Anti-Bacterial and Anti-Inflammatory Properties of Sophoridine and Its Effect on Diarrhea in Mice
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activity of Sophoridine
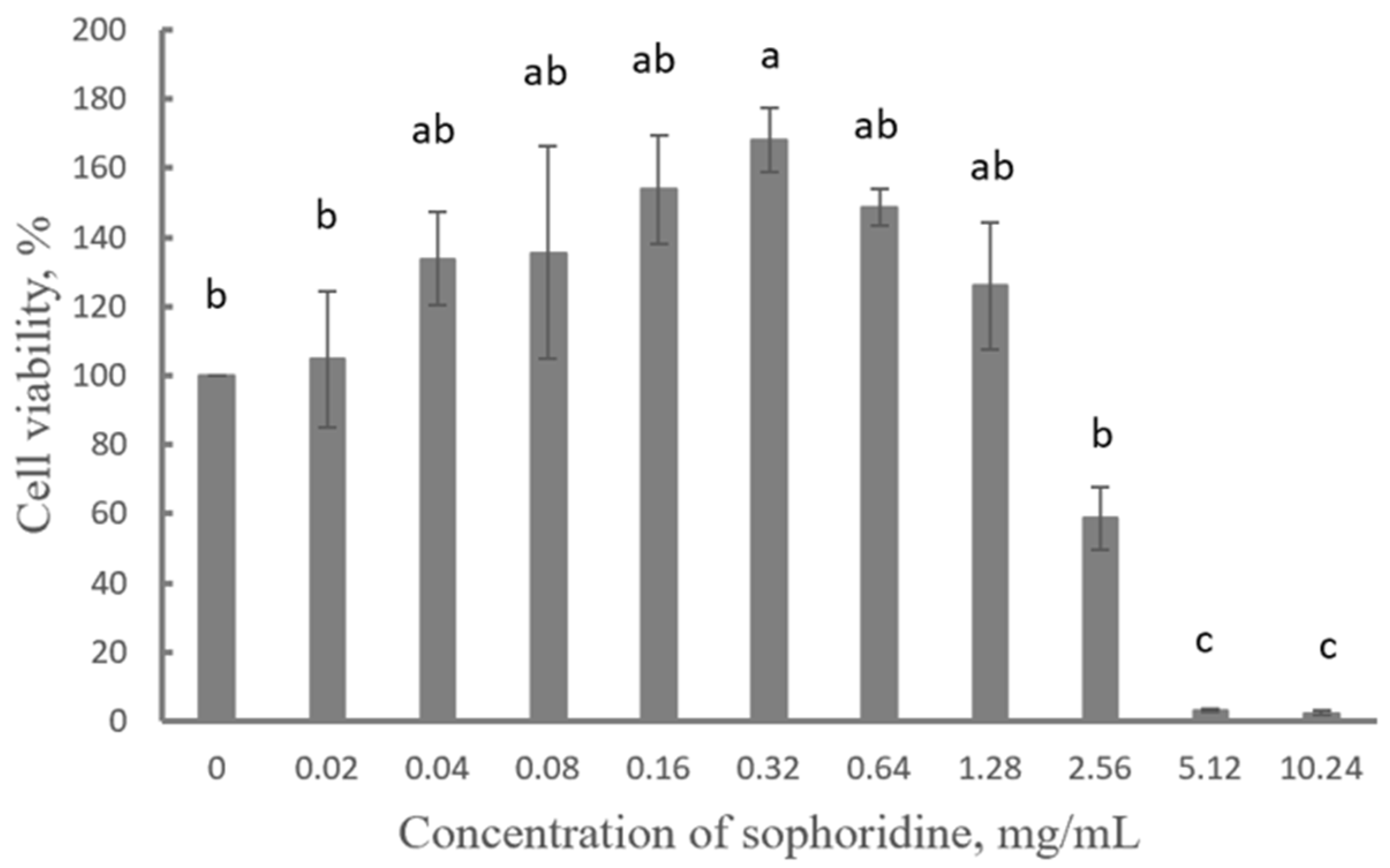
2.2. Cytotoxicity of Sophoridine
2.3. Effect of Sophoridine on NO Release
2.4. Fecal Occult Blood Score
2.5. Serum Inflammatory Cytokines of Mice
2.6. Pathological Changes in Mouse Duodenum
2.7. Effect of Sophoridine on mRNA Expression of NF-κB p65
2.8. Effect of Sophoridine on Protein Expression of NF-κB p65 and Phosphorylated NF-κB p65
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs, Cells, and Animals
4.2. Anti-Microbial Activity of Sophoridine
4.3. Cytotoxicity Evaluation of Sophoridine
4.4. Measurement of Extracellular NO in RAW264.7 Cells Challenged by LPS
4.5. Trial in Mice
4.5.1. Administration
4.5.2. Fecal Occult Blood Detection
4.5.3. Serum Inflammatory Cytokines Analysis
4.5.4. Histological Change in the Duodenum
4.5.5. Expression of NF-κB p65 Assayed by RT-PCR and Western Blot
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LPS | lipopolysaccharide |
| NF-κB | nuclear factor kappa-B |
| TNF-α | tumor necrosis factor-α |
| IL | interleukin |
| E. coli | Escherichia coli |
| S. typhimurium | Salmonella typhimurium |
| S. enteritidis | Salmonella enteritidis |
| S. aureus | Staphylococcus aureus |
References
- GBD Diarrhoeal Diseases Collaborators. Estimates of Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoeal Diseases: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut Microbiota and Diarrhea: An Updated Review. Front. Cell. Infect. Microbiol. 2021, 11, 625210. [Google Scholar] [CrossRef]
- Holland, R. Some Infectious Causes of Diarrhea in Young Farm Animals. Clin. Microbiol. Rev. 1990, 3, 345–375. [Google Scholar] [CrossRef] [PubMed]
- Heuer, C.; Healy, A.; Zerbinii, C. Economic Effects of Exposure to Bovine Viral Diarrhea Virus on Dairy Herds in New Zealand. J. Dairy. Sci. 2007, 90, 5428–5438. [Google Scholar] [CrossRef] [PubMed]
- Mariano, V.; Nardi, A.; Moruzzo, R.; Di Iacovo, F.; Rossignoli, C. In-Farm Cost of an Outbreak of Diarrhoea in Lambs. Small. Ruminant. Res. 2018, 166, 17–21. [Google Scholar] [CrossRef]
- Bäumler, A.; Sperandio, V. Interactions between The Microbiota and Pathogenic Bacteria in the Gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Alvarez-Martinez, F.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An Overview of Their Antibacterial, Antibiotic-Enhancing and Antivirulence Activities. Int. J. Antimicrob. Agent. 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Zacchino, S.A.; Butassi, E.; Liberto, M.D.; Raimondi, M.; Postigo, A.; Sortino, M. Plant Phenolics and Terpenoids as Adjuvants of Antibacterial and Antifungal Drugs. Phytomedicine 2017, 37, 27–28. [Google Scholar] [CrossRef]
