice treatment option for cancer patients.
1. Introduction
Research in recent decades on knowledge related to the occurrence, early diagnosis, development, and complex treatment methods of neoplasia has had a rapid evolution, reduced mortality, and improved the living conditions for millions of patients. However, the work of researchers must continue to answer all the key questions in basic, translational, and clinical cancer research that have not yet been addressed.
1.1. Biological Characteristics of Neoplasms
Neoplasms have six biological features gained during evolution, which provide complexity through the maintenance of proliferative signaling, evasion in the face of suppressive factors, resistance to cell death, immortality through perpetual replication, development of new blood vessels, strong expansion, and distant proliferation, all due to genome instability, which triggers genetic diversity and precipitates the emergence of all these hallmarks and inflammation [1].
1.2. Current Cancer Statistics and Future Projections
According to the latest statistical data from the American Cancer Society, 2,001,140 new cases of cancer and 611,720 deaths caused by this serious disease have been estimated for the US by 2024 [2].
According to the World Health Organization (WHO) and the International Agency for Research on Cancer, global cancer incidence and mortality are projected to expand, and the cancer burden will increase by about 77% by 2050, putting even more pressure on health systems, individuals, and communities [3].
1.3. Challenges in Drug Development and Potential Solutions
The diversity of cancers, some of which are unique, is challenging and requires innovative approaches in oncology pharmaceutical research, development, and manufacturing.
Accelerating new anticancer therapies to benefit patients worldwide must overcome several barriers, the most significant of which are high costs, long development and testing periods, and potentially low success rates. An analysis of the ten most recent anticancer drugs produced by ten US pharmaceutical companies revealed a median time to market of 7.3 years (range: 5.8–15.2 years) and a median cost of $648.0 million (range: $157.3 million–$1.95 billion).
In summary, the drug discovery and development processes involve many years, many failures, a lot of uncertainty, and, additionally, regulations, production, and finding the right patients for testing; if it does not work the first time, it is necessary to learn from mistakes and try again [4,5,6,7]. A promising solution in scientific research and the pharmaceutical industry would be computer-assisted drug discovery, which offers immense advantages for overcoming these obstacles and especially for accelerating the processes. Various machine learning techniques can fundamentally change the stages of drug discovery, from virtual screening to predictive modeling of drug-target interactions.
1.4. Role of Artificial Intelligence (AI) and Quantum Computing in Oncology
The great hope is opened by quantum computing, which is still in its early stages, but which very soon could be applied to find optimal solutions regarding the complicated aspects of molecular simulations and optimization, impossible for classical computers [8].
The hope of addressing the complexity of cancer from the perspective of discovering new drugs and innovative nanotechnologies also involves the use of AI algorithms to process the huge data generated by the evolution of cancer, from a single cell to metastases. The multifaceted nature of cancer as a systemic disease encompasses tumor emergence and development, tumor and immunological microenvironment, senescence, metabolic and nutritional disorders, circadian rhythms, involvement of the nervous system, microbiome and coagulation disorders. AI models of genetic mutations are fundamental for analyzing and interpreting gene-environment interactions, providing molecular oncology with valuable information repositories for cancer prevention, early diagnosis, improvement of quality of life, and provision of targeted therapy. AI provides a diverse palette of computational tools and algorithms, which facilitate the speeding up of drug discovery processes, making the best of nanoformulation development, upgrading manufacturing efficiency, strict quality control and revolutionizing post-market surveillance methodologies [9,10,11].
1.5. Evolution of Cancer Therapies
The historical axis of anticancer therapies began long ago, and some are still administered today. In the second half of the 20th century, the initiation of cytotoxic antitumor therapy in solid and hematological neoplasms through applied chemotherapy constituted a cornerstone in cancer treatment. Advances in the 1980s, as a result of research in cellular and molecular biology, led to the synthesis of molecularly targeted cancer drugs. Innovative studies related to oncogenic genes and the signaling pathways involved have represented a major step forward in cancer treatment. Chemotherapy and targeted therapy have substantially improved the quality of life of patients, survival and the complete disappearance of some tumors. However, the inability to target some key oncogenic mutations, the acquisition of drug resistance after favorable preliminary clinical results, toxicity and other subsequent side effects have represented major barriers.
At the beginning of the 21st century, genetic engineering triggered a new stage in clinical pharmaco-oncology therapy by introducing monoclonal antibodies and immune checkpoint inhibitors for serious or even metastatic neoplasms, which previously offered no hope.
Currently, new cell therapies, antitumor vaccines and innovative biopharmaceutical products have emerged with great hopes for the future [12,13,14].
The up-to-date surgical, radiological and pharmacological treatments used in oncology, although they have reduced the heavy burden of cancer worldwide, have not achieved complete eradication, and have not stopped some relapses, toxic side effects and immune system disorders.
1.6. Innovations in Photodynamic Therapy (PDT)
Current integrative multimodal therapies, such as PDT, photothermal therapy (PTT), photoimmunotherapy, and smart multifunctional platforms loaded with nanodrugs, are innovative solutions in nanomedicine and hold promise for synergistic cancer treatment in the future [15]. PDT, as a cancer treatment method, involves selective sensitization of tissues to light, and has opened the era of nanotechnology [16].
PDT is minimally invasive, does not induce significant adverse reactions, and triggers antitumor immune responses. Although preclinical and clinical results of PDT have been favorable, and it has received U.S. Food and Drug Administration (FDA) approval since 1995 for the treatment of esophageal cancer, it is not yet widely applied in clinics worldwide due to some limitations. These impediments are the reduced penetration of light into the depth of the tumor mass; the biodistribution of photosensitizer (PS) that can also accumulate in healthy cells; insufficient number of reactive oxygen species (ROS) due to hypoxic tumor microenvironment (TME); transient vascular injury and then repair; incomplete tumor destruction, with sub-sequent tumor recurrence; and limited generation of antitumor immune responses. For PSs to become ideal, it is necessary to improve their solubility, photostability, selectivity and phototoxicity by applying the latest nanotechnologies. PDT still needs to be improved by assimilating new nanotechnologies and overcoming all the current limitations mentioned above, to become a first-choice treatment for patients. Combining PDT with other therapies, performed through diverse strategies, can generate cooperative or synergistic effects to increase efficiency and achieve the eradication of the tumor [17,18,19,20] (Figure 1).
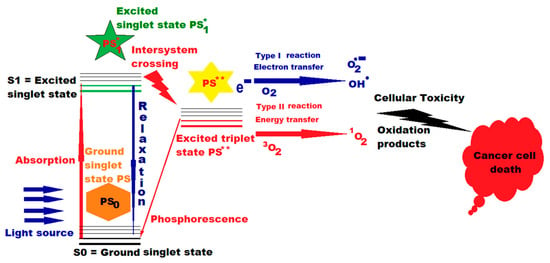
Figure 1.
Action of PDT and photosensitizer (PS) in cancer therapy. A single asterisk * means “excited singlet state”, and two asterisks ** mean “excited triplet state” (Figure 1 was imagined and drawn by L.M.A. using Microsoft Paint 3D for Windows 10, being already published by L.M.A. [21]).
1.7. Future Directions in Cancer Research
Research in recent years has shown that some photosensitizers incorporated into nanotechnologies have demonstrated higher intracellular uptake, superior targeting rates, increased toxicity to cancer cells, accelerated caspase activity, and deoxyribonucleic acid (DNA) cleavage, ultimately triggering apoptosis [21]. PDT as a state-of-the-art medical technology, using blue light (400–470 nm), can also effectively fight bacterial, fungal, and viral infections [22].
Innovative strategies for future research should include the development of new radicals, the design and manufacturing of new light-activatable PSs in the TME, the disruption of the electron transport chain in the mitochondrial membrane by photocatalytic oxidation of NADH in complex I, and the innovation of smart PDT systems [23].
Various therapeutic modalities, including PTT, PDT, X-ray induced PDT (XPDT), immunotherapy, and synergistic therapy, have been extensively explored for effective disease treatment. Each of these approaches offers unique advantages: PTT relies on localized heat generation to destroy diseased cells, XPDT enhances PDT’s applicability by enabling deeper tissue penetration, and immunotherapy strengthens the body’s immune response against pathological conditions. Synergistic therapy, which combines multiple treatment strategies, has emerged as a promising approach to enhance therapeutic outcomes. Among these, PDT has gained particular attention due to its minimally invasive nature, precise tumor targeting, and ability to induce localized cytotoxic effects with minimal damage to surrounding healthy tissues. Furthermore, its compatibility with other therapeutic strategies highlights its crucial role in advancing more effective and personalized treatment approaches (Figure 2 and Figure 3).
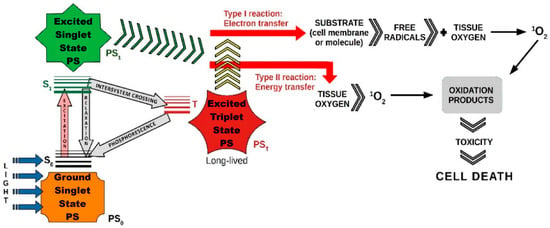
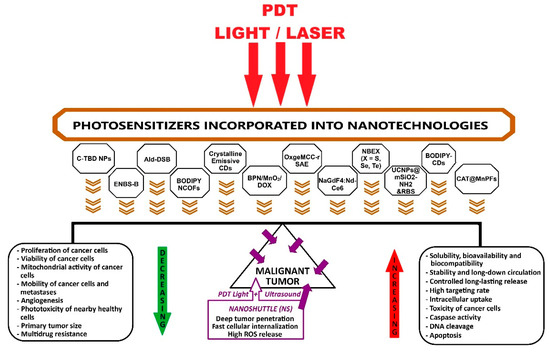
The primary aim of this review was to present a synthesis of current photosensitizers and the nanotechnologies in which they can be incorporated as PDT nanoagents, multifunctional theranostic nanoplatforms, and nanoshuttles successfully applied in preclinical studies of photodynamic therapy, with a focus on solid tumors.
The secondary goal was to highlight studies on PDT applications mainly targeting mitochondria for the elimination of deep malignant tumors.
Starting from the studies reviewed, the final objective is to invite and encourage researchers to contribute through creative approaches to optimizing PDT performance, imagining and designing new high-efficiency platforms for pharmaceutical formulations, smart PDT systems, and nanoshuttles through interdisciplinary approaches, also involving AI tools and computational algorithms to accelerate the discovery processes of innovative bioformulations for cutting-edge applications in molecular oncology and clinical cancer therapy.
2. State-of-the-Art Nano-Photosensitizers, Functional Nanosystems and Nanoscale Delivery Vehicles
Accurate image-guided treatment is of paramount importance in eliminating tumors in clinical applications. Mao et al. investigated an innovative nanotechnology using a chemiexcited photosensitizer, which can be precisely stimulated by hydrogen peroxide (H2O2) within the neoplastic surroundings to produce far-red/near-infrared (FR/NIR) emission and singlet oxygen (1O2). Organic fluorogens with aggregation-induced emission (AIEgen) features have emerged as favored materials for nanoparticle (NP) manufacture. AIEgens exhibit low fluorescence in solutions, but this phenomenon becomes stronger in aggregates due to limited intramolecular motion. Bis [2,4,5-trichloro-6-(pentyloxycarbonyl)phenyl] oxalate (CPPO) and an AIE photosensitizer, TPE-BT-DC (TBD), were carefully designed for aggregation-induced FR/NIR emission and co-encapsulated in pluronic F-127 and soybean oil to form C-TBD nanoparticles (C-TBD NPs). Using these NPs, breast tumors (both primary and metastatic) can be detected clearly through chemiluminescence imaging with a very high signal-to-noise ratio. These NPs serve as specific H2O2 probes for in vivo cancer detection through chemiluminescence imaging. The chemiluminescent signals from tumors and the 1O2 production can be enhanced by the addition of an antitumor drug, β-phenylethyl isothiocyanate (FEITC), which increases the amount of H2O2 at the tumor site for efficient neoplastic therapy. This demonstrates an advanced approach for light-source-free image-guided tumor treatment. C-TBD NPs open a new strategy for smart, accurate, and non-invasive tumor management [24].
