Callus Culture System from Lonicera japonica Thunb Anthers: Light Quality Effects on Callus Quality Evaluation
Abstract
:1. Introduction
2. Results and Discussion
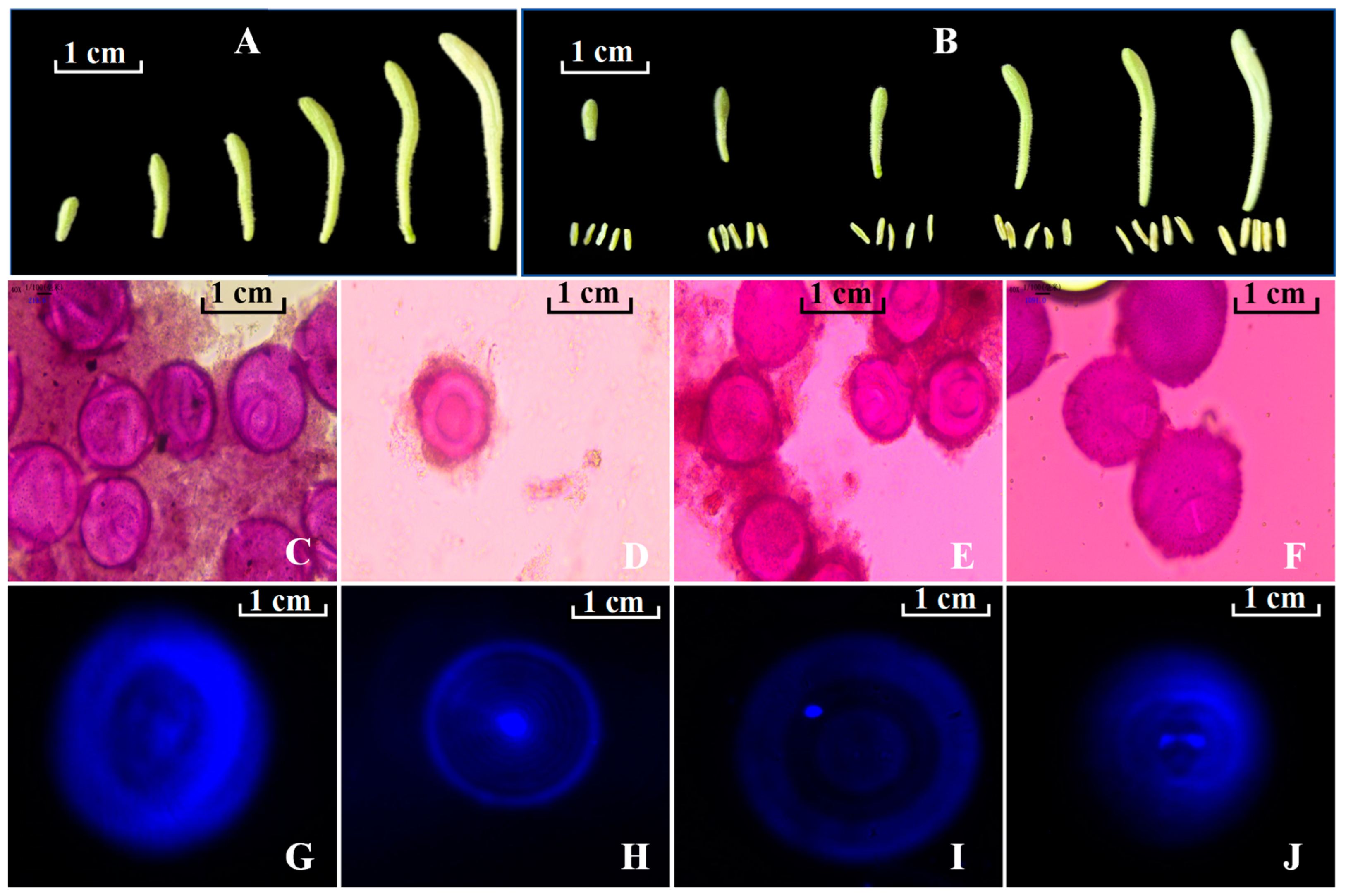
2.1. Association Between Flower Bud/Floret Size and Developmental Stage of Microspores
2.2. Callus Induction
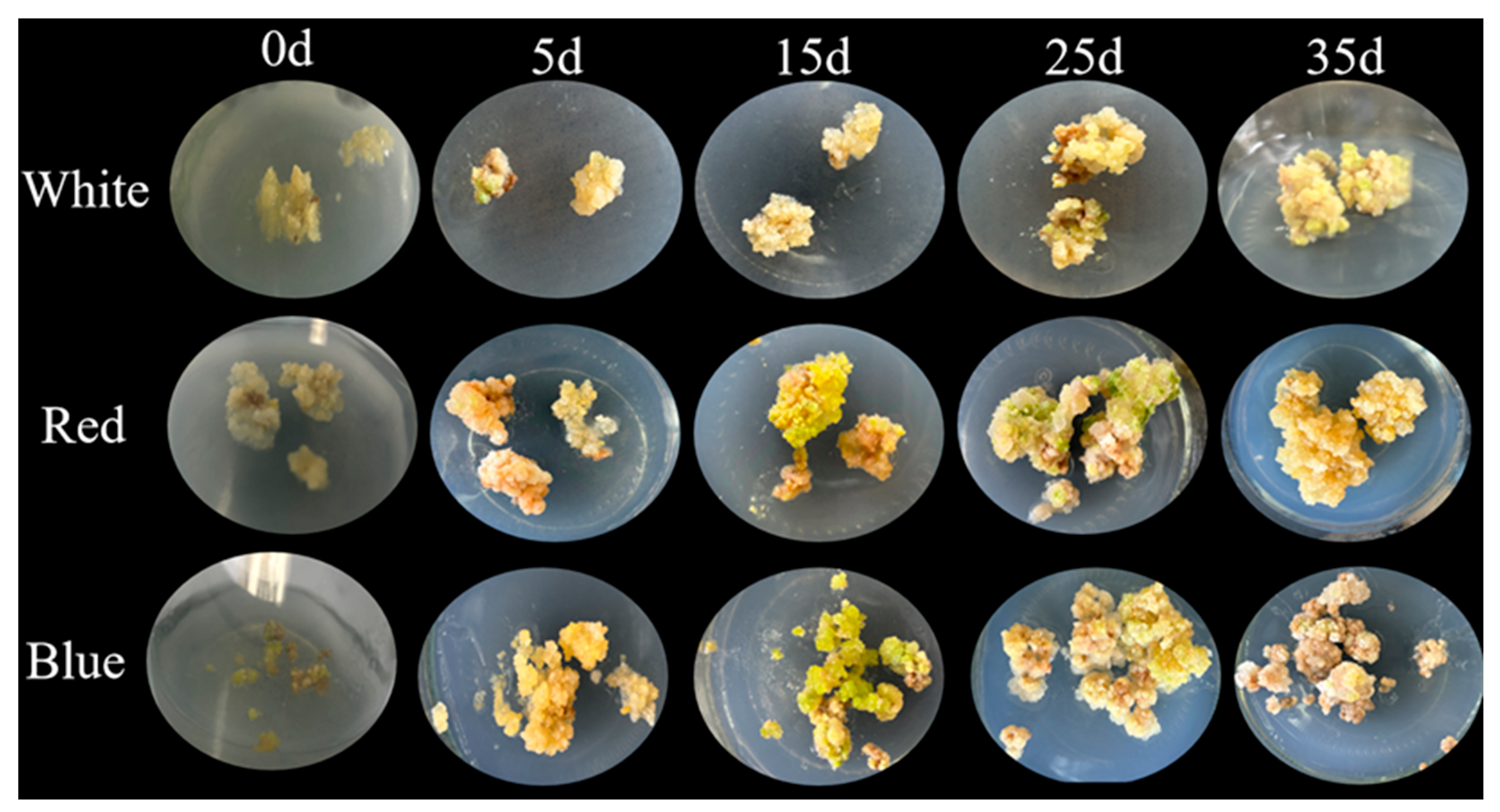
2.3. Callus Proliferation
2.4. Histological Observations
2.5. Influence of Different Light Qualities on the Growth and Development of L. japonica Callus
2.6. Effect of Light Qualities on the Accumulation of Total Phenols, Total Flavonoids, and Soluble Sugars
2.7. Effect of Light Quality on Antioxidant Capacity
2.8. Chromatographic Fingerprint Analysis
2.9. Principal Component Analysis

2.10. Impact of Light Quality on the Content of Chlorogenic and 3,5-Dicaffeoylquinic Acids
3. Material and Methods
3.1. Experiment Materials
3.2. Association Between Flower Bud/Floret Size and Microspore Developmental Stage
3.3. Callus Induction
3.4. Callus Proliferation
3.5. Histomorphological Observation of Callus
3.5.1. Scanning Electron Microscopy (SEM)
3.5.2. Paraffin Sections
3.6. Induction of Callus Proliferation Under Different Light Conditions
3.7. Phytochemical Analysis
3.7.1. Determination of Total Flavonoid, Total Phenolic Content and Soluble Sugar
Total Flavonoid Content
Total Phenolic Content
Soluble Sugar Content
3.7.2. Determination of Antioxidant Capacity
3.8. HPLC Fingerprint Establishment
3.8.1. Chromatographic Conditions
3.8.2. Fingerprint Establishment
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, W.; Zheng, W.; Hu, X.; Xu, X.; Zhang, L.; Tian, J. Variations of Metabolites and Proteome in Lonicera Japonica Thunb. Buds and Flowers under UV Radiation. Biochim. Biophys. Acta BBA-Proteins Proteom. 2017, 1865, 404–413. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae Flos and Lonicerae Flos: A Systematic Review of Ethnopharmacology, Phytochemistry and Pharmacology. Phytochem. Rev. 2020, 19, 1–61. [Google Scholar] [CrossRef]
- Kang, O.H.; Choi, Y.A.; Park, H.J.; Lee, J.Y.; Kim, D.K.; Choi, S.C.; Kim, T.H.; Nah, Y.H.; Yun, K.J.; Choi, S.J.; et al. Inhibition of Trypsin-Induced Mast Cell Activation by Water Fraction of Lonicera japonica. Arch. Pharm. Res. 2004, 27, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, K.I.; Lee, D.H.; Kang, S.R.; Nagappan, A.; Kim, J.A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Hah, Y.S.; et al. Polyphenolic Extract Isolated from Korean Lonicera japonica Thunb. Induce G2/M Cell Cycle Arrest and Apoptosis in HepG2 Cells: Involvements of PI3K/Akt and MAPKs. Food Chem. Toxicol. 2012, 50, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Rhee, H.I.; Kim, J.H.; Yoo, H.; Lee, B.Y.; Um, K.A.; Kim, K.; Noh, J.Y.; Lim, K.M.; Chung, J.H. Anti-Inflammatory and Analgesic Activities of SKLJI, a Highly Purified and Injectable Herbal Extract of Lonicera japonica. Biosci. Biotechnol. Biochem. 2010, 74, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, P.; Liu, H.; Zhang, Y.; Yu, X.; Li, J.; Pu, G.A. Simple, Rapid, and Practical Method for Distinguishing Lonicerae Japonicae Flos from Lonicerae Flos. Molecules 2019, 24, 3455. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, Q.; Li, Y.; Tian, Y.; Zheng, J.; Zhang, J. Exploration of Exogenous Chlorogenic Acid as a Potential Plant Stimulant: Enhancing Physiochemical Properties in Lonicera japonica. Physiol. Mol. Biol. Plants 2024, 30, 453–466. [Google Scholar] [CrossRef]
