Disparity Between Functional and Structural Recovery of Placental Mitochondria After Exposure to Hypoxia
Abstract
1. Introduction
2. Results
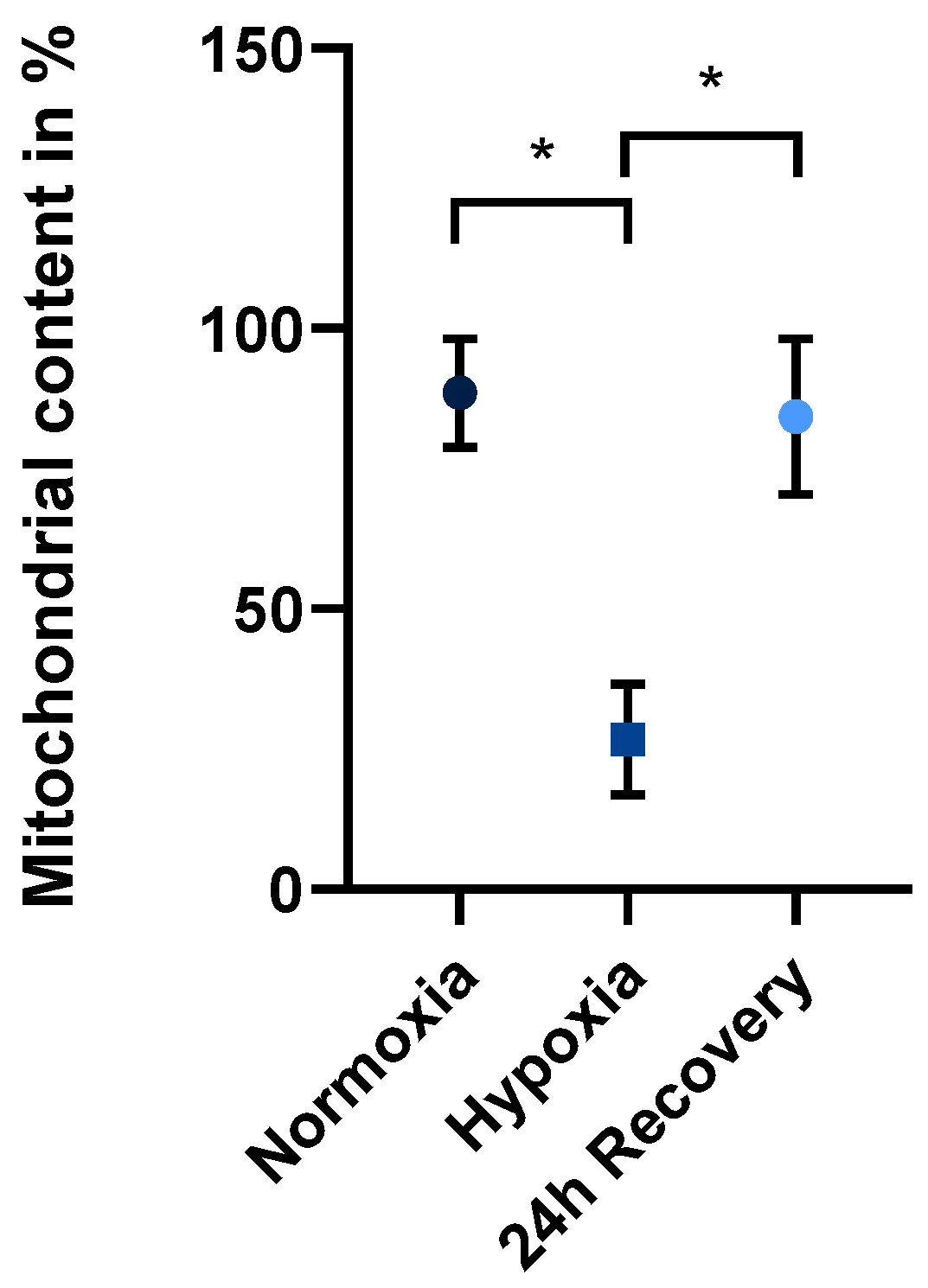
2.1. Mitochondrial Content in Normoxically and Hypoxically Cultured JEG-3 Cells
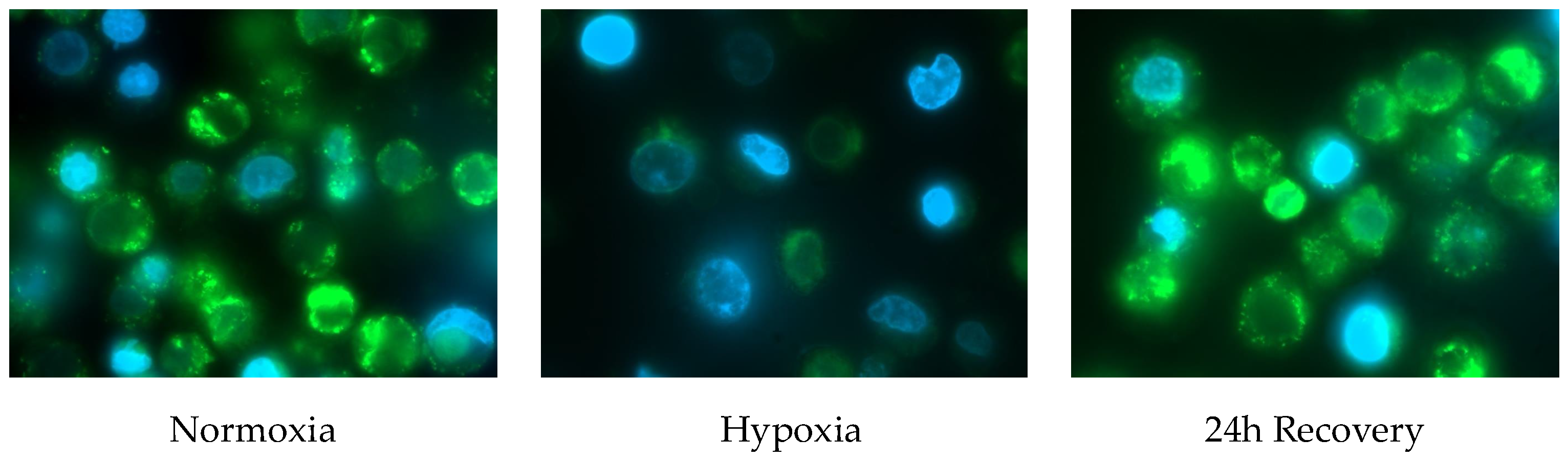
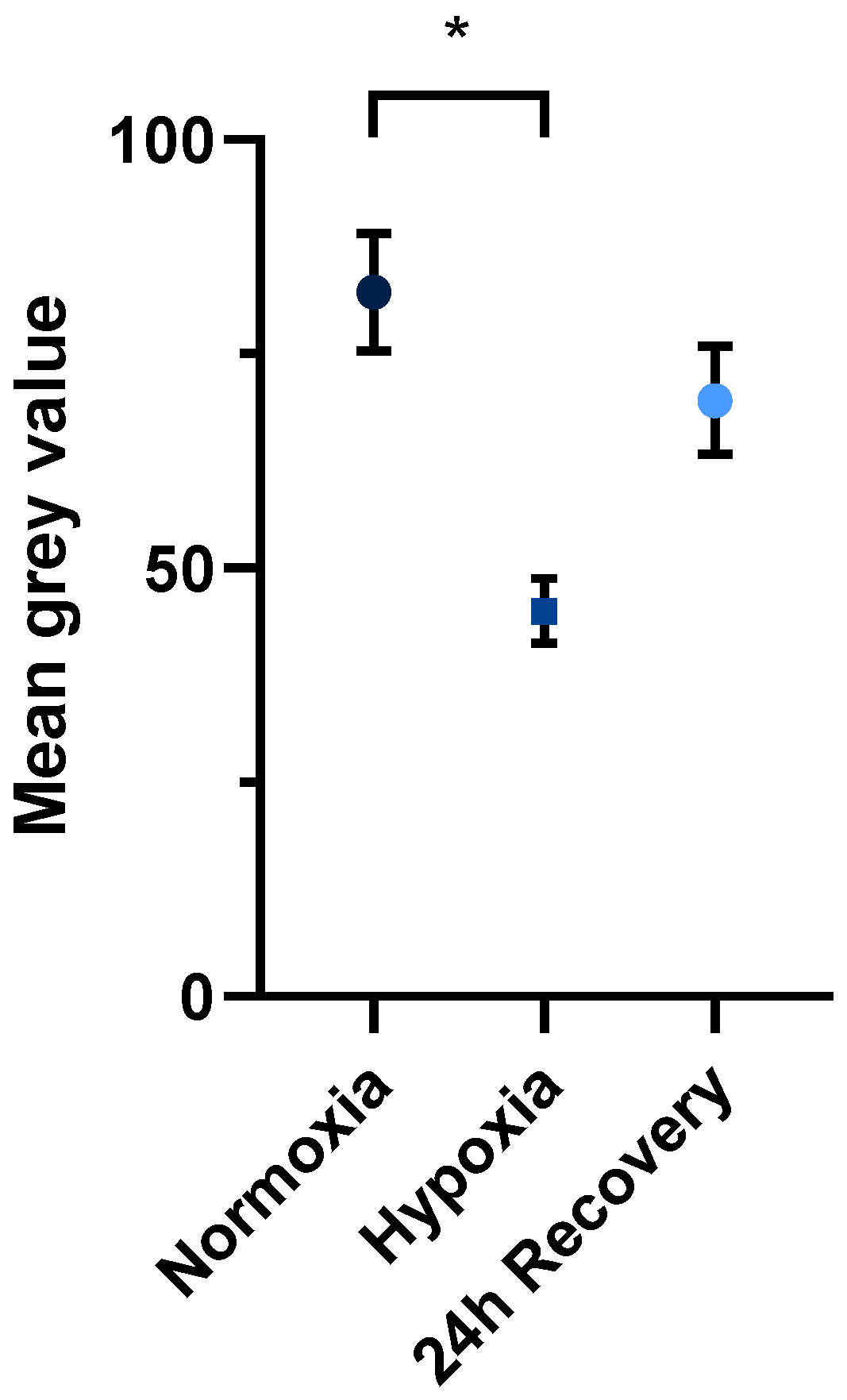
2.2. Fluorescence Microscopy of Normoxically and Hypoxically Cultured JEG-3 Cells
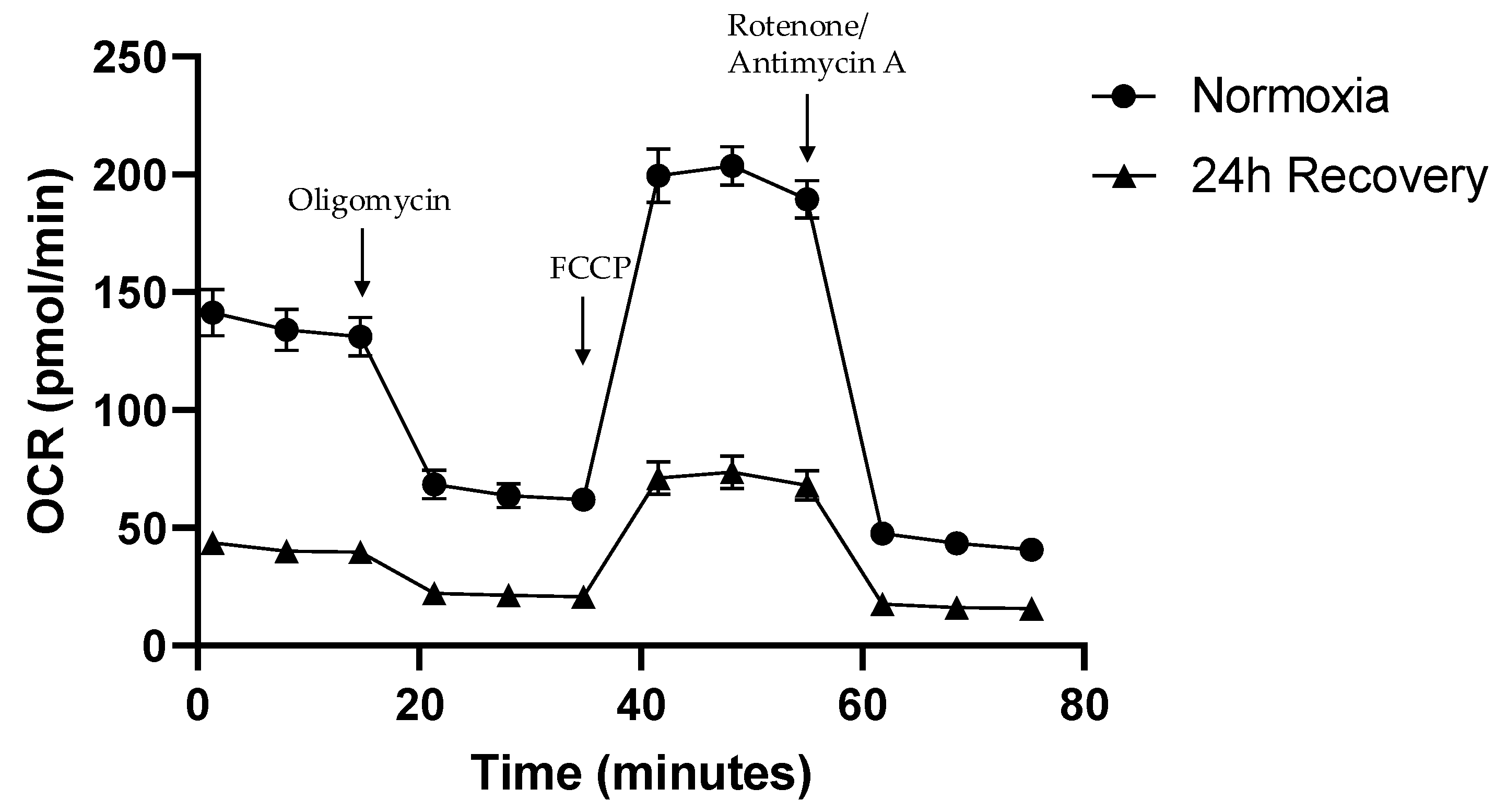
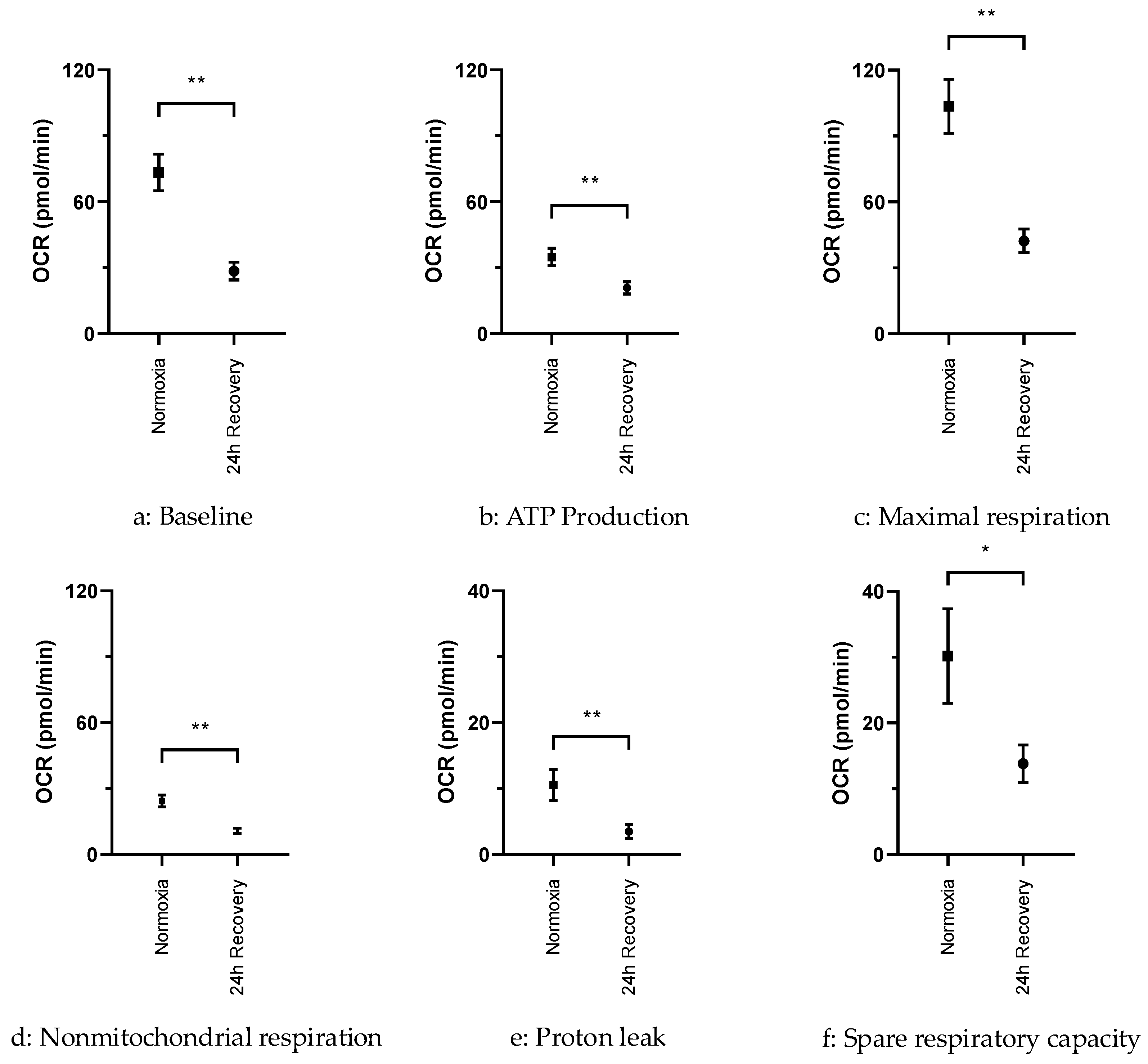
2.3. OCR in Normoxically and Hypoxically Cultured JEG-3 Cells
3. Discussion
3.1. Decrease in Mitochondrial Content After Exposure to Hypoxia
3.2. Restoration of Mitochondrial Content After Re-Exposure to Normoxia
3.3. Persisting Functional Impairment of JEG-3 Cells After Exposure to Hypoxia
3.4. Limitations
4. Materials and Methods
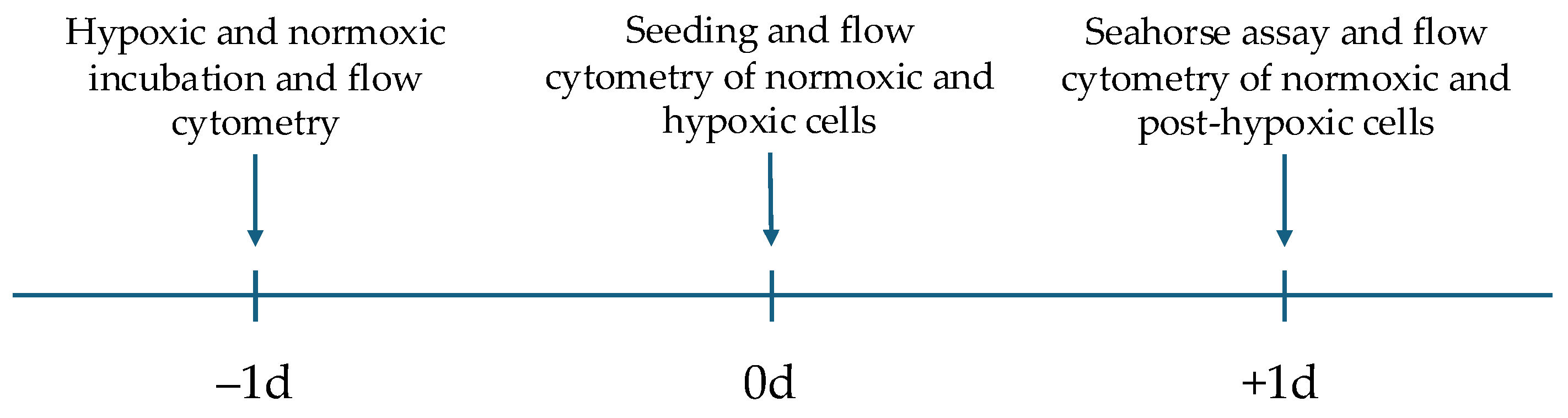
4.1. Cell Culture and Experimental Protocol