2.1. Addressing Oxygen Deficiency in Tumor Environments
A deficiency in the amount of oxygen in nearly all solid tumors is a major limitation of conventional PDT and significantly reduces its curative success. Li et al. developed a molecular superoxide radical (O2–•) generator called ENBS-B, activated by NIR light, to overcome this challenge in hypoxic solid tumors. The authors revealed the O2–• mechanism responsible for antihypoxic reactions and validated its action by targeting hypoxic solid tumor ablation in vivo. This research demonstrates that near-infrared light-initiated molecular superoxide radical generators, paradoxically, under critical hypoxic conditions (only 2% O2), can produce significant O2–• through type I photoreactions. O2–• is partially converted to highly toxic OH· through SOD-mediated cascade processes. These radicals synergistically damage lysosomes inside cells, triggering apoptosis of cancer cells and resulting in potent PDT effectiveness [25].
2.2. Two-Photon Excited Fluorescence for Deeper Tumor Imaging
Sun et al. designed and tested organic dyes that exhibit excellent performance in two-photon excited fluorescence and 1O2 generation, such as turn-on-type two-photon absorption (2PA) dyes, which function even in intracellular environments and are suitable for both imaging and PDT of tumors in deeper tissues. They created an acetyl-terminated distyrylbenzene derivative (Ace-DSB), which quickly converts into aldehyde-terminated molecules (Ald-DSB), displaying a significant increase in 2PA cross-section (δ) values at 760 nm. This transformation activates the 2PA features of the molecules, enabling their use in intracellular conditions. Moreover, Ald-DSB showed a 40-fold higher capacity for 2P generation of 1O2 than Ace-DSB, paving the way for turning-on 2PA molecules for both in vitro and in vivo solid tumor imaging and 2PA-PDT. The turn-on feature was found useful for both two-photon laser confocal scanning microscopy imaging and two-photon excited PDT. The two techniques have been successfully applied to MCF-7 cells and melanoma tumors. This study sets the stage for the intelligent design of 2PA photosensitizers for future translational solid tumor treatments in clinical settings [26].
2.3. Covalent Organic Frameworks (COFs) for Photodynamic Therapy
A new category of organic porous materials, covalent organic frameworks (COFs), has shown promise in heterogeneous catalysis, energy storage, and analytical chemistry, with significant potential in cancer nanotherapeutics. COFs are characterized by porosity, modularity, stability, and metal-free structures, though size and structure control in aqueous dispersibility could improve their use. Guan et al. developed two boron-dipyrromethene (BODIPY)-decorated nanoscale COFs (NCOFs), which were nanocrystallized to be used for PDT of tumors. The two organic photosensitizers (PSs), BODIPY NCOFs, were obtained through Schiff-base precipitation of free end –CHO groups using the “functionalization of bonding defects” (BDF) method. These NCOFs had nanometric size (approximately 110 nm), low toxicity in the dark, and exceptionally high phototoxicity, proven by both in vitro and in vivo tests [27].
2.4. Multifunctional Nanoplatforms for Targeted Tumor Management
Neoplastic tumors are a significant challenge in medical science due to their variety, complexity, and the pressure to discover effective treatments. To address this, Wu et al. developed a multifunctional nanoplatform with intelligent tumor-specific theranostic capabilities for effective cancer management. A nanosponge composed of tightly packed MnO2-laden black phosphorus nanosheets (BPN) was designed as an ingenious, multifaceted theranostic nanocarrier. This platform enables tumor microenvironment (TME) simulation for pH and redox-responsive Magnetic Resonance Imaging (MRI) and synergistically enhances PDT, PTT, and chemotherapy. The (BPN/MnO2) nanoframework serves as a nanomedicine platform for tumor-specific smart diagnosis and autonomous treatment. The authors demonstrated that BPN/MnO2 PDT improves hypoxia by 3.8-fold and enhances PTT by 37% compared to BPNs alone. The MnO2 platform offers many TME-responsive properties, maximizing drug management at the neoplasm site and reducing chemotherapy side effects on healthy cells. An additional payload of doxorubicin (DOX) led to a BPN/MnO2/DOX nanocomposite, which exhibited smart self-controlled drug delivery under TME-specific functional reactions, such as pH, H2O2, and glutathione, with further control by external photoirradiation. This innovation demonstrates the potential of multifunctional nanotheranostics in cancer treatment [28].
2.5. Carbon Dots for Photodynamic Therapy
Carbon dots (CDs) have gained attention as nature-friendly nanomaterials with various applications. However, the development of CDs has faced constraints due to strict reaction conditions and lengthy synthesis steps. Wei et al. synthesized highly crystalline and luminescent CDs for the first time using a “wet chemistry” ion-thermal strategy, which allows the efficient production of CDs from different biochemical intermediates under non-severe conditions at ambient temperature and 210 °C. ZnCl2 was used to facilitate pyrolysis, developing CDs with tunable wavelengths and strong photogeneration of the superoxide radical anion (O2−) for high-tech applications such as PDT and photocatalysis, including the organic photosynthesis of tetrahydroquinoline via photosensitized cyclization [29].
2.6. Oxygen-Generating PDT Nanoagents
The ultimate effect of PDT in solid tumors relies on molecular oxygen in tissues, which must generate increased concentrations of cytotoxic 1O2, a process that is hindered by the hypoxia characteristic of solid neoplasms. Wang et al. developed a secure multifaceted self-assembled PDT nanoagent, namely OxgeMCC-r single-atom enzyme (SAE), consisting of a single-atom ruthenium as the active catalytic site attached in a metal–organic skeleton Mn3[Co(CN)6]2 with encapsulated chlorine e6 (Ce6), which serves as a cata-lase-like nanozyme for oxygen generation. Self-assembly of OxgeMCC-r SAEs requires in-group coordination, hydrophobic, and electrostatic interactions between organic linker, PVP polymer, photosensitizer, and metal ions. Setting up of Mn3[Co(CN)6]2 nanoparticles, referred to as MC, was achieved in a milky colloidal suspension, and these were nearly spherical, with a diameter of approximately 80 nm. Ru3+ was added to the initial solution and the doped Ru nodes were reduced with NaBH4. The newly created Mn3 [Co(CN)6]2-Ru (MC-r) nanoparticles also had a uniform size of approximately 80 nm. Ce6 was selected as the hydrophobic PS, to be integrated within MC or MC-r nanoparticles during the multicomponent self-assembly procedure. This operation gives rise to a nanometer scale matrix that is designed to encase Ce6. OxgeMCC-r SAE is characterized by precise form and structure, steady dimensions and big loading capability. Upon in situ production of O2 through the transformation between endogenous H2O2 and single-atom Ru species forming OxgeMCC-r SAE, the deficiency in the amount of oxygen in the tumor microenvironment is ameliorated. Wang et al.’s study provides a practical demonstration of a self-assembled nanozyme with truly organized and catalytically active single-atom Ru sites for PDT [30].
2.7. Lanthanide-Triplet Near-Infrared (NIR) Sensitization
Zheng et al. designed and tested an innovative procedure to reach high effects for ROS production via lanthanide-triplet NIR sensitization (for deeper penetration), in which lanthanide NP-organic PS nanoconjugates directly shifted the triplet energy from NPs to PSs, evading the intersystem crossing. This technique makes operational energy-effective NIR photosensitization to give rise to cytotoxic 1O2 for in vivo deep-tumor PDT under a low near-infrared power density of only 80 mW/cm2, i.e., 100 times smaller than conventional approaches. The researchers put together a NaGdF4:Nd (50 mol %) -Ce6 donor-acceptor hybrid system and they conducted systematic experiments to examine all of its photophysical properties. Ce6, as a standard PS, has been widely used in PDT studies for various cancer tumors and could be an energy acceptor with the lowest triplet state at 1.14 eV, making NIR photosensitization possible. The authors used an 8 nm NaGdF4 nanocrystal as a host medium in which they integrated Nd3+ as a photon absorber to make the Ce6 triplets sensitive. To demonstrate the influence of energy coupling on triplet relocation, they tested the process of inducing photosensitivity by lanthanide nanocrystals mixing different porphyrin and phthalocyanine derivatives (PpIX, TCPP, Ce6, and ZnPcS), characterized by certain triplet energies. They proved that only TCPP (T 1 = 1.43 eV9), Ce6 (T 1 = 1.14 eV), and ZnPcS (T 1 = 1.13 eV9) could be sensitized with maximum productivity by NaGdF4:Nd nanocrystals, when illuminated at 808 nm. Triplet levels of various porphyrin and phthalocyanine PSs could be successfully occupied by energy delivered from lanthanide NPs, without the necessity to populate the higher excited singlet states, so that the generation of cytotoxic 1O2 is feasible at ultralow NIR irradiation, opening new avenues for deep-tumor PDT [31].
2.8. Oxygen-Independent PDT via RNA-Targeting Photosensitizers
Being accurate and non-invasive, PDT is recognized as an elective treatment option for cancer. Even so, its conventional configuration relies on oxygen and ROS generation to eliminate the cancerous tumors based on type-I and type-II processes. At the same time, the sensitization of PSs, the medicines used against cancer cells, is based on the surrounding O2, which is insufficient in tumors, characterized by a significant lack of molecular oxygen, thus restricting the final curative outcome of PDT. Yao et al. invented a novel category of PSs, NBEX (X = S, Se, Te), which could attach to RNA of the neoplastic cells, and straightly induce the excitation energy to RNA for its breakdown after the irradiation by light, without the need for molecular oxygen, and thus destroying RNA and killing the corresponding neoplastic cells, even under hypoxic conditions, totally independent of O2, i.e., type III mechanisms. Previously, a smart fluorescent dye NBE was prepared, having its origins on the “door bolt” tool, which could identify RNA specifically and keep away from errors, recognizing cancer cells from normal ones due to the high level of RNA in neoplastic cells. The structure of NBEX was like that of NBE. In vivo, NBEX (X = S, Se, Te) could self-assemble into NPs and spontaneously gather into the tumor region within minutes, displaying an outstanding activity for eliminating various tumors and their metastases. At the same time, PDT with NBEX could influence and enhance immune responses to prevent further actions and relapses [32].
2.9. BODIPY-Based Photosensitizers for PDT
Among organic PSs, boron dipyrromethene (BODIPY) derivatives have captured the attention of researchers because they are fluorescent in the NIR with limited width of ab-sorption and emission bands, with very good fluorescence and photostability, increased elimination coefficient, and very good fluorescence quantum output. The implementations of BODIPY derivatives are multiple, both in the pharmaceutical field and in cancer therapy for targeted drug delivery, and as PSs, drug monitoring and tracking, as well as cancer diagnosis and treatment due to their fluorescence properties. Modifying the hydrophilic structure while maintaining the hydrophobic BODIPY as the core would improve the absorption range, would increase water solubility and biocompatibility for better use in living tissues. Various applications have been attempted, but all have proven expensive, difficult to achieve and manufacture, and with uncertain results by embedding hydrophilic protein-based formations, lipidosomes, and PEG substances into the hydrophobic BODIPY core [33,34,35,36].
Lu et al. [37] designed and prepared three new PSs as NIR BODIPY-CDs complexes (BODIPY-α-CD, BODIPY-β-CD, and BODIPY-γ-CD) with superior water solubility and biocompatibility through the click reaction of alkynyl-containing BODIPY and azide-modified cyclodextrin (CD). Because of stronger conjugation and after embodying triazole and CD elements, the fluorescence was displaced to the red region by approximately 90 nm. All three BODIPY derivatives had no cytotoxicity against NIH 3T3 and HeLa tumor cell lines under dark conditions. The BODIPY-β-CD type had the best PDT effect upon NIR irradiation (808 nm) against HeLa cells, with a drastic elimination of up to 80% neo-plastic cells, for a concentration of 100 μg/mL. BODIPY-CDs complexes offer new perspectives in PDT applied to cancer therapy [37].
2.10. Advances in PDT for Glioma and Deep Tumors
Glioma is a cancerous brain tumor and is challenging because its rate is 40% of all brain tumors. It is a virulent, intrusive cancer with a high recurrence and a likely poor course. All conventional means that could be applied, such as surgical operation, radio- and chemotherapy, are unsuccessful in eliminating this kind of tumor in 89% of patients, in relation to its position, the degree of penetration into the surrounding tissues and its size [38,39,40,41].
PDT has arisen as an encouraging novel anti-glioma treatment, but enhancements are needed by overcoming intra-tumoral hypoxia and extending the laser penetration into the neoplastic tissue [42,43].
Li et al. [44] designed and produced upconverted UCNPs@mSiO2-NH2 NPs by thermal breakdown, applying energy resonance transmission and NIR up-conversion. To study and control in time, space, and for accurate measurement of doses of the nitric oxide (NO) released in multiple biological implementations, the authors designed an 808 nm-excited NO photocontrolled release platform, by electrically charging at rest the photosensitive NO donor metal-nitrosyl complex, Roussin’s black salt (RBS), which releases NO after illumination onto UCNPs@mSiO2-NH2 NPs, resulting UCNPs@mSiO2-NH2&RBS with anti-neoplastic consequences [44].
