Genetic Polymorphisms in MHC Classes I and II Predict Outcomes in Metastatic Colorectal Cancer †
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Association of MHC Class I and II SNPs with Clinical Outcomes in the Cetuximab Cohort
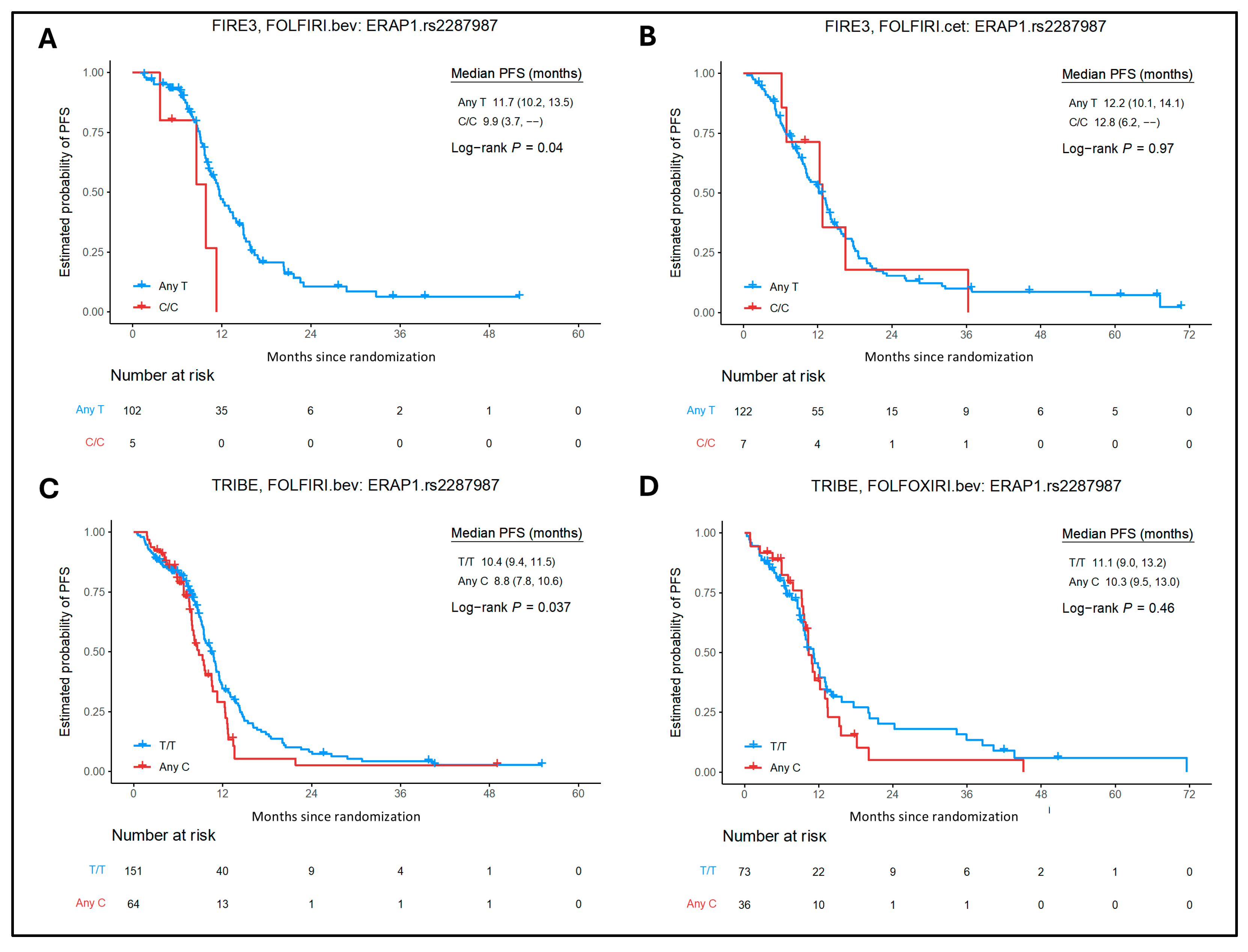
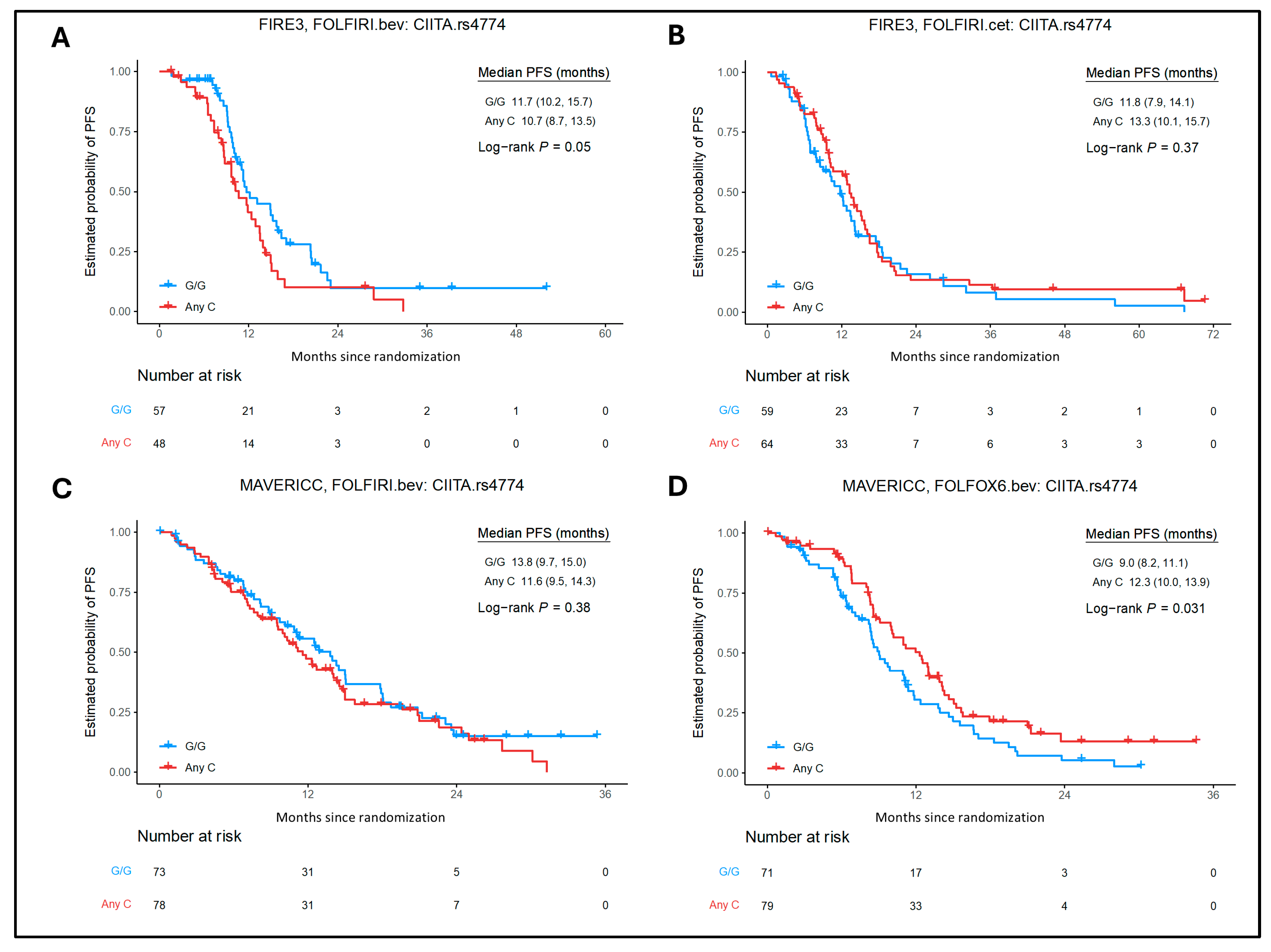
2.3. Association of MHC Class I and II SNPs with Clinical Outcomes in the Bevacizumab Cohorts
2.4. Association of MHC Class I and II SNPs with Clinical Outcomes in FOLFOX6/Bevacizumab Arm of MAVERICC Trial
2.5. Treatment-by-SNP Interaction
2.6. Associations Between Selected SNPs and Gene Expression Level in Colon Tissue from GTEx Analysis
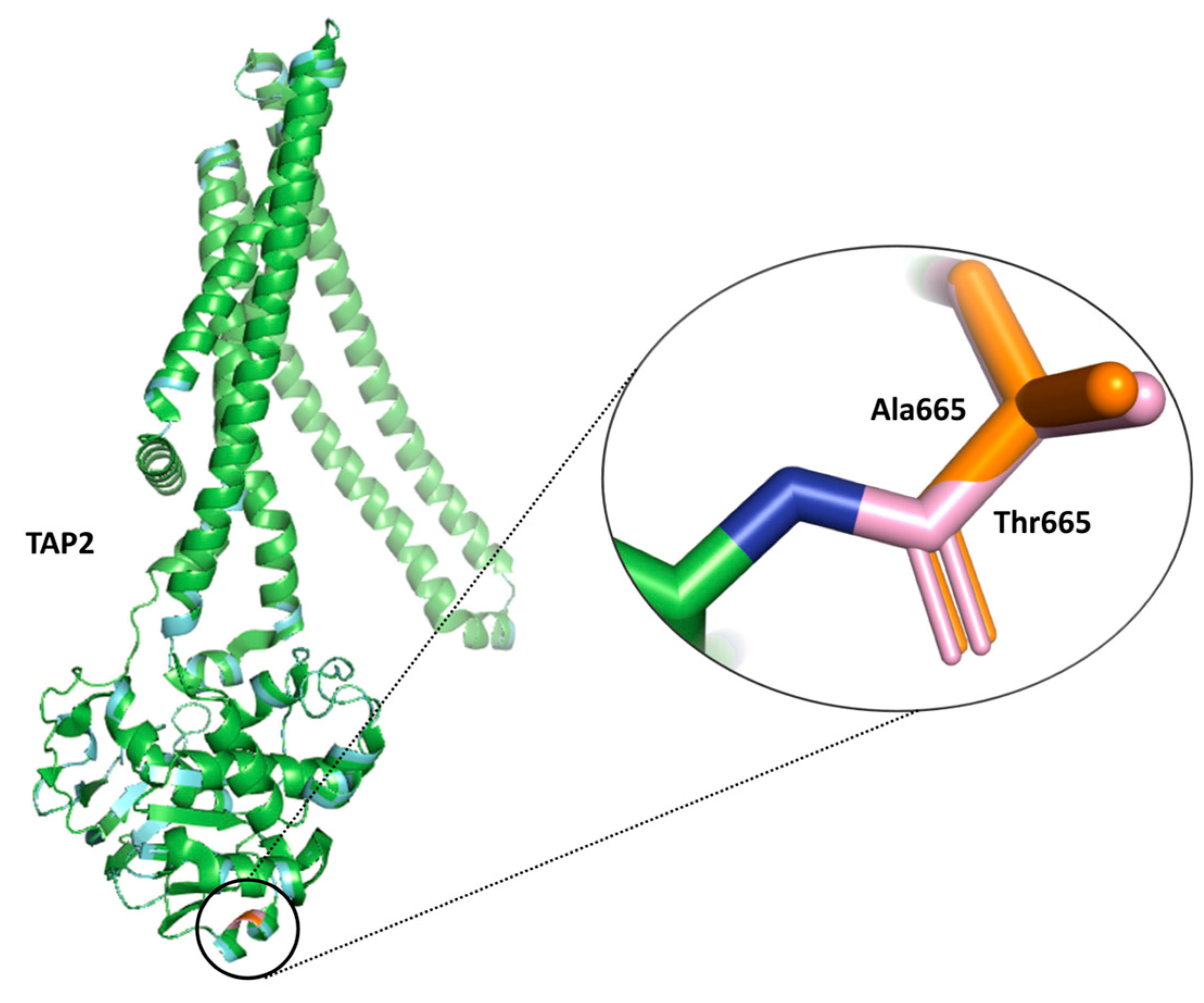
2.7. Impact of Selected SNPs on Protein Function and Stability from SIFT and DUET Analysis
3. Discussion
4. Materials and Methods
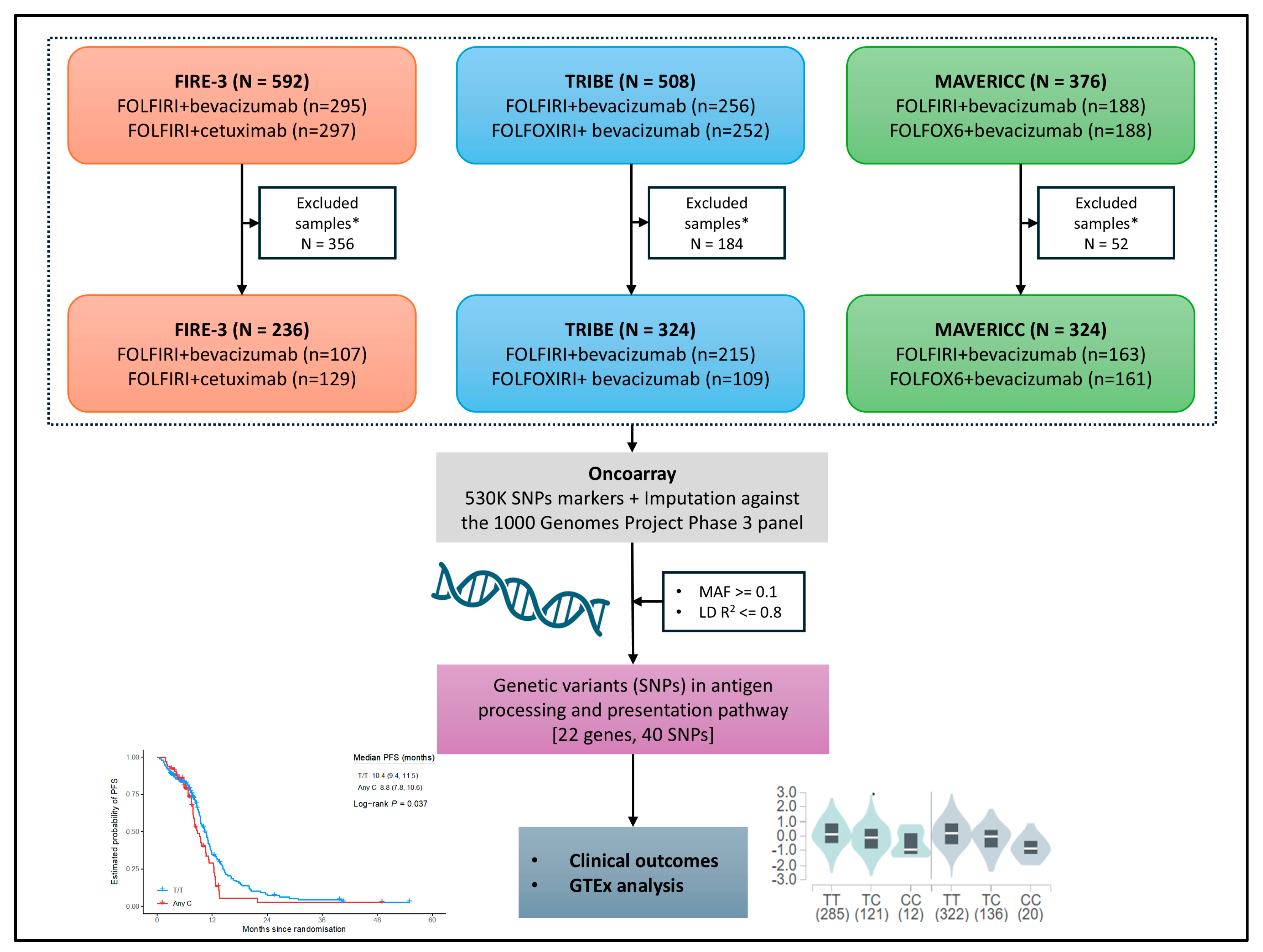
4.1. Patient Samples/Cohorts and Study Design
4.2. Selection of Polymorphisms and Genotyping
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIM | Ancestry informative markers |
| APC | Antigen presenting cells |
| APM | Antigen processing and presentation machinery |
| B2M | Beta-2-microglobulin |
| CRC | Colorectal Cancer |
| ECOG | Eastern Cooperative Oncology Group |
| EGFR | Epidermal growth factor receptor |
| ERAP1/2 | Endoplasmic reticulum aminopeptidases ½ |
| GTEx | Genotype-Tissue Expression |
| HLA | Human Leukocyte Antigens |
| KIRs | Killer cell immunoglobulin-like receptors |
| LD | Linkage disequilibrium |
| MHC | Major Histocompatibility Complex |
| NF-κB | Nuclear factor-kappa B |
| NK | Natural killer cells |
| NSCLC | Non-small cell lung cancer |
| OS | Overall Survival |
| PC | Principal components |
| PFS | Progression free survival |
| PS | Performance status |
| SIFT | Sort Intolerant from Tolerant |
| SNP | Single nucleotide polymorphisms |
| TAP1/2 | Transporter associated with antigen processing 1/2 |
