A DNA-Based Plasmonic Nano-Ruler
Abstract
1. Introduction
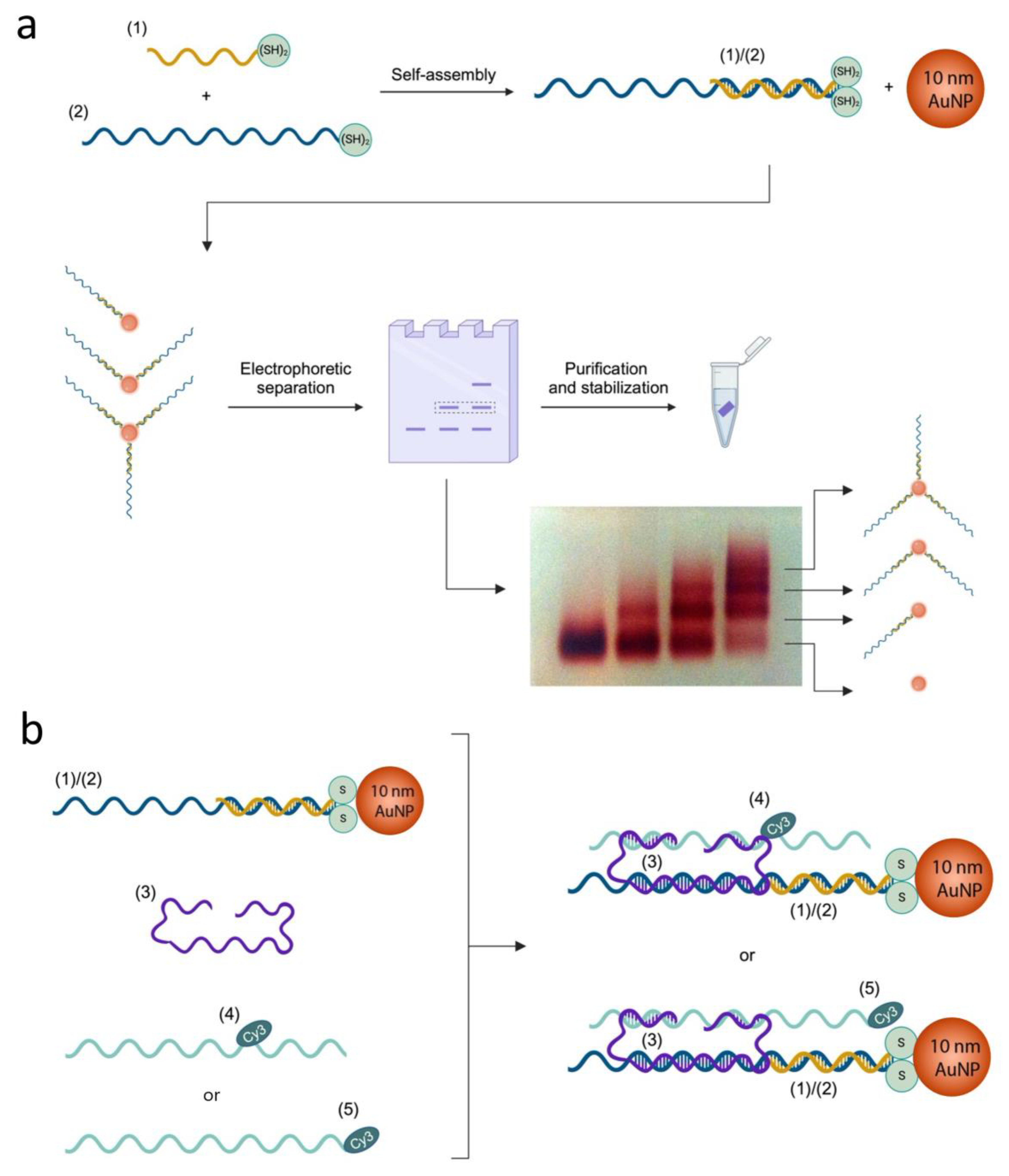
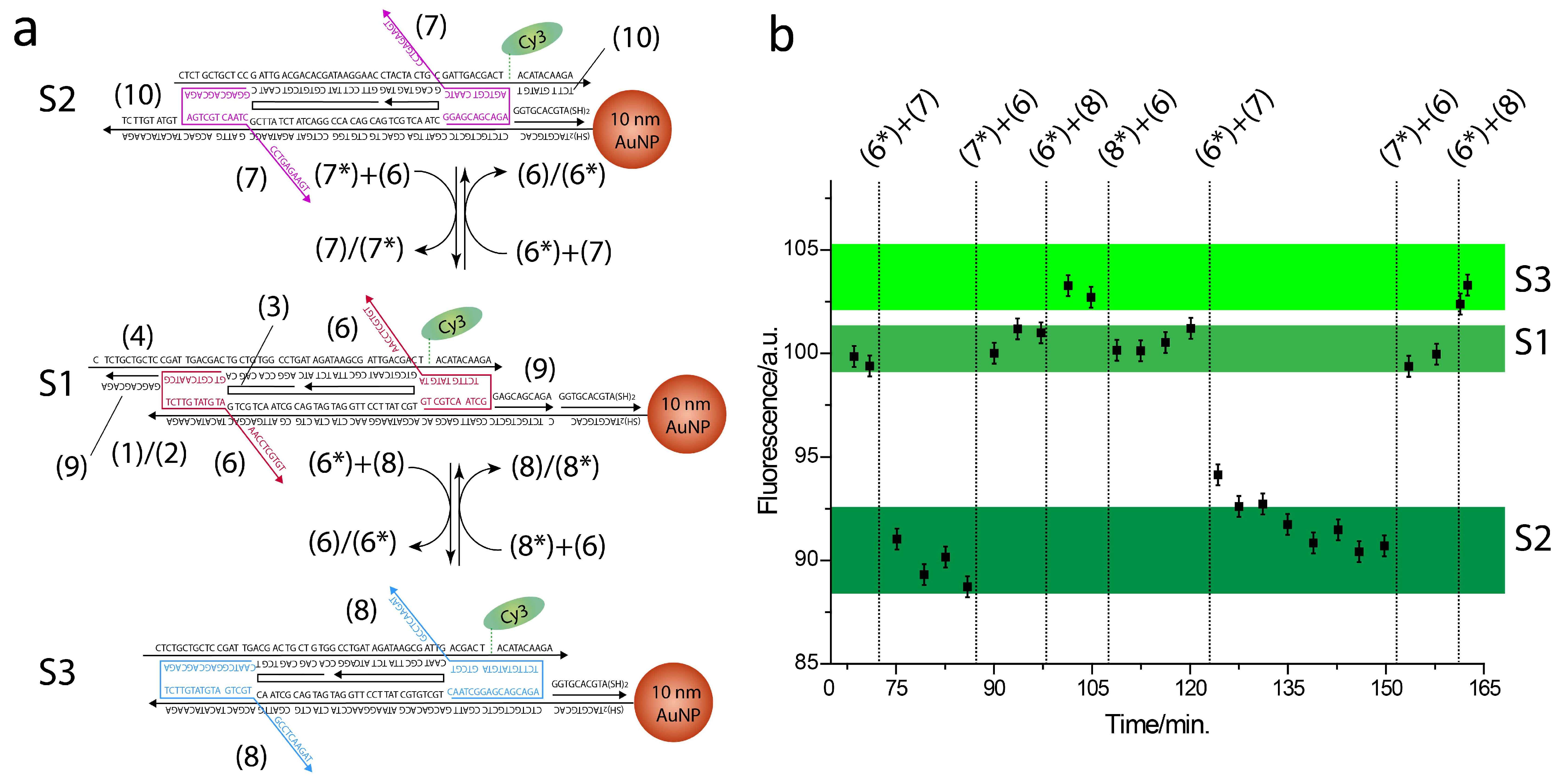
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ssDNA | Single-strand DNA |
| AuNP | Gold nanoparticle |
| Cy3 | Sulfo-Cyanine3 |
References
- Mao, X.; Liu, M.; Li, Q.; Fan, C.; Zuo, X. DNA-Based Molecular Machines. JACS Au 2022, 2, 2381–2399. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ying, J.Y. Reconfigurable A-Motif, i-Motif and Triplex Nucleic Acids for Smart PH-Responsive DNA Hydrogels. Mater. Today 2023, 63, 188–209. [Google Scholar] [CrossRef]
- Mills, A.; Aissaoui, N.; Maurel, D.; Elezgaray, J.; Morvan, F.; Vasseur, J.J.; Margeat, E.; Quast, R.B.; Lai Kee-Him, J.; Saint, N.; et al. A Modular Spring-Loaded Actuator for Mechanical Activation of Membrane Proteins. Nat. Commun. 2022, 13, 3182. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.; Carrascosa Marzo, R.; Bath, J.; Turberfield, A.J. Strategies for Constructing and Operating DNA Origami Linear Actuators. Small 2021, 17, 2007704. [Google Scholar] [CrossRef]
- Michelson, A.; Subramanian, A.; Kisslinger, K.; Tiwale, N.; Xiang, S.; Shen, E.; Kahn, J.S.; Nykypanchuk, D.; Yan, H.; Nam, C.Y.; et al. Three-Dimensional Nanoscale Metal, Metal Oxide, and Semiconductor Frameworks through DNA-Programmable Assembly and Templating. Sci. Adv. 2024, 10, eadl0604. [Google Scholar] [CrossRef]
- Rui Lee, S.; Yu Jie Ong, C.; Yi Wong, J.; Ke, Y.; Dong, Z.; Lim, J.Y.C.; Hu, Y. Supramolecular Oligo-Thymine/Melamine Nanobridge-Driven Macroscopic Engineering: Reprogrammable Hydrogels for Multi-Stimuli Responsive Architectures. Chem. Eng. J. 2024, 497, 154698. [Google Scholar] [CrossRef]
- Rothfischer, F.; Vogt, M.; Kopperger, E.; Gerland, U.; Simmel, F.C. From Brownian to Deterministic Motor Movement in a DNA-Based Molecular Rotor. Nano Lett. 2024, 24, 5224–5230. [Google Scholar] [CrossRef]
- Hildebrandt, L.L.; Preus, S.; Zhang, Z.; Voigt, N.V.; Gothelf, K.V.; Birkedal, V. Single Molecule FRET Analysis of the 11 Discrete Steps of a DNA Actuator. J. Am. Chem. Soc. 2014, 136, 8957–8962. [Google Scholar] [CrossRef]