NO is a vital signaling molecule, with major consequences in pathological activities, especially in cancer, where at high concentrations it generates reactive nitrogen species, which interreact with ROS and result in oxidative and nitrosyl reactions. These latter responses trigger DNA base deamination and enzyme nitrosylation, with important damage to cellular metabolism and, ultimately, cause programmed cell death of neoplastic cells [45,46,47].
In vivo investigations on glioma and chordoma xenograft mouse models demonstrated remarkable suppression of neoplastic cells in the UCNPs@mSiO2-NH2&RBS lot treated with NIR, without major adverse reactions in mice.
The UCNPs@mSiO2-NH2&RBS platform developed by the authors with NO release upon NIR irradiation has great prospects for PDT applied in deep malignant tumors [44].
Nanobiotechnology has advanced greatly and has paved the way for enzyme loading on structured nanomaterials with novel physical and chemical properties, related to the enhancement of catalytic quality and enzyme encapsulation [48]. To increase the potential of PDT, novel enzyme-encapsulated nanomaterials have been developed to target deep tumors with high-performance PS and trigger oxygen generation, which can rely on cata-lase-loaded nanodrugs or nanocarriers [49].
Qiao et al. designed and implemented a multifunctional PS consisting of catalase-loaded manganese-porphyrin frameworks (CAT@MnPFs) to catalytically aid PDT applied to 4T1 breast cancer cell line. The assembly of Mn2+ ions and Protoporphyrin IX (PpIX) occurred in a first step in MnPFs via covalent coordination, and then the catalase was loaded. Under irradiation with red light (wavelength = 650 nm), the porphyrin (protoporphyrin IX) in the CAT@MnPFs structure can transform oxygen (O2) into cytotoxic 1O2, which will trigger the destroying PDT effect. The packed catalase can break down the hydrogen peroxide (H2O2) into O2 in just 600 s, and, consequently, further advances the production of 1O2 through PDT, further enhancing the effects of photodynamic therapy.
Newly designed CAT@MnPFs cover the benefits of PSs and catalase for PDT characterized by evolving oxygen generation processes for the treatment of cells lacking oxygen in their environment, i.e., hypoxic media [50].
A synthesis of the studies presented above regarding some recent nanotechnologies tested in PDT is presented in Table 1.

Table 1.
Experimental studies of nanotechnologies applied in the therapy of deep tumors through PDT.
3. Nano-Photosensitizers in PDT Targeting Mitochondria in Cancer Cells
3.1. Mechanisms of PDT and Challenges in Solid Tumors
Experimental studies conducted in vitro and in vivo, and clinical studies with different PSs have revealed the fate of target cells and their relationship with apoptosis, necrosis, autophagy, and other nonspecific aspects of cell death. Both the mode of action and the efficacy of PDT are facilitated by the release of ROS and correlate directly with the irradiation energy, dose, irradiation cycle, PS accumulation in the tumor, PS metabolism, and tumor hypoxia [51].
One of the limitations of PDT efficacy on solid tumors is hypoxia, which increases due to oxygen consumption during the therapeutic process. Consequently, overcoming this effect of PDT on tumor hypoxia remains a constant concern for researchers.
3.2. Advances in Nano-Photosensitizers for Hypoxia Mitigation
Yang et al. manufactured the Mn3O4@MSNs@IR780 nanoparticle by absorbing the PS IR780 into 90 nm mesoporous silica nanoparticles (MSNs) and coating the surface pores with 5 nm Mn3O4 nanoparticles. Under PDT, this nanoparticle exhibits a durable ability to reduce hypoxia through the catalysis of intratumoral H2O2 and mitochondrial damage targeted by IR780. This nanoparticle generated oxygen, ROS, and inhibited respiration in vitro on the human gastric cancer MKN-45P cell line and in vivo in MKN-45P tumor-bearing xenograft mice, preventing tumor recurrence and leading to a favorable prognosis [52].
Wen et al. [53] designed a nanoplatform with an oxygen regulator, 3-bromopyruvate (3BP), using a core/shell structured poly(lactic-co-glycolic acid) (PLGA) nanoplatform, with 3BP enclosed in the core and IR780 in the shell [3BP@PLGA-IR780], to enhance PDT.
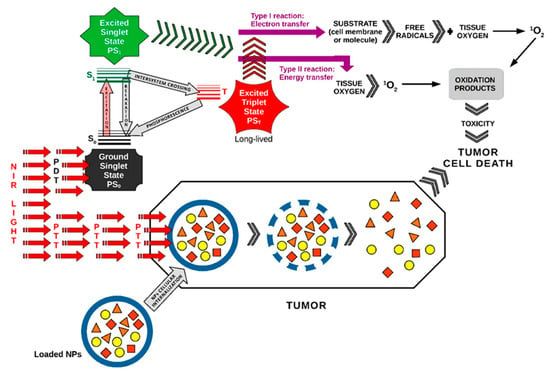
For in vivo evaluation, 4T1-xenograft tumors were intravenously injected with the 3BP@PLGA-IR780 suspension. Experimental studies in 4T1-xenograft mammary tumor mice demonstrated that this nanoplatform has deep tumor penetration capabilities, disrupts tumor cell energy metabolism, reduces oxygen consumption by inhibiting the respiratory chain, enhances ROS generation, and precisely targets mitochondria. This platform could also function as a contrast agent for fluorescence and photoacoustic imaging in image-guided cancer treatment, making it a promising alternative for improving PDT performance [53] (Figure 4).
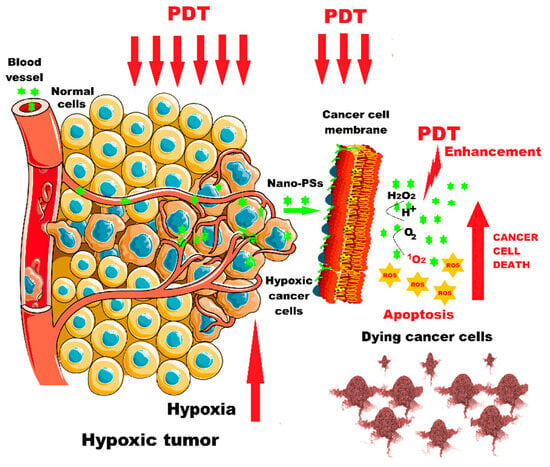
Figure 4.
PDT enhancement in hypoxic tumors using nano-photosensitizers. Nano-PSs = nano-photosensitizers (Figure 4 was imagined and drawn by L.M.A. using Microsoft Paint 3D for Windows 10 and using completely free picture material from SeekPNG.com (accessed on 9 March 2025), for which we are very grateful).
3.3. Novel Photosensitizer Strategies to Improve PDT
Zinc phthalocyanine (ZnPc) photosensitizers, despite their strong absorption at 650–750 nm, high triplet state quantum yields, and good biocompatibility, are rarely used in cancer therapy due to their low solubility and aggregation tendency in aqueous media. Nash et al. developed a novel nanoscale metal-organic layer (nMOL) assembly, ZnOPPc@nMOL, in which ZnOPPc (zinc(II)-2,3,9,10,16,17,23,24-octa(4-carboxyphenyl) phthalocyanine) PS was supported on secondary building units (SBU) of an Hf12 nMOL for PDT.
Upon laser application, SBU-bound ZnOPPc PSs absorbed 700 nm radiation and rapidly released singlet oxygen, preventing aggregation-induced self-quenching. ZnOPPc@nMOL exhibited mitochondrial penetration and tumor growth inhibition exceeding 99%, achieving cure rates of 40–60% in two colon cancer mouse models [54].
Given the remarkable photophysical properties of some PSs, they could be administered as multifunctional theranostic agents that combine PDT with deep penetration capabilities and high imagistics resolution. However, most PSs are not used due to low water solubility, poor specificity, and high toxicity in the dark. Cai et al. designed and encapsulated cyclometalated iridium (III) complexes (Ir) into biocompatible nanoparticles [Ir-NPs] that exhibited aggregation-induced emission (AIE) properties, high photoinduced ROS generation efficiency, two-photon excitation, and high mitochondrial targeting quality. The Ir-NPs disrupted mitochondrial redox homeostasis and in vitro apoptosis in SKOV3 ovarian carcinoma cell line. In vivo studies in mice bearing SKOV3 ovarian carcinoma tumors showed that Ir-NPs targeted, deeply penetrated, and destroyed the tumors with low toxicity in the dark. The authors reported that the Ir-NPs could be used as two-photon imaging agents for tissue visualization down to 300 μm. This multifunctional Ir-NPs nano-complex may serve to improve photodynamic therapy and clinical bioimaging applications [55].
3.4. Mitochondrial Targeting for Enhanced PDT in Breast Cancer
According to WHO statistics from 2022, breast cancer was diagnosed in 2.3 million women worldwide, leading to 670,000 deaths. Of all breast tumor subtypes, triple-negative breast cancer (TNBC) accounts for ~15% of invasive cases and is considered the most aggressive molecular subtype, exhibiting high metastatic potential, treatment resistance, and poor prognosis. While standard treatments include surgery, radiotherapy, chemotherapy, hormonal therapy, and targeted biologicals, major challenges persist due to toxicity, high recurrence rates, and therapeutic resistance [56,57,58].
Consequently, there is an urgent need to design and obtain new drugs or novel load-ing and delivery technologies that improve the clinical response of subjects with triple-negative breast cancer. PDT is an already approved method as an antitumor therapy, appreciated as a promising means of photoactivation, precise tumor targeting and low toxicity. However, due to the low water solubility of current PSs, rapid depletion of the optimal level of ROS due to high accumulations of glutathione (GSH) in TME, the efficacy of PDT and synergistic therapies is limited. Huang et al. designed and obtained the TPP-TK-PPa/DEM NPs nanoplatform which was prepared by self-assembly of the amphiphilic heterodimeric photosensitizer (TPP-TK-PPa) with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycer-ol)-2000] micelles, or DSPE-PEG2000 and the mitochondrial GSH depleting agent diethyl maleate (DEM) for co-delivery in PDT. TPP-TK-PPa/DEM NPs have excellent mitochondrial targeting capabilities and can effectively deliver therapeutic agents to tumor cells and free ROS in mitochondria. At the same time, when administering PDT, TPP-TK-PPa/DEM NPs can increase ROS production in situ and simultaneously reduce ROS consumption by reducing intracellular GSH concentration under the action of slow release of DEM after cellular internalization. The increased release of ROS during PDT produces major changes in the morphology and physiology of the mitochondrial membrane with effects on accelerating cellular apoptosis phenomena. In vitro studies demonstrated that after PDT with TPP-TK-PPa/DEM NPs, remarkable cytotoxicity occurs in the MDA-MB-231 cell line, which is classified as “triple-negative” breast cancer. TPP-TK-PPa/DEM NPs provide a photosensitizer for PDT with a precise and efficient targeting capacity on tumor cells [59].
3.5. Future Perspectives on Mitochondrial Modulation in PDT
Small organic molecules excited by laser irradiation serve as fluorophores, widely used in medical imaging, fluorescence-guided surgery, and diagnostics. Research has recently focused on near-infrared (NIR) fluorophores (700–900 nm), forming the so-called in vivo optical window. Although many compounds have been proposed as PSs, the FDA has only approved four fluorophores for clinical trials: indocyanine green (ICG), methylene blue (MB), 5-aminolevulinic acid (ALA), and pafolacianine (OTL38) [60,61,62].
Bonelli et al. designed an innovative nano-PDT formulation consisting of mitochondria-targeted coumarin-PS mixed into amphoteric polyurethane-polyurea hybrid nanocapsules (NCs). The encapsulation of polyurethane-polyurea hybrid NCs with coumarin-based fluorophores (COUPY 1 and 2) using ECOSTRATAR technology resulted in enhanced photostability, lower dark cytotoxicity, and improved in vitro photoactivity. PDT with NC-COUPY-2 in human HeLa, buffalo green monkey kidney (BGM) cells, and HeLa multicellular tumor spheroids (MTCS) demonstrated strong tumor inhibition, high phototoxicity, mitochondrial damage, ROS production, and apoptosis induction [63].
