The Simultaneous Deletion of pH-Sensing Receptors GPR4 and OGR1 (GPR68) Ameliorates Colitis with Additive Effects on Multiple Parameters of Inflammation
Abstract
1. Introduction
2. Results
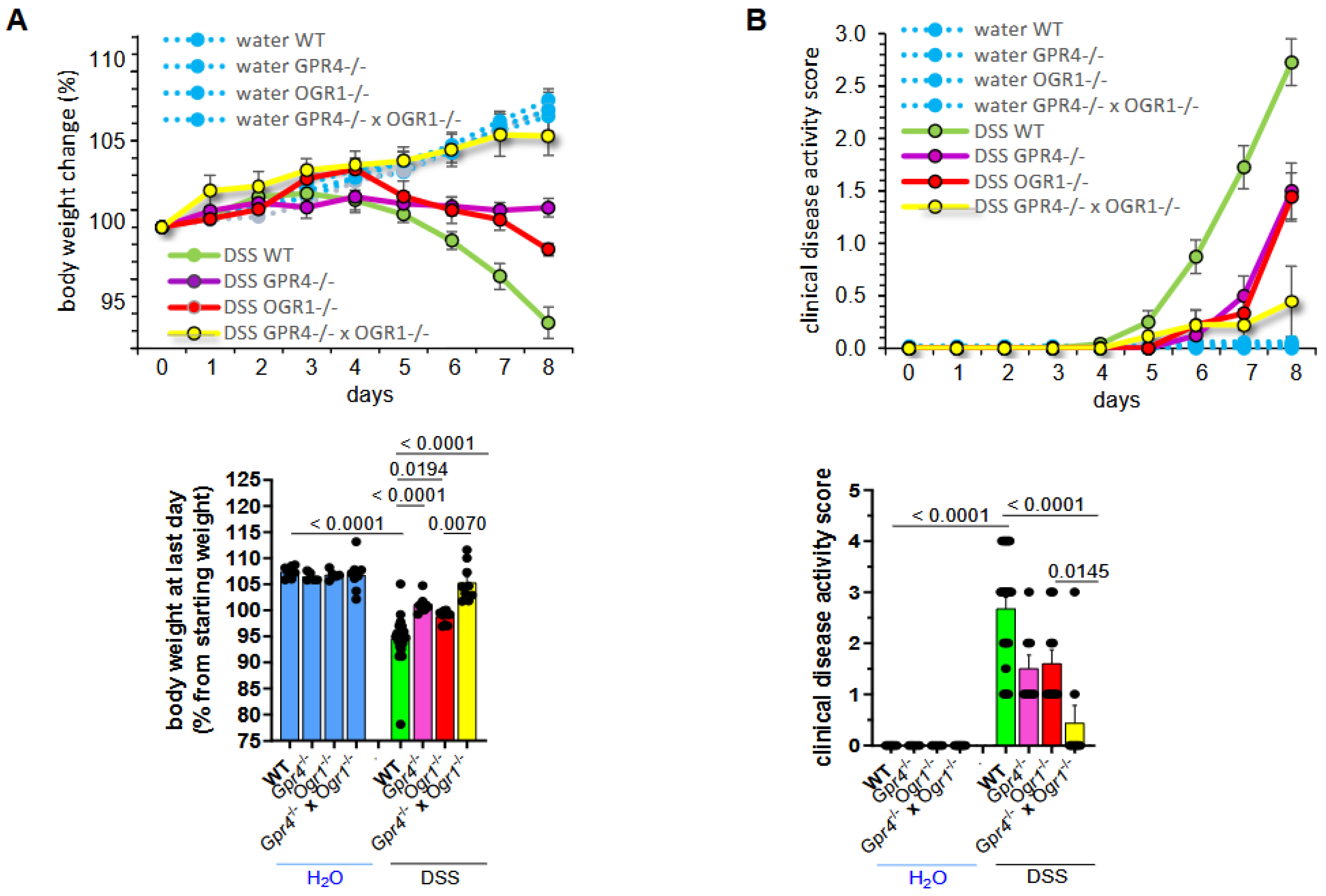
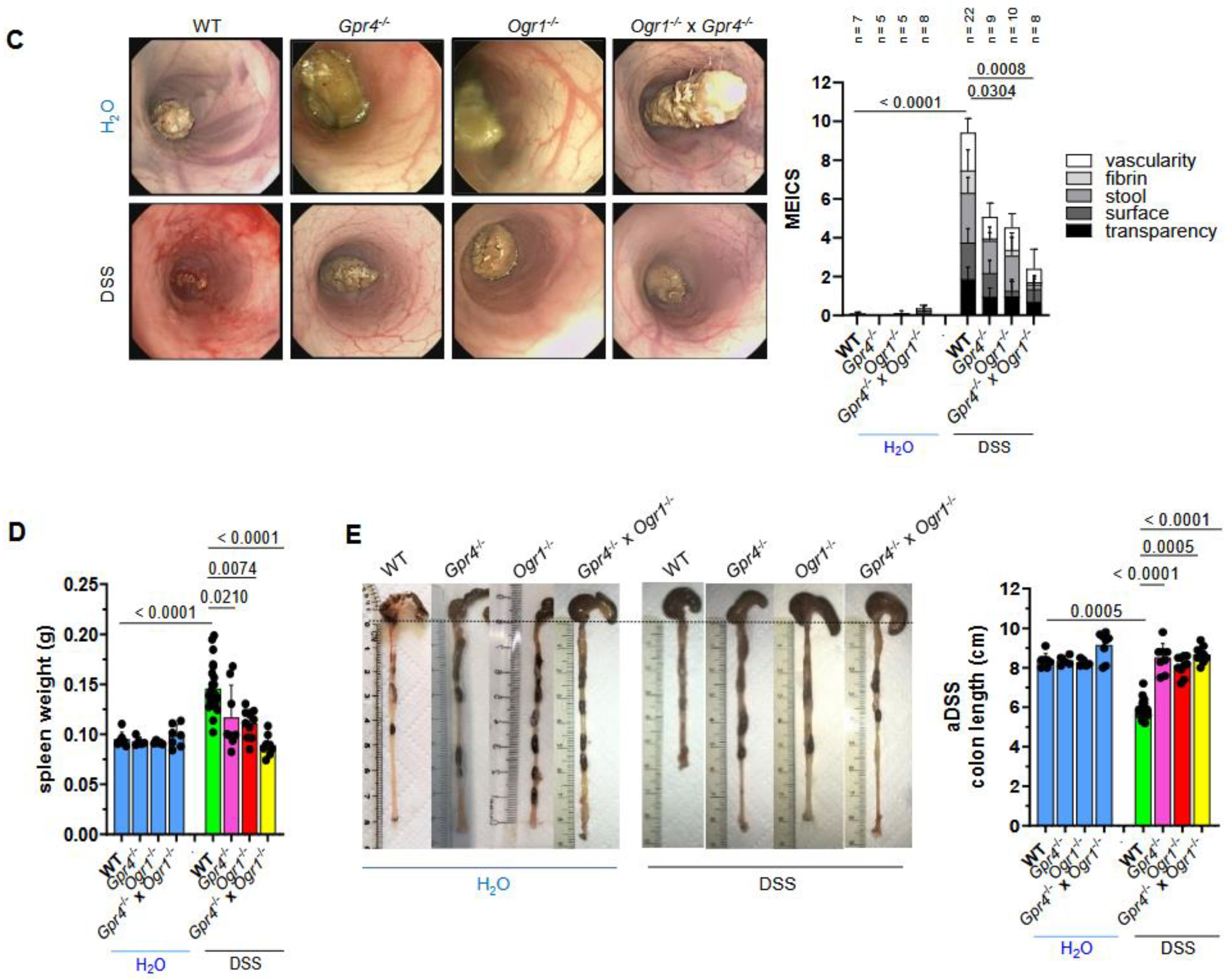
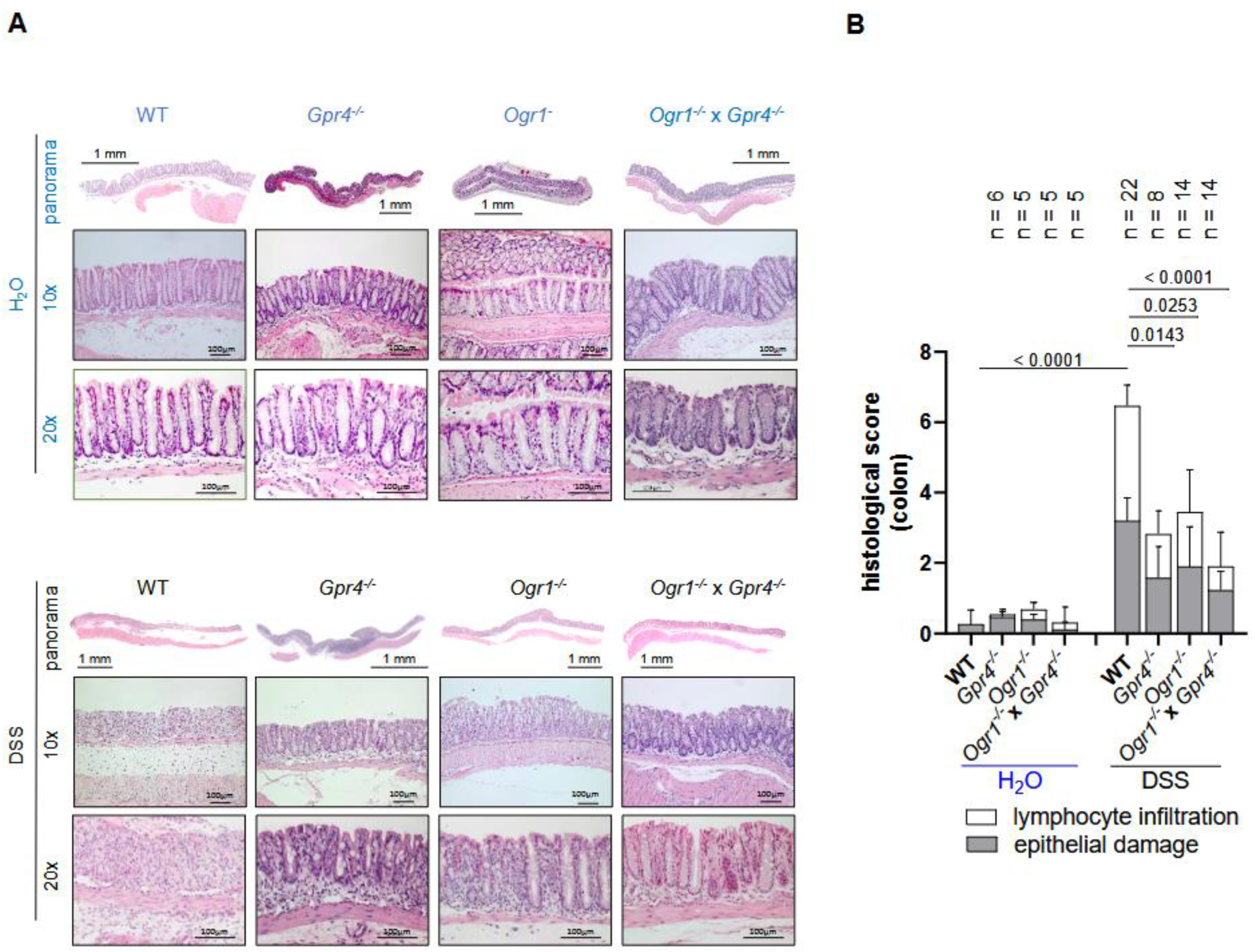
2.1. Simultaneous Loss of GPR4 and OGR1 Reduces Inflammation in DSS-Induced Acute Colitis with Additive Effects on Multiple Parameters
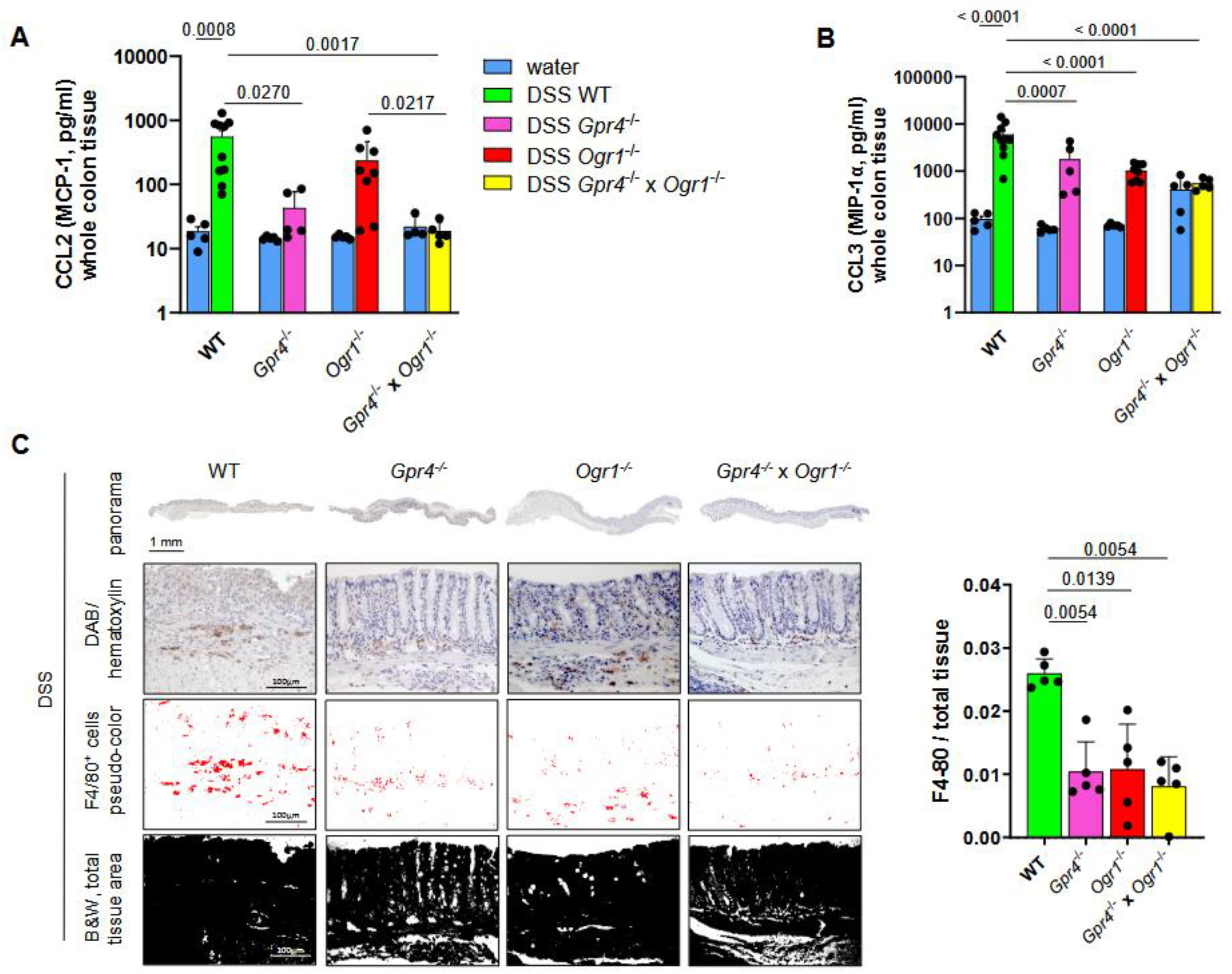
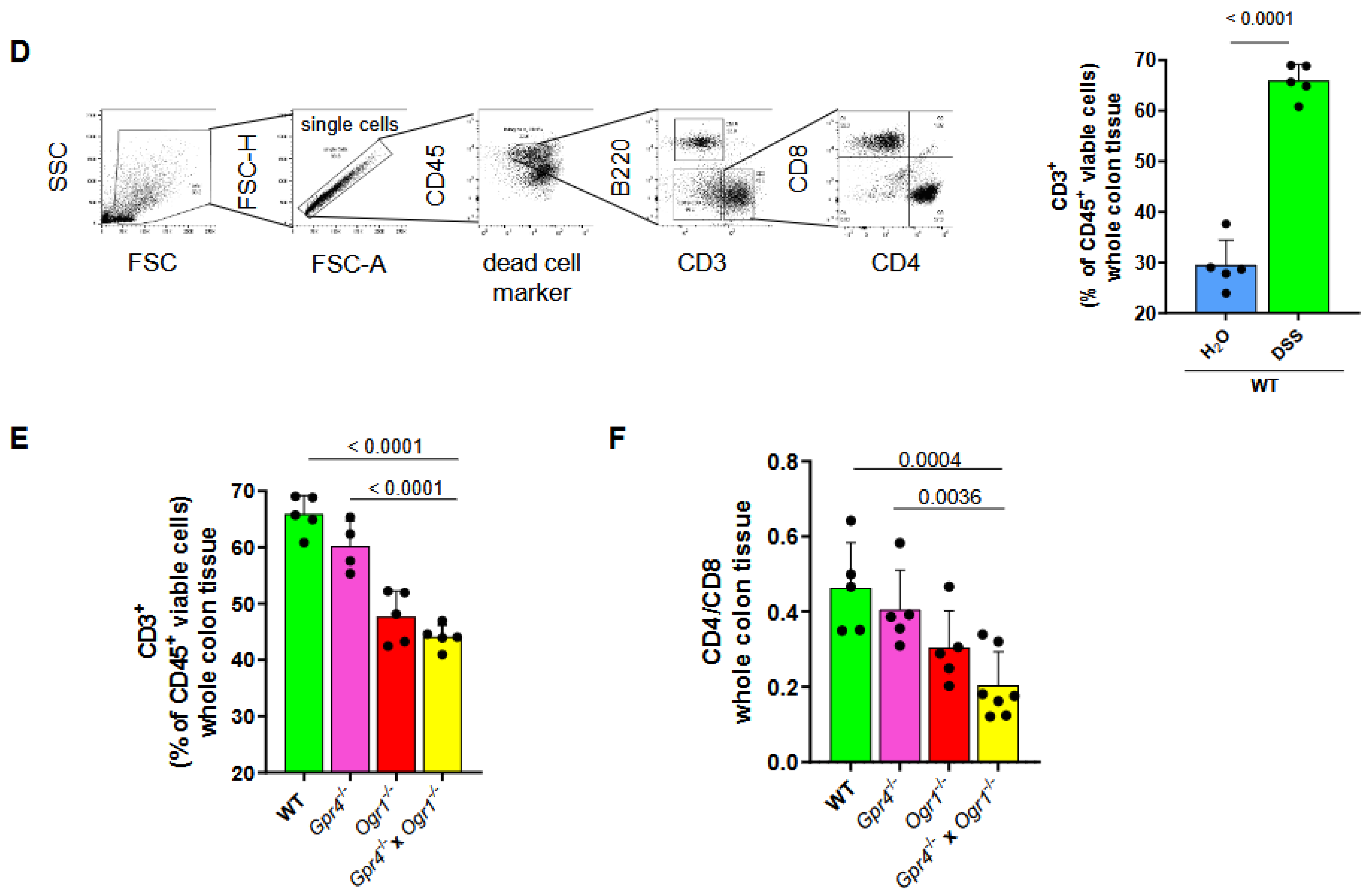
2.2. Loss of Both Gpr4 and Ogr1 Decreases F4/80+ and CD4/CD8 Ratio During Acute Colitis
2.3. The Absence of Both GPR4 and OGR1 Reduces Inflammation in Spontaneous Colitis
2.4. The Absence of Both GPR4 and OGR1 Reduces CD4/CD8 Ratio and Macrophages in Spontaneous Colitis
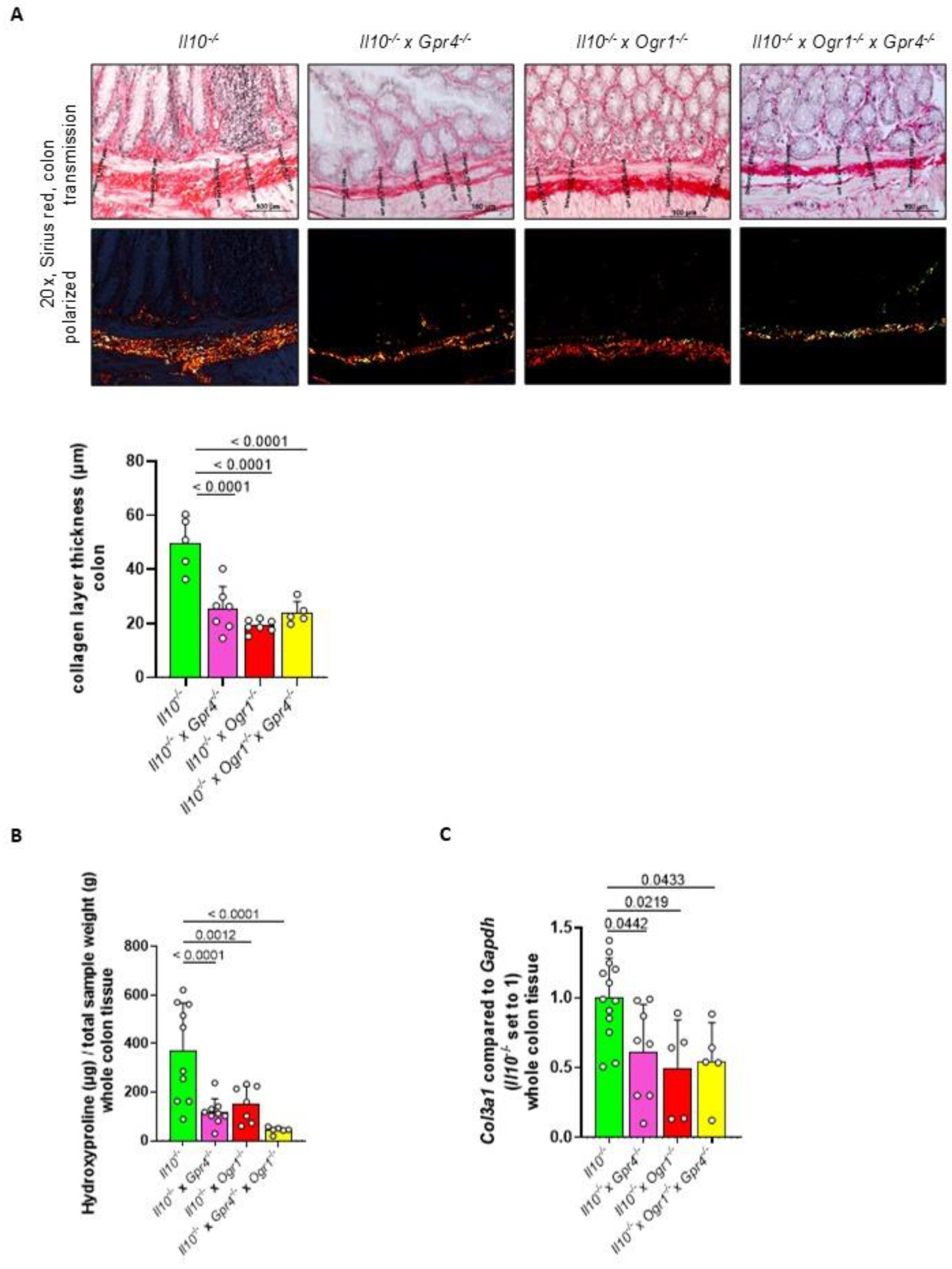
2.5. The Absence of Gpr4 and Ogr1 Reduces Fibrosis in Spontaneous Colitis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. DSS-Induced Acute Colitis
4.3. Spontaneous Il10 Deficient Colitis Model
4.4. Clinical Disease Activity Score
4.5. Assessment of Colonoscopy
4.6. Serum and Tissue Collection for Histology and Molecular Analysis
4.7. Histological Score in Mice
4.8. Flow Cytometry
4.9. Lamina Propria Lymphocytes Isolation
4.10. Splenocyte Isolation
4.11. Compensation Controls
4.12. Immunohistochemistry (IHC) and Densitometry
4.13. Sirius Red Staining and Collagen Layer Thickness
4.14. Hydroxyproline Determination
4.15. Multiplex Cytokines Analysis
4.16. Ribonucleic Acid (RNA) Isolation, Complementary DNA (cDNA) Synthesis, and qPCR
4.17. Western Blot (WB)
4.18. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| cAMP | cyclic adenosine monophosphate |
| CCL4 | chemokine (C-C motif) ligands 4 |
| CD | Crohn’s disease |
| cDNA | complementary DNA |
| DSS | dextran sodium sulfate |