- Allegra, M. Antioxidant and Anti-Inflammatory Properties of Plants Extract. Antioxidants 2019, 8, 549. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- dos Reis Nunes, C.; Arantes, M.B.; de Faria Pereira, S.M.; da Crus, L.L.; de Souza Passos, M.; de Moraes, L.P.; Vieira, I.J.C.; de Oliveira, D.B. Plants as Sources of Anti-Inflammatory Agents. Moleculars 2020, 25, 3726. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Liu, Y.; Su, Z.; Liu, Y. Plant Polypeptides: A Review on Extraction, Isolation, Bioactivities and Prospects. International J. Biolog. Macromol. 2022, 207, 169–178. [Google Scholar] [CrossRef]
- Hilal, B.; Khan, M.M.; Fariduddin, Q. Recent Advancements in Seciphering the Therapeutic Properties of Plant Scondary Metabolites: Phenolics, Terpenes, and Alkaloids. Plant Physiol. Biochem. 2024, 211, 108674. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.G.; Zhao, X.L.; Xu, W.C.; Zhao, X.J.; Wang, J.N.; Lin, X.W.; Sun, T.; Fu, Z.J. Activation of Spinal NK-κB/p65 Contributes to Peripheral Inflammation and Hyperalgesia in Rat Adjuvant-Induced Arthritis. Arthritis Rheumatol. 2014, 66, 896–906. [Google Scholar] [CrossRef]
- Shen, Y.; Teng, L.; Qu, Y.; Liu, J.; Zhu, X.; Chen, S.; Yang, L.; Huang, Y.; Song, Q.; Fu, Q. Anti-Proliferation and Anti-inflammation Effects of Corilagin in Rheumatoid Arthritis by Downregulating NF-κB and MAPK Signaling Pathways. J. Ethnopharmacol. 2022, 284, 114791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, P.Q.; Shen, H. Protective Effects of Berberine Hydrochloride on DSS-induced Ulcerative Colitis in Rats. Int. Immunopharmacol. 2019, 68, 242–251. [Google Scholar] [CrossRef]
- Mockenhaupt, K.; Gonsiewski, A.; Kordula, T. RelB and Neuroinflammation. Cells 2021, 10, 1609. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Yu, S.; Li, C.; Gao, B.; Zhou, X. Cannabidiol Attenuates Periodontal Inflammation through Inhibiting TLR4/NF-κB Pathway. J Periodont Res. 2023, 58, 697–707. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Li, H.; Fan, J.; Shen, J.; Yu, X.; Zhou, Y.; Mao, H. ATG5-Mediated Autophagy Suppresses NF-κB Signaling to Limit Epithelial Inflammatory Response to Kidney Injury. Cell Death Dis. 2019, 10, 253. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Sun, Y.; Yang, C.; Yue, Y. NF-κB Signaling Pathway Mechanism in Cow Intertoe Skin Inflammation Caused by Fusobacterium necrophorum. Front. Cell. Infect. Microbiol. 2023, 13, 1156449. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, H.; Row, K. Simultaneous Quantification of Multiple Alkaloids in Sophora Flavescens Ait and Human Urine by HPLC. Biotechnol. Bioproc. E 2009, 14, 675–679. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Liu, H.; Zhang, G.; Wu, X. Preparation and in Vitro Evaluation of Ethosomal Total Alkaloids of Sophora alopecuroides Loaded by a Transmembrane pH-Gradient Method. AAPS PharmSciTech. 2010, 11, 1350–1358. [Google Scholar] [CrossRef]
