Identification of Cysteine synthase (Cys) Gene Family in Tomato (Solanum lycopersicum) and Functional of SlCys5 in Cold Stress Tolerance
Abstract
1. Introduction
2. Results
2.1. Identification of Cys Family Members in Tomato
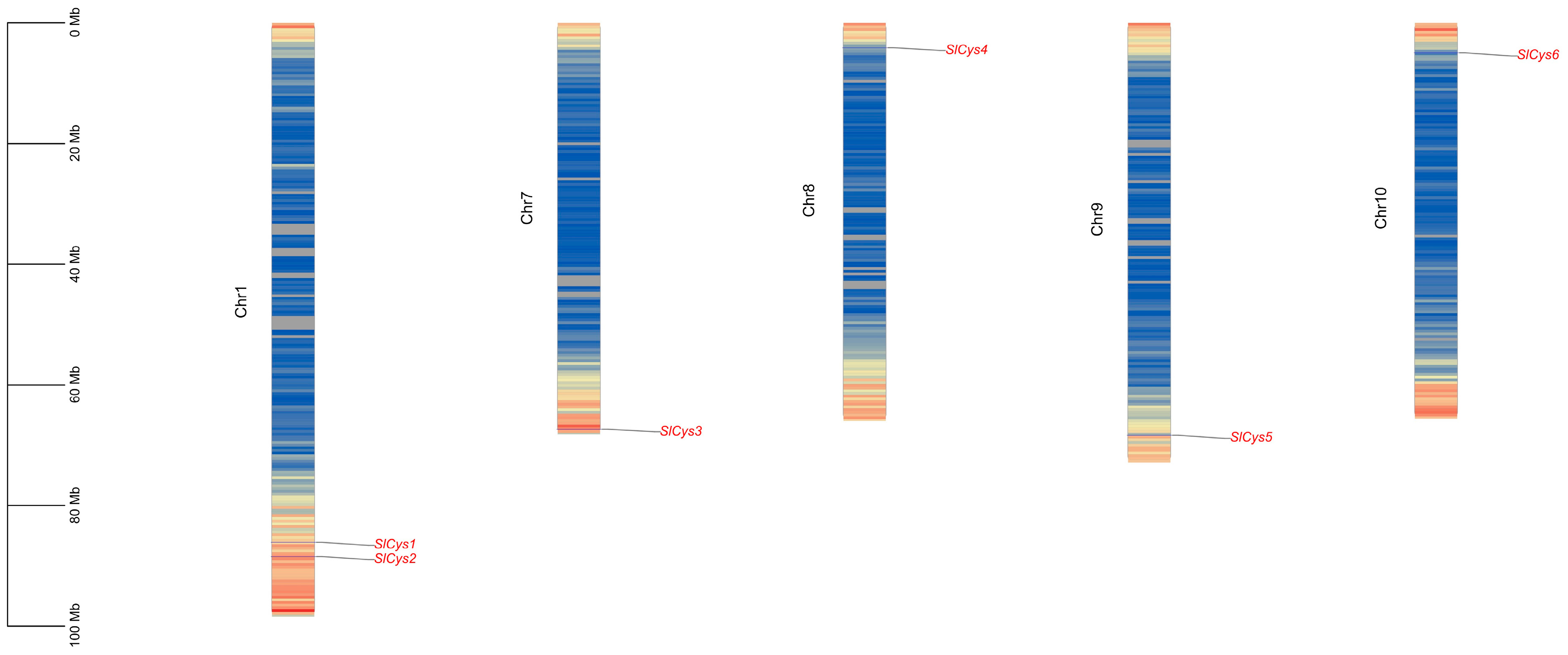
2.2. Localization Analysis of Tomato SlCys Genes on Chromosomes
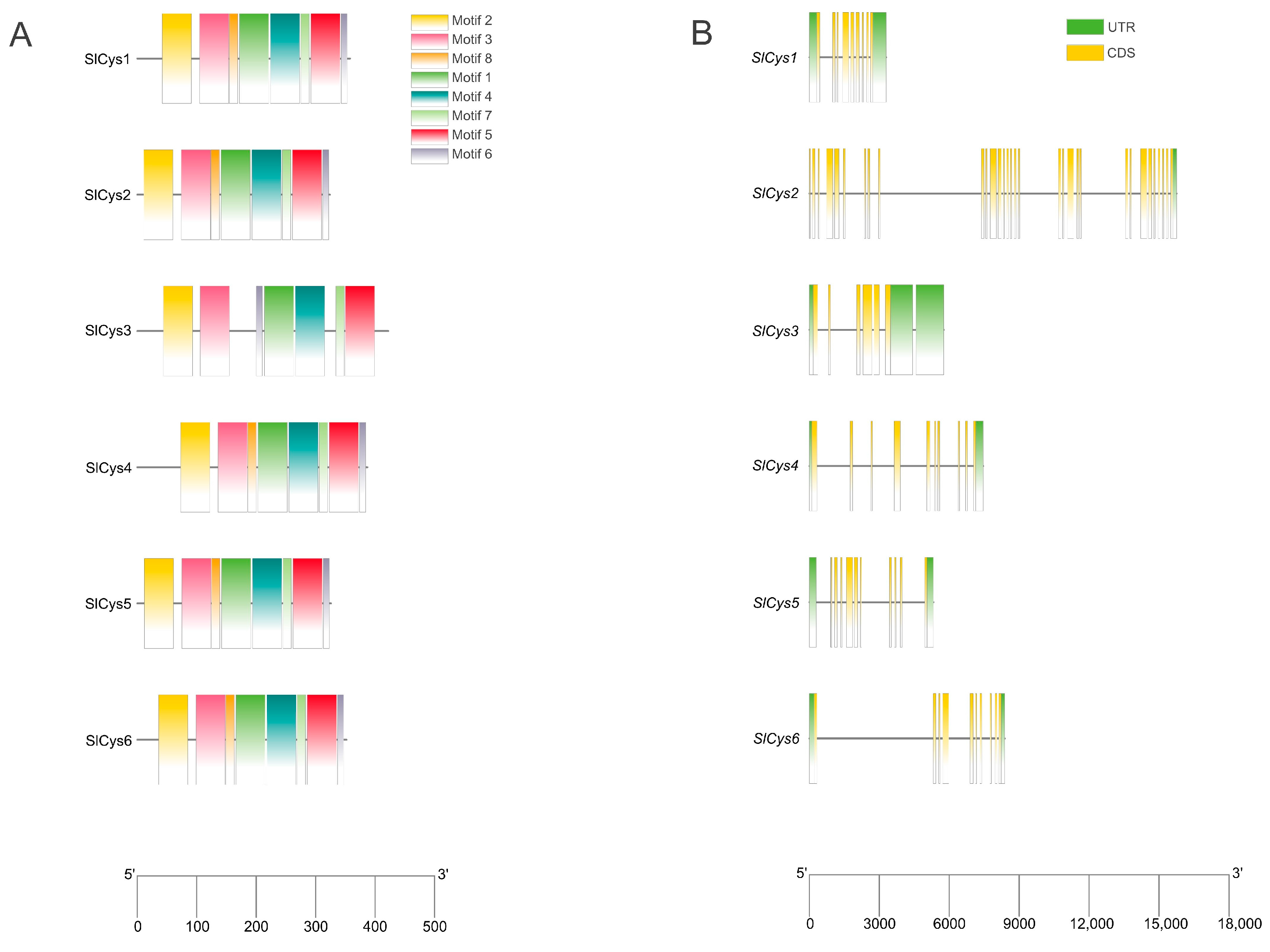
2.3. Analysis of Protein-Conserved Motifs and Gene Structures of SlCys Family Members in Tomato
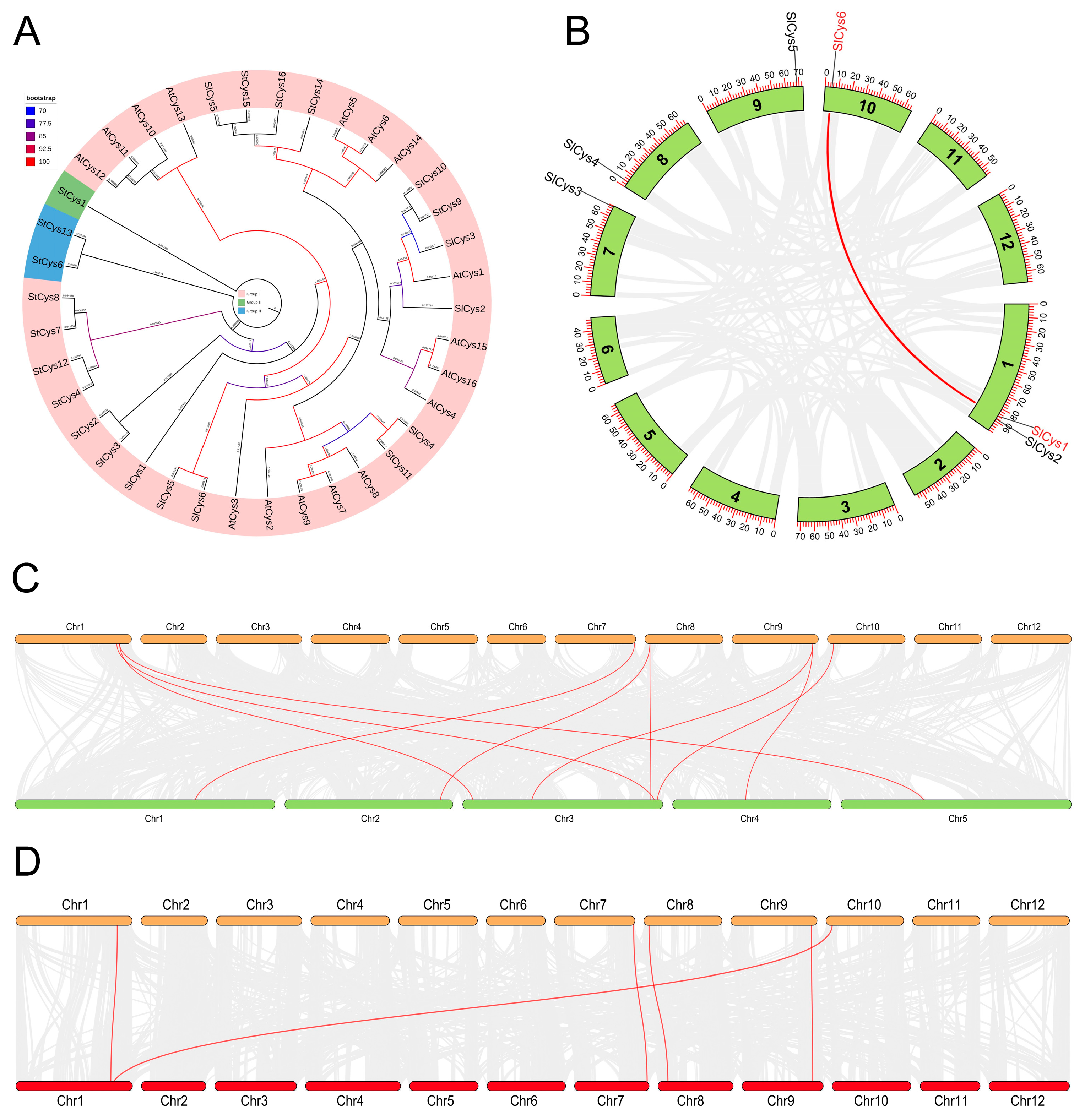
2.4. Phylogenetic Analysis of Cys Proteins and Collinearity Analysis of Cys Family Genes
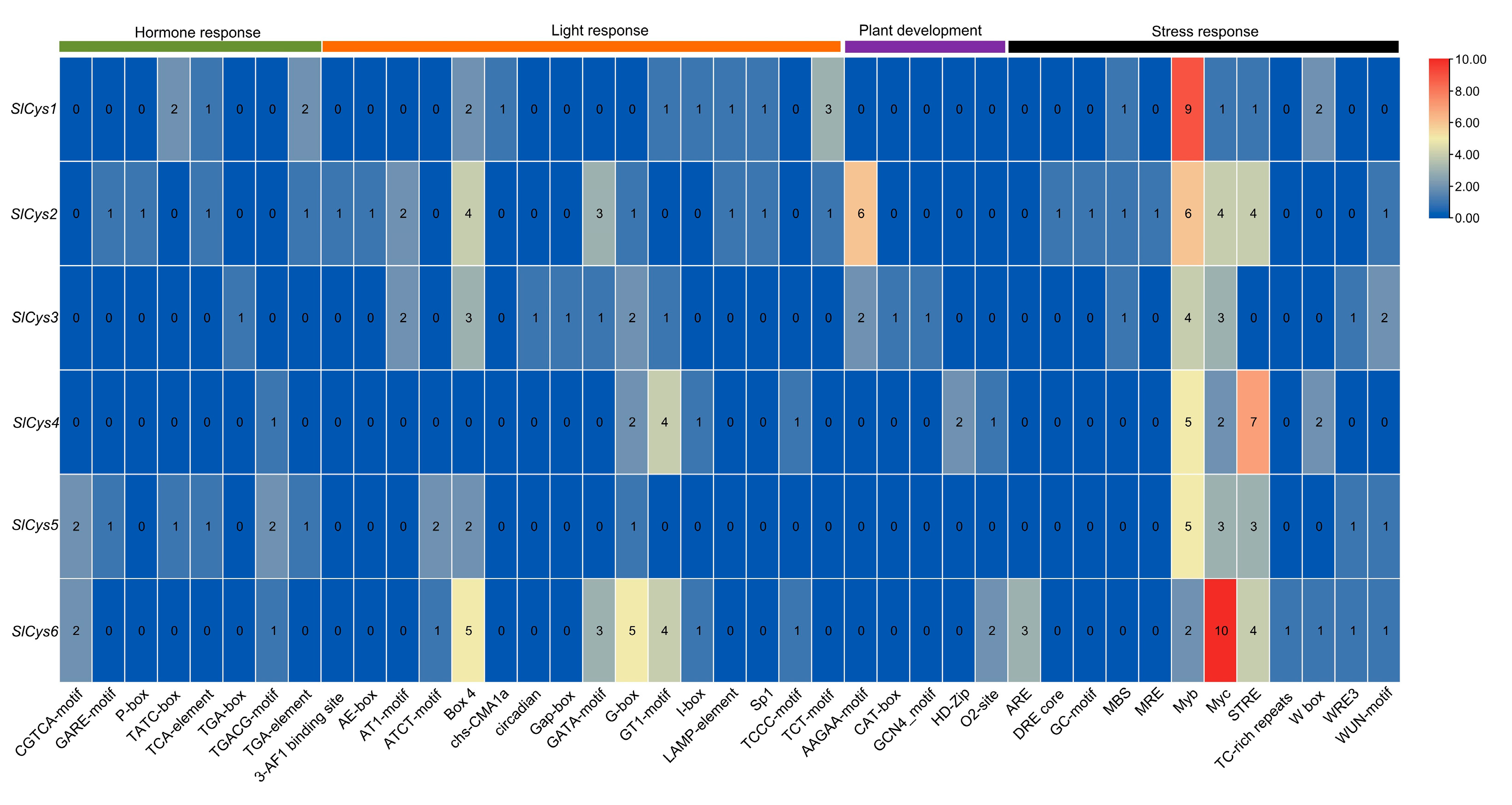
2.5. Analysis of Cis-Acting Elements in Tomato SlCys Promoter
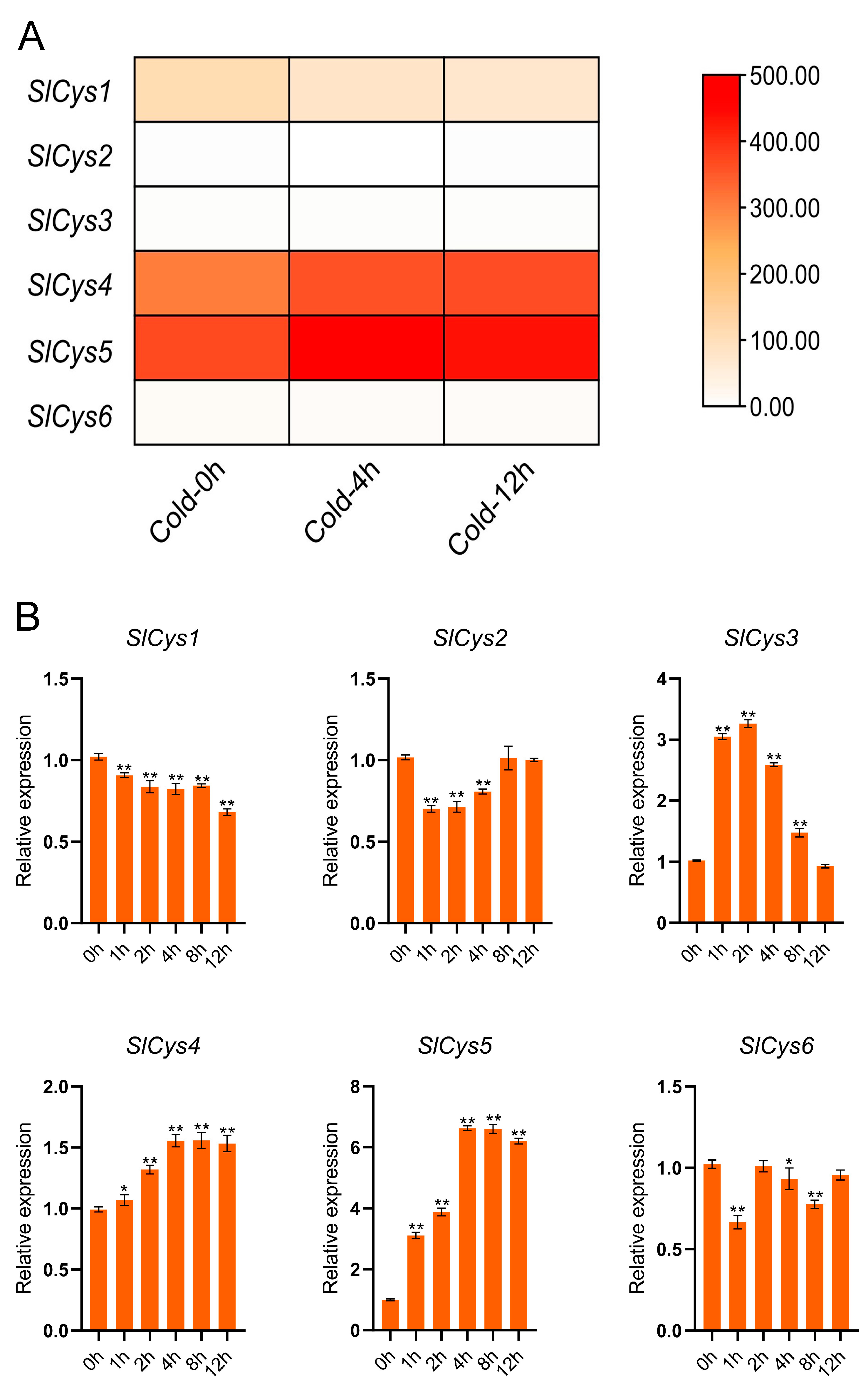
2.6. Transcriptome Data and qRT-PCR Analysis of SlCys Family Genes Under Cold Stress
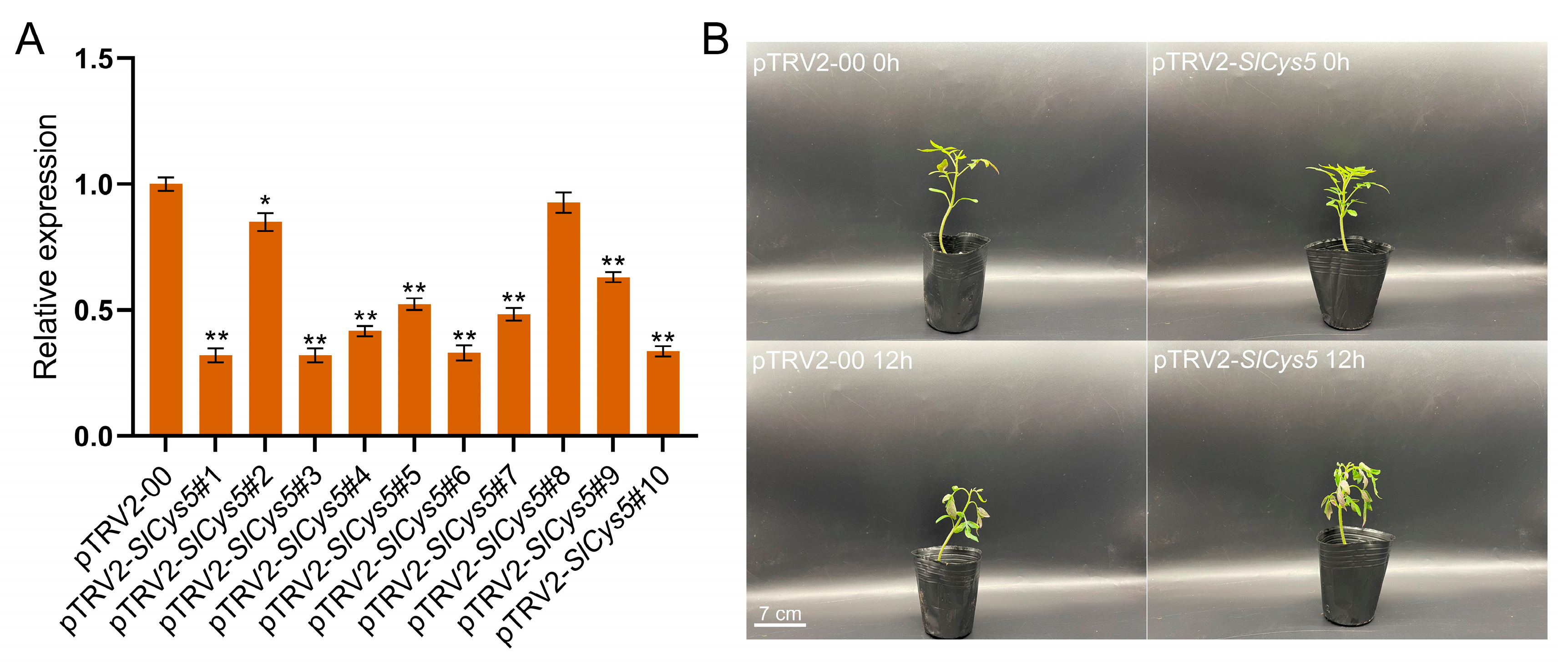
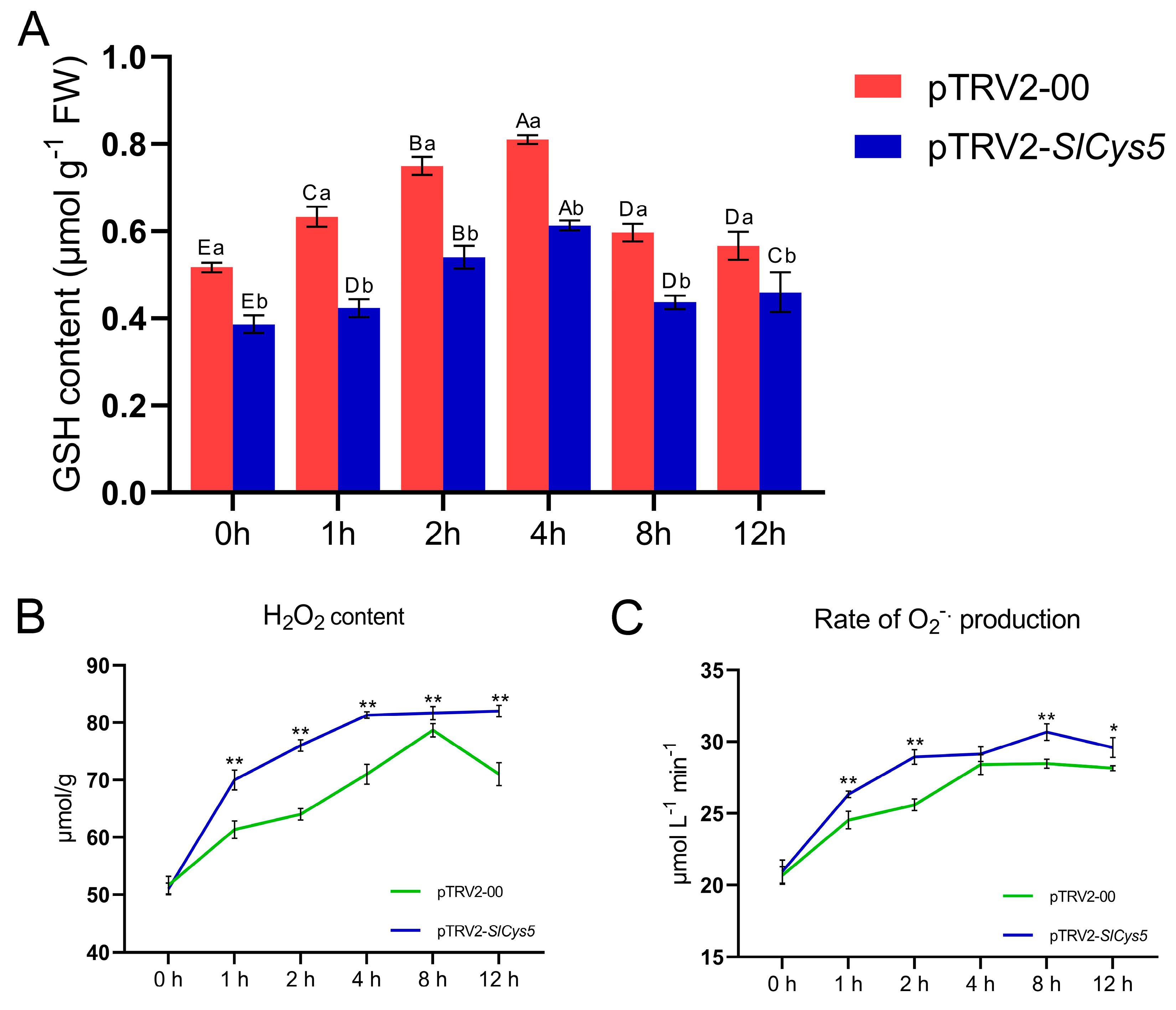
2.7. Silencing of the SlCys5 Gene Reduced Cold Tolerance in Tomato
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Identification and Sequence Analysis of Tomato Cys Family Members
4.3. Chromosomal Localization Analysis of Tomato Cys Family Genes
4.4. Analysis of Conserved Motifs and Gene Structures of Tomato SlCys Family Members
4.5. Phylogenetic Analysis of Cys Family Members