| TAPBP | Tapasin/TAP-binding protein |
| TNBC | Triple negative breast cancer |
| VEGF | Vascular endothelial growth factor |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Mahmood, F.; Akingboye, A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects. Int. J. Mol. Sci. 2020, 21, 5311. [Google Scholar] [CrossRef]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A Guide to Antigen Processing and Presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Tapias, P.; Castiblanco, J.; Anaya, J.M. Major Histocompatibility Complex: Antigen Processing and Presentation. In Autoimmunity: From Bench to Bedside; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Al, E., Eds.; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Evnouchidou, I.; Birtley, J.; Seregin, S.; Papakyriakou, A.; Zervoudi, E.; Samiotaki, M.; Panayotou, G.; Giastas, P.; Petrakis, O.; Georgiadis, D.; et al. A Common Single Nucleotide Polymorphism in Endoplasmic Reticulum Aminopeptidase 2 Induces a Specificity Switch That Leads to Altered Antigen Processing. J. Immunol. 2012, 189, 2383–2392. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.-P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to Checkpoint Blockade Therapy through Inactivation of Antigen Presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K. Single Nucleotide Polymorphisms in the Gene Encoding the Major Histocompatibility Complex Class II Transactivator (CIITA) in Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2005, 64, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Mumphrey, M.B.; Hosseini, N.; Parolia, A.; Geng, J.; Zou, W.; Raghavan, M.; Chinnaiyan, A.; Cieslik, M. Distinct Mutational Processes Shape Selection of MHC Class I and Class II Mutations across Primary and Metastatic Tumors. Cell Rep. 2023, 42, 112965. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Adolf, I.C.; Almars, A.; Dharsee, N.; Mselle, T.; Akan, G.; Nguma, I.J.; Nateri, A.S.; Atalar, F. HLA-G and Single Nucleotide Polymorphism (SNP) Associations with Cancer in African Populations: Implications in Personal Medicine. Genes Dis. 2022, 9, 1220–1233. [Google Scholar] [CrossRef]