- Zhao, X.; Mei, L.; Pei, J.; Liu, Z.; Shao, Y.; Tao, Y.; Zhang, X.; Jiang, L. Sophoridine from Sophora Flower Attenuates Ovariectomy Induced Osteoporosis through the RANKL-ERK-NFAT Pathway. J. Agric. Food Chem. 2017, 65, 9647–9654. [Google Scholar] [CrossRef]
- Ur Rashid, H.; Rasool, S.; Ali, Y.; Khan, K.; Utrera Martines, M.A. Anti-cancer Potential of Sophoridine and its Derivatives: Recent Progress and Future Perspectives. Bioorganic Chem. 2020, 99, 103863. [Google Scholar] [CrossRef]
- Wang, B.; Xu, J.; Wang, H.; Chang, S.; Liu, N. Effect and Mechanism of Sophoridine to Suppress Hepatocellular Carcinoma in Vitro and Vivo. Bomed. Pharmacother. 2017, 95, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, H.; Shao, Y.; Wang, K.; Yin, S.; Qiu, Y.; Wu, H.; Liu, E.; Wang, T.; Gao, X.; et al. Sophoridine Inhibits Human Colorectal Cancer Progression via Targeting MAPKAPK2. Mol. Cancer Res. 2019, 17, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Guan, Q.; Luo, J.; Deng, W.; Liu, J.; Yan, R.; Wang, W. Sophoridine Exerts Tumor-Suppressive Activities via Promoting ESRRG-Mediated Beta-Catenin Degradation in Gastric Cancer. BMC Cancer 2020, 20, 582. [Google Scholar] [CrossRef]
- Zhao, B.; Hui, X.; Zeng, H.; Yin, Y.; Huang, J.; Tang, Q.; Ge, G.; Lei, T. Sophoridine Inhibits the Tumour Growth of Non-Small Lung Cancer by Inducing Macrophages M1 Polarisation via MAPK-mediated Inflammatory Pathway. Front. Oncol. 2021, 11, 634851. [Google Scholar] [CrossRef]
- Huang, X.; Li, B.; Shen, L. Studies on the Anti-Inflammatory Effect and its Mechanisms of Sophoridine. J. Anal. Methods Chem. 2014, 2014, 502626. [Google Scholar] [CrossRef]
- Ren, G.; Ding, G.; Zhang, H.; Wang, H.; Jin, Z.; Yang, G.; Han, Y.; Zhang, X.; Li, G.; Li, W. Antiviral Activity of Sophoridine Against Enterovirus 71 in Vitro. J. Ethnopharm. 2019, 236, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhao, B.; Ju, Y.; Dai, L. Inhibitory Effect of Bis (Piperidine) Alkaloids on Five Environmental Bacterial Strains. J. Nanjing For. Univ. 2001, 81, 81–84. [Google Scholar]
- Cely-Veloza, W.; Kato, M.J.; Coy-Barrera, E. Quinolizdine-type Alkaloids: Chemodiversity, Occurrence, and Bioactivity. ACS. Omega 2019, 8, 27862–27893. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, P.; Mao, Y.; Wang, G. Research Advancements in Pharmacological Activities and Mechanisms of Matrine. Pharmacogn. Mag. 2023, 20, 189–205. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.; Hong, J.; Liu, C.; Zhang, X.; Zheng, J.; Xu, Y.; Ou, Z.; Zheng, J.; Yu, D. Inhibitory Effect of Two Traditional Chinese Medicine Monomers, Berberine and Matrine, on the Quorum Sensing System of Antimicrobial-Resistant Escherichia coli. Front. Microbiol. 2019, 10, 2584. [Google Scholar] [CrossRef]