| EC | endothelial cell |
| Gapdh | glyceraldehyde-3-phosphate dehydrogenase |
| G-CSF | granulocyte colony-stimulating factor |
| FSC | forward scatter |
| GPRs | G protein-coupled receptors |
| GPR4 | G protein-coupled receptor 4 |
| IBD | inflammatory bowel disease |
| HBSS | Hank’s balanced salt solution |
| HE | hematoxylin and eosin |
| IL | interleukin |
| KC | keratinocyte-derived chemokine |
| MCP-1 | monocyte chemoattractant protein-1 |
| MEICS | murine endoscopic index of colitis severity |
| MIP-1α | macrophage inflammatory protein-1α |
| OGR1 | ovarian cancer GPR 1 (GPR68) |
| PBS | phosphate-buffered solution |
| qPCR | real-time quantitative polymerase chain reaction |
| SSC | side scatter |
| Tnf | tumor necrosis factor |
| UC | ulcerative colitis |
| WT | wildtype |
References
- Ludwig, M.G.; Vanek, M.; Guerini, D.; Gasser, J.A.; Jones, C.E.; Junker, U.; Hofstetter, H.; Wolf, R.M.; Seuwen, K. Proton-sensing G-protein-coupled receptors. Nature 2003, 425, 93–98. [Google Scholar] [CrossRef]
- Wyder, L.; Suply, T.; Ricoux, B.; Billy, E.; Schnell, C.; Baumgarten, B.U.; Maira, S.M.; Koelbing, C.; Ferretti, M.; Kinzel, B.; et al. Reduced pathological angiogenesis and tumor growth in mice lacking GPR4, a proton sensing receptor. Angiogenesis 2011, 14, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Z.; Leffler, N.R.; Asch, A.S.; Chi, J.T.; Yang, L.V. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE 2013, 8, e61991. [Google Scholar] [CrossRef] [PubMed]
- Miltz, W.; Velcicky, J.; Dawson, J.; Littlewood-Evans, A.; Ludwig, M.G.; Seuwen, K.; Feifel, R.; Oberhauser, B.; Meyer, A.; Gabriel, D.; et al. Design and synthesis of potent and orally active GPR4 antagonists with modulatory effects on nociception, inflammation, and angiogenesis. Bioorg. Med. Chem. 2017, 25, 4512–4525. [Google Scholar] [CrossRef] [PubMed]