- Balasubramanian, A.; John, T.; Asselin-Labat, M.-L. Regulation of the Antigen Presentation Machinery in Cancer and Its Implication for Immune Surveillance. Biochem. Soc. Trans. 2022, 50, 825–837. [Google Scholar] [CrossRef]
- Anderson, P.; Aptsiauri, N.; Ruiz-Cabello, F.; Garrido, F. HLA Class I Loss in Colorectal Cancer: Implications for Immune Escape and Immunotherapy. Cell Mol. Immunol. 2021, 18, 556–565. [Google Scholar] [CrossRef]
- Griffith, B.D.; Turcotte, S.; Lazarus, J.; Lima, F.; Bell, S.; Delrosario, L.; McGue, J.; Krishnan, S.; Oneka, M.D.; Nathan, H.; et al. MHC Class II Expression Influences the Composition and Distribution of Immune Cells in the Metastatic Colorectal Cancer Microenvironment. Cancers 2022, 14, 4092. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.S.; Ramage, J.M.; Madjd, Z.; Spendlove, I.; Ellis, I.O.; Scholefield, J.H.; Durrant, L.G. Immunosurveillance Is Active in Colorectal Cancer as Downregulation but Not Complete Loss of MHC Class I Expression Correlates with a Poor Prognosis. Int. J. Cancer 2006, 118, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, A.; Sobczyński, M.; Pawełczyk, K.; Porębska, I.; Jasek, M.; Wagner, M.; Niepiekło-Miniewska, W.; Kowal, A.; Dubis, J.; Jędruchniewicz, N.; et al. Polymorphisms of Antigen-Presenting Machinery Genes in Non-Small Cell Lung Cancer: Different Impact on Disease Risk and Clinical Parameters in Smokers and Never-Smokers. Front. Immunol. 2021, 12, 664474. [Google Scholar] [CrossRef]