- Fan, Q.; Wu, J.; Xi, B.; Li, C.; Wang, X.; Li, H. A Screening Model of Antibacterial Agents Based on Escherichia coli Cell-Division Protein. Appl. Sci. 2023, 13, 4493. [Google Scholar] [CrossRef]
- Qiu, M.; Shi, F.; Dai, F.; Song, R.; Wang, S.; You, Y.; Zhao, B. A Reactive Oxygen Species Activation Mechanism Contributes to Sophoridine-induced Apoptosis in Rat Liver BRL-3A cells. J. Ethnopharmacol. 2018, 213, 376–383. [Google Scholar] [CrossRef]
- Yue, Z.; Si, T.; Pan, Z.; Cao, W.; Yan, Z.; Jiang, Z.; Ouyang, H. Sophoridine Suppresses Cell Growth in Human Medulloblastoma through FoxM1, NF-κB and AP-1. Oncol. Lett. 2017, 14, 7941–7946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, D.; Wu, F.; Tu, J.; Song, J.; Xu, M.; Ji, J. Sophoridine Suppresses Lenvatinib-Resistant Hepatocellular Carcinoma Growth by Inhibiting RAS/MEK/ERK Axis via Decreasing VEGFR2 Expression. J. Cell Mol. Med. 2020, 25, 549–560. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, F.; Bai, C.; Yao, C.; Zhong, H.; Zou, C.; Chen, X. Sophoridine Induces Apoptosis and S Phase Arrest via ROS-dependent JNK and ERK Activation in Human Pancreatic Cancer Cells. J. Exp. Clin. Cancer Res. 2017, 36, 124. [Google Scholar] [CrossRef]
- Coleman, J. Nitric Oxide in Immunity and Inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of Nitric Oxide in Inflammation Disease. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Minhas, R.; Bansal, Y.; Bansal, G. Inducible Nitric Oxide Synthase Inhibitors: A Comprehensive Update. Med. Res. Rev. 2020, 40, 823–855. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Roth, J. Monitoring Disease Activity by Stool Analyses: From Occult Blood to Molecular Markers of Intestinal Inflammation and Damage. Gut 2020, 58, 859–868. [Google Scholar] [CrossRef]
- Lima, T.; Silva, O.; Silva, L.; Rocha, T.; Grossi-de-sá, M.; Franco, O.; Leonardecz, E. In Vivo Effects of Cagaita (Eugenia dysenterica, DC.) Leaf Extracts on Diarrhea Treatment. Evid-Based Compl. Alt. 2011, 309390. [Google Scholar] [CrossRef] [PubMed]
- Orsi, P.; Seito, L.; Di Stasi, L. Hymenaea stigonocarpa Mart. ex Hayne: A Tropical Medicinal Plant with Intestinal Anti-Inflammatory Activity in TNBS Model of Intestinal Inflammation in Rats. J. Ethnopharmacol. 2014, 151, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, Y.; Peng, J.; Chen, W.; Lu, L.; Lin, J. Qingjie Fuzheng Granule Attenuates 5-Fluorouracil-Induced Intestinal Mucosal Damage. Biomed. Pharmacother. 2019, 118, 109223. [Google Scholar] [CrossRef]
- Beukema, M.; Ishisono, K.; de Waard, J.; Faas, M.; de Vos, P.; Kitaguchi, K. Pectin Limits Epithelial Barrier Disruption by Citrobacter Rodentium through Anti-Microbial Effects. Food Funct. 2021, 12, 881–891. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, L.; Zhao, Q.; Suo, X.; Hao, Z. Matrine Provides a Protective Effect Against Eimeria Tenella Challenge by Alleviating Intestinal Barrier Damage. Vet. Parasitol. 2023, 319, 109940. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, Y.; Xiao, Z.; Hu, Z.; Zhang, J. Sophocarpine and Matrine Inhibit the Production of Tnf-A and Il-6 in Murine Macrophages and Prevent Cachexia-Related Symptoms Induced by Colon26 Adenocarcinoma in Mice. Int. Immunopharmacol. 2008, 8, 1767–1772. [Google Scholar] [CrossRef]