4.6. Collinearity Analysis of Cys Family Genes
4.7. Analysis of Promoter Cis-Acting Elements of Cys Family Genes in Tomato
4.8. Transcriptome Data and qRT-PCR Analysis of SlCys Genes
4.9. Virus-Induced Silencing of SlCys5
4.10. Physiological Assays
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birke, H.; Haas, F.H.; De Kok, L.J.; Balk, J.; Wirtz, M.; Hell, R. Cysteine biosynthesis, in concert with a novel mechanism, contributes to sulfide detoxification in mitochondria of Arabidopsis thaliana. Biochem. J. 2012, 445, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Khare, R.; Kumar, S.; Shukla, T.; Ranjan, A.; Trivedi, P.K. Differential sulphur assimilation mechanism regulates response of Arabidopsis thaliana natural variation towards arsenic stress under limiting sulphur condition. J. Hazard. Mater. 2017, 337, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Hell, R.; Bork, C.; Bogdanova, N.; Frolov, I.; Hauschild, R. Isolation and characterization of two cDNAs encoding for compartment specific isoforms of O-acetylserine (thiol) lyase from Arabidopsis thaliana. FEBS Lett. 1994, 351, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, F.; Itagaki, S.; Murakoshi, I. Purification and characterization of two forms of cysteine synthase from Allium tuberosum. Phytochemistry 1992, 32, 31. [Google Scholar] [CrossRef]