- Stintzing, S.; Modest, D.P.; Rossius, L.; Lerch, M.M.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; et al. FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab for Metastatic Colorectal Cancer (FIRE-3): A Post-Hoc Analysis of Tumour Dynamics in the Final RAS Wild-Type Subgroup of This Randomised Open-Label Phase 3 Trial. Lancet Oncol. 2016, 17, 1426–1434. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial Therapy with FOLFOXIRI and Bevacizumab for Metastatic Colorectal Cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef]
- Parikh, A.R.; Lee, F.-C.; Yau, L.; Koh, H.; Knost, J.; Mitchell, E.P.; Bosanac, I.; Choong, N.; Scappaticci, F.; Mancao, C.; et al. MAVERICC, a Randomized, Biomarker-Stratified, Phase II Study of MFOLFOX6-Bevacizumab versus FOLFIRI-Bevacizumab as First-Line Chemotherapy in Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 2988–2995. [Google Scholar] [CrossRef]
- Mittal, P.; Battaglin, F.; Yang, Y.; Millstein, J.; Stintzing, S.; Parikh, A.R.; Soni, S.; Lo, J.H.; Torres-Gonzalez, L.; Algaze, S.; et al. Single Nucleotide Polymorphisms (SNPs) in MHC Class I and II Genes to Predict Outcome in Patients (Pts) with Metastatic Colorectal Cancer (MCRC): Data from FIRE-3, MAVERICC, and TRIBE Trials. J. Clin. Oncol. 2023, 41, 3578. [Google Scholar] [CrossRef]
- Wolin, A.; Lahtela, E.L.; Anttila, V.; Petrek, M.; Grunewald, J.; van Moorsel, C.H.M.; Eklund, A.; Grutters, J.C.; Kolek, V.; Mrazek, F.; et al. SNP Variants in Major Histocompatibility Complex Are Associated with Sarcoidosis Susceptibility—A Joint Analysis in Four European Populations. Front. Immunol. 2017, 8, 422. [Google Scholar] [CrossRef]
- McMahon, G.; Ring, S.M.; Davey-Smith, G.; Timpson, N.J. Genome-Wide Association Study Identifies SNPs in the MHC Class II Loci That Are Associated with Self-Reported History of Whooping Cough. Hum. Mol. Genet. 2015, 24, 5930–5939. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, M.; Reyna, J.; Jakubosky, D.; Donovan, M.K.; Bonder, M.-J.; Matsui, H.; Stegle, O.; Nariai, N.; D’Antonio-Chronowska, A.; Frazer, K.A. Systematic Genetic Analysis of the MHC Region Reveals Mechanistic Underpinnings of HLA Type Associations with Disease. Elife 2019, 8, e48476. [Google Scholar] [CrossRef]