The great progress made in the last decades in preclinical research on deciphering the role of mitochondria in cancer cells, and the rapid implementation of precision medicine in clinical pathology have led to an increase in the survival time of cancer patients. It has been observed that mitochondria permanently require oxygen for the metabolic oxidative phosphorylation (OXPHOS) process in order to obtain the energy necessary for cellular survival. Obstruction of the OXPHOS metabolic pathway will lead to disruption of the membrane potential, dysfunction of mitochondrial morphology and respiration, decreased ATP production and increased ROS [64].
3.6. Mitochondrial Complex III and ROS Release
Mitochondrial complex III (CIII, or cytochrome bc1 complex; ubiquinol cytochrome c reductase) on the electron transport chain (ETC) of oxidative phosphorylation is today considered the main point of ROS release in mitochondria and in blocking the upstream electron flow, with the reduction of ROS production in mitochondria, which oxidize the substrates in complex I. Complex III is composed of two centers, one called Qo disposed towards the intermembrane space and the other Qi placed on the inner membrane facing the mitochondrial matrix. Antimycin A is a secondary metabolite produced by Streptomyces bacteria and has a role as an inhibitor of cellular respiration, especially on oxidative phosphorylation. Antimycin A acts on complex III and inhibits the Qi center with consequences on the increase of superoxide production in the Qo center, thus directing oxidants away from the antioxidant defense of the matrix. Once interfered with by the inhibitor, complex III releases ROS into the cytoplasm that will degrade mitochondrial subcellular organelles, leading to cellular dysfunction. Complex III of the mitochondrial electron transport chain (mETC) has the fundamental mission of activating autophagy, unlike antimycin A, which can inhibit autophagy [65,66,67].
3.7. Atovaquone and Its Impact on Mitochondrial Respiration
Atovaquone, an FDA-approved antimalarial drug, is a specific inhibitor of the mitochondrial electron transport chain at complex III, depletes mitochondrial membrane potential, increases oxidative stress in vitro [68], decreases hypoxia in vivo [69], and, in addition, it can improve the antitumor response [70,71,72,73].
3.8. Biomimetic Nanoparticles in Cancer Treatment
Li et al. synthesized a biomimetic nanoparticle considered as an oxygen reservoir in which they included the mitochondrial photosensitizer IR780, atovaquone (ATO), an inhibitor of mitochondrial respiration, and co-loaded them into the red blood cell membrane (RBCm), coated with perfluorocarbon (PFC) with a liposome core, named RBCm@ATO-IR780-PFC liposomes. The authors experimentally used in vitro cell cultures from the human gastric cancer line AGS, and in vivo, mice with tumors induced from CT26 colorectal cancer cells. Biomimetic nanoparticles with ATO under the action of PDT effectively inhibited mitochondrial respiration and reduced endogenous oxygen consumption, and PFC delivered exogenous oxygen in a large amount. These oxygen modulators reversed hypoxia in vitro and in vivo and had prominent antitumor action on targeted mitochondria [74]. Fluorescence imaging-guided PDT is attracting widespread interest due to its unique advantages, including non-invasiveness, high spatiotemporal visibility, minimal side effects, and ease of use compared to conventional therapy procedures [75,76].
3.9. Photosensitizers and Aggregation-Induced Emission (AIE)
Traditional photosensitizers have rigid planar structures, are hydrophobic, and under physiological conditions tend to aggregate, which causes them to lose their fluorescence properties and consequently, their singlet oxygen-emitting power, a process called aggregation-induced quenching (ACQ). This phenomenon has led to research that has observed a bright emission from solid-state luminogenic dyes, a property known as AIEgen. The discovery of these properties of highly fluorescent AIEgens may be useful for monitoring and ameliorating the release of increased ROS and encouraging effects in suppressing cancerous lesions [77,78].
3.10. Design of Organic AIEgens for Cancer Therapy
Zhao et al. advanced a molecular design strategy to produce organic AIEgens to facilitate fluorescence imaging (FLI)-guided diagnosis and highly cost-effective photoinduced specificity in cancer therapy. The authors succeeded in designing four theranostic agents as aggregation-induced emission (AIE)-based photosensitizers (TPAPyTZ, TPAPyTC, TPAPyTM, and TPAPyTI) that have specific AIE properties. TPAPyTZ and TPAPyTC were attested for precise Golgi apparatus (GA) targeting, which could lead to cancer cell death. In vitro and in vivo studies have demonstrated that TPAPyTZ and TPAPyTC possess potent AIE properties, potent type I/II ROS scavenging capabilities, high photostability, precise GA-specific targeting, activation of mitochondrial apoptosis during PDT, and very good imaging quality. AIEgens of the TPAPyTZ and TPAPyTC-type have demonstrated their photodynamic effect in vivo through a percentage of 88%, and, respectively, 69% inhibition in HCT116 bearing-tumor mice. The results provide a promising method for applications of AIEgens for fluorescence imaging-guided PDT [79].
3.11. Mitochondrial Targeting in Cancer Therapy
It has been observed that PDT targeting mitochondria is much more effective in cancer therapy, as mitochondria are the fundamental cellular organelles for cellular respiration and the convergence points of various signals to transduction pathways [80].
3.12. Cytotoxicity of PDT and Targeting Mitochondria
Given the very short nanosecond (10–320 ns) lifetime and narrow diffusion beam (10–55 nm) of ROS, the cytotoxicity of PDT correlates with the area of cellular organelles targeted by PS [81].
Cationic nanocarriers based on triphenylphosphine and pyridinium are the most widely used in the synthesis of PSs for targeting mitochondria, but they still have problems related to rapid elimination, severe toxicity to the immune system, and the existence of the phospholipid bilayer surrounding mitochondria, constituting a barrier to effective action. Therefore, there is a pressing need to design nanocarriers for mitochondrial-targeted PDT that have cost-effective and living system-friendly structures, i.e., non-toxic and free of immunological events [82,83].
Designing DNA-based nanostructures for PS manufacture could overcome the drawbacks associated with mitochondrial-directed PDT. To progress in this field, it is necessary to identify DNA structures that are stable in biological environments and target tumor tissues. Research has shown that DNA nanostructures encoded with the poly-AS1411 aptamer for PS loading would provide durability to the DNA nanostructures and multiple guide probes, thereby improving the intelligent and sensitive recognition of tumor structures for targeted phototherapy [84,85].
3.13. DNA Nanotechnology for Mitochondrial-Targeted PDT
Chen et al. designed and developed a DNA nanoclew encoded with poly-AS1411 aptamer (multifunctional DNA nanoclew-AS-AMD) loaded with mitochondrial-selective cationic PS (APNO) and paramagnetic Mn2+ for MRI and PDT targeting mitochondria. The AS1411 aptamer in AS-AMD was designed to increase tumor targeting accuracy and cellular uptake. Paramagnetic Mn2+ released into the acidic TME enhances MRI performance in tumor tissue, and then precisely targets via PDT. Selectively released cationic APNO targeted the mitochondrial membrane and released ROS that induced in vitro apoptosis in 4T1 breast tumor cells. In vivo research in 4T1-tumor-bearing mice demonstrated a 91.3% decrease in tumor volume and extended survival rate in the AS-AMD group treated with PDT. AS-AMD demonstrated a very good tumor targeting response in the 4T1 tumor-bearing mouse model, substantially improved MRI operation and PDT efficiency. This aptamer-encoded DNA nanotechnology delivery system offers a novel mitochondrial-targeted PDT platform strategy that can be tailored for the therapy of various diseases by changing the sequence of the aptamer region in the DNA fragment. This research proposes a biocompatible and versatile DNA nanoplatform for the delivery of cationic PSs targeting mitochondria and the advancement of novel nanotheranostic agents [86].
3.14. Mitochondria-Targeted Photosensitizers in Cancer Therapy
Because mitochondria produce cellular energy and regulate cellular apoptosis, they have become attractive targets for PS targeting in PDT. As previously discussed, current PSs targeting mitochondria rely on the structure of cationic molecules that will be attracted to the negatively charged mitochondrial membrane. In 2015, Zhang et al. developed the photosensitizer IR700DX-6T that targeted a translocator protein (TSPO) on the mitochondrial membrane of TSPO-positive breast cancer cells (MDA-MB-231). IR700DX-6T induced apoptosis of TSPO-positive breast cancer cells in vitro and exponentially inhibited in vivo tumor growth in MDA-MB-231-bearing mice [87].
TSPO is a protein consisting of 169 amino acids rich in tryptophan, located on the outer membrane of mitochondria, which participates in metabolic and immune regulatory processes, being recently considered as a tumor biomarker, whose expression correlates with tumor aggressiveness and is used for diagnosis in molecular imaging studies and as an attractive therapeutic target in cancer [88,89].
3.15. Colorectal Cancer and PDT
Colorectal cancer is the second leading cause of death from all forms of cancer and ranks third in incidence worldwide. In 2020, there were over 1.9 million new cases of colorectal cancer, of which over 930,000 deaths occurred worldwide. The highest incidence was in Europe, Australia, and New Zealand, with the highest mortality rate in Eastern Europe. It is estimated that by 2040, there will be 3.2 million new cases of colorectal cancer per year with an increase in incidence of 63% and deaths by 73% per year [90,91].
Based on a previous study, Zhou et al. investigated the effects of IR700DX-6T-PDT on CT26 and HCT11 colorectal cancer (CRC) cells, in vitro and in vivo in the BALB/c mouse model with CT26 tumor. The authors demonstrated that IR700DX-6T-PDT potently inhibited CRC proliferation both in vitro and in vivo. At the same time, IR700DX-6T-PDT induced pyroptosis, p38 phosphorylation, and caspase-3-dependent cleavage of gasdermin E (GSDME). The use of decitabine, a methyltransferase inhibitor, reversed GSDME by demethylation and enhanced the effect of IR700DX-6T-PDT. The combination of decitabine, IR700DX-6T-PDT, and an anti-PD-1 antibody overcame the immunosuppressive environment and reduced tumor volume in vivo in experimental animals, thus offering a promising strategy for the therapy of “cold” tumors [92].
3.16. Challenges in PDT Efficacy
The efficacy of PDT in solid cancers is conditioned by two essential aspects: the first would be related to the reduced partial pressure of oxygen due to excessive tumor proliferation and liquefaction necrosis, and the second aspect is related to the diffusion and reduced lifetime of ROS molecules [93,94,95].
Li et al. invented a nanoplatform to target mitochondria and deliver oxygen to improve the efficiency of PDT in eliminating cancerous tumors. This nanoplatform comprises eccentric hollow mesoporous organic silica nanoparticles (EHMONs) equipped with triphenylphosphine (CTPP) molecules for mitochondrial targeting, the chlorin e6 PS and perfluorocarbons (PFCs) that can be loaded with oxygen. The EHMONs-Ce6-CTPP@PFC nanoplatform experimentally used on triple-negative breast cancer cells and in 4T1 tumor-bearing mouse models had good biocompatibility, high mitochondrial targeting capacity, and high destructive effects due to the high singlet oxygen content [96].
3.17. Overcoming Biological Barriers in PDT
In the case of deep tumors, upconversion nanoparticles (UCNPs) are used as nanocarriers for PSs as they increase the efficiency of PDT in these pathologies, but there are still many impediments to overcome biological barriers for effective mitochondrial targeting. In this regard, Liu et al. designed and manufactured photodynamic nanoplatforms targeting CD44 positive cells and mitochondria by self-assembly of conjugated hyaluronic acid-conjugated-methoxy poly(ethylene glycol)-diethylenetriamine-grafted-(chlorin e6-dihydrolipoic acid-(3-carboxypropyl)triphenylphosphine bromide) polymeric ligands (HA-c-mPEG-Deta-g-(Ce6-DHLA-TPP)) and NaErF4:Tm@NaYF4 core–shell UCNPs (referred to as CMPNs). Testing of these CMPNs showed good stability and drug loading capacity as well as enhanced competence in generating singlet oxygen (1O2) under the action of laser light based on the fluorescence resonance energy transfer (FRET) mechanism. In vitro experimental research on the human lung adenocarcinoma cell line A549 proved increased cytotoxicity and substantially reduced viability of CD44 positive cancer cells after using CMPNs, followed by the administration of NIR laser radiation. This research revealed the crucial role facilitated by CD44 positive cells in stimulating endocytosis and the capacity of UCNPs as nanocarriers for CD44 targeting and delivery of Ce6 PS. The study results open up prospects for the innovation of special nanoagents in PDT, which can overcome the current limitations of NIR laser irradiation for targeting mitochondria in deep cancers [97].