- Sun, C.; Teng, Y.; Li, G.; Yoshioka, S.; Yokota, J.; Miyamura, M.; Fang, H.; Zhang, Y. Metabonomics Study of the Protective Effects of Lonicera Japonica Extract on Acute Liver Injury in Dimethylnitrosamine Treated Rats. J. Pharm. Biomed. Anal. 2010, 53, 98–102. [Google Scholar] [CrossRef]
- Wang, T.; Yang, B.; Guan, Q.; Chen, X.; Zhong, Z.; Huang, W.; Zhu, W.; Tian, J. Transcriptional Regulation of Lonicera japonica Thunb. during Flower Development as Revealed by Comprehensive Analysis of Transcription Factors. BMC Plant Biol. 2019, 19, 198. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, Metabolism and Bioavailability of Flavonoids: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, J.; Yang, R.; Fang, L.; Zhang, Y. A Review: The Triterpenoid Saponins and Biological Activities of Lonicera Linn. Molecules 2020, 25, 3773. [Google Scholar] [CrossRef]
- Lai, K.H.; Chen, Y.L.; Lin, M.F.; El-Shazly, M.; Chang, Y.C.; Chen, P.J.; Su, C.H.; Chiu, Y.C.; Illias, A.M.; Chen, C.C.; et al. Lonicerae japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants 2022, 11, 1781. [Google Scholar] [CrossRef]
- Hu, M.; Hu, Z.; Du, L.; Du, J.; Luo, Q.; Xiong, J. Establishment of Cell Suspension Culture of Lonicera japonica Thunb and Analysis Its Major Secondary Metabolites. Ind. Crops Prod. 2019, 137, 98–104. [Google Scholar] [CrossRef]
- Pan, Y.; Li, L.; Xiao, S.; Chen, Z.; Sarsaiya, S.; Zhang, S.; Guang, S.; Liu, H. Callus growth kinetics and accumulation of secondary metabolites of Bletilla striata Rchb.f. using a callus suspension culture. PLoS ONE. 2020, 15, e0220084. [Google Scholar] [CrossRef]
- Buranasudja, V.; Rani, D.; Malla, A.; Kobtrakul, K.; Vimolmangkang, S. Insights into Antioxidant Activities and Anti-Skin-Aging Potential of Callus Extract from Centella asiatica (L.). Sci. Rep. 2021, 11, 13459. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Betekhtin, A.; Rojek, M.; Nowak, K.; Pinski, A.; Milewska-Hendel, A.; Kurczynska, E.; Doonan, J.H.; Hasterok, R. Cell Wall Epitopes and Endoploidy as Reporters of Embryogenic Potential in Brachypodium distachyo Callus Culture. Int. J. Mol. Sci. 2018, 19, 3811. [Google Scholar] [CrossRef]
- Lv, S.; Ding, F.; Zhang, S.; Nosov, A.M.; Kitashov, A.V.; Yang, L. Induction and Suspension Culture of Panax japonicus Callus Tissue for the Production of Secondary Metabolic Active Substances. Plants 2024, 13, 2480. [Google Scholar] [CrossRef]
- Bondarev, N.; Reshetnyak, O.; Bondareva, T.; Il’in, M.; Nosov, N. Impact of cultivation factors in vitro on the growth and the biosynthesis of steviol glycosides in Stevia rebaudiana cell cultures. Physiol. Mol. Biol. Plants 2019, 25, 1091–1096. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xia, L.; Zhou, L.; Liu, W.; Wang, P.; Qing, Z.; Zeng, J. Influence of Different Elicitors on BIA Production in Macleaya cordata. Sci. Rep. 2021, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Tariq, U.; Ali, M.; Abbasi, B.H. Morphogenic and Biochemical Variations under Different Spectral Lights in Callus Cultures of Artemisia absinthium L. J. Photochem. Photobiol. B 2014, 130, 264–271. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Neugart, S.; Toutounchi, P.M.; Brestic, M. Precultivation of young seedlings under different color shades modifies the accumulation of phenolic compounds in Cichorium leaves in later growth phases. Environ. Exp. Bot. 2019, 165, 30–38. [Google Scholar] [CrossRef]
- Dantas, L.A.; Rosa, M.; Resende, E.C.; Silva, F.G.; Pereira, P.S.; Souza, A.C.L.; e Silva, F.H.L.; Neto, A.R. Spectral quality as an elicitor of bioactive compound production in Solanum aculeatissimum JACQ cell suspension. J. Photochem. Photobiol. B 2020, 204, 111819. [Google Scholar] [CrossRef]
- Sobhani Najafabadi, A.; Khanahmadi, M.; Ebrahimi, M.; Moradi, K.; Behroozi, P.; Noormohammadi, N. Effect of different quality of light on growth and production of secondary metabolites in adventitious root cultivation of Hypericum perforatum. Plant Signal. Behav. 2019, 14, 1640561. [Google Scholar] [CrossRef]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-Induced Biochemical Variations in Secondary Metabolite Production and Antioxidant Activity in Callus Cultures of Stevia Rebaudiana (Bert). J. Photochem. Photobiol. B 2016, 154, 51–56. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of Different Spectral Lights with Biomass Accumulation and Production of Antioxidant Secondary Metabolites in Callus Cultures of Medicinally Important Prunella vulgaris L. J. Photochem. Photobiol. B 2016, 159, 1–7. [Google Scholar] [CrossRef]
- Lai, C.C.; Pan, H.; Zhang, J.; Wang, Q.; Que, Q.X.; Pan, R.; Lai, Z.X.; Lai, G.T. Light Quality Modulates Growth, Triggers Differential Accumulation of Phenolic Compounds, and Changes the Total Antioxidant Capacity in the Red Callus of Vitis davidii. J. Agric. Food Chem. 2022, 70, 13264–13278. [Google Scholar] [CrossRef]
- Hayta, S.; Gurel, A.; Akgun, I.H.; Altan, F.; Ganzera, M.; Tanyolac, B.; Bedir, E. Induction of Gentiana cruciata Hairy Roots and Their Secondary Metabolites. Biologia 2011, 66, 618–625. [Google Scholar] [CrossRef]
- Singh, J.; Sabir, F.; Sangwan, R.S.; Narnoliya, L.K.; Saxena, S.; Sangwan, N.S. Enhanced Secondary Metabolite Production and Pathway Gene Expression by Leaf Explants-Induced Direct Root Morphotypes Are Regulated by Combination of Growth Regulators and Culture Conditions in Centella asiatica (L.) Urban. Plant Growth Regul. 2015, 75, 55–66. [Google Scholar] [CrossRef]
- Azeez, H.; Ibrahim, K.; Pop, R.; Pamfil, D.; Hârţa, M.; Bobiș, O. Changes Induced by Gamma Ray Irradiation on Biomass Production and Secondary Metabolites Accumulation in Hypericum Triquetrifolium Turra Callus Cultures. Ind. Crops Prod. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Karakas, F.P. Efficient Plant Regeneration and Callus Induction from Nodal and Hypocotyl Explants of Goji Berry (Lycium barbarum L.) and Comparison of Phenolic Profiles in Calli Formed under Different Combinations of Plant Growth Regulators. Plant Physiol. Biochem. 2020, 146, 384–391. [Google Scholar] [CrossRef]