- Thompson, J.C.; Davis, C.; Deshpande, C.; Hwang, W.-T.; Jeffries, S.; Huang, A.; Mitchell, T.C.; Langer, C.J.; Albelda, S.M. Gene Signature of Antigen Processing and Presentation Machinery Predicts Response to Checkpoint Blockade in Non-Small Cell Lung Cancer (NSCLC) and Melanoma. J. Immunother. Cancer 2020, 8, e000974. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Limagne, E.; Thibaudin, M.; Nuttin, L.; Spill, A.; Derangere, V.; Cattan, V.; Peranzoni, E.; Amellal, N. The Combination of Trifluridine/Tipiracil and Oxaliplatin Induces Immunogenic Cell Death in Microsatellite Stable Colorectal Cancer. Ann. Oncol. 2018, 29, viii186. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, H.; Xiao, X.; Tang, M.; Tian, Z.; Wei, H.; Sun, R.; Zheng, X. Low-Dose Immunogenic Chemotherapeutics Promotes Immune Checkpoint Blockade in Microsatellite Stability Colon Cancer. Front. Immunol. 2022, 13, 1040256. [Google Scholar] [CrossRef]
- Rana, P.S.; Ignatz-Hoover, J.J.; Driscoll, J.J. Targeting Proteasomes and the MHC Class I Antigen Presentation Machinery to Treat Cancer, Infections and Age-Related Diseases. Cancers 2023, 15, 5632. [Google Scholar] [CrossRef] [PubMed]
- Elamin, Y.Y.; Rafee, S.; Toomey, S.; Hennessy, B.T. Immune Effects of Bevacizumab: Killing Two Birds with One Stone. Cancer Microenviron. 2015, 8, 15–21. [Google Scholar] [CrossRef]
- De Aguiar, R.B.; Moraes, J.Z. de Exploring the Immunological Mechanisms Underlying the Anti-Vascular Endothelial Growth Factor Activity in Tumors. Front. Immunol. 2019, 10, 1023. [Google Scholar] [CrossRef]
- Mimura, K.; Kono, K.; Takahashi, A.; Kawaguchi, Y.; Fujii, H. Vascular Endothelial Growth Factor Inhibits the Function of Human Mature Dendritic Cells Mediated by VEGF Receptor-2. Cancer Immunol. Immunother. 2007, 56, 761–770. [Google Scholar] [CrossRef]