3.18. Immune Response in PDT
Immune checkpoint blockade (ICB) is a major opportunity for anti-neoplastic immunotherapy, and immunogenic cell death (ICD) is considered a promising pathway for tumor cell elimination. This process stimulates the adaptive immune response of T cells and the formation of long-term immunological memory. PDT can upregulate the programmed cell death 1 (PD-1)/PD-ligand 1(PD-L1) inhibitors, which will block the immunosuppressive signaling pathway and stimulate antitumor immune responses, leading to the killing of primary tumor tissue and blocking metastasis [98].
One marker of favorable response to immunotherapy is increased expression of PD-L1, which can be induced by hypoxia-inducible factor 1-alpha (HIF-1-alpha). HIF-1α plays an important role in the cellular response to systemic oxygen concentrations and the transcription of over 60 genes, including vascular endothelial growth factor (VEGF) and erythropoietin. HIF-1α, VEGF, and erythropoietin are involved in increased oxygen delivery, cell proliferation, and survival, as well as glucose and iron metabolism. HIF-1α expression can be regulated by PDT through oxidative stress, hypoxia, and ROS levels. It appears that HIF-1α and signaling pathways involved in hypoxia induce PD-L1 expression in the tumor when PDT acts. Tumor-associated M2 macrophages (TAMs) and suppressive tumor immune microenvironment (TIME) facilitate tumor growth and metastasis [99,100,101,102,103].
3.19. PROTAC Nanoplatforms for PDT
Proteolysis-targeting chimera (PROTAC) is designed to target proteins and induce antitumor effects. PROTAC has emerged as a promising acquisition for targeted therapy in various types of cancers, but the beneficial effect is limited by the low distribution and concentration in the tumor. PROTACs are molecules with bifunctional activity consisting of the target protein ligand, a linker, and an E3 ubiquitin-protein ligase that triggers the formation of chains of linked ubiquitin molecules to target the protein for degradation via the ubiquitin-proteasome pathway. PROTACs as heterobifunctional molecules have the primary purpose of inducing the proteasomal degradation, not inhibiting the target [104,105,106].
In this regard, Tong et al. have developed a multifunctional PROTAC-PDT nanoplatform (dBET6@CFMPD) to treat breast cancer and its metastasis. The nanoplatform dBET6@CFMPD is obtained via the self-assembly between dBET6 (small-molecule degrader) and the photosensitizer chlorin e6 (Ce6)-modified MMP-2 (matrix metalloproteinase-2) sensitive peptide (FFRFKGPLGLAGC)-PEG-DSPE conjugates. The multifunctional dBET6@CFMPD nanoplatform was used for mitochondrial targeting via PDT. The experiment was done using murine 4T1 breast cancer cells, 4T1-Luc cells, murine E0771 breast cancer cells, and E0771-Luc cells. A large amount of ROS was generated, strong cytotoxicity, and severe tumor cell death were observed in vitro. The antitumor and anti-metastatic effects were evaluated in vivo on 4T1 and E0771 subcutaneous models of female BALB/c mice indicating the great beneficial potential of dBET6@CFMPD for the treatment of breast cancer models and brain metastasis, by integrating PDT-PROTAC combination therapy and TIME remodeling. The anti-metastatic effect and its mechanism were investigated in vitro and showed an association between bromodomain-containing protein 4 (BRD4) inhibitor and Ce6 that strongly suppressed cell migration. The beneficial anti-metastatic effect of dBET6@CFMPD was supported by three factors: the first was the downregulation of epithelial-mesenchymal transition (EMT) induced by BRD4 degradation; the next was given by MMP-2 which set in motion the formation of nanofibers to limit tumor cell migration; and the last was due to the decrease in cell viability and motility induced by PDT. The authors demonstrated that the multifunctional PROTAC-PDT nanoplatform (dBET6@CFMPD) displayed in vitro and in vivo high biodistribution and retention and could trigger a strong antitumor effect on primary and metastatic tumors, transforming into a valuable nanomedicine with great future applications for combination cancer therapy [107].
3.20. Induction of Immunogenic Cell Death (ICD)
Induction of ICD is considered a promising method for cancer treatment because it triggers immune responses that ultimately lead to tumor cell death or necrosis. PDT is part of the category of innovative approaches in the fight to eradicate tumor formations by stimulating PSs that induce ROS release and initiation of ICD. The photoimmunotherapy response faces several impediments, such as reduced immunogenicity and efficacy due to insufficient ROS production, uneven distribution of the PS, and low oxygen levels in TME [108,109].
Duan et al. developed Rh-PTZ nanoparticles, i.e., a mitochondria-targeted type-I photosensitizer (Rh-PTZ) based on rhodanine, for PDT in a hypoxic environment. The compound Rh-PTZ was fabricated based on a typical D-A configuration with intramolecular charge transfer (ICT) properties. To improve biocompatibility and biostability, the authors encapsulated hydrophobic Rh-PTZ in F127 (polyethylene-polypropylene glycol) and obtained Rh-PTZ nanoparticles (Rh-PTZ NPs), which were then studied for mitochondrial targeting, ROS generation, and induction of ICD activation abilities under PDT. The researchers demonstrated that Rh-PTZ vigorously targets mitochondria, inducing the release of O2–• and OH, thus causing dramatic mitochondrial dysregulation. In vitro study was performed on 4T1 cell culture, where the level of immunofluorescence, ROS, cytotoxicity, intracellular ATP concentrations were monitored, and the antitumor activity was investigated in vivo on a 4T1 cancer model induced in female BALB/c mice. PDT with Rh-PTZ NPs amplified the ICD effect produced by mitochondrial stress with stimulation of the immune response, thus favoring the maturation of dendritic cells, improving the volume of infiltrated CD8+ immune T cells, and attenuating the immunosuppressive TME. The authors developed a novel phenothiazine-based type I PS with mitochondrial targeting of hypoxic tumors, which may provide new opportunities in the development of cancer PDT by improving cancer ICD through mitochondrial oxidative stress [110] (Table 2).

Table 2.
Photosensitizers targeting mitochondria in PDT.
4. Final Remarks and Conclusions
PDT as a form of phototherapy involves light and photosensitizing chemicals, PSs, which in combination with molecular oxygen trigger the death of cancer cells through phototoxicity. This relatively new and promising method has two major impediments: the photosensitizers, which do not always effectively fulfill their intended role; and secondly, the TME, which, being hypoxic, dramatically limits the effects of this therapy. Given the special characteristics manifested by its non-invasive nature, spatiotemporal control, negligible side effects, and low risk of drug resistance, PDT has aroused the interest of clinicians and is already approved for the therapy of skin, breast, lung, esophageal, head, and neck cancers, etc., [111,112].
4.1. Recent Advancements in PDT: New Approaches
Production of high cytotoxic singlet oxygen, even though it has many applications, including PDT, is effective in the liquid phase but less efficacious in solids, for example, in nanomaterials. Samperi et al. assembled a mixture of supramolecular materials that contained a PS entrapped in highly organized nanofibers and proved by scientific experiments their remarkable ability to generate (1O2). By microscopy of super-resolution radial fluctuations (SRRF), the tetrakis(4-carboxyphenyl)porphyrin was studied after being embodied within the fibers of only a few nanometers of a bis-imidazolium gelator, a procedure capable of exactly determining the location of the chromophore. High-efficiency (1O2) was given rise from the hybrid nanofibers upon irradiation, compared to the dissolved porphyrin, resulting in a maximum productivity with a starting 14-fold superior value from fibers, as detected experimentally compared to porphyrin. This experiment opens new avenues for the easy preparation of similar, highly versatile hybrid supramolecular nanomaterials, much more efficient than currently dissolved PSs, performing multiple functions to generate highly efficient (1O2) in PDT, as well as in other laser medical applications [113].
4.2. Future Directions and Innovations in PDT
The invention of advanced photosensitizers, as shown in Table 1, which are easier to produce, have an extended absorption scale and biocompatibility, better selectivity, and enhanced efficiency, is a pressing need for improving PDT in deep tumor therapy.
In addition, recent research shows the possibility of preparing inventive organic shuttle-like nanoassemblies, i.e., nanoshuttles (NSs) with different shapes by isomeric photosensitizers, which exhibit clear crystalline features and ordered molecular packing, enhancing in vivo PDT with an 81% inhibition rate on tumor growth. The NSs have demonstrated excellent targeting and deep tumor penetration capacity, and fast cellular internalization with high ROS release. These NSs pave the way for the development of high-performance platforms for PDT applied in cancer therapy [114].
An inspirational diagram of the state-of-the-art nano-photosensitizers, functional nanosystems, nanoscale delivery vehicles, and PDT-integrated nanoshuttles discussed in this review, which await successful application in clinical cancer therapy, is depicted in Figure 5.
4.3. The Role of Mitochondria in Cancer Cells and PDT
Great progress in the past decades in preclinical research on deciphering the role of mitochondria in cancer cells and the rapid implementation of precision medicine in clinical pathology have led to increased survival times for cancer patients. As shown in mammalian studies, mitochondria play a crucial role in cellular power supply by providing ATP from the tricarboxylic acid cycle (TCA) and oxidative phosphorylation. It has been observed that mitochondria constantly require oxygen for the metabolic process of phosphorylation to obtain the energy necessary for cellular survival. Obstruction of the OXPHOS metabolic pathway will lead to disruption of the membrane potential, dysfunction of mitochondrial morphology and respiration, decreased ATP production, and increased ROS [115,116].
4.4. Mechanisms of ROS Production and Its Impact on Cellular Health
Mitochondria are versatile and critical metabolic structures not only for energy production but also for modulating cellular immunity through several mechanisms, which generate cell apoptosis, ROS signaling, and DNA-dependent mitochondrial immune activation. These events are regulated by mitochondrial dynamics and mitochondrial damage-associated molecular patterns (mtDAMPs), complex phenomena by which mitochondria change, including their length and connectivity [117].
Production of ROS inside a cell can be achieved under the influence of several factors such as electron leakage during aerobic respiration, after inflammatory processes mediated by macrophages, under the action of ultraviolet rays, and many other external or stress stimuli. More and more publications have reported that mitochondrial ROS (mROS) plays a highly significant role in maintaining cellular, tissue, and whole organism homeostasis. mROS can modulate many signaling pathways and can stimulate the expressions of adaptation and defense of the organism in stress situations. mROS modulates fundamental physiological transformations related to cellular differentiation, proliferation, aging, and apoptosis. Increased ROS concentration, known as oxidative stress, will give rise to lipid peroxidation, protein carbonylation (the introduction of aldehyde or ketone carbonyl groups into the protein structure), DNA damage, and other changes, which will lead to pathologies such as senescence, metabolic, neurological, and other diseases, including cancer. Dysregulation of mitochondrial function will stimulate retrograde signaling pathways through specialized molecules in mitochondria such as ROS, calcium, oncometabolites, and exported mitochondrial DNA (mtDNA), as well as stress response pathways for the initiation, progression, and metastasis of cancer. Disruption of mitochondrial energy metabolism could eliminate the potential anticancer capacity of the immune system and amplify the phenomenon of cancer cell migration in the tumor microenvironment. All these recently proven aspects have inspired promising therapeutic strategies targeting mitochondria for the dissipation of malignant tumors through PDT [118,119].
4.5. PDT with Mitochondrial Targeting: A Promising Approach for Cancer Treatment
This is why PDT with specific photosensitizers targeting mitochondria is considered much more effective in cancer therapy, as mitochondria represent the fundamental cellular organelles for cellular respiration and the convergence points of various signals to cellular transduction pathways. PDT possesses a remarkable capacity in eliminating cancerous and non-oncological tumors through mechanisms of cell death induction, immune adjustment, etc. Since there is no protocol with specific criteria for each type of cancer for the use of a certain light source, a certain photosensitizer, and the appropriate dose, a percentage of patients do not respond satisfactorily to this treatment method and relapse or present metastases. On the other hand, in PDT, the fundamental pathophysiological mechanisms for cell death, which occur either through apoptosis, autophagy, or necrosis, are still not fully investigated. Currently, several references related to cell death pathways have appeared on this topic, such as necroptosis, mitotic catastrophe, paraptosis, and pyroptosis. A special type of cell death is ICD, which can also cause an immune response induced by antigens from destroyed cells, which in turn trigger very beneficial antitumor effects. The induced cell death pattern is conditioned by the laser or light dose, the subcellular site targeted by the PS, and the way in which it influences signaling pathways. Based on the above and the mechanisms of action, several treatment strategies are proposed by designing specifically targeted PSs and associating other therapeutic modalities such as nano-drugs, immunotherapy, and vaccines with the aim of inducing ICD, which would bring additional benefits to patients [116,120,121].