- Zhang, Z.; Olsen, E.M.; Kryger, M.; Voigt, N.V.; Tørring, T.; Gültekin, E.; Nielsen, M.; Mohammadzadegan, R.; Andersen, E.S.; Nielsen, M.M.; et al. A DNA Tile Actuator with Eleven Discrete States. Angew. Chem. Int. Ed. 2011, 50, 3983–3987. [Google Scholar] [CrossRef]
- Simmel, F.C.; Yurke, B.; Singh, H.R. Principles and Applications of Nucleic Acid Strand Displacement Reactions. Chem. Rev. 2019, 119, 6326–6369. [Google Scholar] [CrossRef]
- Turberfield, A.J.; Yurke, B.; Mills, A.P.; Simmel, F.C.; Neumann, J.L. A DNA-Fuelled Molecular Machine Made of DNA. Nature 2000, 406, 605–608. [Google Scholar] [CrossRef]
- Kuzyk, A.; Schreiber, R.; Zhang, H.; Govorov, A.O.; Liedl, T.; Liu, N. Reconfigurable 3D Plasmonic Metamolecules. Nat. Mater. 2014, 13, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, Q.; Elshaer, M.R.; Knorr, B.; Chaikin, P.; Zhu, G. Tri-State Logic Computation by Activating DNA Origami Chains. Nanoscale 2024, 16, 11991–11998. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, S.; Klein, M.; Orbach, R.; Willner, I.; Remacle, F.; Levine, R.D. Continuous Variables Logic via Coupled Automata Using a DNAzyme Cascade with Feedback. Chem. Sci. 2017, 8, 2161–2168. [Google Scholar] [CrossRef]
- Soukarie, D.; Nocete, L.; Bittner, A.M.; Santiago, I. DNA Data Storage in Electrospun and Melt-Electrowritten Composite Nucleic Acid-Polymer Fibers. Mater. Today Bio 2024, 24, 100900. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, S.; Geng, C.; Zheng, Y.; Bai, S.; Cao, X.; Liu, K.; Yang, Y.; Lu, C.; Jiang, X. Multiplexed Random Access Approach to DNA Microspheres for High-Capacity Data Storage. Adv. Funct. Mater. 2024, 34, 2408852. [Google Scholar] [CrossRef]
- Mo, F.; Li, C.; Sun, J.; Lin, X.; Yu, S.; Wang, F.; Liu, X.; Li, J. Programming Fast DNA Amplifier Circuits with Versatile Toehold Exchange Pathway. Small 2024, 20, 2402914. [Google Scholar] [CrossRef]