- Maharani, A.; Fanata, W.I.D.; Laeli, F.N.; Kim, K.M.; Handoyo, T. Callus Induction and Regeneration from Anther Cultures of Indonesian Indica Black Rice Cultivar. J. Crop Sci. Biotechnol. 2020, 23, 21–28. [Google Scholar] [CrossRef]
- Germanà, M.A. Anther Culture for Haploid and Doubled Haploid Production. Plant Cell Tissue Organ Cult. 2011, 104, 283–300. [Google Scholar] [CrossRef]
- Bhatia, R.; Dey, S.S.; Sood, S.; Sharma, K.; Sharma, V.K.; Parkash, C.; Kumar, R. Optimizing Protocol for Efficient Microspore Embryogenesis and Doubled Haploid Development in Different Maturity Groups of Cauliflower (B. oleracea Var. Botrytis L.) in India. Euphytica 2016, 212, 439–454. [Google Scholar] [CrossRef]
- Kumar, K.R.; Singh, K.P.; Bhatia, R.; Raju, D.V.S.; Panwar, S. Optimising Protocol for Successful Development of Haploids in Marigold (Tagetes spp.) through in Vitro Androgenesis. Plant Cell Tissue Organ Cult. 2019, 138, 11–28. [Google Scholar] [CrossRef]
- Alan, A.R.; Celebi-Toprak, F.; Lachin, A.; Yildiz, D.; Gozen, V.; Besirli, G. Doubled Haploid Broccoli (Brassica olearacea Var. Italica) Plants from Anther Culture. In Doubled Haploid Technology: Volume 2: Hot Topics, Apiaceae, Brassicaceae, Solanaceae; Segui-Simarro, J.M., Ed.; Springer US: New York, NY, USA, 2021; pp. 201–216. [Google Scholar] [CrossRef]
- Baliyan, N.; Srivastava, A.; Rao, M.; Mishra, A.K.; Bharti, H.; Khar, A.; Mangal, M. Correlation of Stages of Microsporogenesis with Bud and Anther Morphology in Pepper Genotypes through DAPI Staining with Different Levels of Mordant in Cytological fixative. Protoplasma 2024, 261, 367–376. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, X.; Wang, Y.; Feng, H.; Ren, X.; Liu, H.; Liu, W. Carbohydrate Metabolism and Gene Regulation during Anther Development in an Androdioecious Tree, Tapiscia sinensis. Ann. Bot. 2017, 120, 967–977. [Google Scholar] [CrossRef]
- Yuliani, F.; Dewi, W.S.; Yunus, A.; Siswanto, U. The study of artemisinin content in callus Artemisia annua L. cultures elicited with endophytic Fungi aspergillus sp. Molecules 2018, 13, 155. [Google Scholar] [CrossRef]
- Zayova, E.; Nedev, T.; Petrova, D.; Zhiponova, M.; Kapchina, V.; Chaneva, G. Tissue Culture Applications of Artemisia annua L. Callus for Indirect Organogenesis and Production Phytochemical. Plant Tissue Cult. Biotechnol. 2020, 30, 97–106. [Google Scholar] [CrossRef]
- Iiyama, C.M.; Sartoratto, A.; Cardoso, J.C. Tissue-Specific and Novel Secondary Metabolites Identified by SPME/GC-MS in Artemisia annua Calli Cultured under Different Wavelength. Ind. Crops Prod. 2024, 208, 117767. [Google Scholar] [CrossRef]
- Wu, S.; Zu, Y.; Wu, M. High Yield Production of Salidroside in the Suspension Culture of Rhodiola sachalinensis. J. Biotechnol. 2003, 106, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.; Naik, N.; Ngangkham, U.; Verma, R.L.; Katara, J.L.; Singh, O.N.; Samantaray, S. Doubled Haploids Generated through Anther Culture from an Elite Long Duration Rice Hybrid, CRHR32: Method Optimization and Molecular Characterization. Plant Biotechnol. 2016, 33, 177–186. [Google Scholar] [CrossRef]