4.6. Challenges and Advances in PDT for Solid Tumors
One of the problems related to the efficiency of PDT applied in solid tumors is tumor hypoxia, aggravated especially by oxygen consumption during the therapeutic process, the rapid depletion of the optimal level of ROS due to high accumulations of GSH in the tumor microenvironment, and for these reasons, therefore, advancing these aspects is a permanent concern of researchers. Another impediment related to PDT is that most PSs put into practice have the inconvenience of being poorly soluble in water, with low specificity, and high toxicity in the dark.
Traditional PSs have inflexible planar structures, and, as mentioned, they are hydrophobic, and under physiological conditions, they tend to agglomerate with the loss of fluorescence properties and the ability to release singlet oxygen, a process known as aggregation-induced quenching. It is precisely this phenomenon that has prompted research that has observed that there is a bright emission of luminogenic dyes in the solid state, a property known as aggregation-induced emission (AIEgens). The discovery of these properties of highly fluorescent AIEgens may be useful for monitoring and ameliorating the release of an increased amount of ROS and encouraging effects in suppressing cancer lesions.
To improve the properties of PSs, there is currently great interest among researchers in finding nanocarriers such as inorganic nanoparticles (nonporous and mesoporous silica NPs, carbon-based NPs, quantum dots, and noble metal-based plasmonic NPs) and biodegradable organic NPs and their radiolabeled forms: liposomes, albumin-based nanoparticles, dendrimers, and polymeric nanoparticles, etc.
4.7. Future Directions: Mitochondrial-Targeted Nano-Photosensitizers
Mitochondrial-targeted nano-photosensitizers revealed in this review (see Table 2), designed with increased water solubility capabilities to extend the wavelength limits of deep tumor targeting by PDT, improve the specific delivery of PSs for certain tumor categories, and increase the efficiency of PDT in hypoxic TME, are highly promising, opening the way for coupling with other complex treatment procedures in molecular oncology.
This review should inspire researchers to contribute through creative approaches to optimizing PDT performance, imagining and designing new high-efficiency platforms for pharmaceutical formulations, smart PDT systems, and nanoshuttles for cutting-edge applications in molecular oncology and clinical cancer therapy.
4.8. Future Perspectives
Looking ahead, future advancements should focus on overcoming these limitations by developing novel PSs, including mitochondrial-targeted nano-photosensitizers. These new PSs, designed for increased solubility and targeted delivery, offer the potential for improved outcomes, particularly in challenging environments like hypoxic tumors. Additionally, combining PDT with other cancer therapies, such as chemotherapy, immunotherapy, and targeted molecular therapies, could provide synergistic effects that enhance therapeutic efficacy, reduce resistance, and improve patient survival.
The integration of PDT with combination therapies is a promising avenue for future research. By leveraging the strengths of each therapeutic modality, these approaches can help address the multifaceted nature of cancer, including tumor heterogeneity, resistance mechanisms, and the complex tumor microenvironment. For example, the use of PDT in conjunction with immunotherapies could stimulate immune responses while also directly killing cancer cells. Furthermore, nanotechnology offers the potential to design smart delivery systems that can selectively target tumors, reduce systemic toxicity, and maximize the effects of PDT.
Ultimately, continued innovation and interdisciplinary collaboration are essential to refine PDT protocols and develop novel combination strategies that will make PDT a cornerstone in modern cancer treatment regimens. With these efforts, PDT has the potential to become a first-choice treatment, providing patients with more effective, personalized, and less invasive options in the fight against cancer.
Author Contributions
Conceptualization of the review article, L.M.A. and C.A.; validation, L.M.A. and G.L.; writing—original draft preparation, L.M.A. and C.A.; software for Figs., L.M.A.; writing—review and editing, G.L. and L.M.A.; reorganization and management, G.L.; supervision, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data (selected literature) presented in this review article are available on request from the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Ace-DSB | acetal-terminated distyrylbenzene derivative |
| ACQ | aggregation-induced quenching |
| AI | artificial intelligence |
| AIE | aggregation-induced emission |
| AIEgens | aggregation-induced emission luminogens |
| ALA | 5-aminolevulinic acid |
| Ald-DSB | aldehyde-terminated molecules |
| AS-AMD | poly-AS1411 aptamer |
| ATO | atovaquone |
| ATP | adenosine triphosphate |
| BDF | bonding defects functionalization |
| BET | extra-terminal domain |
| BGM | buffalo green monkey kidney |
| BODIPY | boron-dipyrromethene |
| BODIPY-CDs complexes | BODIPY-α-CD, BODIPY-β-CD, and BODIPY-γ-CD where CD = cyclodextrin |
| 3BP | 3-bromopyruvate |
| BPN | black phosphorus nanosheets |
| BPN/MnO2 | nanosponge from tightly packed MnO2-laden BPN |
| BPN/MnO2/DOX | BPN/MnO2 nanocomposite loaded with DOX |
| BRD4 | bromodomain-containing protein 4 |
| CAT@MnPFs | catalase-loaded manganese-porphyrin frameworks |
| CD | cyclodextrin |
| CDs | carbon dots |
| Ce6 | chlorin e6 |
| CIII | mitochondrial complex III; cytochrome bc1 complex; ubiquinol cytochrome c reductase; |
| CMPNs | self-assembly of hyaluronic acid-conjugated-methoxy poly(ethylene glycol)-diethylenetriamine-grafted-(chlorin e6-dihydrolipoic acid-(3-carboxypropyl)triphenylphosphine bromide) polymeric ligands (HA-c-mPEG-Deta-g-(Ce6-DHLA-TPP)) and NaErF4:Tm@NaYF4 core-shell UCNPs |
| COFs | covalent organic frameworks |
| COUPY 1 | coumarin-based fluorophores 1 |
| COUPY 2 | coumarin-based fluorophores 2 |
| CPPO | bis [2,4,5-trichloro-6-(pentyloxycar-bonyl)phenyl]oxalate |
| C-TBD NPs | C-TBD nanoparticles |
| CTPP | triphenylphosphine |
| dBET6 | small-molecule degrader |
| DCs | dendritic cells |
| DEM | diethyl maleate |
| DNA | deoxyribonucleic acid |
| DOX | doxorubicin |
| EHMONs | eccentric hollow mesoporous organic silica NPs |
| EMT | epithelial-mesenchymal transition |
| ENBS-B | molecular superoxide radical (O2–•) generator |
| ETC | electro-transport chain |
| FDA | U.S. Food and Drug Administration |
| FEITC | β-phenylethyl isothiocyanate |
| FFRKGPLGLAGC-PEG-DSPE | DSPE-PEG2000-modified transformable peptides |
| FLI | fluorescence imaging |
| FRET | fluorescence resonance energy transfer |
| FR/NIR | far-red/near-infrared |
| GSDME | gasdermin E |
| GSH | glutathione |
| H2O2 | hydrogen peroxide |
| HeLa | cervical cancer cell line containing human papillomavirus 18 (HPV-18) |
| HIF-1-alpha | hypoxia-inducible factor 1-alpha |
| HPV-18 | human papillomavirus 18 |
| ICB | immune checkpoint blockade |
| ICD | immunogenic cell death |
| ICG | indocyanine green |
| Ir | iridium |
| IR780 | IR-780 dye for near-infrared fluorescence imaging/photosensitizer |
| MB | methylene blue |
| MC | Mn3 [Co(CN) 6]2 |
| MC-r | Mn3 [Co(CN) 6]2 -Ru |
| MCF-7 | human breast cancer cell line |
| MDA-MB-231 | TSPO-positive breast cancer cells |
| mETC | mitochondrial electron transport chain |
| MKN-45 cells | human gastric adenocarcinoma cell line |
| MRI | magnetic resonance imaging |
| mROS | mitochondrial ROS |
| MSNs | mesoporous silica nanoparticles |
| Mn3O4@MSNs@IR780 | NPs-PS (IR780)—90 nm MSNs—capping surface pores 5 nm Mn3O4 NPs |
| mtDAMPs | mitochondrial damage-associated molecular patterns |
| mtDNA | mitochondrial DNA |
| MTCS | HeLa multicellular tumor spheroids |
| NaGdF4:Nd | nanocrystals |
| NBEX (X = S, Se, Te) | type-III PS designed to bind to RNA |
| NCs | nanocapsules |
| NCOFs | boron-dipyrromethene (BODIPY)-decorated nanoscale COFs |
| NIR | near infrared |
| nMOL | nanoscale metal-organic layer |
| NO | nitric oxide |
| NP | nanoparticle |
| NPs | nanoparticles |
| NSs | nanoshuttles |
| 1O2 | singlet oxygen |
| OTL38 | pafolacianine |
| OxgeMCC-r SAE | OxgeMCC-r single-atom enzyme (SAE) |
| OXPHOS | oxidative phosphorylation |
| 2PA | two-photon absorption |
| PD-1 | programmed cell death-1 |
| PD-L1 | programmed death ligand 1 |
| PDT | photodynamic therapy |
| PFC | perfluorocarbon |
| PFCs | perfluorocarbons |
| PLGA | polylactic-co-glycolic acid |
| PpIX | Protoporphyrin IX |
| PROTAC | proteolysis-targeting chimeras |
| PTT | photothermal therapy |
| RBCm | red blood cell membrane |
| RBS | Roussin’s black salt |
| Rh-PTZ NPs | Rh-PTZ nanoparticles |
| ROS | reactive oxygen species |
| SAE | single-atom enzyme |
| SBU | secondary building units |
| SRRF | super-resolution radial fluctuations |
| TAMs | tumor-associated M2 macrophages |
| TBD | TPE-BT-DC |
| TCA | tricarboxylic acid cycle |
| TIME | tumor immune microenvironment |
| TME | tumor microenvironment |
| TNBC | triple-negative breast cancer |
| TPP-TK-PPa | amphiphilic heterodimeric photosensitizer |
| TSPO | translocator protein |
| UCNPs | upconversion nanoparticles |
| UCNPs@mSiO2-NH2&RBS | platform with nitric oxide release upon NIR irradiation |
| US | United States |
| VEGF | vascular endothelial growth factor |
| WHO | World Health Organization |
| ZnPc | zinc phthalocyanine |
| ΔΨm | mitochondrial membrane potential |
| Increased | ↑ |
| Decreased | ↓ |
| Present | + |
| Absent/Missing | - |
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2024. Basic Cancer Facts. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf (accessed on 27 December 2024).
- World Health Organization. Eastern Mediterranean Region. Available online: https://www.emro.who.int/media/news/world-cancer-day-2024.html (accessed on 27 December 2024).