- Koprivova, A.; Kopriva, S. Hormonal control of sulfate uptake and assimilation. Plant Mol. Biol. 2016, 91, 617–627. [Google Scholar] [CrossRef]
- Cui, W.; Chen, H.; Zhu, K.; Jin, Q.; Xie, Y.; Cui, J.; Xia, Y.; Zhang, J.; Shen, W. Cadmium-induced hydrogen sulfide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (homo) glutathione and reactive oxygen species homeostases. PLoS ONE 2014, 9, e109669. [Google Scholar] [CrossRef]
- Cui, W.; Li, L.; Gao, Z.; Wu, H.; Xie, Y.; Shen, W. Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J. Exp. Bot. 2012, 63, 5521–5534. [Google Scholar] [CrossRef]
- Kawashima, C.G.; Noji, M.; Nakamura, M.; Ogra, Y.; Suzuki, K.T.; Saito, K. Heavy metal tolerance of transgenic tobacco plants over-expressing cysteine synthase. Biotechnol. Lett. 2004, 26, 153–157. [Google Scholar] [CrossRef]
- Dominguez-Solis, J.R.; Gutiérrez-Alcalá, G.; Romero, L.C.; Gotor, C. The cytosolic O-acetylserine (thiol) lyase gene is regulated by heavy metals and can function in cadmium tolerance. J. Biol. Chem. 2001, 276, 9297–9302. [Google Scholar] [CrossRef]
