Enhanced Anti-Cancer Potential: Investigating the Combined Effects with Coriolus versicolor Extract and Phosphatidylinositol 3-Kinase Inhibitor (LY294002) In Vitro
Abstract
:1. Introduction
2. Results
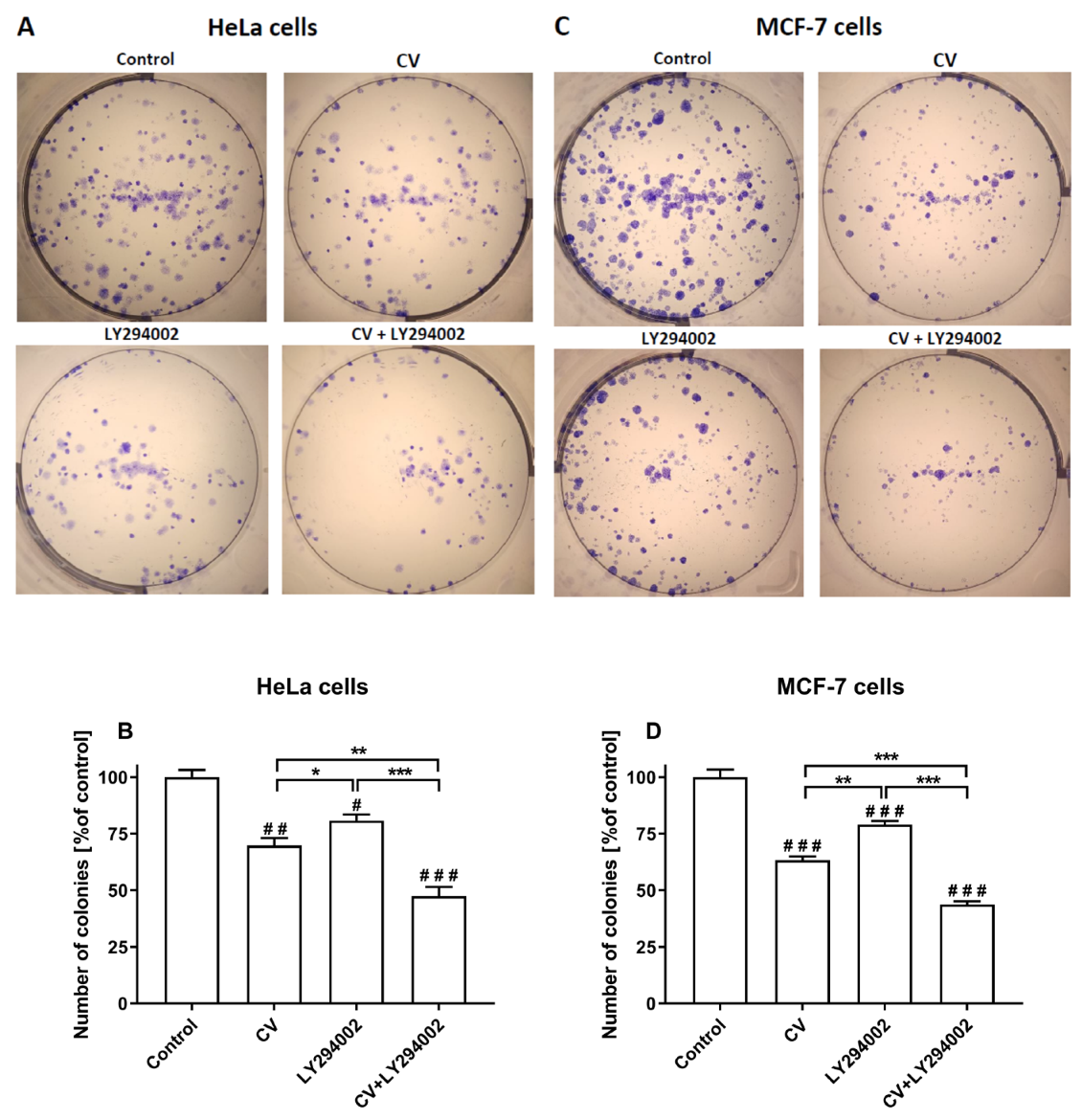
2.1. LY294002 and CV Extract Have Additive Cytotoxic Effects Against Cancer Cells
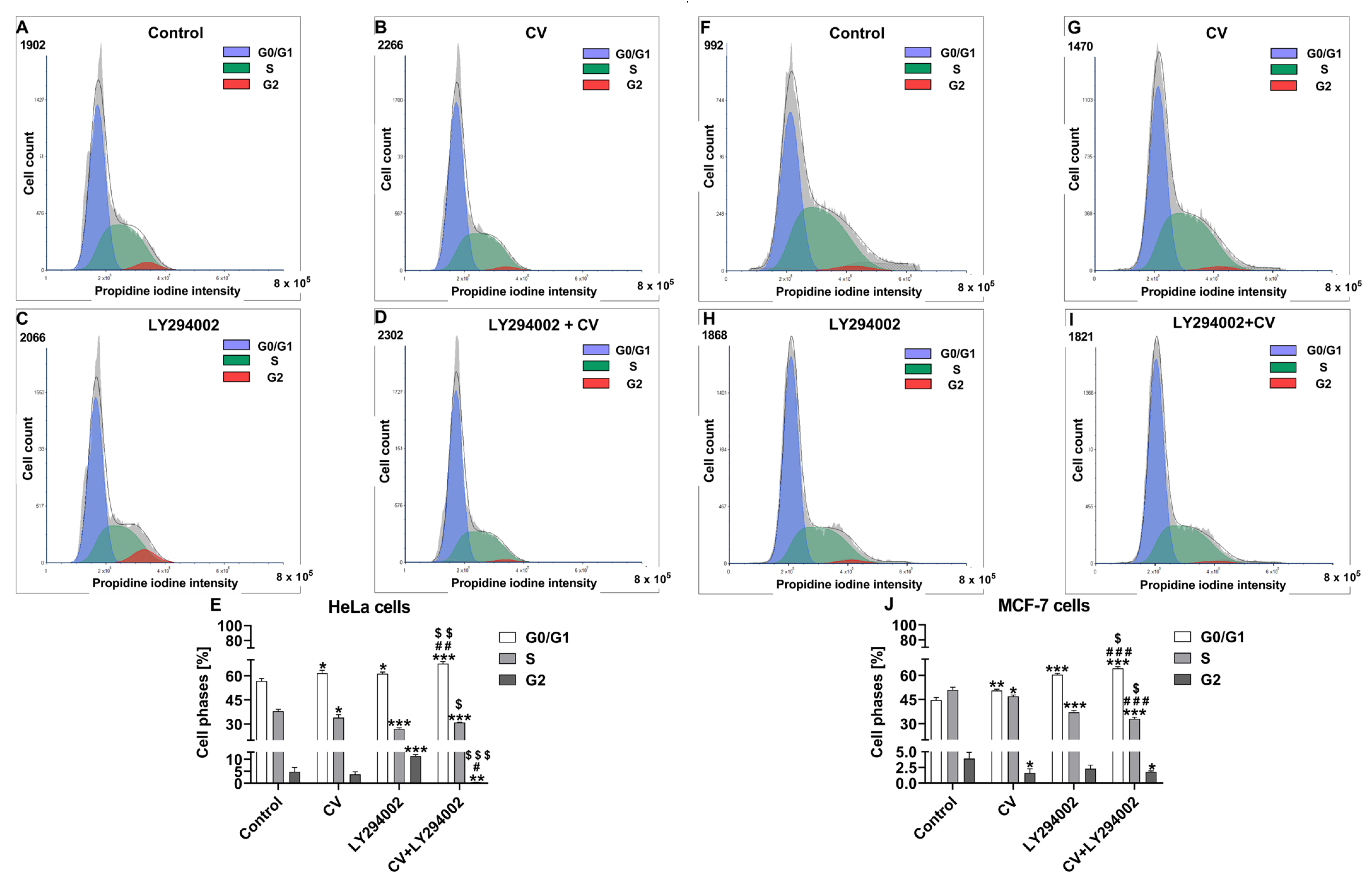
2.2. Co-Treatment of Cancer Cells with LY294002 and CV Extract Increases Cell Cycle Arrest at the G0/G1 Phase
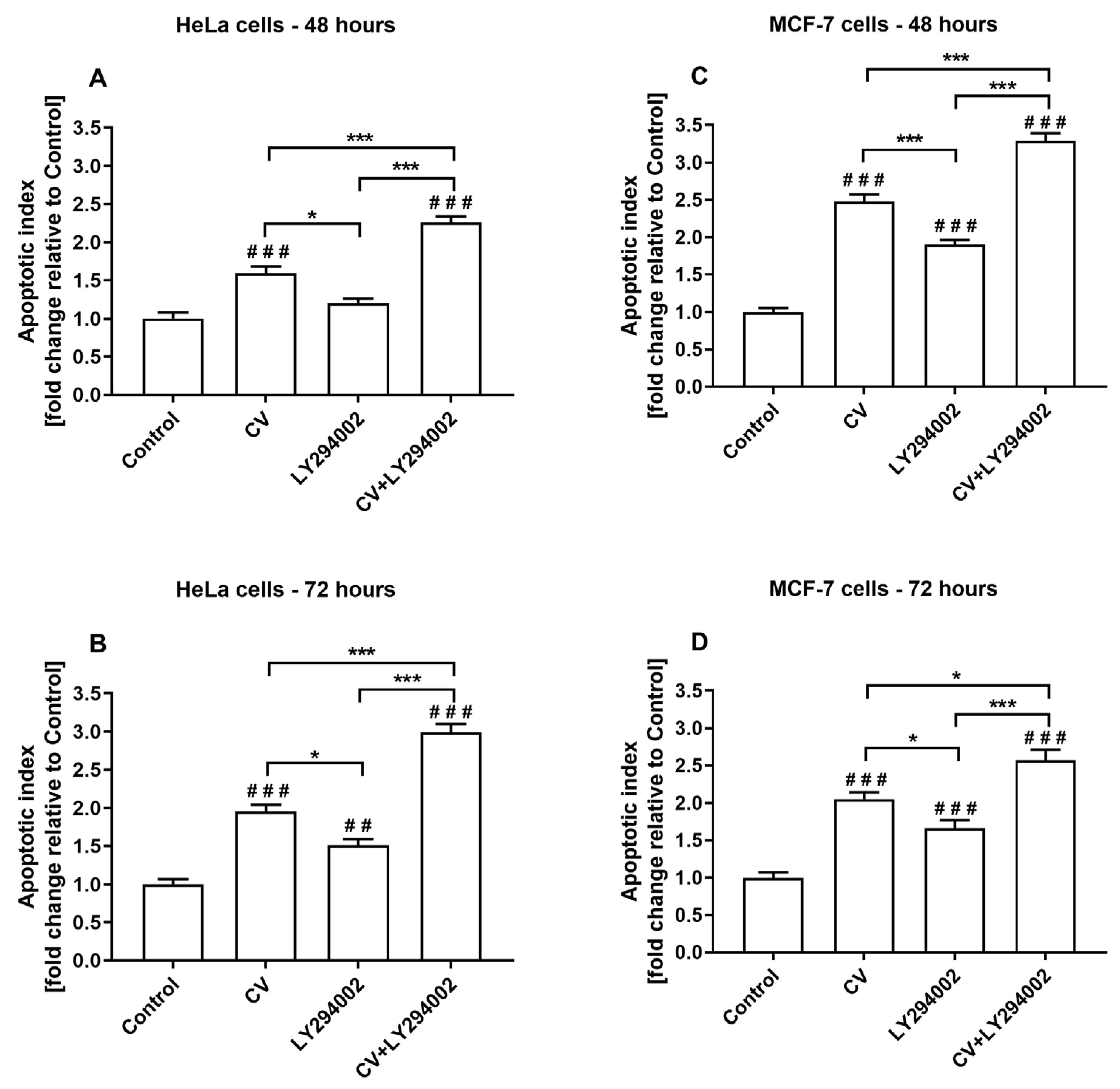
2.3. Co-Treatment of Cancer Cells with LY294002 and CV Extract Increases Cell Apoptosis
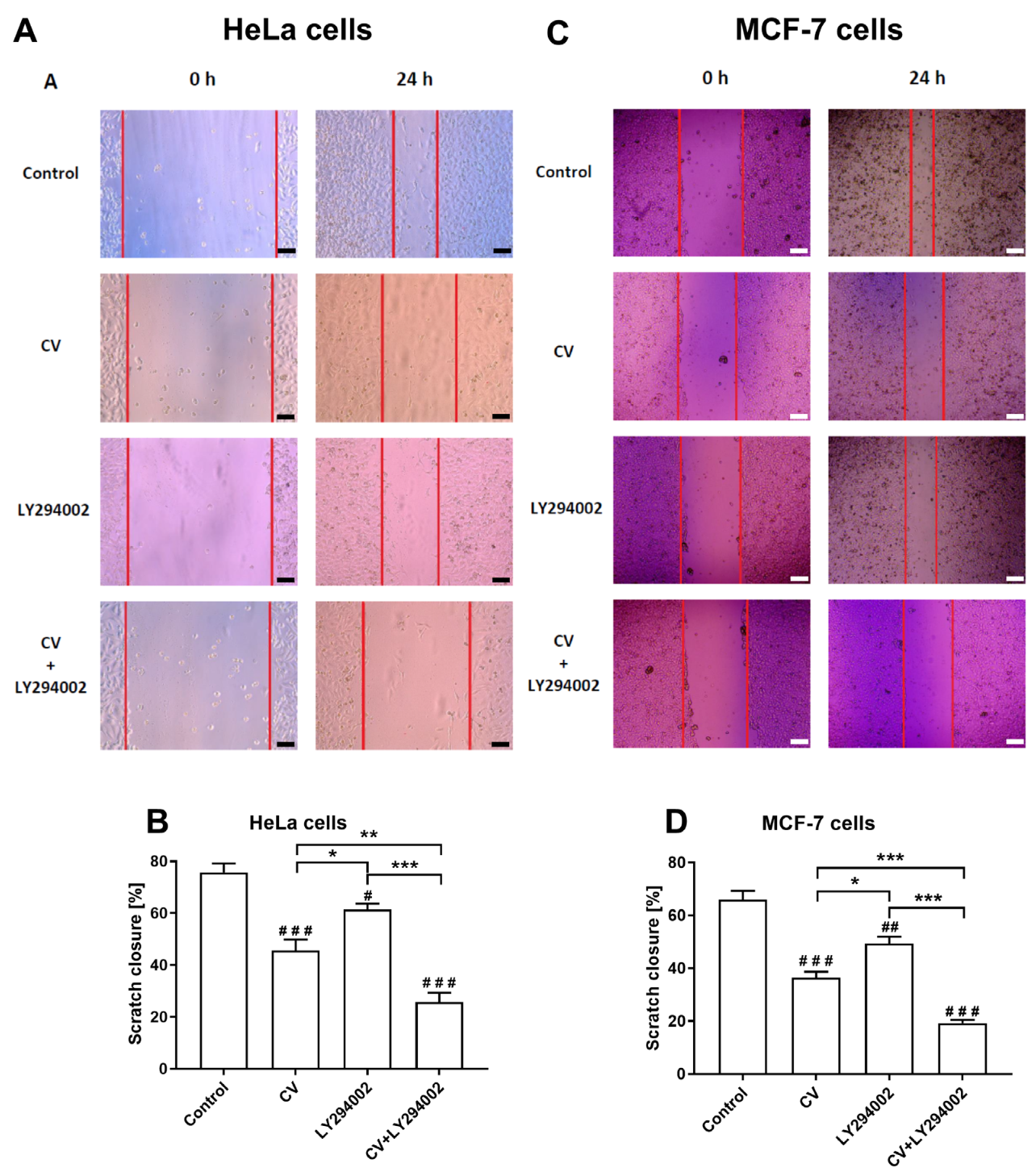
2.4. LY294002 Increases the Anti-Migratory Activity of the CV Extract
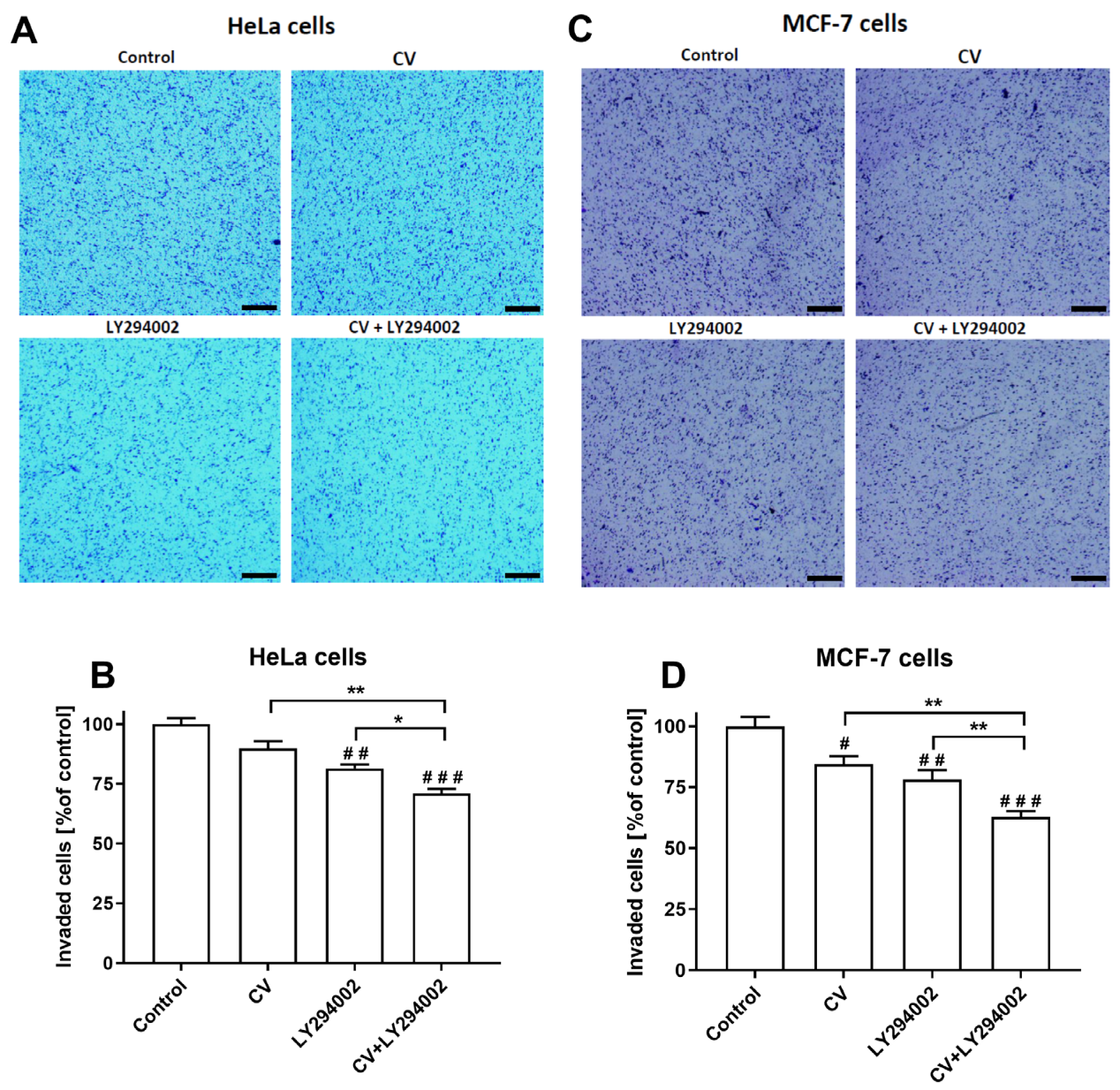
2.5. LY294002 Decreases the Invasive Ability of the Cancer Cells Stimulated with the CV Extract
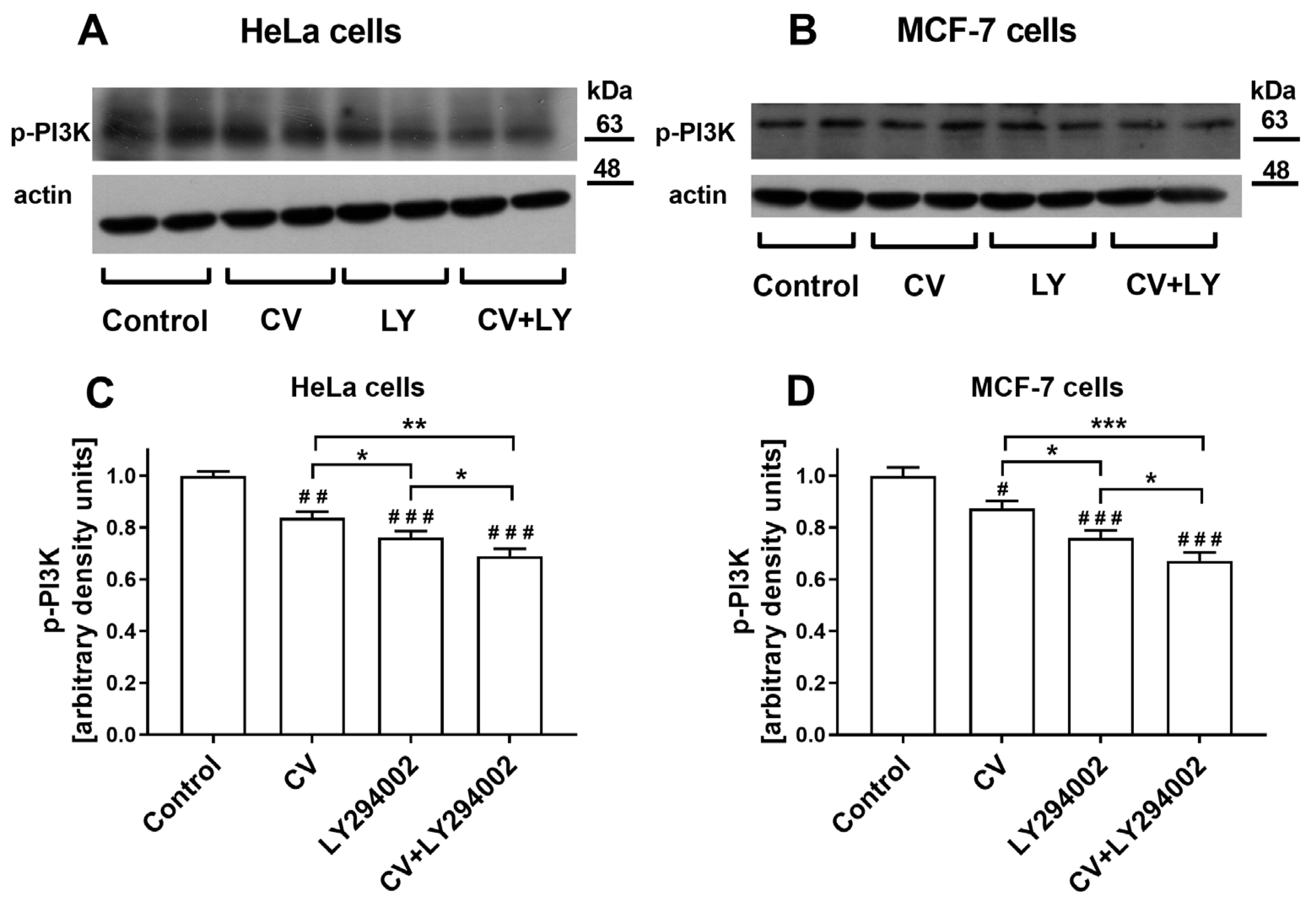