- Boucher, Y.; Kumar, A.S.; Posada, J.M.; Gjini, E.; Pfaff, K.; Lipschitz, M.; Lako, A.; Duda, D.G.; Rodig, S.J.; Hodi, F.S.; et al. Bevacizumab Improves Tumor Infiltration of Mature Dendritic Cells and Effector T-Cells in Triple-Negative Breast Cancer Patients. NPJ Precis. Oncol. 2021, 5, 62. [Google Scholar] [CrossRef]
- Kallingal, A.; Olszewski, M.; Maciejewska, N.; Brankiewicz, W.; Baginski, M. Cancer Immune Escape: The Role of Antigen Presentation Machinery. J. Cancer Res. Clin. Oncol. 2023, 149, 8131–8141. [Google Scholar] [CrossRef]
- Bufalieri, F.; Fruci, D.; Di Marcotullio, L. ERAP1 as an Emerging Therapeutic Target for Medulloblastoma. Trends in Cancer 2022, 8, 4–8. [Google Scholar] [CrossRef] [PubMed]
- James, E.; Bailey, I.; Sugiyarto, G.; Elliott, T. Induction of Protective Antitumor Immunity through Attenuation of ERAAP Function. J. Immunol. 2013, 190, 5839–5846. [Google Scholar] [CrossRef] [PubMed]
- Joyce, P.; Quibell, M.; Shiers, J.; Tong, C.; Clark, K.; Ternette, N.; Anderton, K.; Sette, J.; Paes, W.; Leishman, A. First-in-Class Inhibitors of ERAP1 Alter the Immunopeptidome of Cancer, Driving a Differentiated T Cell Response Leading to Tumor Growth Inhibition. J. Immunother. Cancer 2021, 9, A583. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Yan, Z.; Dai, S.; Liu, S.; Wang, X.; Wang, J.; Zhang, X.; Shi, L.; Yao, Y. Polymorphisms in Endoplasmic Reticulum Aminopeptidase Genes Are Associated with Cervical Cancer Risk in a Chinese Han Population. BMC Cancer 2020, 20, 341. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Navarro, C.; López de Castro, J.A. ERAP1 Structure, Function and Pathogenetic Role in Ankylosing Spondylitis and Other MHC-Associated Diseases. Mol. Immunol. 2014, 57, 12–21. [Google Scholar] [CrossRef]
- Pollack, B.P.; Sapkota, B.; Cartee, T.V. Epidermal Growth Factor Receptor Inhibition Augments the Expression of MHC Class I and II Genes. Clin. Cancer Res. 2011, 17, 4400–4413. [Google Scholar] [CrossRef]
