Peripheral Blood Mononuclear Cells Cytokine Profile in a Patient with Toxic Epidermal Necrolysis Triggered by Lamotrigine and COVID-19: A Case Study
Abstract
1. Introduction
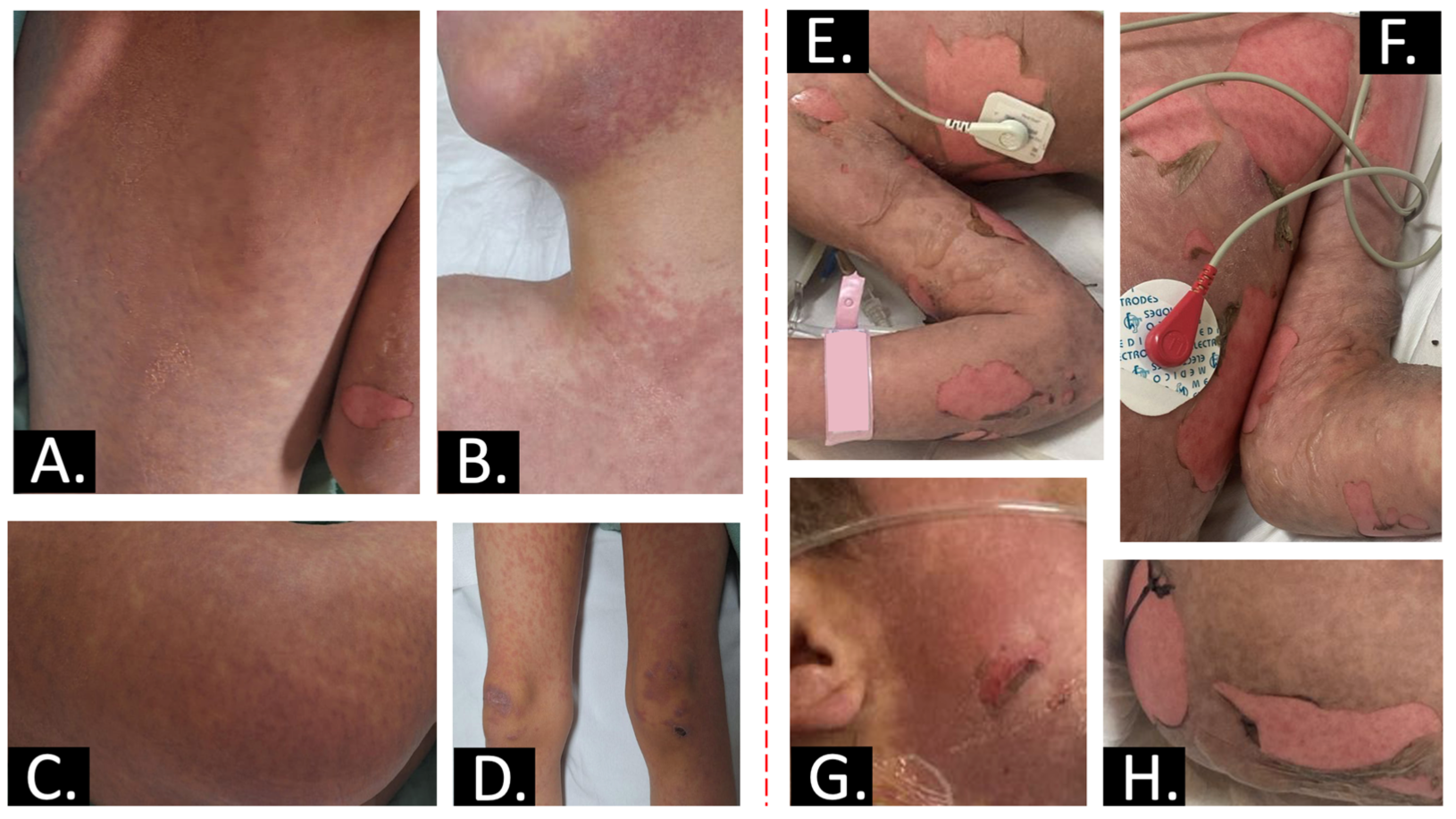
2. Case Report
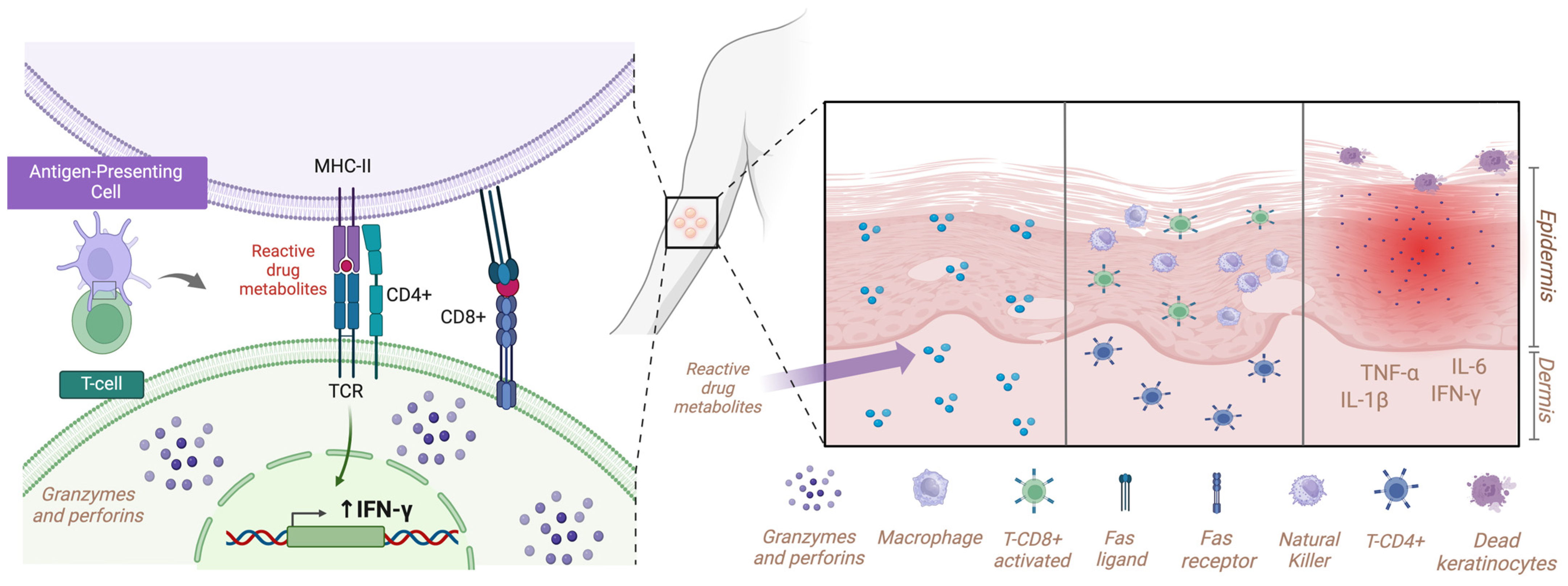
3. Discussion
Study Limitations and Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bastuji-Garin, S.; Rzany, B.; Stern, R.S.; Shear, N.H.; Naldi, L.; Roujeau, J.C. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch. Dermatol. 1993, 129, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Frantz, R.; Huang, S.; Are, A.; Motaparthi, K. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management. Medicina 2021, 57, 895. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, P.; Mockenhaupt, M.; Panzer, R.; Emmert, S. Erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis–diagnosis and treatment. J. Dtsch. Dermatol. Ges. 2020, 18, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Madariaga, C.; Aviles-Ku, D.C.; Carrillo-Lugo, M.S.; Pacheco-Pino, P.A.; Bobadilla-Rosado, L.O.; Méndez-Domínguez, N. Características epidemiológicas de las hospitalizaciones secundarias a síndrome de Stevens-Johnson y necrólisis epidérmica tóxica en México. Dermatol. Rev. Mex. 2022, 66, 654–662. [Google Scholar] [CrossRef]
- Mockenhaupt, M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert Rev. Clin. Immunol. 2011, 7, 803–813. [Google Scholar] [CrossRef]
- Borrelli, E.P.; Lee, E.Y.; Descoteaux, A.M.; Kogut, S.J.; Caffrey, A.R. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia 2018, 59, 2318–2324. [Google Scholar] [CrossRef]
- Duong, T.A.; Valeyrie-Allanore, L.; Wolkenstein, P.; Chosidow, O. Severe cutaneous adverse reactions to drugs. Lancet 2017, 390, 1996–2011. [Google Scholar] [CrossRef]
- Garg, V.K.; Buttar, H.S.; Bhat, S.A.; Ainur, N.; Priya, T.; Kashyap, D.; Tuli, H.S. Stevens-johnson Syndrome and Toxic Epidermal Necrolysis: An Overview of Diagnosis, Therapy Options and Prognosis of Patients. Recent Adv. Inflamm. Allergy Drug Discov. 2023, 17, 110–120. [Google Scholar] [CrossRef]