- Jamil, S.Z.M.R.; Rohani, E.R.; Baharum, S.N.; Noor, N.M. Metabolite Profiles of Callus and Cell Suspension Cultures of Mangosteen. 3 Biotech. 2018, 8, 322. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Jeong, B.R. Callus Induction and Browning Suppression in Tree Peony Paeonia ostii ‘Fengdan’. Hortic. Environ. Biotechnol. 2020, 61, 591–600. [Google Scholar] [CrossRef]
- Dai, L.; Han, S.; Zhang, Y.; Hao, D. Genetic Architecture of Embryogenic Callus Induction in Maize from the Perspective of Population Genomics. Plant Cell Tissue Organ Cult. 2022, 150, 345–359. [Google Scholar] [CrossRef]
- Binte Mostafiz, S.; Wagiran, A. Efficient Callus Induction and Regeneration in Selected Indica Rice. Agronomy 2018, 8, 77. [Google Scholar] [CrossRef]
- Corral-Martínez, P.; Siemons, C.; Horstman, A.; Angenent, G.C.; de Ruijter, N.; Boutilier, K. Live Imaging of Embryogenic Structures in Brassica Napus Microspore Embryo Cultures Highlights the Developmental Plasticity of Induced Totipotent Cells. Plant Reprod. 2020, 33, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, A.; Qin, M.; Qin, X.; Yang, S.; Su, S.; Sun, Y.; Zhang, L. Direct and Indirect Somatic Embryogenesis Induction in Camellia oleifera Abel. Front. Plant Sci. 2021, 12, 644389. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, B.; Ma, D.; Guo, S.; Liao, F.; Li, Z. Study on the Induction of Somatic Embryogenesis and Morphological Structural Changes during the Development of Handeliodendron bodinieri L. Plant Cell Tissue Organ Cult. 2024, 156, 62. [Google Scholar] [CrossRef]
- Kong, E.Y.Y.; Biddle, J.; Kalaipandian, S.; Adkins, S.W. Coconut Callus Initiation for Cell Suspension Culture. Plants 2023, 12, 968. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, D.; Cui, X.; Li, M.; Jin, L.; Paré, P.W.; Li, M.; Wei, J. In Vitro Bioactive Metabolite Production and Plant Regeneration of Medicinal Plant Angelica sinensis. Ind. Crops Prod. 2023, 194, 116276. [Google Scholar] [CrossRef]
- Fang, H.; Dong, Y.; Zhou, R.; Wang, Q.; Duan, Q.; Wang, C.; Bao, Y.; Xu, S.; Lang, X.; Gai, S.; et al. Optimization of the Induction, Germination, and Plant Regeneration System for Somatic Embryos in Apomictic Walnut (Juglans regia L.). Plant Cell Tissue Organ Cult. 2022, 150, 289–297. [Google Scholar] [CrossRef]
- Zhao, X.; Lian, Y.; Jin, Z.; Zhang, X.; Yan, Y.; Fan, S. Shoot Organogenesis and Somatic Embryogenesis in Leaf Tissue of Pulsatilla tongkangensis Y.N. Lee & T.C. Lee. Plant Biotechnol. Rep. 2022, 16, 389–400. [Google Scholar] [CrossRef]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic Compounds in Antimicrobial Therapy. J. Med. Food 2017, 20, 1031–1038. [Google Scholar] [CrossRef]
- Foss, K.; Przybyłowicz, K.E.; Sawicki, T. Antioxidant Activity and Profile of Phenolic Compounds in Selected Herbal Plants. Plant Foods Hum. Nutr. 2022, 77, 383–389. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Wang, W.; Zhang, L.; Wang, C.; Zhang, Q.; Wang, J.; Du, M.; Sheng, L.; Hu, D. MdCIbHLH1 Modulates Sugar Metabolism and Accumulation in Apple Fruits by Coordinating Carbohydrate Synthesis and Allocation. Hortic. Plant J. 2024, 11, 578–592. [Google Scholar] [CrossRef]