- Jasso, G.J.; Jaiswal, A.; Varma, M.; Laszewski, T.; Grauel, A.; Omar, A.; Silva, N.; Dranoff, G.; Porter, J.A.; Mansfield, K.; et al. Colon stroma mediates an inflammation-driven fibroblastic response controlling matrix remodeling and healing. PLoS Biol. 2022, 20, e3001532. [Google Scholar] [CrossRef]
- de Valliere, C.; Cosin-Roger, J.; Baebler, K.; Schoepflin, A.; Mamie, C.; Mollet, M.; Schuler, C.; Bengs, S.; Lang, S.; Scharl, M.; et al. pH-Sensing G Protein-Coupled Receptor OGR1 (GPR68) Expression and Activation Increases in Intestinal Inflammation and Fibrosis. Int. J. Mol. Sci. 2022, 23, 1419. [Google Scholar] [CrossRef]
- Tobo, M.; Tomura, H.; Mogi, C.; Wang, J.Q.; Liu, J.P.; Komachi, M.; Damirin, A.; Kimura, T.; Murata, N.; Kurose, H.; et al. Previously postulated “ligand-independent” signaling of GPR4 is mediated through proton-sensing mechanisms. Cell. Signal. 2007, 19, 1745–1753. [Google Scholar] [CrossRef]
- Tomura, H.; Mogi, C.; Sato, K.; Okajima, F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: A novel type of multi-functional receptors. Cell. Signal. 2005, 17, 1466–1476. [Google Scholar] [CrossRef]
- Li, J.; Guo, B.; Wang, J.; Cheng, X.; Xu, Y.; Sang, J. Ovarian cancer G protein coupled receptor 1 suppresses cell migration of MCF7 breast cancer cells via a Galpha12/13-Rho-Rac1 pathway. J. Mol. Signal. 2013, 8, 6. [Google Scholar] [CrossRef]
- Wei, W.C.; Bianchi, F.; Wang, Y.K.; Tang, M.J.; Ye, H.; Glitsch, M.D. Coincidence Detection of Membrane Stretch and Extracellular pH by the Proton-Sensing Receptor OGR1 (GPR68). Curr. Biol. 2018, 28, 3815–3823.e4. [Google Scholar] [CrossRef]