- Varol, F.; Can, Y.Y.; Sahin, E.; Durak, C.; Kilic, A.; Sahin, C.; Gursoy, F.; Akin, T. The role of treatment with plasma exchange therapy in two pediatric toxic epidermal necrolysis cases related to COVID-19. J. Clin. Apher. 2022, 37, 516–521. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Hong, H.S.; Hsih, M.S.; Yang, L.C.; Ho, H.C.; Wu, J.Y.; Chen, Y.T. Medical genetics: A marker for Stevens-Johnson syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef]
- Posadas, S.J.; Padial, A.; Torres, M.J.; Mayorga, C.; Leyva, L.; Sanchez, E.; Alvarez, J.; Romano, A.; Juarez, C.; Blanca, M. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J. Allergy Clin. Immunol. 2002, 109, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Metbulut, A.P.; Ozkaya Parlakay, A.; Bayhan, G.I.; Kanik Yuksek, S.; Gulhan, B.; Sengul Emeksiz, Z.; Senel, E.; Dibek Misirlioglu, E. Evaluation of cutaneous symptoms in children infected with COVID-19. Pediatr. Allergy Immunol. 2021, 32, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, A.O.S.; Karaatmaca, B.; Tiftik, M.; Cinel, G.; Senel, E. COVID-19 presenting like Steven Johnson Syndrome in a pediatric patient. Authorea 2020, 1–4. [Google Scholar] [CrossRef]
- Zou, H.; Daveluy, S. Toxic epidermal necrolysis and Stevens-Johnson syndrome after COVID-19 infection and vaccination. Australas. J. Dermatol. 2023, 64, e1–e10. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Update–31 August 2020. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update (accessed on 31 August 2020).
- Roujeau, J.C.; Stern, R.S. Severe adverse cutaneous reactions to drugs. N. Engl. J. Med. 1994, 331, 1272–1285. [Google Scholar] [CrossRef]
- Pavlos, R.; Mallal, S.; Phillips, E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics 2012, 13, 1285–1306. [Google Scholar] [CrossRef]
- Jouhar, L.; Yahya, M.; Elsiddiq, S. Toxic Epidermal Necrolysis associated with COVID-19 infection: A case report. Clin. Case Rep. 2022, 10, e05565. [Google Scholar] [CrossRef]
- Westly, E.D.; Wechsler, H.L. Toxic epidermal necrolysis. Granulocytic leukopenia as a prognostic indicator. Arch. Dermatol. 1984, 120, 721–726. [Google Scholar] [CrossRef]