- Padariya, M.; Kote, S.; Mayordomo, M.; Dapic, I.; Alfaro, J.; Hupp, T.; Fahraeus, R.; Kalathiya, U. Structural Determinants of Peptide-Dependent TAP1-TAP2 Transit Passage Targeted by Viral Proteins and Altered by Cancer-Associated Mutations. Comput. Struct. Biotechnol. J. 2021, 19, 5072–5091. [Google Scholar] [CrossRef]
- Henle, A.M.; Nassar, A.; Puglisi-Knutson, D.; Youssef, B.; Knutson, K.L. Downregulation of TAP1 and TAP2 in Early Stage Breast Cancer. PLoS ONE 2017, 12, e0187323. [Google Scholar] [CrossRef]
- Dai, D.; Chen, Y.; Ru, P.; Zhou, X.; Tao, J.; Ye, H.; Hong, Q.; Tang, L.; Pan, G.; Lin, D.; et al. Significant Association between TAP2 Polymorphisms and Rheumatoid Arthritis: A Meta-Analysis. Diagn. Pathol. 2014, 9, 129. [Google Scholar] [CrossRef]
- Wieczorek, E.; Jablonowski, Z.; Lesicka, M.; Jablonska, E.; Kutwin, P.; Reszka, E.; Garstka, M.A. Genetic Contributions of MHC Class I Antigen Processing and Presentation Pathway to Bladder Cancer Risk and Recurrence. Neoplasma 2022, 69, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Pollack, B.P. EGFR Inhibitors, MHC Expression and Immune Responses: Can EGFR Inhibitors Be Used as Immune Response Modifiers? Oncoimmunology 2012, 1, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Shallak, M.; Celesti, F.; Accolla, R.S. Unveiling the Hidden Treasury: CIITA-Driven MHC Class II Expression in Tumor Cells to Dig up the Relevant Repertoire of Tumor Antigens for Optimal Stimulation of Tumor Specific CD4+ T Helper Cells. Cancers 2020, 12, 3181. [Google Scholar] [CrossRef]
