Patient-Derived Colorectal Cancer Extracellular Matrices Modulate Cancer Cell Stemness Markers
Abstract
1. Introduction
2. Results and Discussion
2.1. Distribution Profiles of Colon Cancer Cells Differ on Normal vs. Tumor Matrices from Colorectal Cancer Patients
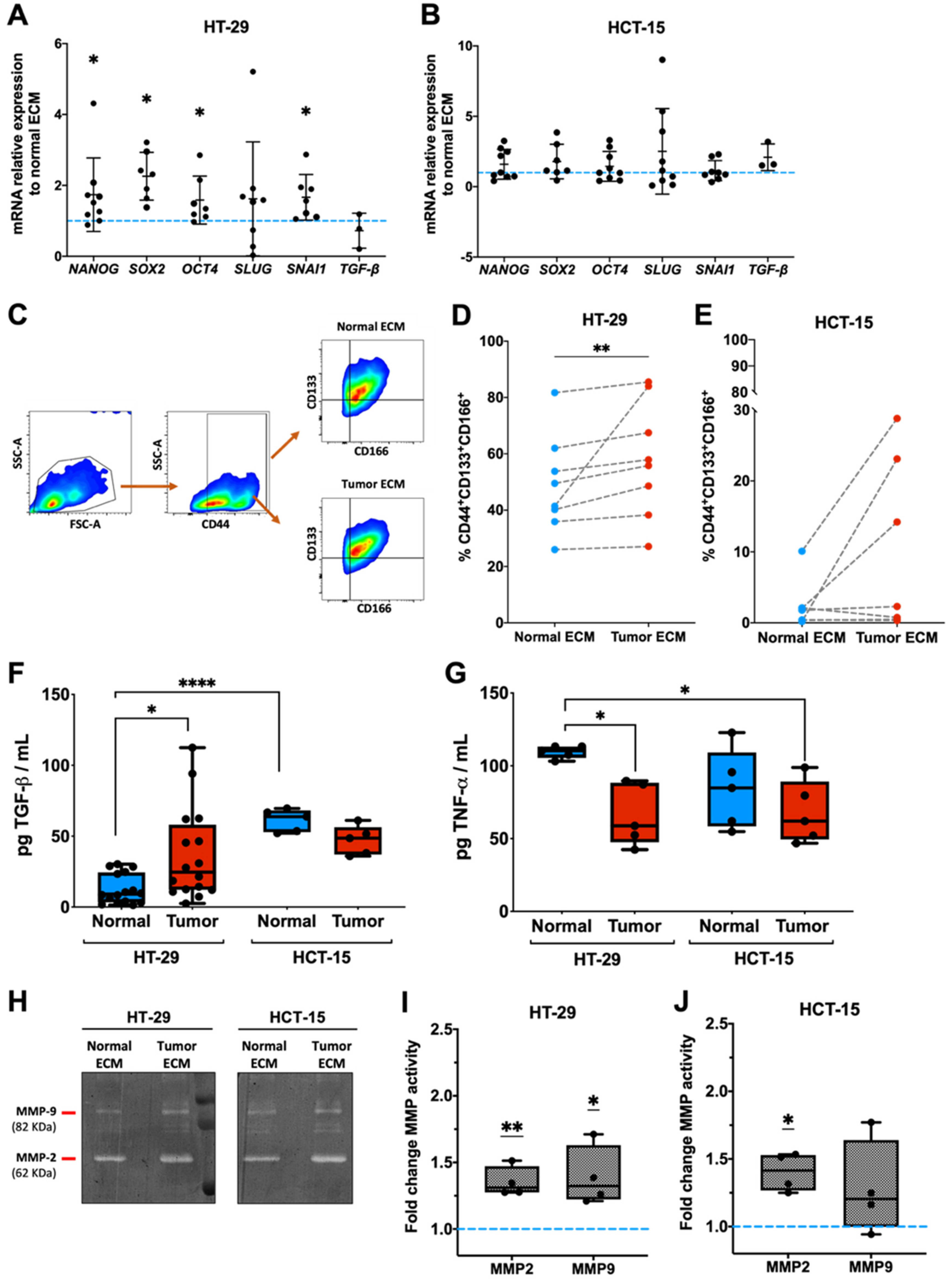
2.2. Tumor Matrices Induce HT-29 Colon Cancer Cells Stemness and Proteolysis
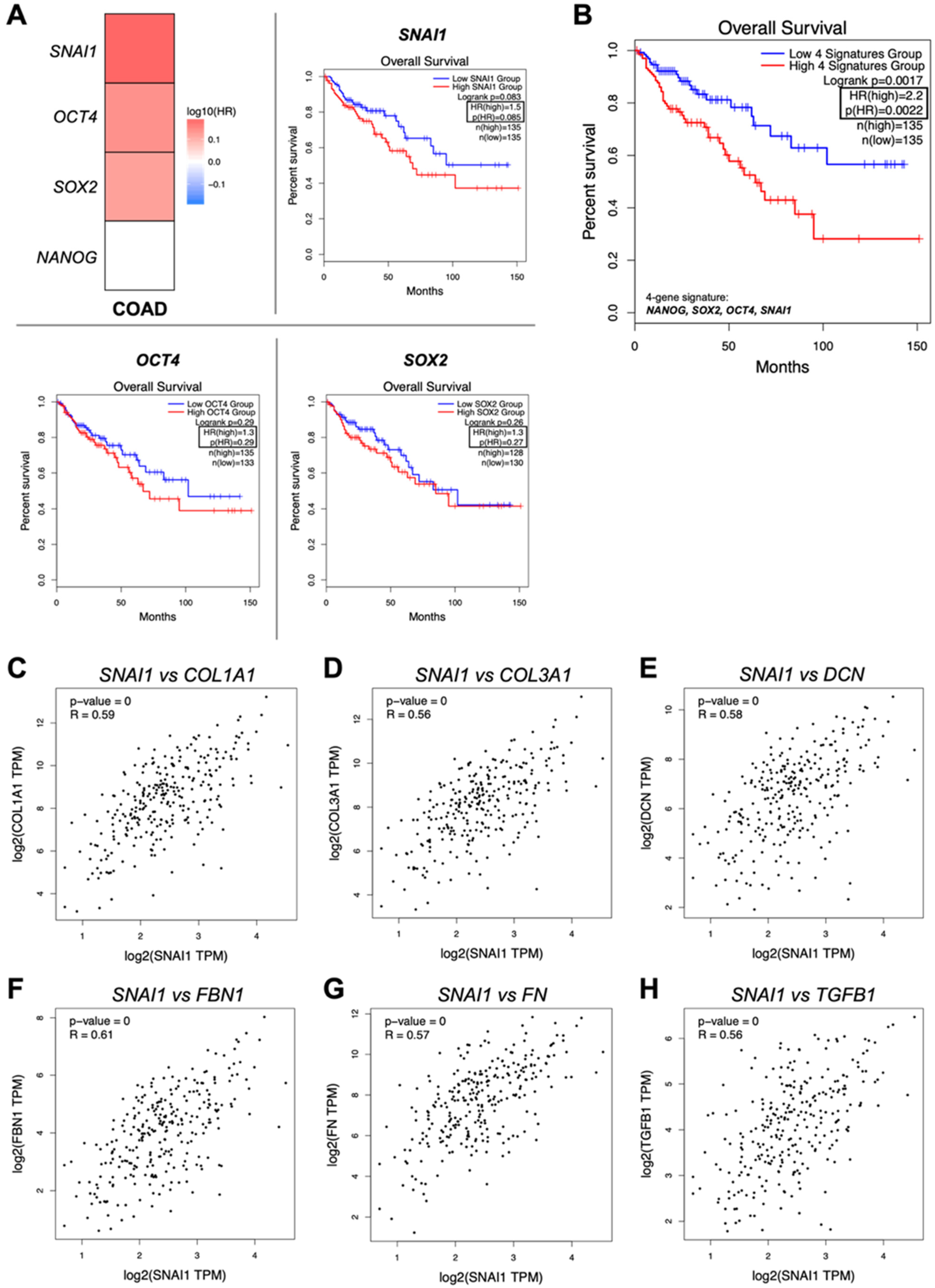
2.3. The Stemness Signature Triggered by Tumor Decellularized Matrices Is Associated with Patients’ Worse Overall Survival
3. Materials and Methods
3.1. Clinical Samples and Ethics Statement
3.2. Cell Lines and Culture Conditions
3.3. Tissue Decellularization
3.4. Tissue Recellularization
3.5. Hematoxylin and Eosin Staining
3.6. Masson’s Trichrome Staining
3.7. DAPI Staining
3.8. Flow Cytometry
3.9. Enzyme-Linked Immunosorbent Assay (ELISA)
3.10. RNA Extraction
3.11. Quantitative Real-Time PCR
3.12. Gelatin Zymography
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSC | Cancer stem cell |
| CRC | Colorectal Cancer |
| ECM | Extracellular matrix |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Uttam, S.; Stern, A.M.; Sevinsky, C.J.; Furman, S.; Pullara, F.; Spagnolo, D.; Nguyen, L.; Gough, A.; Ginty, F.; Taylor, D.L.; et al. Spatial domain analysis predicts risk of colorectal cancer recurrence and infers associated tumor microenvironment networks. Nat. Commun. 2020, 11, 3515–3528. [Google Scholar] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar]
- Cañellas-Socias, A.; Cortina, C.; Hernando-Momblona, X.; Palomo-Ponce, S.; Mulholland, E.J.; Turon, G.; Mateo, L.; Conti, S.; Roman, O.; Sevillano, M.; et al. Metastatic recurrence in colorectal cancer arises from residual EMP1+ cells. Nature 2022, 611, 603–613. [Google Scholar]
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A.; Baiocchi, M. Colorectal Cancer Stem Cells: An Overview of Evolving Methods and Concepts. Cancers 2021, 13, 5910. [Google Scholar] [CrossRef]
- Vaiopoulos, A.G.; Kostakis, I.D.; Koutsilieris, M.; Papavassiliou, A.G. Colorectal Cancer Stem Cells. Stem Cells 2012, 30, 363–371. [Google Scholar]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.J. The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 2019, 7, 86. [Google Scholar]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar]