- Ferrandiz-Pulido, C.; Garcia-Patos, V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch. Dis. Child. 2013, 98, 998–1003. [Google Scholar] [CrossRef]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, S.; Li, S.; Pan, Y.; Ding, Y. Biologic TNF-alpha inhibitors in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: A systemic review. J. Dermatol. Treat. 2020, 31, 66–73. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Reference | Hospitalization Days | |||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | ||
| Red blood cells | |||||
| Erythrocyte (106/μL) | 3.0–5.3 | 3.87 | 3.61 | NT | 3.62 |
| Hemoglobin (g/dL) | 12.6–16.0 | 11.6 * | 11.1 * | NT | 11.1 * |
| Hematocrit (%) | 42.0–51.0 | 35.3 * | 33.1 * | NT | 33.9 * |
| Mean corpuscular volume (fL) | 80.0–100.0 | 91.2 | 91.7 | NT | 93.6 |
| MCH (pg/cell) | 27.0–32.0 | 30 | 30.7 | NT | 30.7 |
| MCHC (g/dL) | 32.0–36.0 | 32.9 | 33.5 | NT | 32.7 |
| Red cell distribution width (%) | 11.0–17.0 | 14.1 | 14.3 | NT | 14 |
| Platelets (103/μL) | 150.0–400.0 | 150 | 119 * | NT | 162 |
| Mean platelet volume (fL) | 7.0–11.0 | 9.1 | 9.6 | NT | 8.3 |
| White blood cells | |||||
| Leukocytes (103/μL) | 5.0–10.0 | 6.38 | 3.9 * | NT | 3.6 * |
| Neutrophils (%) | 37.0–75 | 82.5 * | 74.2 | NT | 50.7 |
| Lymphocytes (%) | 17.0–45.0 | 10.7 * | 16.4 * | NT | 35.7 |
| Monocyte (%) | 2.0–12.0 | 6.4 | 9.4 | NT | 13.6 * |
| Eosinophils (%) | 1.0–7.0 | 0.2 * | NT | NT | NT |
| Basophils (%) | 0.3–2.0 | 0.2 * | NT | NT | NT |
| Leukocyte absolute values | |||||
| Lymphocytes (103/μL) | 1.0–5.0 | 0.68 * | 0.6 * | NT | 1.3 |
| Neutrophils (103/μL) | 2.0–8.0 | 5.27 | 2.9 | NT | 1.8 * |
| Monocyte (103/μL) | 0.1–1.0 | 0.41 | 0.4 | NT | 0.5 |
| Eosinophils (103/μL) | 0.0–0.4 | 0 | NT | NT | NT |
| Basophils (103/μL) | 0.0–0.2 | 0 | NT | NT | NT |
| Leukocyte differential | |||||
| Lymphocytes (%) | NA | 17 * | NT | NT | NT |
| Monocyte (%) | NA | 9 | NT | NT | NT |
| Segmented neutrophils (%) | NA | 40 * | NT | NT | NT |
| Bands (%) | NA | 34 * | NT | NT | NT |
| Eosinophils (%) | NA | 0 | NT | NT | NT |
| Basophils (%) | NA | 0 | NT | NT | NT |
| Coagulation parameters | |||||
| Prothrombin time (seconds) | 11.0–14.0 | 22.9 * | NT | 15.7 * | NT |