- Julius, B.T.; Slewinski, T.L.; Baker, R.F.; Tzin, V.; Zhou, S.; Bihmidine, S.; Jander, G.; Braun, D.M. Maize Carbohydrate Partitioning Defective1 Impacts Carbohydrate Distribution, Callose Accumulation, and Phloem Function. J. Exp. Bot. 2018, 69, 3917–3931. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of Light Quality on Growth, Secondary Metabolites Production and Antioxidant Activity in Callus Culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B 2018, 183, 258–265. [Google Scholar] [CrossRef]
- Fan, X.; Zang, J.; Xu, Z.; Guo, S.; Jiao, X.; Liu, X.; Gao, Y. Effects of Different Light Quality on Growth, Chlorophyll Concentration and Chlorophyll Biosynthesis Precursors of Non-Heading Chinese Cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Hassanpour, H. Potential Impact of Red-Blue LED Light on Callus Growth, Cell Viability, and Secondary Metabolism of Hyoscyamus reticulatus. In Vitro Cell. Dev. Biol.-Plant. 2022, 58, 256–265. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Li, S.; Chen, X.; Lu, C. Comparative Analysis of Phenolic Compound Profiles, Antioxidant Capacities, and Expressions of Phenolic Biosynthesis-Related Genes in Soybean Microgreens Grown under Different Light Spectra. J. Agric. Food Chem. 2019, 67, 13577–13588. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Nazir, M.; Ullah, M.A.; Younas, M.; Siddiquah, A.; Shah, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Light-Mediated Biosynthesis of Phenylpropanoid Metabolites and Antioxidant Potential in Callus Cultures of Purple Basil (Ocimum basilicum L. Var Purpurascens). Plant Cell Tissue Organ Cult. 2020, 142, 107–120. [Google Scholar] [CrossRef]
- Biswal, B.; Jena, B.; Giri, A.K.; Acharya, L. Monochromatic Light Elicited Biomass Accumulation, Antioxidant Activity, and Secondary Metabolite Production in Callus Culture of Operculina turpethum (L.). Plant Cell Tissue Organ Cult. 2022, 149, 123–134. [Google Scholar] [CrossRef]
- Azad, M.O.; Kim, W.W.; Park, C.H.; Cho, D.H. Effect of Artificial LED Light and Far Infrared Irradiation on Phenolic Compound, Isoflavones and Antioxidant Capacity in Soybean (Glycine max L.) Sprout. Foods 2018, 7, 174. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Dai, Z.; Ma, S. Quality Analysis of Long Dan Xie Gan Pill by a Combination of Fingerprint and Multicomponent Quantification with Chemometrics Analysis. J. Anal. Methods Chem. 2018, 2018, 4105092. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Y.; Li, P.; Tang, Z.; Guo, Y.; Wen, L.; Yu, H.; Cui, F.; Hu, F. Study on Environmental Factors Affecting the Quality of Codonopsis Radix Based on MaxEnt Model and All-in-One Functional Factor. Sci. Rep. 2023, 13, 20726. [Google Scholar] [CrossRef]
- Liang, X.; Guo, Z.C.; Wang, L.; Li, R.C.; Lin, W.W. Nearly Optimal Stochastic Approximation for Online Principal Subspace Estimation. Sci. China Math. 2023, 66, 1087–1122. [Google Scholar] [CrossRef]
- Luo, G.; Chen, G.; Tian, L.; Qin, K.; Qian, S.E. Minimum Noise Fraction versus Principal Component Analysis as a Preprocessing Step for Hyperspectral Imagery Denoising. Can. J. Remote Sens. 2016, 42, 106–116. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 2020th ed.; China Medical Science Press: Beijing, China, 2020.