- Murphy-Ullrich, J.E.; Sage, E.H. Revisiting the matricellular concept. Matrix Biol. 2014, 37, 1–14. [Google Scholar]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Lu, P.; Werb, Z. The Role of Stroma in Tumor Development. Cancer J. 2015, 21, 250–253. [Google Scholar]
- Dogan, E.; Kisim, A.; Bati-Ayaz, G.; Kubicek, G.J.; Pesen-Okvur, D.; Miri, A.K. Cancer stem cells in tumor modeling: Challenges and future directions. Adv. Nanobiomed Res. 2021, 1, 2100017. [Google Scholar]
- Pinto, M.L.; Rios, E.; Silva, A.C.; Neves, S.C.; Caires, H.R.; Pinto, A.T.; Durães, C.; Carvalho, F.A.; Cardoso, A.P.; Santos, N.C.; et al. Decellularized human colorectal cancer matrices polarize macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via CCL18. Biomaterials 2017, 124, 211–224. [Google Scholar]
- Marques-Magalhães, Â.; Cruz, T.; Costa, Â.M.; Estêvão, D.; Rios, E.; Canão, P.A.; Velho, S.; Carneiro, F.; Oliveira, M.J.; Cardoso, A.P. Decellularized colorectal cancer matrices as bioactive scaffolds for studying tumor-stroma interactions. Cancers 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.B.; Carvalho, F.A.; Matafome, P.N.; Soares, R.A.; Santos, N.C.; Travasso, R.D.; Oliveira, M.J. Mice with Type 2 diabetes present significant alterations in their tissue biomechanical properties and histological features. Biomedicines 2021, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Rodrigues, S.C.; Caldeira, J.; Nunes, A.M.; Sampaio-Pinto, V.; Resende, T.P.; Oliveira, M.J.; Barbosa, M.A.; Thorsteinsdóttir, S.; Nascimento, D.S. Three-dimensional scaffolds of fetal decellularized hearts exhibit enhanced potential to support cardiac cells in comparison to the adult. Biomaterials 2016, 104, 52–64. [Google Scholar]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines - a resource for biomedical studies. Mol. Cancer 2017, 16, 1–16. [Google Scholar]
- Cao, J.; Wu, X.; Qin, X.; Li, Z. Uncovering the Effect of Passage Number on HT29 Cell Line Based on the Cell Metabolomic Approach. J. Proteome Res. 2021, 20, 1582–1590. [Google Scholar]
- Parkinson, G.T.; Salerno, S.; Ranji, P.; Håkansson, J.; Bogestål, Y.; Wettergren, Y.; Ståhlberg, A.; Lindskog, E.B.; Landberg, G. Patient-derived scaffolds as a model of colorectal cancer. Cancer Med. 2021, 10, 867–882. [Google Scholar] [CrossRef]