- Ning, H.; Zhang, C.; Yao, Y.; Yu, D. Overexpression of a soybean O-acetylserine (thiol) lyase-encoding gene GmOASTL4 in tobacco increases cysteine levels and enhances tolerance to cadmium stress. Biotechnol. Lett. 2010, 32, 557–564. [Google Scholar] [CrossRef]
- Alvarez, C.; Calo, L.; Romero, L.C.; GarcilA, I.; Gotor, C. An O-acetylserine (thiol) lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol. 2010, 152, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, M.A.; Galmés, J.; Moreno, I.; Mullineaux, P.M.; Gotor, C.; Romero, L.C. Photosynthetic adaptation to length of day is dependent on S-sulfocysteine synthase activity in the thylakoid lumen. Plant Physiol. 2012, 160, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Nakamura, T.; Kusano, T.; Sano, H. Three Arabidopsis genes encoding proteins with differential activities for cysteine synthase and beta-cyanoalanine synthase. Plant Cell Physiol. 2000, 41, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Song, T.; Yu, J.; Zhang, W.; Hou, X.; Ling, Z.K.; Cui, G. Genome-wide investigation of the cysteine synthase gene family shows that overexpression of CSase confers alkali tolerance to alfalfa (Medicago sativa L.). Front. Plant Sci. 2021, 12, 792862. [Google Scholar] [CrossRef]
