Polydatin Alleviates Cyclophosphamide-Induced Mouse Immunosuppression by Promoting Splenic Lymphocyte Proliferation and Thymic T Cell Development and Differentiation
Abstract
:1. Introduction
2. Results
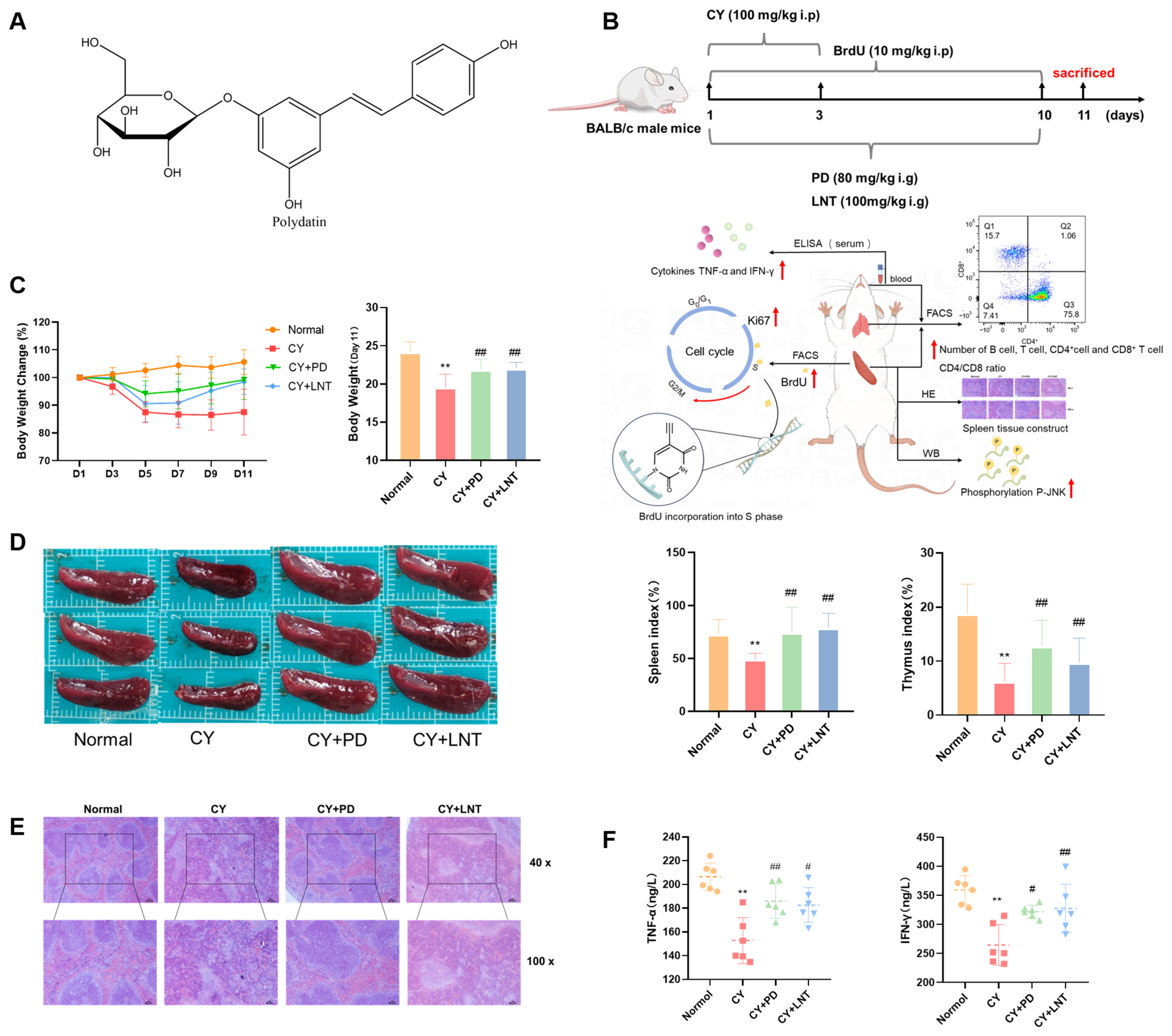
2.1. PD Inhibited CY-Induced Immunosuppression in Mice
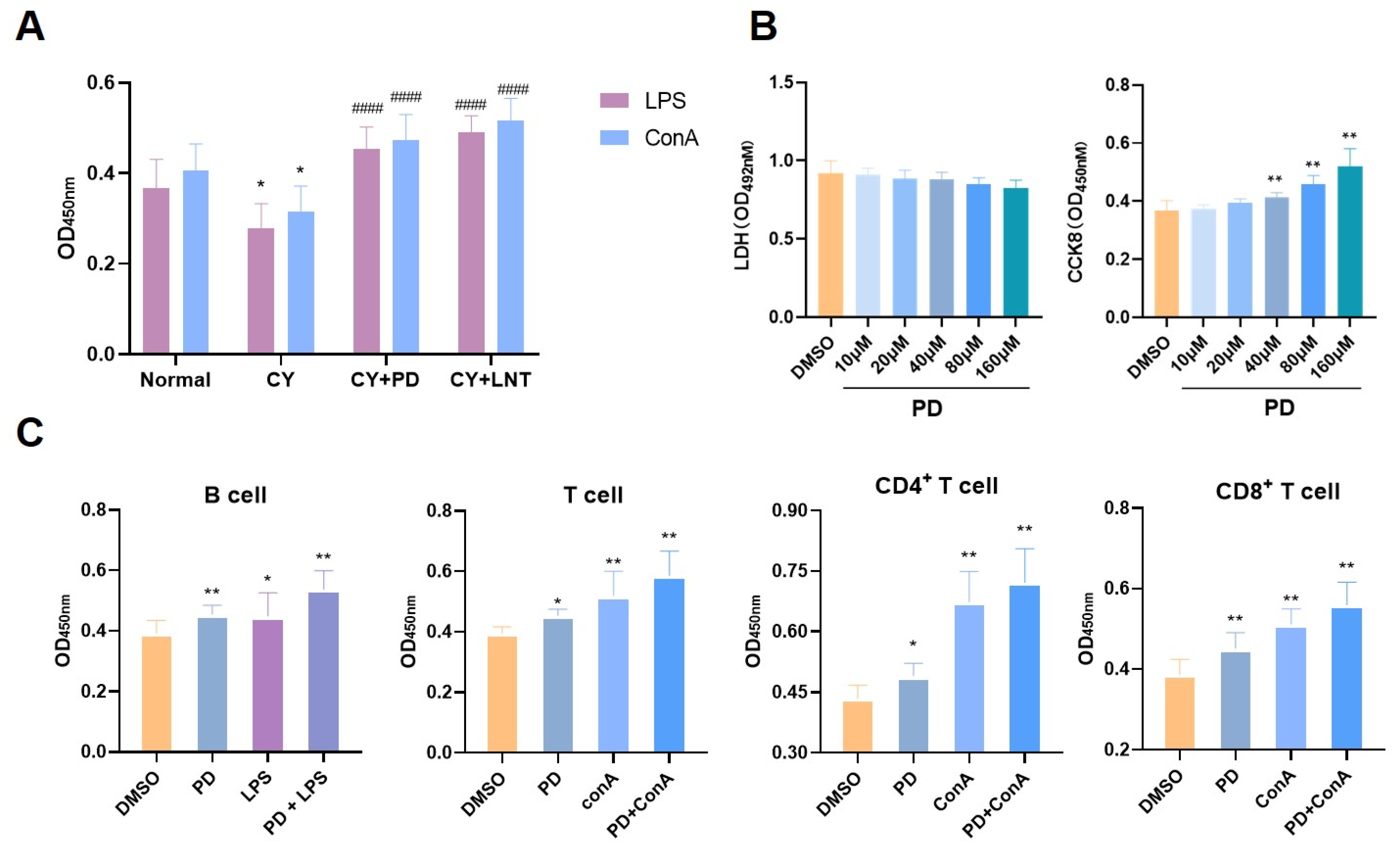
2.2. PD Promoted Splenic Lymphocyte Proliferation In Vivo and In Vitro
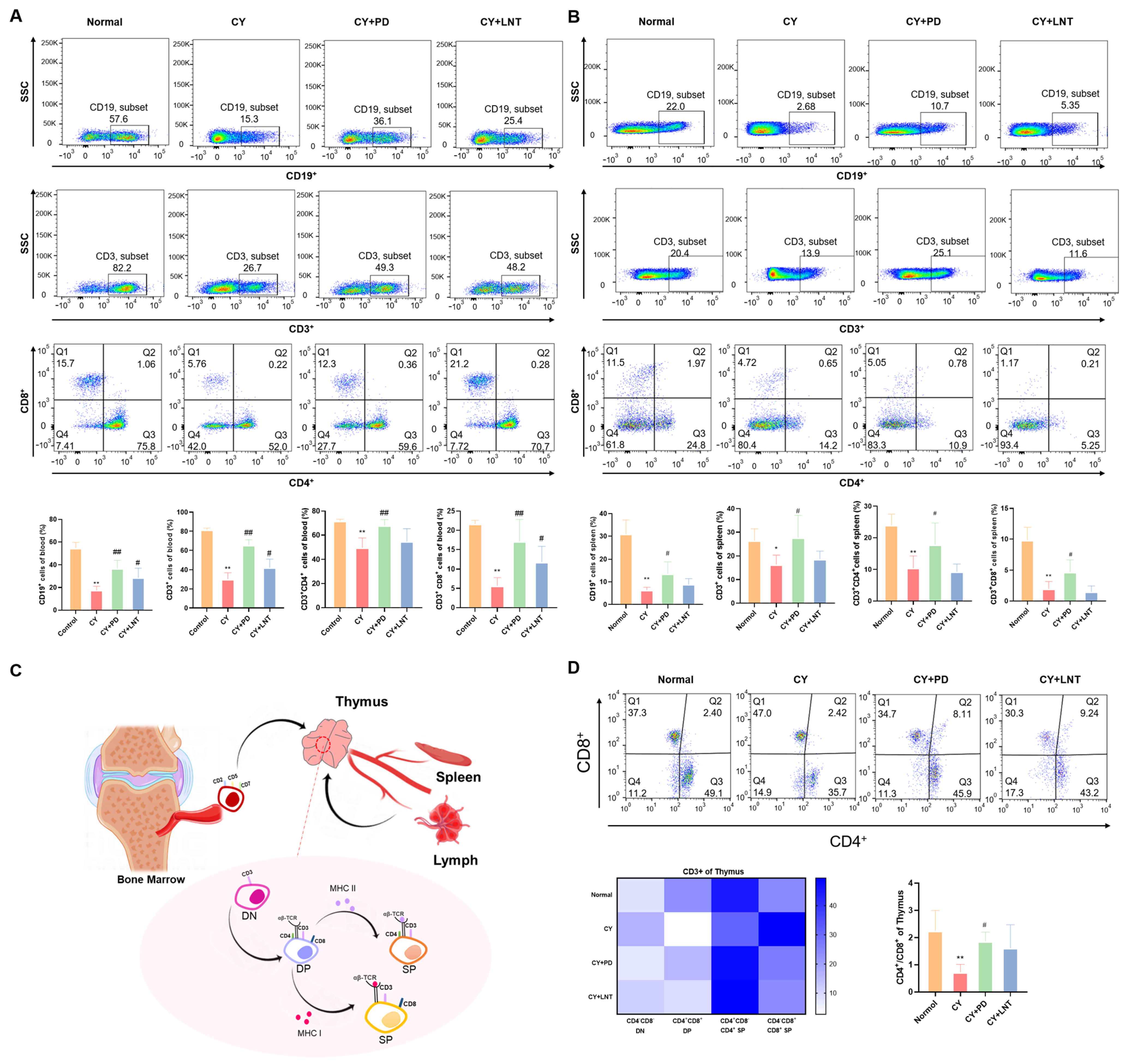
2.3. PD Inhibited the Abnormal Percentages of Lymphocyte Subsets in the Peripheral, Spleen, and Thymus Isolated from CY-Induced Immunosuppressed Mice
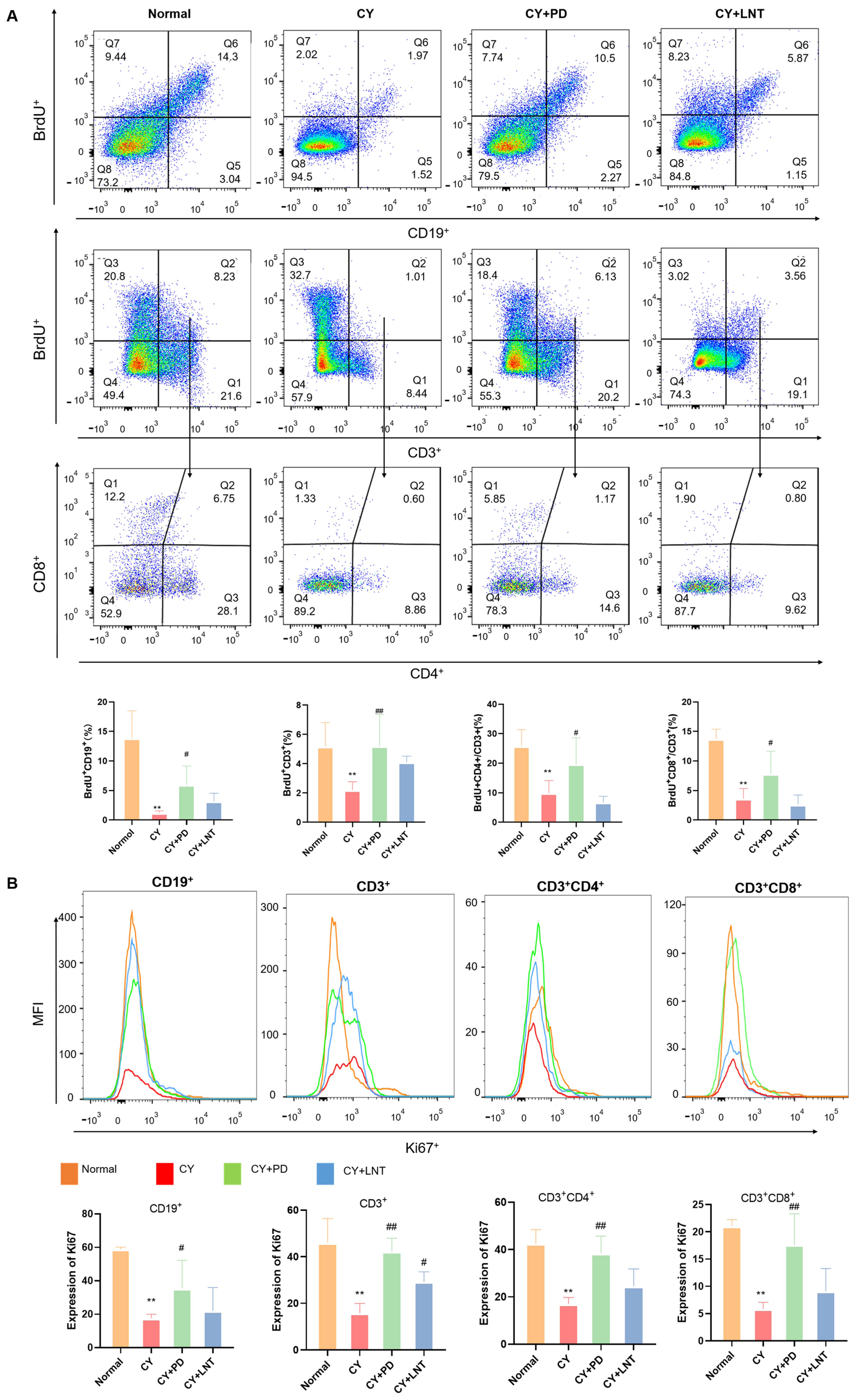
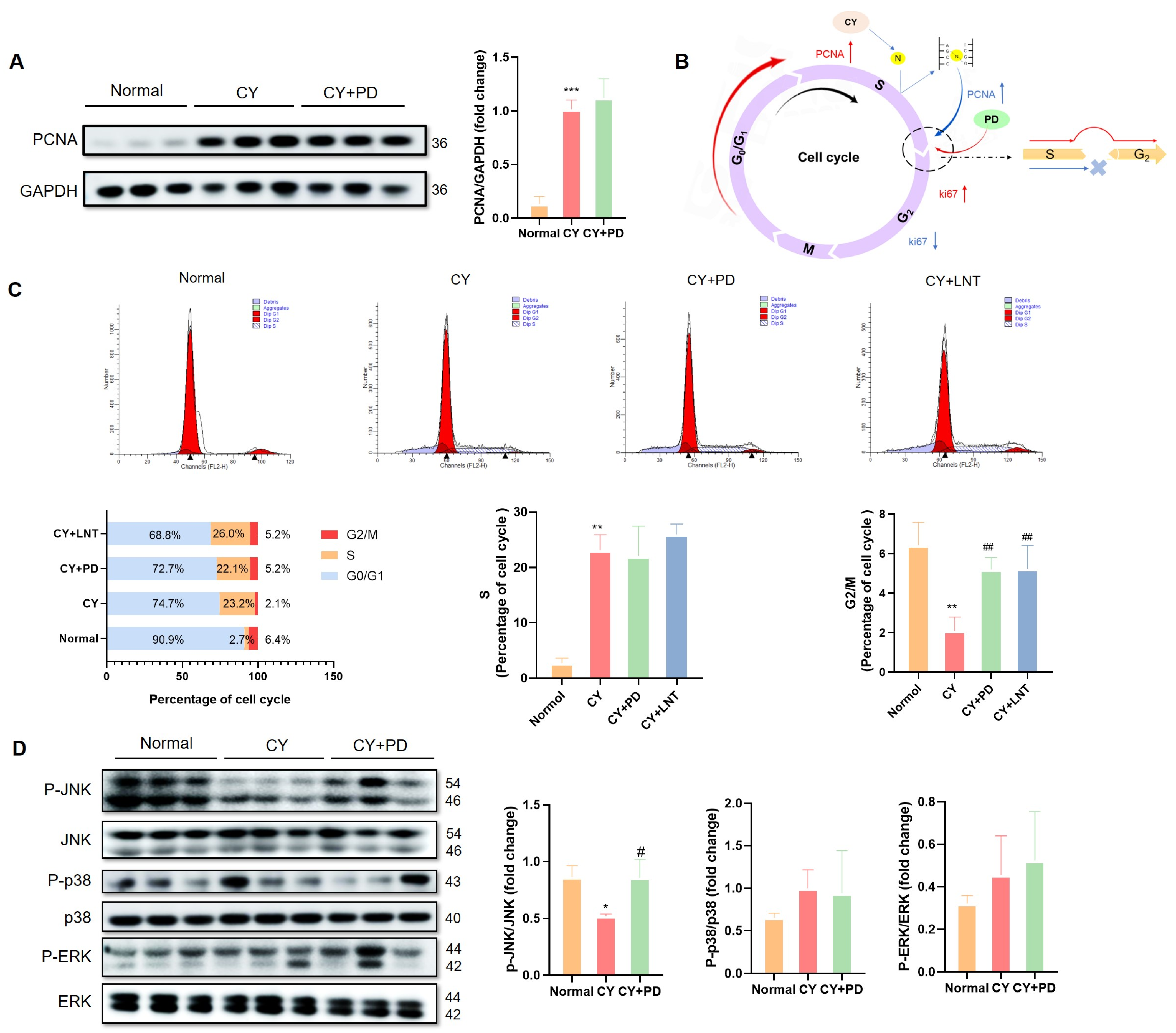
2.4. PD Promoted the Proliferation of Splenic Lymphocytes in CY-Induced Immunosuppressed Mice
2.5. PD Promoted the Restoration of Lymphocyte Numbers in the G2/M Phase
3. Discussion
4. Materials and Methods
4.1. Drug Preparation
4.2. Animals
4.3. CY-Induced Immunosuppression Model
4.4. Hematoxylin and Eosin Staining
4.5. Assessment of Serum TNF-α and IFN-γ by ELISA
4.6. Splenic Lymphocyte Subset Selection and Culture
4.7. Splenic Lymphocyte Proliferation and Cytotoxicity Assay
4.8. Flow Cytometry
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurley, L.H. DNA and its associated processes as targets for cancer therapy. Nat. Rev. Cancer 2002, 2, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wang, X.L.; He, D.H.; Cheng, Y.X. Protection against chemotherapy- and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Gress, R.E.; Deeks, S.G. Reduced thymus activity and infection prematurely age the immune system. J. Clin. Investig. 2009, 119, 2884–2887. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.H.; Zhu, X.S.; Xu, H.Y.; Zhao, Z.X. Characterization and protective effect of polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, L.; Polák, S.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Li, Q.L.; Yuan, Q.; Jiang, N.; Zhang, Y.W.; Su, Z.W.; Lv, L.; Sang, X.Y.; Chen, R.; Feng, Y.; Chen, Q.J. Dihydroartemisinin regulates immune cell heterogeneity by triggering a cascade reaction of CDK and MAPK phosphorylation. Signal Transduct. Target. Ther. 2022, 7, 222. [Google Scholar] [CrossRef]
- Wang, X.G.; Chen, Z.G.; Mishra, A.K.; Silva, A.; Ren, W.H.; Pan, Z.G.; Wang, J.H. Chemotherapy-induced differential cell cycle arrest in B-cell lymphomas affects their sensitivity to Wee1 inhibition. Haematologica 2018, 103, 466–476. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef]
- Keswani, T.; Mitra, S.; Bhattacharyya, A. Copper-Induced Immunotoxicity Involves Cell Cycle Arrest and Cell Death in the Liver. Environ. Toxicol. 2015, 30, 411–421. [Google Scholar] [CrossRef]
- Zeng, W.; Song, X.; Zhang, Y.; Xiao, S.; Wang, Y. Research Progress on Pharmacological Activities and Pharmacokinetics of Gentiopicroside. Jiangxi J. Tradit. Chin. Med. 2014, 45, 69–71. (In Chinese) [Google Scholar]
- Li, S.-H.; Li, J.-Y.; Wu, K.-L.; Xu, J.-J.; Chang, G.-J.; Wang, W.-Q.; Zhang, Y.-L.; Ma, Z.-Q.; Wang, W.-L.; Li, S.-D. Progress in the Pharmacological Action and Molecular Mechanism of Gentiopicroside. J. Kunming Med. Univ. 2020, 41, 5. (In Chinese) [Google Scholar]
- Lanzilli, G.; Cottarelli, A.; Nicotera, G.; Guida, S.; Ravagnan, G.; Fuggetta, M.P. Anti-inflammatory Effect of Resveratrol and Polydatin by In Vitro IL-17 Modulation. Inflammation 2012, 35, 240–248. [Google Scholar] [CrossRef]