| International normalized ratio (%) | 0.93–1.5 | 1.77 * | NT | 1.18 | NT |
| PTT (seconds) | 26.0–40.0 | 40.7 * | NT | 35.4 | NT |
| D dimer (μg/mL) | <0.50 | 1.37 | NT | NT | NT |
| Fibrinogen (mg/dL) | 200–400 | 274 | NT | NT | NT |
| Biochemical parameters | |||||
| Ferritin (ng/mL) | 30–400 | 397.6 | NT | NT | NT |
| Glucose (mg/dL) | 70.0–110.0 | 147 * | 112 * | 126 * | 121 * |
| Urea (mg/dL) | 15.0–43.0 | 18.7 | 18.3 | 17.7 | 20.8 |
| Serum creatinine (mg/dL) | 0.7–1.5 | 0.36 | 0.27 * | 0.25 * | 0.28 * |
| Blood urea nitrogen (mg/dL) | 7.0–20 | 9 | 8.6 | NT | 10 |
| Sodium (mmol/L) | 135.0–148.0 | 140 | 138.3 | 137 | 146 |
| Potassium (mmol/L) | 3.5–5.3 | 3.04 * | 3.9 | 3.57 | 4.15 |
| Chlorine (mmol/L) | 98.0–107.0 | 106.5 | 108.7 * | 106.5 | 110.4 * |
| Calcium (mg/dL) | 8.4–10.2 | 7.15 | 9.2 | 7.27 * | 7.81 * |
| Phosphorous (mg/dL) | 2.5–4.5 | 3.2 | 3.3 | 2.99 | 3.48 |
| Magnesium (mg/dL) | 1.6–2.3 | 1.7 | 1.6 | 1.74 | 1.98 |
| Bilirubin total (mg/dL) | 0.2–1.3 | 0.19 | 0.19 | NT | 0.22 |
| Direct bilirubin (mg/dL) | 0.0–0.4 | 0.1 | 0.09 | NT | 0.13 |
| Indirect bilirubin (mg/dL) | 0.0–1.0 | 0.09 | 0.1 | NT | 0.09 |
| AST (U/L) | 10.0–42.0 | 55 * | 67.4 * | NT | 63.4 * |
| ALP (U/L) | 38.0–126.0 | 98 | 83.7 | NT | 95 |
| ALT (U/L) | 0.0–42.0 | 18.9 | 27.1 | NT | 35.2 |
| LDH (U/L) | 135.0–225.0 | 712 * | 512 * | NT | 451 * |
| Globulin (mg/dL) | 2–3.3 | 1.96 * | 2.99 | NT | 2.5 |
| Albumin (g/dL) | 3.8–5.10 | 3.02 * | 2.99 * | 2.65 * | 2.94 * |
| Albumin globulin ratio | 1.10–18.10 | 1.54 | 0.94 * | NT | 1.18 |
| Total protein (g/dL) | 6.4–8.3 | 4.98 * | 5.8 * | NT | 5.44 * |
| CKMB (U/L) | 7.0–25.0 | 437.2 * | NT | NT | NT |
| CK (U/L) | 20.0–180.0 | 311 * | NT | NT | NT |
| Triglycerides (mg/dL) | 40.0–160.0 | NT | 78 | NT | 86 |
| Total cholesterol (mg/dL) | 50.0–200.0 | NT | 91 | NT | 121 |
| HDL cholesterol (mg/dL) | 40.0–45.0 | NT | 38.5 * | NT | 46.1 |
| LDL cholesterol (mg/dL) | 50.0–172.0 | NT | 75.4 | NT | 94.6 |
| VLDL cholesterol (mg/dL) | 45.0–65.0 | NT | 15.6 * | NT | 17.2 * |
| C reactive protein (mg/dL) | 0.25–0.65 | NT | NT | NT | 11.98 * |
| Procalcitonine (ng/mL) | <0.5 | NT | NT | NT | 0.781 |