- Akbudak, M.A.; Filiz, E.; Uylas, S. Identification of O-acetylserine (thiol) lyase (OASTL) genes in sorghum (Sorghum bicolor) and gene expression analysis under cadmium stress. Mol. Biol. Rep. 2019, 46, 343–354. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Lu, J.; Tian, B.; Liu, X.; Yang, G.; Pei, Y. Cloning and functional analysis of four O-Acetylserine (thiol) lyase family genes from foxtail millet. Plant Physiol. Biochem. 2019, 139, 325–332. [Google Scholar] [CrossRef]
- Carolina, C.; Francisco, A.C. Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci. 2005, 169, 75–82. [Google Scholar]
- Goulas, E.; Schubert, M.; Kieselbach, T.; Kleczkowski, L.A.; Gardeström, P.; Schröder, W.; Hurry, V. The chloroplast lumen and stromal proteomes of Arabidopsis thaliana show differential sensitivity to short-and long-term exposure to low temperature. Plant J. 2006, 47, 720–734. [Google Scholar] [CrossRef]
- Stitt, M.; Hurry, V. A plant for all seasons: Alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr. Opin. Plant Biol. 2002, 5, 199–206. [Google Scholar] [CrossRef]
- Strand, A.; Hurry, V.; Henkes, S.; Huner, N.; Gustafsson, P.; Gardestrom, P.; Stitt, M. Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol. 1999, 119, 1387–1398. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Mao, Z.; Liu, W.; Jiang, S.; Xu, H.; Su, M.; Zhang, J.; Wang, N.; Zhang, Z.; et al. Ethylene increases the cold tolerance of apple via the MdERF1B-MdCIbHLH1 regulatory module. Plant J. 2021, 106, 379–393. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Feng, N.; Zheng, D.; Liu, M.; Zhou, H.; Zhang, R.; Huang, X.; Huang, A. Exogenous Hemin enhances the antioxidant defense system of rice by regulating the AsA-GSH cycle under NaCl stress. PeerJ 2024, 12, e17219. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D.; Pilon, M.; Pilon-Smits, E.A.H. The functions of NifS-like proteins in plant sulfur and selenium metabolism. Plant Sci. 2007, 174, 117–123. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, L.; Tang, Z.; Sun, S.; Ma, J.F.; Huang, X.-Y.; Zhao, F.-J. OASTL-A1 functions as a cytosolic cysteine synthase and affects arsenic tolerance in rice. J. Exp. Bot. 2020, 71, 3678–3689. [Google Scholar] [CrossRef]
- Choi, Y.E.; Kwon, K.W.; Lee, J.C.; Woo, S.Y. Expression of the rice cytoplasmic cysteine synthase gene in tobacco reduces ozone-induced damage. Plant Biotechnol. Rep. 2007, 1, 93–100. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Li, Z.; Zou, X.; Jiang, C.; Zhu, J.; Zhang, Y.; Han, Y.; Liu, C.; Hao, J. Genome-wide analysis of ABF gene family in lettuce (Lactuca sativa L.) reveals the negative roles of LsABF1 in thermally induced bolting. J. Hortic. Sci. Biotechnol. 