- Lo Muzio, L.; Bizzoca, M.E.; Ravagnan, G. New intriguing possibility for prevention of coronavirus pneumonitis: Natural purified polyphenols. Oral. Dis. 2022, 28, 899–903. [Google Scholar] [CrossRef]
- Liu, B.; Guo, D. Research progress of gentiopicroside in the treatment of immune diseases. China J. Tradit. Chin. Med. Pharm. 2024, 39, 5999–6002. (In Chinese) [Google Scholar]
- Liu, Z.W.; Chen, C.X.; Jin, R.M.; Shi, G.Q.; Song, C.Q.; Hu, Z.B. Studies on liver-protection and promoting bile secretion of gentiopicroside. Chin. Herb. Med. 2002, 33, 47–50. (In Chinese) [Google Scholar] [CrossRef]
- Li, R.; Li, J.Z.; Huang, Y.J.; Li, H.; Yan, S.S.; Lin, J.X.; Chen, Y.; Wu, L.M.; Liu, B.; Wang, G.S.; et al. Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int. J. Biol. Sci. 2018, 14, 1411–1425. [Google Scholar] [CrossRef]
- Shah, M.A.; Hamid, A.; Faheem, H.I.; Rasul, A.; Baokbah, T.A.S.; Haris, M.; Yousaf, R.; Saleem, U.; Iqbal, S.; Alves, M.S.; et al. Uncovering the Anticancer Potential of Polydatin: A Mechanistic Insight. Molecules 2022, 27, 7175. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.Y.; Zhang, K.X.; Liu, Q.D.; Ma, T.Y.; Tan, L.; Ma, L.Q. Protective Effects of Polydatin from Grapes and Houtt. on Damaged Macrophages Treated with Acetaminophen. Nutrients 2022, 14, 2077. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Zhang, C.M.; Cai, Z.M.; Chen, L.G.; Chen, Z.L.; Zhao, K.; Qiao, S.A.; Wang, Y.C.; Meng, L.J.; et al. Anti-Influenza Effect and Mechanisms of Lentinan in an ICR Mouse Model. Front. Cell. Infect. Microbiol. 2022, 12, 892864. [Google Scholar] [CrossRef]