- Wiley, S.Z.; Sriram, K.; Liang, W.; Chang, S.E.; French, R.; McCann, T.; Sicklick, J.; Nishihara, H.; Lowy, A.M.; Insel, P.A. GPR68, a proton-sensing GPCR, mediates interaction of cancer-associated fibroblasts and cancer cells. FASEB J. 2018, 32, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J.; Christensen, L.A.; Jacobsen, B.A.; Rasmussen, S.N. Very low intraluminal colonic pH in patients with active ulcerative colitis. Dig. Dis. Sci. 1993, 38, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Press, A.G.; Hauptmann, I.A.; Hauptmann, L.; Fuchs, B.; Fuchs, M.; Ewe, K.; Ramadori, G. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 1998, 12, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Lardner, A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001, 69, 522–530. [Google Scholar] [CrossRef]
- Hanly, E.J.; Aurora, A.A.; Shih, S.P.; Fuentes, J.M.; Marohn, M.R.; De Maio, A.; Talamini, M.A. Peritoneal acidosis mediates immunoprotection in laparoscopic surgery. Surgery 2007, 142, 357–364. [Google Scholar] [CrossRef]
- Mogi, C.; Tobo, M.; Tomura, H.; Murata, N.; He, X.D.; Sato, K.; Kimura, T.; Ishizuka, T.; Sasaki, T.; Sato, T.; et al. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J. Immunol. 2009, 182, 3243–3251. [Google Scholar] [CrossRef]
- Brokelman, W.J.; Lensvelt, M.; Borel Rinkes, I.H.; Klinkenbijl, J.H.; Reijnen, M.M. Peritoneal changes due to laparoscopic surgery. Surg. Endosc. 2011, 25, 1–9. [Google Scholar] [CrossRef]
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Franke, A.; McGovern, D.P.; Barrett, J.C.; Wang, K.; Radford-Smith, G.L.; Ahmad, T.; Lees, C.W.; Balschun, T.; Lee, J.; Roberts, R.; et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 2010, 42, 1118–1125. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Zeitz, J.; Biedermann, L. The Search for Causative Environmental Factors in Inflammatory Bowel Disease. Dig. Dis. 2016, 34 (Suppl. S1), 48–55. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H. Treatment of inflammatory bowel disease from the immunological perspective. Immunol. Med. 2020, 43, 79–86. [Google Scholar] [CrossRef]