2025, 100, 53–67. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, F.; Berhe, M.; Zhou, R.; Li, D.; Li, H.; Yang, L.; Zhou, T.; Zhang, Y.; Wang, L.; et al. Genome-wide identification, classification, and expression profiling of LAC gene family in sesame. BMC Plant Biol. 2024, 24, 1254. [Google Scholar] [CrossRef]
- Baloch, A.A.; Kakar, K.U.; Rais, S.; Nawaz, Z.; Almoneafy, A.A.; Raza, A.M.; Khan, S.; Ullah, R. Genome-wide analysis of CNGC gene family in Brassica juncea (L.) Czern reveals key targets for stress resistance and crop improvement. Plant Gene 2025, 41, 100487. [Google Scholar] [CrossRef]
- Lian, C.; Li, Q.; Yao, K.; Zhang, Y.; Meng, S.; Yin, W.; Xia, X. Populus trichocarpa PtNF-YA9, a multifunctional transcription factor, regulates seed germination, abiotic stress, plant growth and development in Arabidopsis. Front. Plant Sci. 2018, 9, 954. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, e681. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yan, P.; Zeng, Y.; Shen, W.; Tuo, D.; Li, X.; Zhou, P. Nimble cloning: A simple, versatile, and efficient system for standardized molecular cloning. Front. Bioeng. Biotechnol. 2019, 7, e460. [Google Scholar] [CrossRef]