- Senekovič, J.; Ciringer, T.; Ambrožič-Dolinšek, J.; Islamčević Razboršek, M. The Effect of Combined Elicitation with Light and Temperature on the Chlorogenic Acid Content, Total Phenolic Content and Antioxidant Activity of Berula erecta in Tissue Culture. Plants 2024, 13, 1463. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effects of Light Sources on Major Flavonoids and Antioxidant Activity in Common Buckwheat Sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, W.; Xia, J.; Yu, H.; Wang, Q.; Pang, F.; Zhao, M. Metabolomic and Transcriptomic Analyses Reveal That Blue Light Promotes Chlorogenic Acid Synthesis in Strawberry. J. Agric. Food Chem. 2020, 68, 12485–12492. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Fukuda, N.; Yoshida, H.; Fujiuchi, N.; Yano, R.; Kusano, M. Effects of Light Quality, Photoperiod, CO2 Concentration, and Air Temperature on Chlorogenic Acid and Rutin Accumulation in Young Lettuce Plants. Plant Physiol. Biochem. 2022, 186, 290–298. [Google Scholar] [CrossRef]
- Popielarska-Konieczna, M.; Kozieradzka-Kiszkurno, M.; Świerczyńska, J.; Góralski, G.; Ślesak, H.; Bohdanowicz, J. Ultrastructure and Histochemical Analysis of Extracellular Matrix Surface Network in Kiwifruit Endosperm-Derived Callus Culture. Plant Cell Rep. 2008, 27, 1137–1145. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Chen, X.; Dong, L.; Zheng, Y.; Wu, M.; Ding, Q.; Xu, S.; Ding, C.; Liu, W. Establishment of Callus Induction System, Histological Evaluation and Taxifolin Production of Larch. Plant Cell Tissue Organ Cult. 2021, 147, 467–475. [Google Scholar] [CrossRef]
- Li, M.; Lv, M.; Yang, D.; Wei, J.; Xing, H.; Paré, P.W. Temperature-Regulated Anatomical and Gene-Expression Changes in Sinopodophyllum hexandrum Seedlings. Ind. Crops Prod. 2020, 152, 112479. [Google Scholar] [CrossRef]
- Liu, X.; Luo, M.; Li, M.; Wei, J. Depicting Precise Temperature and Duration of Vernalization and Inhibiting Early Bolting and Flowering of Angelica sinensis by Freezing Storage. Front. Plant Sci. 2022, 13, 853444. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Lei, D.; Zhou, X.; Wang, H.; Lu, J.; Lin, Y.; Zhang, Y.; Wang, Y.; He, W.; Li, M.; et al. Effect of Different Culture Conditions on Anthocyanins and Related Genes in Red Pear Callus. Agronomy 2023, 13, 2032. [Google Scholar] [CrossRef]
- Lay, M.M.; Karsani, S.A.; Mohajer, S.; Abd Malek, S.N. Phytochemical Constituents, Nutritional Values, Phenolics, Flavonols, Flavonoids, Antioxidant and Cytotoxicity Studies on Phaleria macrocarpa (Scheff.) Boerl Fruits. BMC Complement. Altern. Med. 2014, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, J.; Cai, S.; Chen, X.; Quan, X.; Zhang, G. Association Mapping for Total Polyphenol Content, Total Flavonoid Content and Antioxidant Activity in Barley. BMC Genom. 2018, 19, 81. [Google Scholar] [CrossRef]
- Villamarin-Raad, D.A.; Lozano-Puentes, H.S.; Chitiva, L.C.; Costa, G.M.; Díaz-Gallo, S.A.; Díaz-Ariza, L.A. Changes in Phenolic Profile and Total Phenol and Total Flavonoid Contents of Guadua Angustifolia kunth Plants under Organic and Conventional Fertilization. ACS Omega 2023, 8, 41223–41231. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A Method for Routine Measurements of Total Sugar and Starch Content in Woody Plant Tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Jha, D.K.; Panda, L.; Ramaiah, S.; Anbarasu, A. Evaluation and Comparison of Radical Scavenging Properties of Solvent Extracts from Justicia Adhatoda Leaf Using DPPH Assay. Appl. Biochem. Biotechnol. 2014, 174, 2413–2425. [Google Scholar] [CrossRef]
- Sethi, S.; Joshi, A.; Arora, B.; Bhowmik, A.; Sharma, R.R.; Kumar, P. Significance of FRAP, DPPH, and CUPRAC Assays for Antioxidant Activity Determination in Apple Fruit Extracts. Eur. Food Res. Technol. 2020, 246, 591–598. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Cheng, J.; Guo, F.; Wang, L.; Li, Z.; Zhou, C.; Wang, H.; Liang, W.; Jiang, X.; Chen, Y.; Dong, P. Evaluating the impact of ecological factors on the quality and habitat distribution of Lonicera japonica Flos using HPLC and the MaxEnt model. Front. Plant Sci. 2024, 15, 1397939. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liang, T.; Fu, Q.; Xiao, Y.S.; Feng, J.T.; Ke, Y.X.; Liang, X.M. Fingerprint analysis of Ligusticum chuanxiong using hydrophilic interaction chromatography and reversed-phase liquid chromatography. J. Chromatogr. A 2009, 1216, 2136–2141. [Google Scholar] [CrossRef] [PubMed]