- Ji, X.Y.; Su, L.; Zhang, P.; Yue, Q.L.; Zhao, C.; Sun, X.; Li, K.L.; Liu, X.L.; Zhang, S.; Zhao, L. Lentinan improves intestinal inflammation and gut dysbiosis in antibiotics-induced mice. Sci. Rep. 2022, 12, 19609. [Google Scholar] [CrossRef] [PubMed]
- Kupfahl, C.; Geginat, G.; Hof, H. Lentinan has a stimulatory effect on innate and adaptive immunity against murine infection. Int. Immunopharmacol. 2006, 6, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, H.Y.; Yu, J.; Qu, C.H.; Wang, Q.; Yang, S.; Ma, S.C.; Ni, J. Quality control and immunological activity of lentinan samples produced in China. Int. J. Biol. Macromol. 2020, 159, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, S.W.; Luo, S.; Liao, H.F.; Wang, Y.Y.; Deng, X.L.; Ma, F.L.; Ma, C.W.; Zhou, L. Identification of genes underlying the enhancement of immunity by a formula of lentinan, pachymaran and tremelia polysaccharides in immunosuppressive mice. Sci. Rep. 2018, 8, 10082. [Google Scholar] [CrossRef]
- Nowell, C.S.; Farley, A.M.; Blackburn, C.C. Thymus organogenesis and development of the thymic stroma. Methods Mol. Biol. 2007, 380, 125–162. [Google Scholar]
- De Obaldia, M.E.; Bhandoola, A. Transcriptional Regulation of Innate and Adaptive Lymphocyte Lineages. Annu. Rev. Immunol. 2015, 33, 607–642. [Google Scholar] [CrossRef]
- Klein, L.; Kyewski, B.; Allen, P.M.; Hogquist, K.A. Positive and negative selection of the T cell repertoire: What thymocytes see (and don’t see). Nat. Rev. Immunol. 2014, 14, 377–391. [Google Scholar] [CrossRef]
- Shah, D.K.; Zúñiga-Pflücker, J.C. An Overview of the Intrathymic Intricacies of T Cell Development. J. Immunol. 2014, 192, 4017–4023. [Google Scholar] [CrossRef]