- Müller, M.; Hermann, P.C.; Liebau, S.; Weidgang, C.; Seufferlein, T.; Kleger, A.; Perkhofer, L. The role of pluripotency factors to drive stemness in gastrointestinal cancer. Stem Cell Res. 2016, 16, 349–357. [Google Scholar]
- Vasefifar, P.; Motafakkerazad, R.; Maleki, L.A.; Najafi, S.; Ghrobaninezhad, F.; Najafzadeh, B.; Alemohammad, H.; Amini, M.; Baghbanzadeh, A.; Baradaran, B. Nanog, as a key cancer stem cell marker in tumor progression. Gene 2022, 827, 146448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, S.M.; Chen, S.Y.; Chen, J.X.; Wang, Z.Q.; Wang, Y.D.; Zheng, H. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by beta-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021, 12, 449. [Google Scholar] [PubMed]
- Wu, Y.D.; Zhou, B.H.P. Snail more than EMT. Cell Adhes. Migr. 2010, 4, 199–203. [Google Scholar]
- Zou, W.; Zhang, Y.; Bai, G.; Zhuang, J.; Wei, L.; Wang, Z.; Sun, M.; Wang, J. siRNA-induced CD44 knockdown suppresses the proliferation and invasion of colorectal cancer stem cells through inhibiting epithelial-mesenchymal transition. J. Cell. Mol. Med. 2022, 26, 1969–1978. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Han, S.; Yang, W.; Zong, S.; Li, H.; Liu, S.; Li, W.; Shi, Q.; Hou, F. Clinicopathological, prognostic and predictive value of CD166 expression in colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 64373–64384. [Google Scholar]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar]
- Pang, M.F.; Siedlik, M.J.; Han, S.; Stallings-Mann, M.; Radisky, D.C.; Nelson, C.M. Tissue stiffness and hypoxia modulate the Integrin-Linked Kinase ILK to control breast cancer stem-like cells. Cancer Res. 2016, 76, 5277–5287. [Google Scholar]
- Tan, F.; Huang, Y.; Pei, Q.; Liu, H.; Pei, H.; Zhu, H. Matrix stiffness mediates stemness characteristics via activating the Yes-associated protein in colorectal cancer cells. J. Cell. Biochem. 2019, 120, 2213–2225. [Google Scholar] [CrossRef]
- Ju, S.Y.; Chiou, S.H.; Su, Y. Maintenance of the stemness in CD44+ HCT-15 and HCT-116 human colon cancer cells requires miR-203 suppression. Stem Cell Res. 2014, 12, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kikushige, Y.; Miyawaki, K.; Kunisaki, Y.; Mizuno, S.; Takenaka, K.; Tamura, S.; Okumura, Y.; Ito, M.; Ariyama, H.; et al. Dedifferentiation process driven by TGF-beta signaling enhances stem cell properties in human colorectal cancer. Oncogene 2019, 38, 780–793. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, M.K.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011, 71, 4707–4719. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, A.; Yuan, Q.; Chen, W.; Zhong, H.; Teng, M.; Xu, C.; Qiu, Y.; Cao, J. NF-κB/Twist axis is involved in chysin inhibition of ovarian cancer stem cell features induced by co-treatment of TNF-α and TGF-β. Int. J. Clin. Exp. Pathol. 2019, 12, 101–112. [Google Scholar]
- Liu, Z.W.; Zhang, Y.M.; Zhang, L.Y.; Zhou, T.; Li, Y.Y.; Zhou, G.C.; Miao, Z.M.; Shang, M.; He, J.P.; Ding, N. Duality of interactions between TGF-β and TNF-α during tumor formation. Front. Immunol. 2022, 12, 810286. [Google Scholar] [CrossRef]
- Cabal-Hierro, L.; O’Dwyer, P.J. TNF signaling through RIP1 kinase enhances SN38-induced death in colon adenocarcinoma. Mol. Cancer Res. 2017, 15, 395–404. [Google Scholar] [CrossRef]
- Yamane, K.; Ihn, H.; Asano, Y.; Jinnin, M.; Tamaki, K. Antagonistic effects of TNF-alpha on TGF-beta signaling through down-regulation of TGF-beta receptor type II in human dermal fibroblasts. J. Immunol. 2003, 171, 3855–3862. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Suto, M.J. Thrombospondin-1 regulation of latent TGF-β activation: A therapeutic target for fibrotic disease. Matrix Biol. 2018, 68, 28–43. [Google Scholar] [CrossRef]
- Baulida, J.; Diaz, V.M.; de Herreros, A.G. Snail1: A transcriptional factor controlled at multiple levels. J. Clin. Med. 2019, 8, 757. [Google Scholar] [CrossRef]
- You, H.N.; Ding, W.; Rountree, C.B. Epigenetic regulation of cancer stem cell marker CD133 by Transforming Growth Factor-beta. Hepatology 2010, 51, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.G.; Arnold, S.A.; Jiang, M.; Palmer, T.D.; Ketova, T.; Merkel, A.; Pickup, M.; Samaras, S.; Shyr, Y.; Moses, H.L.; et al. ALCAM/CD166 is a TGF-beta-responsive marker and functional regulator of prostate cancer metastasis to bone. Cancer Res. 2014, 74, 1404–1415. [Google Scholar] [PubMed]
- Xu, R.H.; Sampsell-Barron, T.L.; Gu, F.; Root, S.; Peck, R.M.; Pan, G.J.; Yu, J.; Antosiewicz-Bourget, J.; Tian, S.; Stewart, R. NANOG is a direct target of TGF beta/Activin-mediated SMAD signaling in human ESCs. Cell Stem Cell 2008, 3, 196–206. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Whittaker, C.A.; Carr, S.A.; Tanabe, K.K.; Hynes, R.O. Extracellular matrix signatures of human primary metastatic colon cancers and their metastases to liver. BMC Cancer 2014, 14, 518. [Google Scholar]