2.6. Co-Treatment of Cancer Cells with LY294002 and CV Extract Potentiated Inhibition of Phospho-PI3K Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of CV Extract and LY294002 Solution
4.3. Cell Viability
4.4. Colony Formation Assay
4.5. Apoptosis Assay
4.6. Cell Cycle Analysis
4.7. Scratch Assay
4.8. Invasion Assay
4.9. Western Blot Analysis
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CV | Coriolus versicolor |
| LY294002 | phosphatidylinositol-3-kinase inhibitor |
| PI3K | phosphatidylinositol-3-kinase |
| p-PI3K | phospho- phosphatidylinositol-3-kinase |
References
- Jing, Y.; Zhang, S.; Li, M.; Ma, Y.; Zheng, Y.; Zhang, D.; Wu, L. Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus versicolor. Foods 2022, 11, 2126. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from Mushroom: Health Attributes and Food Industry Applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewski, T.; Pawlikowska, M.; Sobocińska, J.; Wrotek, S. COVID-19 and Cancer Diseases-The Potential of Coriolus versicolor Mushroom to Combat Global Health Challenges. Int. J. Mol. Sci. 2023, 24, 4864. [Google Scholar] [CrossRef]
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory Properties of Coriolus versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef]
- He, Z.; Lin, J.; He, Y.; Liu, S. Polysaccharide-Peptide from Trametes versicolor: The Potential Medicine for Colorectal Cancer Treatment. Biomedicines 2022, 10, 2841. [Google Scholar] [CrossRef]
- Maehara, Y.; Tsujitani, S.; Saeki, H.; Oki, E.; Yoshinaga, K.; Emi, Y.; Morita, M.; Kohnoe, S.; Kakeji, Y.; Yano, T.; et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN(®)): Review of development and future perspectives. Surg. Today 2012, 42, 8–28. [Google Scholar] [CrossRef]
- Torkelson, C.J.; Sweet, E.; Martzen, M.R.; Sasagawa, M.; Wenner, C.A.; Gay, J.; Putiri, A.; Standish, L.J. Phase 1 Clinical Trial of Trametes versicolor in Women with Breast Cancer. ISRN Oncol. 