- Sahoo, M.; Ceballos-Olvera, I.; del Barrio, L.; Re, F. Role of the Inflammasome, IL-1β, and IL-18 in Bacterial Infections. Sci. World J. 2011, 11, 2027–2050. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The Role of Interleukin-1 in General Pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Shouval, D.; Ouahed, J.; Biswas, A.; Goettel, J.; Horwitz, C.; Muise, A.; Snapper, S. Interleukin 10 Receptor Signaling: Master Regulator of Intestinal Mucosal Homeostasis in Mice and Humans. Adv. Immunol. 2014, 122, 177–210. [Google Scholar] [PubMed]
- Morhardt, T.; Hayashi, A.; Ochi, T.; Ouiros, M.; Kitamoto, S.; Nagao-kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Kar, J.; et al. IL-10 Produced by Macrophages Regulates Epithelial Integrity in the Small Intestine. Sci. Rep. 2019, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Ochi, T.; Feng, Y.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Teitelbaum, D.; Kamada, N. Diet-dependent, microbiota-independent regulation of IL-10-producing lamina propria macrophages in the small intestine. Sci. Rep. 2016, 6, 27634. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, J.; Tang, Y.; Peng, Q.; Zhang, L.; Ma, X.; Xu, N.; Wei, J.; Han, H. Sophoridine Inhibits Endotoxin-Induced Acute Lung Injury by Enhancing Autophagy of Macrophage and Reducing Inflammation. J. Leucocyte. Biol. 2022, 112, 115–125. [Google Scholar] [CrossRef]
- Luster, A.; Alon, R.; Andrian, U. Immune Cell Migration in Inflammation: Present and Future Therapeutic Targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Alon, R. Leukocyte Migration into Inflamed Tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Huang, L.; Xie, J.N.; Liang, J.P.; Li, Y.H.; Zhou, Y. Sophoridine Inhibits NF-kappaB Signaling Pathway Activation in Kidney Tissue of Endotoxemia Mice (Abstract). Yao Xue Xue Bao 2011, 46, 1072–1077. [Google Scholar]
- Zhu, J.; Wan, H.; Xiong, Q. Antioxidant Effect of Sophoridine on Lung Injury Induced by Lipopolysaccharide in Mice and Its Influence on NF-KB Expression. Chin. J. Exp. Tradit. Med. Formulae 2011, 20, 192–196. [Google Scholar]
- Ren, L.; Li, X.; Jing, S. Effect of Sophoridine on Proliferation and Signaling Transduction of NF-κB in Human Pancreatic Cancer Capan-1 Cells. Chin. Hosp. Pharm. J. 2017, 37, 1576–1579. [Google Scholar]
- Li, Y.; Chen, L.; Pu, R.; Zhou, L.; Zhou, X.; Li, X. Effects of a Matrine and Sophoridine Containing Herbal Compound Medicine (AH-05) on Liver Cancer. Nat. Prod. Commun. 2020, 7, 15. [Google Scholar] [CrossRef]