- Prasad, V.; Mailankody, S. Research and Development Spending to Bring a Single Cancer Drug to Market and Revenues After Approval. JAMA Intern. Med. 2017, 177, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Mohs, R.C.; Greig, N.H. Drug discovery and development: Role of basic biological research. Alzheimer’s Dement. 2017, 3, 651–657. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sinica. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Wang, F.; Ruan, D.Y.; Xu, R.H. Challenges and opportunities in oncology drug development and clinical research in China. Cell 2024, 187, 1578–1583. [Google Scholar] [CrossRef]
- Pei, Z. Computer-aided drug discovery: From traditional simulation methods to language models and quantum computing. Cell Rep. Phys. Sci. 2024, 5, 102334. [Google Scholar] [CrossRef]
- Huanbutta, K.; Burapapadh, K.; Kraisit, P.; Sriamornsak, P.; Ganokratanaa, T.; Suwanpitak, K.; Sangnim, T. Artificial intelligence-driven pharmaceutical industry: A paradigm shift in drug discovery, formulation development, manufacturing, quality control, and post-market surveillance. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2024, 203, 106938. [Google Scholar] [CrossRef]
- Nissan, N.; Allen, M.C.; Sabatino, D.; Biggar, K.K. Future Perspective: Harnessing the Power of Artificial Intelligence in the Generation of New Peptide Drugs. Biomolecules 2024, 14, 1303. [Google Scholar] [CrossRef]
- Swanton, C.; Bernard, E.; Abbosh, C.; André, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing cancer complexity: Hallmarks of systemic disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef]
- McAleer, S. A history of cancer and its treatment: Presidential Address to the Ulster Medical Society. 7th October 2021. Ulst. Med. J. 2022, 91, 124–129. [Google Scholar]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Synergistic Nanomedicine: Photodynamic, Photothermal and Photoimmune Therapy in Hepatocellular Carcinoma: Fulfilling the Myth of Prometheus? Int. J. Mol. Sci. 2023, 24, 8308. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Grin, M.; Suvorov, N.; Ostroverkhov, P.; Pogorilyy, V.; Kirin, N.; Popov, A.; Sazonova, A.; Filonenko, E. Advantages of Combined Photodynamic Therapy in the Treatment of Oncological Diseases. Biophys. Rev. 2022, 14, 941–963. [Google Scholar] [CrossRef]
- Huis in ‘t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Mariño-Ocampo, N.; Dibona-Villanueva, L.; Escobar-Álvarez, E.; Guerra-Díaz, D.; Zúñiga-Núñez, D.; Fuentealba, D.; Robinson-Duggon, J. Recent Photosensitizer Developments, Delivery Strategies and Combination-based Approaches for Photodynamic Therapy. Photochem. Photobiol. 2023, 2, 469–497. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Litscher, G. Curcumin and Photobiomodulation in Chronic Viral Hepatitis and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 7150. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Meng, C.; Feng, F. Recent advances for enhanced photodynamic therapy: From new mechanisms to innovative strategies. Chem. Sci. 2024, 15, 12234–12257. [Google Scholar] [CrossRef]
- Mao, D.; Wu, W.; Ji, S.; Chen, C.; Hu, F.; Kong, D.; Ding, D.; Liu, B. Chemiluminescence-Guided Cancer Therapy Using a Chemiexcited Photosensitizer. Chem 2017, 3, 991–1007. [Google Scholar] [CrossRef]
- Li, M.; Xia, J.; Tian, R.; Wang, J.; Fan, J.; Du, J.; Long, S.; Song, X.; Foley, J.W.; Peng, X. Near-Infrared Light-Initiated Molecular Superoxide Radical Generator: Rejuvenating Photodynamic Therapy against Hypoxic Tumors. J. Am. Chem. Soc. 2018, 140, 14851–14859. [Google Scholar] [CrossRef]
- Sun, C.L.; Li, J.; Wang, X.Z.; Shen, R.; Liu, S.; Jiang, J.Q.; Li, T.; Song, Q.W.; Liao, Q.; Fu, H.B.; et al. Rational Design of Organic Probes for Turn-On Two-Photon Excited Fluorescence Imaging and Photodynamic Therapy. Chem 2019, 5, 600–616. [Google Scholar] [CrossRef]
- Guan, Q.; Fu, D.D.; Li, Y.A.; Kong, X.M.; Wei, Z.Y.; Li, W.Y.; Zhang, S.J.; Dong, Y.B. BODIPY-Decorated Nanoscale Covalent Organic Frameworks for Photodynamic Therapy. iScience 2019, 14, 180–198. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, G.; Gong, K.; Wang, J.; Ge, X.; Liu, X.; Guo, S.; Wang, F. MnO2-Laden Black Phosphorus for MRI-Guided Synergistic PDT, PTT, and Chemotherapy. Matter 2019, 1, 496–512. [Google Scholar] [CrossRef]
- Wei, S.M.; Feng, K.; Li, C.; Xie, N.; Wang, Y.; Yang, X.L.; Chen, B.; Tung, C.H.; Wu, L.Z. ZnCl2 Enabled Synthesis of Highly Crystalline and Emissive Carbon Dots with Exceptional Capability to Generate O2.–. Matter 2020, 2, 495–506. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H.; et al. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 2020, 11, 357. [Google Scholar] [CrossRef]
- Zheng, B.; Zhong, D.; Xie, T.; Zhou, J.; Li, W.; Ilyas, A.; Lu, Y.; Zhou, M.; Deng, R. Near-infrared photosensitization via direct triplet energy transfer from lanthanide nanoparticles. Chem 2021, 7, 1615–1625. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, J.; Long, S.; Zhao, X.; Li, H.; Du, J.; Shao, K.; Peng, X. The concept and examples of type-III photosensitizers for cancer photodynamic therapy. Chem 2022, 8, 197–209. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, B.; Liu, Y.; Hu, D.; Sheng, Z.; Zhang, X.; Yuan, Z. Molecular Engineering of Near-Infrared Light-Responsive BODIPY-Based Nanoparticles with Enhanced Photothermal and Photoacoustic Efficiencies for Cancer Theranostics. Theranostics 2019, 9, 5315–5331. [Google Scholar] [CrossRef]
- Dartar, S.; Ucuncu, M.; Karakus, E.; Hou, Y.; Zhao, J.; Emrullahoglu, M. BODIPY-Vinyl Dibromides as Triplet Sensitisers for Photodynamic Therapy and Triplet-Triplet Annihilation Upconversion. Chem. Commun. 2021, 57, 6039–6042. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Huang, C.; Zhao, S.; Zhu, S.; Liu, R.; Zhu, H. Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging. Molecules 2023, 28, 2997. [Google Scholar] [CrossRef]
- Das, S.; Dey, S.; Patra, S.; Bera, A.; Ghosh, T.; Prasad, B.; Sayala, K.D.; Maji, K.; Bedi, A.; Debnath, S. BODIPY-Based Molecules for Biomedical Applications. Biomolecules 2023, 13, 1723. [Google Scholar] [CrossRef]
- Lu, B.; Lu, X.; Mu, M.; Meng, S.; Feng, Y.; Zhang, Y. Novel near-infrared BODIPY-cyclodextrin complexes for photodynamic therapy. Heliyon 2024, 10, e26907. [Google Scholar] [CrossRef]
- Lee, J.H.; Wee, C.W. Treatment of Adult Gliomas: A Current Update. Brain Neurorehabil. 2022, 15, e24. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma targeted therapy: Insight into future of molecular approaches. Mol. Cancer. 2022, 21, 39. [Google Scholar] [CrossRef]
- Simon, M.; Hagemann, A.; Gajadin, S.; Signorelli, F.; Vincent, A.J.P.E. Surgical treatment for insular gliomas. A systematic review and meta-analysis on behalf of the EANS neuro-oncology section. Brain Spine 2024, 4, 102828. [Google Scholar] [CrossRef]
- Lucke-Wold, B.; Rangwala, B.S.; Shafique, M.A.; Siddiq, M.A.; Mustafa, M.S.; Danish, F.; Nasrullah, R.M.U.; Zainab, N.; Haseeb, A. Focus on current and emerging treatment options for glioma: A comprehensive review. World J. Clin. Oncol. 2024, 15, 482–495. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Xiang, M.; Zhou, Q.; Shi, Z.; Wang, X.; Li, M.; Jia, Y.; Li, S.; Yang, F.; Wang, W.; Chen, T.; et al. A Review of Light Sources and Enhanced Targeting for Photodynamic Therapy. Curr. Med. Chem. 2021, 28, 6437–6457. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.H.; Fa, X.M.; Liu, M.S.; Wang, Q.L.; Zeng, T.F.; Chen, R.Z.; Ou, J.; Xia, X.W. Preliminary investigation of nitric oxide release from upconverted nanoparticles excited at 808 nm near-infrared for brain tumors. Heliyon 2024, 10, e33576. [Google Scholar] [CrossRef]
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled Delivery of Nitric Oxide for Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 279–303. [Google Scholar] [CrossRef]
- Yu, H.; Tiemuer, A.; Yao, X.; Zuo, M.; Wang, H.Y.; Liu, Y.; Chen, X. Mitochondria-specific near-infrared photoactivation of peroxynitrite upconversion luminescent nanogenerator for precision cancer gas therapy. Acta Pharm. Sin. B 2024, 14, 378–391. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Q.; Zhou, Z.; Bai, H.; Xiao, N.; Xie, J.; Li, C. Nitric oxide-based multi-synergistic nanomedicine: An emerging therapeutic for anticancer. J. Nanobiotechnol. 2024, 22, 674. [Google Scholar] [CrossRef]
- Qamar, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Franco, M.; Iqbal, H. Enzyme-loaded nanostructured materials for the degradation of environmental pollutants. Curr. Opin. Environ. Sci. Health 2022, 30, 100400. [Google Scholar] [CrossRef]
- Du, B.; Tung, C.H. Enzyme-Assisted Photodynamic Therapy Based on Nanomaterials. ACS Biomater. Sci. Eng. 2020, 6, 2506–2517. [Google Scholar] [CrossRef]
- Qiao, Y.; Tang, X.; Qiuju, X.; Zhang, G. Enzyme-loaded manganese-porphyrin metal-organic nanoframeworks for oxygen-evolving photodynamic therapy of hypoxic cells. Heliyon 2024, 10, e33902. [Google Scholar] [CrossRef]
- Darvin, P.; Chandrasekharan, A.; Varadarajan, S.N.; Chandrasekhar, L.; Maliakkal, R.T.; SM, J.S.; Varghese Jancy, S.; Santhoshkumar, T.R. Mitochondria targeted redox GFP reveals time and dose dependent onset and progression of mitochondrial oxidation with diverging cell death decisions during photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 31, 101921. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Ai, S.; Sun, J.; Mai, X.; Guan, W. Self-generating oxygen enhanced mitochondrion-targeted photodynamic therapy for tumor treatment with hypoxia scavenging. Theranostics 2019, 9, 6809–6823. [Google Scholar] [CrossRef]
- Wen, J.; Luo, Y.; Gao, H.; Zhang, L.; Wang, X.; Huang, J.; Shang, T.; Zhou, D.; Wang, D.; Wang, Z.; et al. Mitochondria-targeted nanoplatforms for enhanced photodynamic therapy against hypoxia tumor. J. Nanobiotechnol. 2021, 19, 440. [Google Scholar] [CrossRef]
- Nash, G.T.; Luo, T.; Lan, G.; Ni, K.; Kaufmann, M.; Lin, W. Nanoscale Metal-Organic Layer Isolates Phthalocyanines for Efficient Mitochondria-Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143, 2194–2199. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.N.; Ma, W.; Yang, Y.; Chen, G.; Fu, H.; Cui, C.; Yu, Z.; Wang, X. Multifunctional AIE iridium (III) photosensitizer nanoparticles for two-photon-activated imaging and mitochondria targeting photodynamic therapy. J. Nanobiotechnol. 2021, 19, 254. [Google Scholar] [CrossRef]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.; Wang, M.; Yu, X.; Guo, J.; Sun, T.; Li, X.; Yao, L.; Dong, H.; Xu, Y. Metabolic Reprogramming in Triple-Negative Breast Cancer. Front. Oncol. 2020, 10, 428. [Google Scholar] [CrossRef]
- World Health Organization. Breast Cancer, 13 March 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 15 January 2025).
- Huang, Z.; Li, D.; Gou, F.; Xian, T.; Hu, S.H.; Xu, J.; Luo, F.Y.; Chen, Z.Z.; Wang, C.B.; Zang, M.Y. Mitochondria-targeted photosensitizer based nanoplatform loading glutathione inhibitor for enhanced breast cancer photodynamic therapy. Colloids Surf. B Biointerfaces 2022, 220, 112956. [Google Scholar] [CrossRef]
- Seah, D.; Cheng, Z.; Vendrell, M. Fluorescent Probes for Imaging in Humans: Where Are We Now? ACS Nano 2023, 17, 19478–19490. [Google Scholar] [CrossRef]
- Cytalux: Package Insert/Prescribing Info. Drugs.com. Last Updated on 17 October 2024. Available online: https://www.drugs.com/pro/cytalux.html (accessed on 18 January 2025).
- Ge, Y.; O’Shea, D.F. Review of Clinically Assessed Molecular Fluorophores for Intraoperative Image Guided Surgery. Molecules 2024, 29, 5964. [Google Scholar] [CrossRef]
- Bonelli, J.; Ortega-Forte, E.; Rovira, A.; Bosch, M.; Torres, O.; Cuscó, C.; Rocas, J.; Ruiz, J.; Marchán, V. Improving Photodynamic Therapy Anticancer Activity of a Mitochondria-Targeted Coumarin Photosensitizer Using a Polyurethane-Polyurea Hybrid Nanocarrier. Biomacromolecules 2022, 23, 2900–2913. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Yoon, J. Organelle-Targeted Photosensitizers for Precision Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 19543–19571. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef]
- Ma, X.; Jin, M.; Cai, Y.; Xia, H.; Long, K.; Liu, J.; Yu, Q.; Yuan, J. Mitochondrial electron transport chain complex III is required for antimycin A to inhibit autophagy. Chem. Biol. 2011, 18, 1474–1481. [Google Scholar] [CrossRef]
- Antimycin A from Streptomyces sp. Available online: https://www.sigmaaldrich.com/RO/en/product/sigma/a8674?srsltid=AfmBOoomBUZa_KNALz9iXKTqRrwX7X0lOYTphd0y_7SWH41O6E9GHb5a (accessed on 21 January 2025).