- Jones, R.J.; Barber, J.P.; Vala, M.S.; Collector, M.I.; Kaufmann, S.H.; Ludeman, S.M.; Colvin, O.M.; Hilton, J. Assessment of aldehyde dehydrogenase in viable cells. Blood 1995, 85, 2742–2746. [Google Scholar] [CrossRef]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef]
- Huang, B.X.; Liu, J.X.; Meng, L.Y.; Li, Y.H.; He, D.W.; Ran, X.; Chen, G.X.; Guo, W.J.; Kan, X.C.; Fu, S.P.; et al. Polydatin Prevents Lipopolysaccharide (LPS)-Induced Parkinson’s Disease via Regulation of the AKT/GSK3β-Nrf2/NF-κB Signaling Axis. Front. Immunol. 2018, 9, 2527. [Google Scholar] [CrossRef] [PubMed]
- Schimith, L.E.; dos Santos, M.G.; Arbo, B.D.; André-Miral, C.; Muccillo-Baisch, A.L.; Hort, M.A. Polydatin as a therapeutic alternative for central nervous system disorders: A systematic review of animal studies. Phytother. Res. 2022, 36, 2852–2877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef]
- Chen, L.B.; Musa, A.E. Boosting immune system against cancer by resveratrol. Phytother. Res. 2021, 35, 5514–5526. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Chaudhry, M.S.; Velardi, E.; Dudakov, J.A.; van den Brink, M.R. Thymus: The next (re)generation. Immunol. Rev. 2016, 271, 56–71. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zheng, M.X.; Zhou, M.L.; Zhou, L.M.; Ge, X.; Pang, N.; Li, H.C.; Li, X.Y.; Li, M.D.; Zhang, J.; et al. Lentinan Supplementation Protects the Gut-Liver Axis and Prevents Steatohepatitis: The Role of Gut Microbiota Involved. Front. Nutr. 2022, 8, 803691. [Google Scholar] [CrossRef]