- Milanovic, M.; Kracht, M.; Schmitz, L. The Cytokine-Induced Conformational Switch of Nuclear Factor κB p65 is Mediated by p65 Phosphorylation. Biochem. J. 2014, 457, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ma, L.; Chen, T.; Wang, J. Sophorolipid Suppresses LPS-induced Inflammation in RAW264.7 Cells Through the NF-κB Signaling Pathway. Molecules 2022, 27, 5037. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Protocol 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Riss, T.; Moravec, R.; Niles, A.; Duellman, S.; Benink, H.; Worzella, T.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Neri, B.; Mossa, M.; Scucchi, L.; Sena, G.; Palmieri, G.; Biancone, L. Histological Scores in Inflammatory Bowel Disease. J. Dig. Dis. 2021, 22, 9–22. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 22−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kurien, B.; Scofield, R. Western Blotting. Methods 2006, 38, 283–293. [Google Scholar] [CrossRef]





| Item | E. coli CACC1515 | E. coli CVCC195 | S. typhimurium ATCC14028 | S. enteritidis CVCC3377 | S. aureus ATCC43300 |
|---|---|---|---|---|---|
| MIC, mg/mL | 5.12 | 5.12 | 10.24 | 5.12 | 10.24 |
| MBC, mg/mL | 10.24 | 10.24 | >10.24 | 10.24 | >10.24 |
| C | M | P | LS | MS | HS | |
|---|---|---|---|---|---|---|
| d 1 | - | ++ | + | + | ++ | ++ |
| d 2 | - | ++ | ++ | ++ | ++ | ++ |
| d 3 | - | +++ | +++ | +++ | +++ | +++ |
| d 4 | - | +++ | + | ++ | ++ | ++ |
| d 5 | - | ++ | - | ++ | + | - |
| d 6 | - | ++ | - | - | - | - |
| Group | Inflammatory Cell Infiltration | Epithelial Change | Villous Integration | Total Score |
|---|---|---|---|---|
| C | 0 | 0 | 0 | 0 |
| M | 3 | 1 | 1 | 5 |
| P | 0 | 0 | 0 | 0 |
| HS | 1 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Tao, H.; Fan, Q.; Wang, Z.; Han, B.; Wang, X.; Wang, J. Anti-Bacterial and Anti-Inflammatory Properties of Sophoridine and Its Effect on Diarrhea in Mice. Int. J. Mol. Sci. 2025, 26, 2122. https://doi.org/10.3390/ijms26052122
Wang J, Tao H, Fan Q, Wang Z, Han B, Wang X, Wang J. Anti-Bacterial and Anti-Inflammatory Properties of Sophoridine and Its Effect on Diarrhea in Mice. International Journal of Molecular Sciences. 2025; 26(5):2122. https://doi.org/10.3390/ijms26052122
Chicago/Turabian StyleWang, Jiaxue, Hui Tao, Qiuyu Fan, Zhenlong Wang, Bing Han, Xiumin Wang, and Jingquan Wang. 2025. "Anti-Bacterial and Anti-Inflammatory Properties of Sophoridine and Its Effect on Diarrhea in Mice" International Journal of Molecular Sciences 26, no. 5: 2122. https://doi.org/10.3390/ijms26052122
APA StyleWang, J., Tao, H., Fan, Q., Wang, Z., Han, B., Wang, X., & Wang, J. (2025). Anti-Bacterial and Anti-Inflammatory Properties of Sophoridine and Its Effect on Diarrhea in Mice. International Journal of Molecular Sciences, 26(5), 2122. https://doi.org/10.3390/ijms26052122