- Wang, Y.; de Valliere, C.; Imenez Silva, P.H.; Leonardi, I.; Gruber, S.; Gerstgrasser, A.; Melhem, H.; Weber, A.; Leucht, K.; Wolfram, L.; et al. The Proton-activated Receptor GPR4 Modulates Intestinal Inflammation. J. Crohns Colitis 2018, 12, 355–368. [Google Scholar] [CrossRef]
- Sanderlin, E.J.; Leffler, N.R.; Lertpiriyapong, K.; Cai, Q.; Hong, H.; Bakthavatchalu, V.; Fox, J.G.; Oswald, J.Z.; Justus, C.R.; Krewson, E.A.; et al. GPR4 deficiency alleviates intestinal inflammation in a mouse model of acute experimental colitis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 569–584. [Google Scholar] [CrossRef]
- de Valliere, C.; Wang, Y.; Eloranta, J.J.; Vidal, S.; Clay, I.; Spalinger, M.R.; Tcymbarevich, I.; Terhalle, A.; Ludwig, M.G.; Suply, T.; et al. G Protein-coupled pH-sensing Receptor OGR1 Is a Regulator of Intestinal Inflammation. Inflamm. Bowel Dis. 2015, 21, 1269–1281. [Google Scholar] [CrossRef]
- de Valliere, C.; Babler, K.; Busenhart, P.; Schwarzfischer, M.; Maeyashiki, C.; Schuler, C.; Atrott, K.; Lang, S.; Spalinger, M.R.; Scharl, M.; et al. A Novel OGR1 (GPR68) Inhibitor Attenuates Inflammation in Murine Models of Colitis. Inflamm. Intest. Dis. 2021, 6, 140–153. [Google Scholar] [CrossRef]
- Weder, B.; Schefer, F.; van Haaften, W.T.; Patsenker, E.; Stickel, F.; Mueller, S.; Hutter, S.; Schuler, C.; Baebler, K.; Wang, Y.; et al. New Therapeutic Approach for Intestinal Fibrosis Through Inhibition of pH-Sensing Receptor GPR4. Inflamm. Bowel Dis. 2022, 28, 109–125. [Google Scholar] [CrossRef]
- Sanderlin, E.J.; Marie, M.; Velcicky, J.; Loetscher, P.; Yang, L.V. Pharmacological inhibition of GPR4 remediates intestinal inflammation in a mouse colitis model. Eur. J. Pharmacol. 2019, 852, 218–230. [Google Scholar] [CrossRef]