- Stanisavljevic, M.; Krizkova, S.; Vaculovicova, M.; Kizek, R.; Adam, V. Quantum Dots-Fluorescence Resonance Energy Transfer-Based Nanosensors and Their Application. Biosens. Bioelectron. 2015, 74, 562–574. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon Dots: Synthesis, Formation Mechanism, Fluorescence Origin and Sensing Applications. Green. Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A Review on Syntheses, Properties, Characterization and Bioanalytical Applications of Fluorescent Carbon Dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Yukawa, H.; Baba, Y. In Vivo Fluorescence Imaging and the Diagnosis of Stem Cells Using Quantum Dots for Regenerative Medicine. Anal. Chem. 2017, 89, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lee, J.U.; Kim, Y.; Kim, G.; Jung, Y.; Jo, A.; Park, M.; Lee, S.; Lah, J.D.; Park, J.; et al. A Magnetically Powered Nanomachine with a DNA Clutch. Nat. Nanotechnol. 2024, 19, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Sun, M. Tip-Enhanced Photoluminescence Spectroscopy of Monolayer MoS_2. Photonics Res. 2017, 5, 745–749. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, J.; Zhang, W.; Jiang, L.; Yi, D.; Rujiralai, T.; Ma, J. Broadband Single-Molecule Fluorescence Enhancement Based on Self-Assembled Ag@Au Dimer Plasmonic Nanoantennas. Nanoscale 2022, 14, 17550–17560. [Google Scholar] [CrossRef]
- Sönnichsen, C.; Reinhard, B.M.; Liphardt, J.; Alivisatos, A.P. A Molecular Ruler Based on Plasmon Coupling of Single Gold and Silver Nanoparticles. Nat. Biotechnol. 2005, 23, 741–745. [Google Scholar] [CrossRef]
- Acuna, G.P.; Bucher, M.; Stein, I.H.; Steinhauer, C.; Kuzyk, A.; Holzmeister, P.; Schreiber, R.; Moroz, A.; Stefani, F.D.; Liedl, T.; et al. Distance Dependence of Single-Fluorophore Quenching by Gold Nanoparticles Studied on DNA Origami. ACS Nano 2012, 6, 3189–3195. [Google Scholar] [CrossRef]
- Li, J.F.; Li, C.Y.; Aroca, R.F. Plasmon-Enhanced Fluorescence Spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef]
- Raab, M.; Jusuk, I.; Molle, J.; Buhr, E.; Bodermann, B.; Bergmann, D.; Bosse, H.; Tinnefeld, P. Using DNA Origami Nanorulers as Traceable Distance Measurement Standards and Nanoscopic Benchmark Structures. Sci. Rep. 2018, 8, 1780. [Google Scholar] [CrossRef]
- Lo, P.K.; Karam, P.; Aldaye, F.A.; McLaughlin, C.K.; Hamblin, G.D.; Cosa, G.; Sleiman, H.F. Loading and Selective Release of Cargo in DNA Nanotubes with Longitudinal Variation. Nat. Chem. 2010, 2, 319–328. [Google Scholar] [CrossRef]