- Liu, F.; Wang, H.M.; Wang, T.S.; Zhang, Y.M.; Zhu, X. The efficacy of thymosin α1 as immunomodulatory treatment for sepsis: A systematic review of randomized controlled trials. BMC Infect. Dis. 2016, 16, 488. [Google Scholar] [CrossRef]
- Liu, Y.G.; Yang, A.H.; Qu, Y.D.; Wang, Z.Q.; Zhang, Y.Q.; Liu, Y.; Wang, N.; Teng, L.R.; Wang, D. Ameliorative effects of polysaccharides against cyclophosphamide-induced immunosuppression related to Nrf2/HO-1 signaling in BALB/c mice. Int. J. Biol. Macromol. 2018, 116, 8–15. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Chen, X.Y.; Yi, R.K.; Li, G.J.; Sun, P.; Qian, Y.; Zhao, X. Immunomodulatory Effect of Tremella Polysaccharides against Cyclophosphamide-Induced Immunosuppression in Mice. Molecules 2018, 23, 239. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-R.; Sung, S.-K.; Jang, M.; Lim, T.-G.; Cho, C.-W.; Han, C.-J.; Hong, H.-D. Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng Meyer). Int. J. Biol. Macromol. 2018, 116, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.M.; Lu, J.; Wang, J.B.; Chen, L.; Wang, Y.; Li, L.; Miao, L.; Zhang, H. Prevention of Cyclophosphamide-Induced Immunosuppression in Mice with Traditional Chinese Medicine Xuanfei Baidu Decoction. Front. Pharmacol. 2021, 12, 730567. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Patel, S.K.; Sahu, S.R.; Kumari, P. ‘PIPs’ in DNA polymerase: PCNA interaction affairs. Biochem. Soc. Trans. 2020, 48, 2811–2822. [Google Scholar] [CrossRef]
- Kubota, T.; Katou, Y.; Nakato, R.; Shirahige, K.; Donaldson, A.D. Replication-Coupled PCNA Unloading by the Elg1 Complex Occurs Genome-wide and Requires Okazaki Fragment Ligation. Cell Rep. 2015, 12, 774–787. [Google Scholar] [CrossRef]