4.2. Flow Cytometry and Fluorescence Microscopy
4.3. Analysis of Oxidative Phosphorylation Activity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IUGR | Intrauterine growth restriction |
| FGR | Fetal growth restriction |
| DMEM | Dulbecco’s modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| OCR | Oxygen consumption rate |
| PI | Propium iodide |
| DAPI | 4’,6-diamidino-2-phenylindole |
| Mito | Mitochondrial |
| FCCP | Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone |
| ATP | Adenosine triphosphate |
| SEM | Standard error of mean |
References
- Albu, A.R.; Anca, A.F.; Horhoianu, V.V.; Horhoianu, I.A. Predictive factors for intrauterine growth restriction. J. Med. Life 2014, 7, 165–171. [Google Scholar] [PubMed]
- Kehl, S.; Dotsch, J.; Hecher, K.; Schlembach, D.; Schmitz, D.; Stepan, H.; Gembruch, U. Intrauterine Growth Restriction. Guideline of the German Society of Gynecology and Obstetrics (S2k-Level, AWMF Registry No. 015/080, October 2016). Geburtshilfe Frauenheilkd 2017, 77, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A. The IUGR newborn. Semin. Perinatol. 2008, 32, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef] [PubMed]
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar] [CrossRef]
- Murray, A.J. Oxygen delivery and fetal-placental growth: Beyond a question of supply and demand? Placenta 2012, 33 (Suppl. 2), e16–e22. [Google Scholar] [CrossRef] [PubMed]
- Singer, D. The human fetus and metabolic adaptations to hypoxia. In Hypoxic Respiratory Failure in the Newborn—From Origins to Clinical Management; Dakshinamurti, S., Abman, S.H., Cheung, P.Y., Lakshminrusimha, S., McNamara, P.J., Milsom, W.K., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 6–11. [Google Scholar] [CrossRef]