2012, 2012, 251632. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, M.; Jiang, Y.; Liu, Y.; Luo, H.; Hao, C.; Zeng, P.; Zhang, L. Preclinical and clinical studies of Coriolus versicolor polysaccharopeptide as an immunotherapeutic in China. Discov. Med. 2017, 23, 207–219. [Google Scholar]
- Pilkington, K.; Wieland, L.S.; Teng, L.; Jin, X.Y.; Storey, D.; Liu, J.P. Coriolus (Trametes) versicolor mushroom to reduce adverse effects from chemotherapy or radiotherapy in people with colorectal cancer. Cochrane Database Syst. Rev. 2022, 11, CD012053. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J.W. Mushroom Polysaccharide-Assisted Anticarcinogenic Mycotherapy: Reviewing Its Clinical Trials. Molecules 2022, 27, 4090. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Castel, P.; Toska, E.; Engelman, J.A.; Scaltriti, M. The present and future of PI3K inhibitors for cancer therapy. Nat. Cancer 2021, 2, 587–597. [Google Scholar] [CrossRef]
- Wang, Y.; Kuramitsu, Y.; Baron, B.; Kitagawa, T.; Tokuda, K.; Akada, J.; Maehara, S.I.; Maehara, Y.; Nakamura, K. PI3K inhibitor LY294002, as opposed to wortmannin, enhances AKT phosphorylation in gemcitabine-resistant pancreatic cancer cells. Int. J. Oncol. 2017, 50, 606–612. [Google Scholar] [CrossRef]
- Meng, D.; He, W.; Zhang, Y.; Liang, Z.; Zheng, J.; Zhang, X.; Zheng, X.; Zhan, P.; Chen, H.; Li, W.; et al. Development of PI3K inhibitors: Advances in clinical trials and new strategies (Review). Pharmacol. Res. 2021, 173, 105900. [Google Scholar] [CrossRef]
- Abdallah, M.E.; El-Readi, M.Z.; Althubiti, M.A.; Almaimani, R.A.; Ismail, A.M.; Idris, S.; Refaat, B.; Almalki, W.H.; Babakr, A.T.; Mukhtar, M.H.; et al. Tamoxifen and the PI3K Inhibitor: LY294002 Synergistically Induce Apoptosis and Cell Cycle Arrest in Breast Cancer MCF-7 Cells. Molecules 2020, 25, 3355. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Ozturk, M. Therapeutic potential of the PI3K inhibitor LY294002 and PARP inhibitor Talazoparib combination in BRCA-deficient triple negative breast cancer cells. Cell. Signal. 2022, 91, 110229. [Google Scholar] [CrossRef]
- Huang, A.; Zeng, P.; Li, Y.; Lu, W.; Lai, Y. LY294002 Is a Promising Inhibitor to Overcome Sorafenib Resistance in FLT3-ITD Mutant AML Cells by Interfering with PI3K/Akt Signaling Pathway. Front. Oncol. 2021, 11, 782065. [Google Scholar] [CrossRef]
- Badinloo, M.; Esmaeili Mahani, S. Combined Application of Phosphoinositide 3-Kinase and Mammalian Target of Rapamycin Inhibitors Suppresses Cell Growth and Promotes Apoptosis in Human Lung Cancer Cell Lines. Int. J. Cancer. Manag. 2016, 9, e3433. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2021; Biological Evaluation of Medical Devices. Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2021.