- Watanabe, S.; Hayashi, H.; Haratani, K.; Shimizu, S.; Tanizaki, J.; Sakai, K.; Kawakami, H.; Yonesaka, K.; Tsurutani, J.; Togashi, Y.; et al. Mutational Activation of the Epidermal Growth Factor Receptor Down-regulates Major Histocompatibility Complex Class I Expression via the Extracellular Signal-regulated Kinase in Non–Small Cell Lung Cancer. Cancer Sci. 2019, 110, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Xiao, Y.; Loupakis, F.; Kawanishi, N.; Wang, J.; Battaglin, F.; Soni, S.; Zhang, W.; Mancao, C.; Salhia, B.; et al. Immunogenic Cell Death Pathway Polymorphisms for Predicting Oxaliplatin Efficacy in Metastatic Colorectal Cancer. J. ImmunoTher. Cancer 2020, 8, e001714. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, P.; Battaglin, F.; Yang, Y.; Soni, S.; Stintzing, S.; Parikh, A.R.; Ashouri, K.; Algaze, S.; Jayachandran, P.; Torres-Gonzalez, L.; et al. Genetic Polymorphisms in MHC Classes I and II Predict Outcomes in Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 2556. https://doi.org/10.3390/ijms26062556
Mittal P, Battaglin F, Yang Y, Soni S, Stintzing S, Parikh AR, Ashouri K, Algaze S, Jayachandran P, Torres-Gonzalez L, et al. Genetic Polymorphisms in MHC Classes I and II Predict Outcomes in Metastatic Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(6):2556. https://doi.org/10.3390/ijms26062556
Chicago/Turabian StyleMittal, Pooja, Francesca Battaglin, Yan Yang, Shivani Soni, Sebastian Stintzing, Aparna R. Parikh, Karam Ashouri, Sandra Algaze, Priya Jayachandran, Lesly Torres-Gonzalez, and et al. 2025. "Genetic Polymorphisms in MHC Classes I and II Predict Outcomes in Metastatic Colorectal Cancer" International Journal of Molecular Sciences 26, no. 6: 2556. https://doi.org/10.3390/ijms26062556
APA StyleMittal, P., Battaglin, F., Yang, Y., Soni, S., Stintzing, S., Parikh, A. R., Ashouri, K., Algaze, S., Jayachandran, P., Torres-Gonzalez, L., Zhang, W., Cremolini, C., Heinemann, V., Millstein, J., Singh, I. K., & Lenz, H.-J. (2025). Genetic Polymorphisms in MHC Classes I and II Predict Outcomes in Metastatic Colorectal Cancer. International Journal of Molecular Sciences, 26(6), 2556. https://doi.org/10.3390/ijms26062556