- Mando, C.; De Palma, C.; Stampalija, T.; Anelli, G.M.; Figus, M.; Novielli, C.; Parisi, F.; Clementi, E.; Ferrazzi, E.; Cetin, I. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E404–E413. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E.; Wakx, A.; Fouyet, S.; Dutot, M.; Rat, P. JEG-3 placental cells in toxicology studies: A promising tool to reveal pregnancy disorders. Anat. Cell Biol. 2021, 54, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Drwal, E.; Rak, A.; Gregoraszczuk, E. Co-culture of JEG-3, BeWo and syncBeWo cell lines with adrenal H295R cell line: An alternative model for examining endocrine and metabolic properties of the fetoplacental unit. Cytotechnology 2018, 70, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Rothbauer, M.; Patel, N.; Gondola, H.; Siwetz, M.; Huppertz, B.; Ertl, P. A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci. Rep. 2017, 7, 5892. [Google Scholar] [CrossRef]
- Vangrieken, P.; Al-Nasiry, S.; Bast, A.; Leermakers, P.A.; Tulen, C.B.M.; Janssen, G.M.J.; Kaminski, I.; Geomini, I.; Lemmens, T.; Schiffers, P.M.H.; et al. Hypoxia-induced mitochondrial abnormalities in cells of the placenta. PLoS ONE 2021, 16, e0245155. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Liu, Y.; Cui, L.; Wu, W.B.; Quinn, M.J.; Menon, R.; Zhang, H.J. Hypoxic effects on the mitochondrial content and functions of the placenta in fetal growth restriction. Placenta 2021, 114, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, I.; Gkikas, I.; Tavernarakis, N. Hypoxia and Selective Autophagy in Cancer Development and Therapy. Front. Cell Dev. Biol. 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B. Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017, 12, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.H.; Lala, P.K. Mechanism of control of trophoblast invasion in situ. J. Cell Physiol. 1991, 148, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Diers, A.R.; Vayalil, P.K.; Oliva, C.R.; Griguer, C.E.; Darley-Usmar, V.; Hurst, D.R.; Welch, D.R.; Landar, A. Mitochondrial