- Standish, L.J.; Wenner, C.A.; Sweet, E.S.; Bridge, C.; Nelson, A.; Martzen, M.; Novack, J.; Torkelson, C. Trametes versicolor mushroom immune therapy in breast cancer. J. Soc. Integr. Oncol. 2008, 6, 122–128. [Google Scholar] [PubMed]
- Roca-Lema, D.; Martinez-Iglesias, O.; Fernández de Ana Portela, C.; Rodríguez-Blanco, A.; Valladares-Ayerbes, M.; Díaz-Díaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In Vitro Anti-proliferative and Anti-invasive Effect of Polysaccharide-rich Extracts from Trametes Versicolor and Grifola Frondosa in Colon Cancer Cells. Int. J. Med. Sci. 2019, 16, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Extract from the Coriolus versicolor Fungus as an Anti-Inflammatory Agent with Cytotoxic Properties against Endothelial Cells and Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9063. [Google Scholar] [CrossRef]
- Winder, M.; Bulska-Będkowska, W.; Chudek, J. The use of Hericium erinaceus and Trametes versicolor extracts in supportive treatment in oncology. Acta Pharm. 2021, 71, 1–16. [Google Scholar] [CrossRef]
- Lowenthal, R.; Taylor, M.; Gidden, J.A.; Heflin, B.; Lay, J.O., Jr.; Avaritt, N.; Tackett, A.J.; Urbaniak, A. The mycelium of the Trametes versicolor synn. Coriolus versicolor (Turkey tail mushroom) exhibit anti-melanoma activity in vitro. Biomed. Pharmacother. 2023, 161, 114424. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Curigliano, G.; Shah, R.R. Safety and Tolerability of Phosphatidylinositol-3-Kinase (PI3K) Inhibitors in Oncology. Drug. Saf. 2019, 42, 247–262. [Google Scholar] [CrossRef]
- Coleman, N.; Moyers, J.T.; Harbery, A.; Vivanco, I.; Yap, T.A. Clinical Development of AKT Inhibitors and Associated Predictive Biomarkers to Guide Patient Treatment in Cancer Medicine. Pharmgenomics. Pers. Med. 2021, 14, 1517–1535. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Dual Effect of the Extract from the Fungus Coriolus versicolor on Lipopolysaccharide-Induced Cytokine Production in RAW 264.7 Macrophages Depending on the Lipopolysaccharide Concentration. J. Inflamm. Res. 2022, 15, 3599–3611. [Google Scholar] [CrossRef]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259–29274. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.P.; Frederick, B.; Jacobsen, J.R.; Chapnick, D.; Su, T.T. A High Throughput Screen with a Clonogenic Endpoint to Identify Radiation Modulators of Cancer. Radiat. Res. 2023, 199, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.R.; Licchetta, R.; Mirabilii, S.; Scarpari, M.; Parroni, A.; Fabbri, A.A.; Cescutti, P.; Reverberi, M.; Fanelli, C.; Tafuri, A. Preclinical Antileukemia Activity of Tramesan: A Newly Identified Bioactive Fungal Metabolite. Oxid. Med. Cell. Longev. 2017, 2017, 5061639. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Medina, E.; Berruguilla, E.; Romero, I.; Algarra, I.; Collado, A.; Garrido, F.; Garcia-Lora, A. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer 2008, 8, 78. [Google Scholar] [CrossRef]
- Wan, J.M.; Sit, W.H.; Yang, X.; Jiang, P.; Wong, L.L. Polysaccharopeptides Derived from Coriolus Versicolor Potentiate the S-Phase Specific Cytotoxicity of Camptothecin (CPT) on Human Leukemia HL-60 Cells. Chin. Med. 2010, 5, 16. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, P.; Park, S.; Wu, J.M. Induction of cell cycle changes and modulation of apoptogenic/anti-apoptotic and extracellular signaling regulatory protein expression by water extracts of I’m-Yunity (PSP). BMC Complement. Altern. Med. 2006, 6, 30. [Google Scholar] [CrossRef]
- Lu, X.X.; Cao, L.Y.; Chen, X.; Xiao, J.; Zou, Y.; Chen, Q. PTEN Inhibits Cell Proliferation, Promotes Cell Apoptosis, and Induces Cell Cycle Arrest via Downregulating the PI3K/AKT/hTERT Pathway in Lung Adenocarcinoma A549 Cells. Biomed. Res. Int. 2016, 2016, 2476842. [Google Scholar] [CrossRef]
- Pucci, B.; Kasten, M.; Giordano, A. Cell cycle and apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Lau, C.B.; Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.H.; Chow, M.S. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci. 2004, 75, 797–808. [Google Scholar] [CrossRef]
- Hirahara, N.; Fujioka, M.; Edamatsu, T.; Fujieda, A.; Sekine, F.; Wada, T.; Tanaka, T. Protein-bound polysaccharide-K (PSK) induces apoptosis and inhibits proliferation of promyelomonocytic leukemia HL-60 cells. Anticancer. Res. 2011, 31, 2733–2738. [Google Scholar]
- Luo, K.W.; Yue, G.G.; Ko, C.H.; Lee, J.K.; Gao, S.; Li, L.F.; Li, G.; Fung, K.P.; Leung, P.C.; Lau, C.B. In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fan, D.; Zhou, G.; Li, X.; Deng, H. Phosphatidylinositol 3-kinase inhibitor(LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 34. [Google Scholar] [CrossRef] [PubMed]