- Engers, D.W.; Lindsley, C.W. Allosteric modulation of Class C GPCRs: A novel approach for the treatment of CNS disorders. Drug Discov. Today Technol. 2013, 10, e269–e276. [Google Scholar] [CrossRef]
- Matsingos, C.; Howell, L.A.; McCormick, P.J.; Fornili, A. Elucidating the Activation Mechanism of the Proton-sensing GPR68 Receptor. J. Mol. Biol. 2024, 436, 168688. [Google Scholar] [CrossRef] [PubMed]
- Tobo, A.; Tobo, M.; Nakakura, T.; Ebara, M.; Tomura, H.; Mogi, C.; Im, D.S.; Murata, N.; Kuwabara, A.; Ito, S.; et al. Characterization of Imidazopyridine Compounds as Negative Allosteric Modulators of Proton-Sensing GPR4 in Extracellular Acidification-Induced Responses. PLoS ONE 2015, 10, e0129334. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, N.; Benabbas, C.; Vidal, S.; Daryadel, A.; Bourgeois, S.; Velic, A.; Ludwig, M.G.; Seuwen, K.; Wagner, C.A. The proton-activated G protein coupled receptor OGR1 acutely regulates the activity of epithelial proton transport proteins. Cell Physiol. Biochem. 2012, 29, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Fantini, M.C.; Wirtz, S.; Nikolaev, A.; Kiesslich, R.; Lehr, H.A.; Galle, P.R.; Neurath, M.F. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 2005, 54, 950–954. [Google Scholar] [CrossRef]
- Obermeier, F.; Kojouharoff, G.; Hans, W.; Scholmerich, J.; Gross, V.; Falk, W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin. Exp. Immunol. 1999, 116, 238–245. [Google Scholar] [CrossRef]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef]
- Rittie, L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. Methods Mol. Biol. 2017, 1627, 395–407. [Google Scholar] [CrossRef]
- Tomura, H.; Wang, J.Q.; Komachi, M.; Damirin, A.; Mogi, C.; Tobo, M.; Kon, J.; Misawa, N.; Sato, K.; Okajima, F. Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J. Biol. Chem. 2005, 280, 34458–34464. [Google Scholar] [CrossRef]