- Coates, J.T.T.; Rodriguez-Berriguete, G.; Puliyadi, R.; Ashton, T.; Prevo, R.; Wing, A.; Granata, G.; Pirovano, G.; McKenna, G.W.; Higgins, G.S. The anti-malarial drug atovaquone potentiates platinum-mediated cancer cell death by increasing oxidative stress. Cell Death Discov. 2020, 6, 110. [Google Scholar] [CrossRef]
- Ashton, T.M.; Fokas, E.; Kunz-Schughart, L.A.; Folkes, L.K.; Anbalagan, S.; Huether, M.; Kelly, C.J.; Pirovano, G.; Buffa, F.M.; Hammond, E.M.; et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat. Commun. 2016, 7, 12308. [Google Scholar] [CrossRef]
- Fry, M.; Pudney, M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 1992, 43, 1545–1553. [Google Scholar] [CrossRef]
- Mather, M.W.; Darrouzet, E.; Valkova-Valchanova, M.; Cooley, J.W.; McIntosh, M.T.; Daldal, F.; Vaidya, A.B. Uncovering the molecular mode of action of the antimalarial drug atovaquone using a bacterial system. J. Biol. Chem. 2005, 280, 27458–27465. [Google Scholar] [CrossRef]
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial pharmacology and therapeutics of atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985. [Google Scholar] [CrossRef]
- Chen, A.; Yu, Z.; Ma, N.; Lu, X.; Zhang, Y.; Xu, W.; Wang, Y.; Xie, J.; Qin, Y.; Mo, G.; et al. Atovaquone enhances antitumor efficacy of TCR-T therapy by augmentation of ROS-induced ferroptosis in hepatocellular carcinoma. Cancer Immunol. Immunother. 2024, 73, 49. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Li, Z.; Li, D.; Lu, X.; Ai, S.; Dong, Y.; Liu, S.; Wu, J.; Guan, W. Oxygen tank for synergistic hypoxia relief to enhance mitochondria-targeted photodynamic therapy. Biomater. Res. 2022, 26, 47. [Google Scholar] [CrossRef]
- Zeng, F.; Fan, Z.; Li, S.; Li, L.; Sun, T.; Qiu, Y.; Nie, L.; Huang, G. Tumor Microenvironment Activated Photoacoustic-Fluorescence Bimodal Nanoprobe for Precise Chemo-immunotherapy and Immune Response Tracing of Glioblastoma. ACS Nano 2023, 17, 19753–19766. [Google Scholar] [CrossRef]
- Luo, H.; Gao, S. Recent advances in fluorescence imaging-guided photothermal therapy and photodynamic therapy for cancer: From near-infrared-I to near-infrared-II. J. Control Release. 2023, 362, 425–445. [Google Scholar] [CrossRef]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards more accurate bioimaging of drug nanocarriers: Turning aggregation-caused quenching into a useful tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef]
- Yanhong Duo, Y.; Xiang, Z.; Gao, G.; Luo, G.; Tang, Z.B. Biomedical application of aggregation-induced emission luminogen-based fluorescent sensors. TrAC Trends Anal. Chem. 2023, 167, 117252. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, X.; Shang, R.; Chen, H.; Tan, N. A structure-guided strategy to design Golgi apparatus-targeted type-I/II aggregation-induced emission photosensitizers for efficient photodynamic therapy. Acta Biomater. 2024, 183, 235–251. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.; Kim, J.; Kim, B.; Lee, J.; Kim, Y.; Li, M.; Kang, H.; Kim, J.S. Cancer therapeutics based on diverse energy sources. Chem. Soc. Rev. 2022, 51, 8201–8215. [Google Scholar] [CrossRef]
- Ganji, C.; Muppala, V.; Khan, M.; Nagaraju, P.G.; Farran, B. Mitochondrial-targeted nanoparticles: Delivery and therapeutic agents in cancer. Drug Discov. Today 2023, 28, 103469. [Google Scholar] [CrossRef]
- Long, X.; Liu, M.; Nan, Y.; Chen, Q.; Xiao, Z.; Xiang, Y.; Ying, X.; Sun, J.; Huang, Q.; Ai, K. Revitalizing Ancient Mitochondria with Nano-Strategies: Mitochondria-Remedying Nanodrugs Concentrate on Disease Control. Adv. Mater. 2024, 3, e2308239. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Li, F.; Liu, C.; Huang, M.; Li, J.; Gao, F.; Ruan, X.; Yang, D. An Energy-Storing DNA-Based Nanocomplex for Laser-Free Photodynamic Therapy. Adv. Mater. 2022, 34, e2109920. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Yang, S.; Zhang, R.; Guo, J.; Yang, D. Energy-storing DNA-based hydrogel remodels tumor microenvironments for laser-free photodynamic immunotherapy. Biomaterials 2024, 309, 122620. [Google Scholar] [CrossRef]
- Chen, M.; Xu, H.; Chang, P.; Li, X.; Liu, S.; Xu, L.; Xu, K.; Cheng, G. An intelligent poly aptamer-encoded DNA nanoclew for tumor site activated mitochondria-targeted photodynamic therapy and MR imaging. Mater. Today Bio. 2024, 29, 101318. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; Ling, X.; Shao, P.; Wang, X.; Edwards, W.B.; Bai, M. Tumor mitochondria-targeted photodynamic therapy with a translocator protein (TSPO)-specific photosensitizer. Acta Biomater. 2015, 28, 160–170. [Google Scholar] [CrossRef]
- Betlazar, C.; Middleton, R.J.; Banati, R.; Liu, G.J. The Translocator Protein (TSPO) in Mitochondrial Bioenergetics and Immune Processes. Cells 2020, 9, 512. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Papadopoulos, V. The mitochondrial translocator protein (TSPO, 18 kDa): A key multifunctional molecule in liver diseases. Biochimie 2024, 224, 91–103. [Google Scholar] [CrossRef]
- WHO. Colorectal Cancer. 11 July 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 27 January 2025).
- Wang, L.; Geng, H.; Liu, Y.; Liu, L.; Chen, Y.; Wu, F.; Liu, Z.; Ling, S.; Wang, Y.; Zhou, L. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm 2023, 4, e343. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Wang, B.; Wang, P.; Li, D.; Cao, T.; Zhang, D.; Han, H.; Bai, M.; Wang, X.; et al. Mitochondria-targeted photodynamic therapy triggers GSDME-mediated pyroptosis and sensitizes anti-PD-1 therapy in colorectal cancer. J. Immunother. Cancer 2024, 12, e008054. [Google Scholar] [CrossRef]
- Shi, L.; Hu, F.; Duan, Y.; Wu, W.; Dong, J.; Meng, X.; Zhu, X.; Liu, B. Hybrid Nanospheres to Overcome Hypoxia and Intrinsic Oxidative Resistance for Enhanced Photodynamic Therapy. ACS Nano 2020, 14, 2183–2190. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics 2021, 11, 3278–3300. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, K.; He, Q.; Gu, X.; Jiang, C.; Wu, J. Hypoxia signaling in cancer: Implications for therapeutic interventions. MedComm 2023, 4, e203. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Tao, J.; Su, X.; Zhu, F.; Lu, W.; Han, X.; Dang, M.; Weng, L. Mitochondria-Targeting and Oxygen Self-Supplying Eccentric Hollow Nanoplatform for Enhanced Breast Cancer Photodynamic Therapy. Therapy. Bioinorg. Chem. Appl. 2024, 2024, 6618388. [Google Scholar] [CrossRef]
- Liu, Z.H.; Mo, X.W.; Jiang, W.; Liu, C.; Yin, Y.; Yang, H.Y.; Fu, Y. Multifunctional hyaluronic acid ligand-assisted construction of CD44- and mitochondria-targeted self-assembled upconversion nanoparticles for enhanced photodynamic therapy. Dalton Trans. 2024, 53, 16885–16895. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, G.; Wu, H.; Liu, C.; Zhan, Y.; Qiu, Y.; Shou, C.; Gao, F.; Zhang, J.; Yin, P.; et al. Photodynamic therapy synergizes with PD-L1 checkpoint blockade for immunotherapy of CRC by multifunctional nanoparticles. Mol. Ther. 2021, 29, 2931–2948. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Moralès, O.; Mordon, S.; Delhem, N.; Boleslawski, E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers 2021, 13, 5176. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cheng, Y.; Ma, X.; Jin, Z.; Zhang, Q.; Wang, G. Photodynamic therapy upregulates expression of HIF-1α and PD-L1 in related pathways and its clinical relevance in non-small-cell lung cancer. Eur. J. Med. Res. 2024, 29, 230. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, Y.; Li, X.; Luo, L.; You, J. Nanoplatform-enhanced photodynamic therapy for the induction of immunogenic cell death. J. Control Release 2024, 365, 1058–1073. [Google Scholar] [CrossRef]
- HIF1A. Wikipedia, The Free Encyclopedia. Available online: https://en.wikipedia.org/wiki/HIF1A (accessed on 31 January 2025).
- Konstantinidou, M.; Li, J.; Zhang, B.; Wang, Z.; Shaabani, S.; Ter Brake, F.; Essa, K.; Dömling, A. PROTACs- a game-changing technology. Expert. Opin. Drug Discov. 2019, 14, 1255–1268. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef]
- Li, X.; Pu, W.; Zheng, Q.; Ai, M.; Chen, S.; Peng, Y. Proteolysis-targeting chimeras (PROTACs) in cancer therapy. Mol. Cancer 2022, 21, 99. [Google Scholar] [CrossRef]
- Tong, F.; Wang, Y.; Xu, Y.; Zhou, Y.; He, S.; Du, Y.; Yang, W.; Lei, T.; Song, Y.; Gong, T.; et al. MMP-2-triggered, mitochondria-targeted PROTAC-PDT therapy of breast cancer and brain metastases inhibition. Nat. Commun. 2024, 15, 10382. [Google Scholar] [CrossRef]
- Yu, H.; Chen, B.; Huang, H.; He, Z.; Sun, J.; Wang, G.; Gu, X.; Tang, B.Z. AIE-Active Photosensitizers: Manipulation of Reactive Oxygen Species Generation and Applications in Photodynamic Therapy. Biosensors 2022, 12, 348. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhang, X.; Liu, Y.; Si, L.; Jiang, S.; Wang, A.; Che, X.; Chen, J.; Hu, J. Multi-pathway oxidative stress amplification via controllably targeted nanomaterials for photoimmunotherapy of tumors. J. Nanobiotechnol. 2025, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Li, L.; Zhan, Q.; Chen, J.; Li, Q.; Liu, R.; Tu, Y. Mitochondria-Targeting Type-I Photodynamic Therapy Based on Phenothiazine for Realizing Enhanced Immunogenic Cancer Cell Death via Mitochondrial Oxidative Stress. Int. J. Nanomed. 2025, 20, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Huang, B.; Hasnain Nawaz, M.; Zhang, W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Coord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Samperi, M.; Limón, D.; Amabilino, D.B.; Pérez García, L. Enhancing Singlet Oxygen Generation by Self-Assembly of a Porphyrin Entrapped in Supramolecular Fibers. Cell Rep. Phys. Sci. 2020, 1, 100030. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, Q.; Yang, J.; Zhao, Z.; Gao, A.; Chen, S.; Zhang, Y.; Meng, L.; Dang, D. Shuttle-like nanoassemblies by isomeric photosensitizers to enhance ROS generation and tumor penetration for photodynamic therapy. Matter 2024, 7, 4342–4355. [Google Scholar] [CrossRef]
- Lee, H.C.; Wei, Y.H. Mitochondrial role in life and death of the cell. J. Biomed. Sci. 2000, 7, 2–15. [Google Scholar] [CrossRef]
- Wang, S.F.; Tseng, L.M.; Lee, H.C. Role of mitochondrial alterations in human cancer progression and cancer immunity. J. Biomed. Sci. 2023, 30, 61. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID. Int. J. Mol. Sci. 2023, 24, 17198. [Google Scholar] [CrossRef]
- Antonucci, S.; Di Lisa, F.; Kaludercic, N. Mitochondrial reactive oxygen species in physiology and disease. Cell Calcium. 2021, 94, 102344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyás, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial Reactive Oxygen Species in Infection and Immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef]
- Li, H.; Shen, J.; Zheng, C.; Zhu, P.; Yang, H.; Huang, Y.; Mao, X.; Yang, Z.; Hu, G.; Chen, Y. Cell death: The underlying mechanisms of photodynamic therapy for skin diseases. Interdiscip. Med. 2025, e20240057. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).