bioenergetics of metastatic breast cancer cells in response to dynamic changes in oxygen tension: Effects of HIF-1alpha. PLoS ONE 2013, 8, e68348. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies Inc. Agilent Seahorse XF Cell Mito Stress Test Kit User Guide. Available online: https://www.agilent.com/cs/library/usermanuals/public/XF_Cell_Mito_Stress_Test_Kit_User_Guide.pdf (accessed on 24 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierla, J.R.; Pagerols Raluy, L.; Trochimiuk, M.; Trah, J.; Petrosyan, M.; Velasquez, L.N.; Schumacher, U.; Singer, D.; Heiter, J. Disparity Between Functional and Structural Recovery of Placental Mitochondria After Exposure to Hypoxia. Int. J. Mol. Sci. 2025, 26, 2956. https://doi.org/10.3390/ijms26072956
Sierla JR, Pagerols Raluy L, Trochimiuk M, Trah J, Petrosyan M, Velasquez LN, Schumacher U, Singer D, Heiter J. Disparity Between Functional and Structural Recovery of Placental Mitochondria After Exposure to Hypoxia. International Journal of Molecular Sciences. 2025; 26(7):2956. https://doi.org/10.3390/ijms26072956
Chicago/Turabian StyleSierla, Jonathan R., Laia Pagerols Raluy, Magdalena Trochimiuk, Julian Trah, Mariam Petrosyan, Lis N. Velasquez, Udo Schumacher, Dominique Singer, and Julia Heiter. 2025. "Disparity Between Functional and Structural Recovery of Placental Mitochondria After Exposure to Hypoxia" International Journal of Molecular Sciences 26, no. 7: 2956. https://doi.org/10.3390/ijms26072956
APA StyleSierla, J. R., Pagerols Raluy, L., Trochimiuk, M., Trah, J., Petrosyan, M., Velasquez, L. N., Schumacher, U., Singer, D., & Heiter, J. (2025). Disparity Between Functional and Structural Recovery of Placental Mitochondria After Exposure to Hypoxia. International Journal of Molecular Sciences, 26(7), 2956. https://doi.org/10.3390/ijms26072956