- Xu, Y.; Casey, G. Identification of human OGR1, a novel G protein-coupled receptor that maps to chromosome 14. Genomics 1996, 35, 397–402. [Google Scholar] [CrossRef]



| Appearance | 0 1 2 3 4 | normal (normal well-groomed, smooth fur, clean orifices of the body, clear eyes) marginal (slightly disordered fur, eyes not clotted) minor (dull fur, untended, some orifices of the body marginally clotted) moderate (rough coat, some orifices of the body clotted) elevated (orifices clearly clotted, abnormal body posture) |
| behavior/activity | 0 1 2 3 4 | normal (social interaction, curiosity, grouped sleeping with others, reacts to touch) marginal deviation (reduced movement when cage is opened, reduced interest) mild (less active, but still in the group) moderate (increased inactivity, keeps isolated) elevated (almost no movement) |
| Pain | 0 1 2 3 | no (relaxed body and moving behavior) minor (active, but seldom belly tension observed, no hunching, normal eyes) mild (occasionally wide gait, belly tension more frequent, hunching) severe (frequent belly tension, hunched back, breathing frequency elevated, body temperature reduced, squinted eyes) |
| Stool consistency | 0 1 2 3 4 | solid soft, but formed pellets soft, no pellets formed very soft, but still with solid particles liquid |
| Blood in stool | 0 1 2 | no blood in stool sporadically visible (but dry) traces of blood in stool blood in stool or in cage clearly detectable fresh blood in cage clearly visible or rectal blood loss clearly visible |
| Weight loss | 0 1 2 3 4 5 | stable or weight gain <5% 5–10% 11–15% 16–20% ≥20% |
| Thickening of the colon | 0 1 2 3 | transparent moderate marked non-transparent |