- Kubota, T.; Myung, K.; Donaldson, A.D. Is PCNA unloading the central function of the Elg1/ATAD5 replication factor C-like complex? Cell Cycle 2013, 12, 2570–2579. [Google Scholar] [CrossRef]
- Kubota, T.; Nishimura, K.; Kanemaki, M.T.; Donaldson, A.D. The Elg1 Replication Factor C-like Complex Functions in PCNA Unloading during DNA Replication. Mol. Cell 2013, 50, 273–280. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.W.; Yang, C.; Tian, C.Z.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- Moldovan, G.L.; Pfander, B.; Jentsch, S. PCNA, the maestro of the replication fork. Cell 2007, 129, 665–679. [Google Scholar] [CrossRef]
- Li, X.; Wichai, N.; Wang, J.; Liu, X.; Yan, H.; Wang, Y.; Luo, M.; Zhou, S.; Wang, K.; Li, L.; et al. Regulation of innate and adaptive immunity using herbal medicine: Benefits for the COVID-19 vaccination. Acupunct. Herb. Med. 2022, 2, 196–206. [Google Scholar] [CrossRef]
- Novoszel, P.; Drobits, B.; Holcmann, M.; Fernandes, C.D.; Tschismarov, R.; Derdak, S.; Decker, T.; Wagner, E.F.; Sibilia, M. The AP-1 transcription factors c-Jun and JunB are essential for CD8α conventional dendritic cell identity. Cell Death Differ. 2021, 28, 2404–2420. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.C.; Bosselut, R. Decision checkpoints in the thymus. Nat. Immunol. 2010, 11, 666–673. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Larraneta, E.; González-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-Based Nanoparticles Improve the Oral Bioavailability of Resveratrol and Its Anti-inflammatory Effects in a Mouse Model of Endotoxic Shock. J. Agric. Food Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Ramawat, K.G. Polysaccharides Bioactivity and