- Kirkland, S.C. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br. J. Cancer 2009, 101, 320–326. [Google Scholar] [PubMed]
- You, Y.; Zheng, Q.; Dong, Y.; Xie, X.; Wang, Y.; Wu, S.; Zhang, L.; Wang, Y.; Xue, T.; Wang, Z.; et al. Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells. Oncotarget 2016, 7, 32221–32231. [Google Scholar]
- Liu, G.; Wang, B.; Li, S.; Jin, Q.; Dai, Y. Human breast cancer decellularized scaffolds promote epithelial-to-mesenchymal transitions and stemness of breast cancer cells in vitro. J. Cell. Physiol. 2019, 234, 9447–9456. [Google Scholar]
- Gentilin, E.; D’Angelo, E.; Agostini, M.; Astolfi, L. Decellularized normal and cancer tissues as tools for cancer research. Cancer Gene Ther. 2022, 29, 879–888. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques-Magalhães, Â.; Monteiro-Ferreira, S.; Canão, P.A.; Rios, E.; Costa, Â.M.; Castro, F.; Velho, S.; Paredes, J.; Carneiro, F.; Oliveira, M.J.; et al. Patient-Derived Colorectal Cancer Extracellular Matrices Modulate Cancer Cell Stemness Markers. Int. J. Mol. Sci. 2025, 26, 2890. https://doi.org/10.3390/ijms26072890
Marques-Magalhães Â, Monteiro-Ferreira S, Canão PA, Rios E, Costa ÂM, Castro F, Velho S, Paredes J, Carneiro F, Oliveira MJ, et al. Patient-Derived Colorectal Cancer Extracellular Matrices Modulate Cancer Cell Stemness Markers. International Journal of Molecular Sciences. 2025; 26(7):2890. https://doi.org/10.3390/ijms26072890
Chicago/Turabian StyleMarques-Magalhães, Ângela, Sara Monteiro-Ferreira, Pedro Amoroso Canão, Elisabete Rios, Ângela Margarida Costa, Flávia Castro, Sérgia Velho, Joana Paredes, Fátima Carneiro, Maria José Oliveira, and et al. 2025. "Patient-Derived Colorectal Cancer Extracellular Matrices Modulate Cancer Cell Stemness Markers" International Journal of Molecular Sciences 26, no. 7: 2890. https://doi.org/10.3390/ijms26072890
APA StyleMarques-Magalhães, Â., Monteiro-Ferreira, S., Canão, P. A., Rios, E., Costa, Â. M., Castro, F., Velho, S., Paredes, J., Carneiro, F., Oliveira, M. J., & Cardoso, A. P. (2025). Patient-Derived Colorectal Cancer Extracellular Matrices Modulate Cancer Cell Stemness Markers. International Journal of Molecular Sciences, 26(7), 2890. https://doi.org/10.3390/ijms26072890