| Changes in the vascular pattern | 0 1 2 3 | normal moderate marked bleeding |
| Fibrin visible | 0 1 2 3 | none little marked extreme |
| Granularity of the mucosal surface | 0 1 2 3 | none moderate marked extreme |
| Stool consistency | 0 1 2 3 | normal and solid still shaped unshaped spread |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, F.; Schuler, C.; Ruiz, P.A.; Perren, L.; Malagola, E.; de Vallière, C.; Seuwen, K.; Hausmann, M.; Rogler, G. The Simultaneous Deletion of pH-Sensing Receptors GPR4 and OGR1 (GPR68) Ameliorates Colitis with Additive Effects on Multiple Parameters of Inflammation. Int. J. Mol. Sci. 2025, 26, 1552. https://doi.org/10.3390/ijms26041552
Foti F, Schuler C, Ruiz PA, Perren L, Malagola E, de Vallière C, Seuwen K, Hausmann M, Rogler G. The Simultaneous Deletion of pH-Sensing Receptors GPR4 and OGR1 (GPR68) Ameliorates Colitis with Additive Effects on Multiple Parameters of Inflammation. International Journal of Molecular Sciences. 2025; 26(4):1552. https://doi.org/10.3390/ijms26041552
Chicago/Turabian StyleFoti, Federica, Cordelia Schuler, Pedro A. Ruiz, Leonie Perren, Ermanno Malagola, Cheryl de Vallière, Klaus Seuwen, Martin Hausmann, and Gerhard Rogler. 2025. "The Simultaneous Deletion of pH-Sensing Receptors GPR4 and OGR1 (GPR68) Ameliorates Colitis with Additive Effects on Multiple Parameters of Inflammation" International Journal of Molecular Sciences 26, no. 4: 1552. https://doi.org/10.3390/ijms26041552
APA StyleFoti, F., Schuler, C., Ruiz, P. A., Perren, L., Malagola, E., de Vallière, C., Seuwen, K., Hausmann, M., & Rogler, G. (2025). The Simultaneous Deletion of pH-Sensing Receptors GPR4 and OGR1 (GPR68) Ameliorates Colitis with Additive Effects on Multiple Parameters of Inflammation. International Journal of Molecular Sciences, 26(4), 1552. https://doi.org/10.3390/ijms26041552