Biotechnology; Springer International Publishing: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Zhu, H. Cell proliferation assay by flow cytometry (BrdU and PI Staining). Bio-Protocol 2012, 2, e198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Yan, H.; Liu, X.; Xu, X.; Zhao, W.; Zhang, J.; Wang, M.; Liu, Y.; Miao, L. Polydatin Alleviates Cyclophosphamide-Induced Mouse Immunosuppression by Promoting Splenic Lymphocyte Proliferation and Thymic T Cell Development and Differentiation. Int. J. Mol. Sci. 2025, 26, 2800. https://doi.org/10.3390/ijms26062800
Sun N, Yan H, Liu X, Xu X, Zhao W, Zhang J, Wang M, Liu Y, Miao L. Polydatin Alleviates Cyclophosphamide-Induced Mouse Immunosuppression by Promoting Splenic Lymphocyte Proliferation and Thymic T Cell Development and Differentiation. International Journal of Molecular Sciences. 2025; 26(6):2800. https://doi.org/10.3390/ijms26062800
Chicago/Turabian StyleSun, Na, Huimin Yan, Xiuping Liu, Xingdi Xu, Wei Zhao, Jing Zhang, Meng Wang, Yuxuan Liu, and Lin Miao. 2025. "Polydatin Alleviates Cyclophosphamide-Induced Mouse Immunosuppression by Promoting Splenic Lymphocyte Proliferation and Thymic T Cell Development and Differentiation" International Journal of Molecular Sciences 26, no. 6: 2800. https://doi.org/10.3390/ijms26062800
APA StyleSun, N., Yan, H., Liu, X., Xu, X., Zhao, W., Zhang, J., Wang, M., Liu, Y., & Miao, L. (2025). Polydatin Alleviates Cyclophosphamide-Induced Mouse Immunosuppression by Promoting Splenic Lymphocyte Proliferation and Thymic T Cell Development and Differentiation. International Journal of Molecular Sciences, 26(6), 2800. https://doi.org/10.3390/ijms26062800