- Ren, S.; Ren, L.; Wei, B.; Liu, Y.; Yang, J.; Li, J.; Wang, L. DNA-Templated Fabrication of Metal Nanostructures with Special Shapes. Adv. Sens. Energy Mater. 2024, 4, 100133. [Google Scholar] [CrossRef]
- Cecconello, A.; Cencini, A.; Rilievo, G.; Tonolo, F.; Litti, L.; Vianello, F.; Willner, I.; Magro, M. Chiroplasmonic DNA Scaffolded “Fusilli” Structures. Nano Lett. 2024, 24, 5944–5951. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Jiang, C.; Gou, D.; Long, Y. Surface Plasmon-Assisted Fluorescence Enhancing and Quenching: From Theory to Application. ACS Appl. Bio Mater. 2021, 4, 4684–4705. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhang, T.; Li, W.; Hou, L.; Liu, J.; Gong, Q. Single-Molecule Spontaneous Emission in the Vicinity of an Individual Gold Nanorod. J. Phys. Chem. C 2011, 115, 15822–15828. [Google Scholar] [CrossRef]
- Mertens, H.; Koenderink, A.F.; Polman, A. Plasmon-Enhanced Luminescence near Noble-Metal Nanospheres: Comparison of Exact Theory and an Improved Gersten and Nitzan Model. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 115123. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cencini, A.; Bortoluzzi, M.; Rilievo, G.; Tonolo, F.; Vianello, F.; Magro, M.; Cecconello, A. A DNA-Based Plasmonic Nano-Ruler. Int. J. Mol. Sci. 2025, 26, 2557. https://doi.org/10.3390/ijms26062557
Cencini A, Bortoluzzi M, Rilievo G, Tonolo F, Vianello F, Magro M, Cecconello A. A DNA-Based Plasmonic Nano-Ruler. International Journal of Molecular Sciences. 2025; 26(6):2557. https://doi.org/10.3390/ijms26062557
Chicago/Turabian StyleCencini, Aura, Mary Bortoluzzi, Graziano Rilievo, Federica Tonolo, Fabio Vianello, Massimiliano Magro, and Alessandro Cecconello. 2025. "A DNA-Based Plasmonic Nano-Ruler" International Journal of Molecular Sciences 26, no. 6: 2557. https://doi.org/10.3390/ijms26062557
APA StyleCencini, A., Bortoluzzi, M., Rilievo, G., Tonolo, F., Vianello, F., Magro, M., & Cecconello, A. (2025). A DNA-Based Plasmonic Nano-Ruler. International Journal of Molecular Sciences, 26(6), 2557. https://doi.org/10.3390/ijms26062557