| Stage | Induction Rate (%) | Browning Rate (%) | Contamination Rate (%) |
|---|---|---|---|
| Tetrad stage | 13 ± 0.40 c | 72 ± 0.29 a | 23 ± 0.84 a |
| Early uninucleate stage | 38 ± 0.70 b | 52 ± 0.89 b | 10 ± 0.37 ab |
| Late-uninucleate stage | 78 ± 0.29 a | 15 ± 0.27 c | 7 ± 0.30 b |
| Binucleate stage | 12 ± 0.25 c | 85 ± 0.31 a | 3 ± 0.15 b |
| Test No. | A(6-BA) mg·L−1 | B(NAA) mg·L−1 | C(2,4-D) mg·L−1 | D(KT) mg·L−1 | Induction Rate (%) | Callus Characteristics |
|---|---|---|---|---|---|---|
| I | 1(0.5) | 1(0.5) | 1(0.5) | 1(0.2) | 17 ± 0.30 e | Yellowish-white, watery |
| II | 1(0.5) | 2(1.0) | 3(1.5) | 2(0.5) | 49 ± 0.38 c | Light brown, loose |
| III | 1(0.5) | 3(1.5) | 2(1.0) | 3(0.8) | 69 ± 0.38 b | green, slightly brown, less friable |
| IV | 2(1.0) | 1(0.5) | 3(1.5) | 3(0.8) | 31 ± 0.31 d | Yellow-white, loose |
| V | 2(1.0) | 2(1.0) | 2(1.0) | 1(0.2) | 64 ± 0.34 b | White-green, slightly brown, compact |
| VI | 2(1.0) | 3(1.5) | 1(0.5) | 2(0.5) | 81 ± 0.23 a | Yellow, friable |
| VII | 3(1.5) | 1(0.5) | 2(1.0) | 2(0.5) | 9 ± 0.23 e | White-brown, slightly watery |
| VIII | 3(1.5) | 2(1.0) | 1(0.5) | 3(0.8) | 18 ± 0.25 e | Yellowish-white, slightly watery |
| IX | 3(1.5) | 3(1.5) | 3(1.5) | 1(0.2) | 70 ± 0.33 b | White-green, compact |
| Analytical Method | A(6-BA) mg·L−1 | B(NAA) mg·L−1 | C(2,4-D) mg·L−1 | D(KT) mg·L−1 |
|---|---|---|---|---|
| ANOVA analysis | ||||
| Df | 2 | 2 | 2 | 2 |
| MS | 0.52 | 2.22 | 0.105 | 0.093 |
| p value | 0.0001 ** | 0.0001 ** | 0.0001 ** | 0.0001 ** |
| Range analysis | ||||
| k1 | 0.45 | 0.19 | 0.39 | 0.50 |
| k2 | 0.59 | 0.44 | 0.47 | 0.46 |
| k3 | 0.32 | 0.73 | 0.50 | 0.39 |
| Rk | 0.27 | 0.54 | 0.11 | 0.11 |
| Best level | A2 | B3 | C3 | D1 |
| NO. | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1 | ||||||||||||
| S2 | 0.976 | 1 | |||||||||||
| S3 | 0.984 | 0.982 | 1 | ||||||||||
| S4 | 0.983 | 0.987 | 0.995 | 1 | |||||||||
| S5 | 0.935 | 0.907 | 0.896 | 0.919 | 1 | ||||||||
| S6 | 0.972 | 0.929 | 0.962 | 0.955 | 0.939 | 1 | |||||||
| S7 | 0.843 | 0.805 | 0.808 | 0.833 | 0.928 | 0.856 | 1 | ||||||
| S8 | 0.956 | 0.985 | 0.975 | 0.977 | 0.856 | 0.897 | 0.755 | 1 | |||||
| S9 | 0.956 | 0.948 | 0.93 | 0.946 | 0.922 | 0.896 | 0.776 | 0.936 | 1 | ||||
| S10 | 0.934 | 0.969 | 0.955 | 0.953 | 0.803 | 0.859 | 0.722 | 0.992 | 0.902 | 1 | |||
| S11 | 0.939 | 0.972 | 0.958 | 0.959 | 0.822 | 0.868 | 0.723 | 0.996 | 0.92 | 0.997 | 1 | ||
| S12 | 0.843 | 0.77 | 0.822 | 0.805 | 0.861 | 0.913 | 0.847 | 0.704 | 0.721 | 0.658 | 0.653 | 1 | |
| R | 0.991 | 0.975 | 0.983 | 0.987 | 0.959 | 0.979 | 0.887 | 0.95 | 0.943 | 0.921 | 0.927 | 0.872 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Guo, F.; Liang, W.; Wang, H.; Chen, Y.; Dong, P. Callus Culture System from Lonicera japonica Thunb Anthers: Light Quality Effects on Callus Quality Evaluation. Int. J. Mol. Sci. 2025, 26, 2351. https://doi.org/10.3390/ijms26052351
Cheng J, Guo F, Liang W, Wang H, Chen Y, Dong P. Callus Culture System from Lonicera japonica Thunb Anthers: Light Quality Effects on Callus Quality Evaluation. International Journal of Molecular Sciences. 2025; 26(5):2351. https://doi.org/10.3390/ijms26052351
Chicago/Turabian StyleCheng, Jiali, Fengxia Guo, Wei Liang, Hongyan Wang, Yuan Chen, and Pengbin Dong. 2025. "Callus Culture System from Lonicera japonica Thunb Anthers: Light Quality Effects on Callus Quality Evaluation" International Journal of Molecular Sciences 26, no. 5: 2351. https://doi.org/10.3390/ijms26052351
APA StyleCheng, J., Guo, F., Liang, W., Wang, H., Chen, Y., & Dong, P. (2025). Callus Culture System from Lonicera japonica Thunb Anthers: Light Quality Effects on Callus Quality Evaluation. International Journal of Molecular Sciences, 26(5), 2351. https://doi.org/10.3390/ijms26052351