- Elstner, E.F.; Adelheid, H. Inhibition of nitrite formation from hydroxylammoniumch loride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, R.; Gao, Y.; Yang, X.; Li, X.; Zhu, C.; Mo, F.; Li, K. Identification of Cysteine synthase (Cys) Gene Family in Tomato (Solanum lycopersicum) and Functional of SlCys5 in Cold Stress Tolerance. Int. J. Mol. Sci. 2025, 26, 2801. https://doi.org/10.3390/ijms26062801
Lv R, Gao Y, Yang X, Li X, Zhu C, Mo F, Li K. Identification of Cysteine synthase (Cys) Gene Family in Tomato (Solanum lycopersicum) and Functional of SlCys5 in Cold Stress Tolerance. International Journal of Molecular Sciences. 2025; 26(6):2801. https://doi.org/10.3390/ijms26062801
Chicago/Turabian StyleLv, Rui, Yan Gao, Xueying Yang, Xin Li, Chengyu Zhu, Fulei Mo, and Kuihua Li. 2025. "Identification of Cysteine synthase (Cys) Gene Family in Tomato (Solanum lycopersicum) and Functional of SlCys5 in Cold Stress Tolerance" International Journal of Molecular Sciences 26, no. 6: 2801. https://doi.org/10.3390/ijms26062801
APA StyleLv, R., Gao, Y., Yang, X., Li, X., Zhu, C., Mo, F., & Li, K. (2025). Identification of Cysteine synthase (Cys) Gene Family in Tomato (Solanum lycopersicum) and Functional of SlCys5 in Cold Stress Tolerance. International Journal of Molecular Sciences, 26(6), 2801. https://doi.org/10.3390/ijms26062801