- Novikov, N.M.; Zolotaryova, S.Y.; Gautreau, A.M.; Denisov, E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 2021, 124, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, B.; Li, J.; Lu, H. LY294002 inhibits leukemia cell invasion and migration through early growth response gene 1 induction independent of phosphatidylinositol 3-kinase-Akt pathway. Biochem. Biophys. Res. Commun. 2008, 377, 187–190. [Google Scholar] [CrossRef]
- Shukla, S.; Maclennan, G.T.; Hartman, D.J.; Fu, P.; Resnick, M.I.; Gupta, S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer 2007, 121, 1424–1432. [Google Scholar] [CrossRef]
- Duarte, A.; Silveira, G.G.; Soave, D.F.; Costa, J.P.O.; Silva, A.R. The Role of the LY294002—A Non-Selective Inhibitor of Phosphatidylinositol 3-Kinase (PI3K) Pathway- in Cell Survival and Proliferation in Cell Line SCC-25. Asian. Pac. J. Cancer. Prev. 2019, 20, 3377–3383. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, L.B.; Peng, A.F.; Wang, T.F.; Long, X.H.; Gao, S.; Zhou, R.P.; Liu, Z.L. LY294002 inhibits the malignant phenotype of osteosarcoma cells by modulating the phosphatidylinositol 3-kinase/Akt/fatty acid synthase signaling pathway in vitro. Mol. Med. Rep. 2015, 11, 1352–1357. [Google Scholar] [CrossRef]
- Teranishi, F.; Takahashi, N.; Gao, N.; Akamo, Y.; Takeyama, H.; Manabe, T.; Okamoto, T. Phosphoinositide 3-kinase inhibitor (wortmannin) inhibits pancreatic cancer cell motility and migration induced by hyaluronan in vitro and peritoneal metastasis in vivo. Cancer Sci. 2009, 100, 770–777. [Google Scholar] [CrossRef]
- Zhang, H.; Morisaki, T.; Matsunaga, H.; Sato, N.; Uchiyama, A.; Hashizume, K.; Nagumo, F.; Tadano, J.; Katano, M. Protein-bound polysaccharide PSK inhibits tumor invasiveness by down-regulation of TGF-beta1 and MMPs. Clin. Exp. Metastasis 2000, 18, 343–352. [Google Scholar] [CrossRef]
- Matsunaga, K.; Ohhara, M.; Oguchi, Y.; Iijima, H.; Kobayashi, H. Antimetastatic effect of PSK, a protein-bound polysaccharide, against the B16-BL6 mouse melanoma. Invasion Metastasis 1996, 16, 27–38. [Google Scholar]
- Esposito, A.; Viale, G.; Curigliano, G. Safety, Tolerability, and Management of Toxic Effects of Phosphatidylinositol 3-Kinase Inhibitor Treatment in Patients with Cancer: A Review. JAMA Oncol. 2019, 5, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewski, T.; Sobocińska, J.; Maciejewski, B.; Spisz, P.; Walczak-Skierska, J.; Pomastowski, P.; Wrotek, S. In vitro treatment of triple-negative breast cancer cells with an extract from the Coriolus versicolor mushroom changes macrophage properties related to tumorigenesis. Immunol. Res. 2025, 73, 14. [Google Scholar] [CrossRef] [PubMed]

| Time | Sample | IC50 | ||
|---|---|---|---|---|
| HeLa | MCF-7 | A549 | ||
| 24 h | CV | >250 | >250 | >250 |
| CV + LY294002 | >250 | >250 | >250 | |
| 48 h | CV | >250 | >250 | >250 |
| CV + LY294002 | 215.5 ± 7.6 ** | 239.4 ± 9.9 | >250 | |
| 72 h | CV | 225.2 ± 11.2 | 208.6 ± 7.8 | >250 |
| CV + LY294002 | 153.0 ± 4.5 *** | 133.4 ± 3.9 *** | 244.7 ± 6.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejewski, T.; Sobocińska, J.; Maciejewski, B.; Slovakova, M.; Wrotek, S. Enhanced Anti-Cancer Potential: Investigating the Combined Effects with Coriolus versicolor Extract and Phosphatidylinositol 3-Kinase Inhibitor (LY294002) In Vitro. Int. J. Mol. Sci. 2025, 26, 1556. https://doi.org/10.3390/ijms26041556
Jędrzejewski T, Sobocińska J, Maciejewski B, Slovakova M, Wrotek S. Enhanced Anti-Cancer Potential: Investigating the Combined Effects with Coriolus versicolor Extract and Phosphatidylinositol 3-Kinase Inhibitor (LY294002) In Vitro. International Journal of Molecular Sciences. 2025; 26(4):1556. https://doi.org/10.3390/ijms26041556
Chicago/Turabian StyleJędrzejewski, Tomasz, Justyna Sobocińska, Bartosz Maciejewski, Marcela Slovakova, and Sylwia Wrotek. 2025. "Enhanced Anti-Cancer Potential: Investigating the Combined Effects with Coriolus versicolor Extract and Phosphatidylinositol 3-Kinase Inhibitor (LY294002) In Vitro" International Journal of Molecular Sciences 26, no. 4: 1556. https://doi.org/10.3390/ijms26041556
APA StyleJędrzejewski, T., Sobocińska, J., Maciejewski, B., Slovakova, M., & Wrotek, S. (2025). Enhanced Anti-Cancer Potential: Investigating the Combined Effects with Coriolus versicolor Extract and Phosphatidylinositol 3-Kinase Inhibitor (LY294002) In Vitro. International Journal of Molecular Sciences, 26(4), 1556. https://doi.org/10.3390/ijms26041556