| Cytokine | Type of Immune Response | Types of Producing Cells | Expected Level | Observed Level | Possible Causes of/Possible Explanations for Discrepancy |
|---|---|---|---|---|---|
| IL-1a | Innate | Monocytes, macrophages | ↑ | ↑ | Severe inflammation from TEN |
| IL-1b | Innate | Monocytes, macrophages | ↑ | ↑ | Severe inflammation from TEN |
| IL-6 | Innate | Monocytes, macrophages, T cells | ↑ | ↓ | Acute inflammation from TEN and COVID-19/individual variability, disease phase, viral effect |
| IL-8 | Innate | Monocytes, macrophages, T cells | ↑ | ↑ | Inflammation and neutrophil attraction |
| NFkβ | Innate | All cells | ↑ | ↑ | Pro-inflammatory genes regulation |
| IL-2 | Adaptive | T cells | ↑ | ND | T cell activation/Immunosuppression or individual variability |
| IL-4 | Adaptive | Th2, mast cells | Variable | ↓ | Th2 response/immunosuppression, individual variability |
| IL-5 | Adaptive | Th2, eosinophils | Variable | ↑ | Th2 response and eosinophils/inflammation and Th2 response |
| IL-10 | Adaptive | Monocytes, macrophages, Th2 | ↑ | ND | Anti-inflammatory regulation/insufficient regulatory response |
| IL-12 | Adaptive, Antiviral | Dendritic cells, macrophages | ↑ | ↓ | Antiviral and Th1 response/viral evasion, individual variability |
| INFα | Antiviral | Dendritic cells, macrophages | ↑ | ↑ | Antiviral response |
| INFβ | Antiviral | Dendritic cells, fibroblasts | ↑ | ↑ | Antiviral response |
| INFγ | Antiviral, Adaptive | T cells, NK cells | ↑ | ↑ | Antiviral response and macrophage activation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Fierro, M.L.; Garza-Veloz, I.; Zorrilla-Alfaro, S.M.; Campuzano-Garcia, A.E.; Rodriguez-Borroel, M. Peripheral Blood Mononuclear Cells Cytokine Profile in a Patient with Toxic Epidermal Necrolysis Triggered by Lamotrigine and COVID-19: A Case Study. Int. J. Mol. Sci. 2025, 26, 1374. https://doi.org/10.3390/ijms26031374
Martinez-Fierro ML, Garza-Veloz I, Zorrilla-Alfaro SM, Campuzano-Garcia AE, Rodriguez-Borroel M. Peripheral Blood Mononuclear Cells Cytokine Profile in a Patient with Toxic Epidermal Necrolysis Triggered by Lamotrigine and COVID-19: A Case Study. International Journal of Molecular Sciences. 2025; 26(3):1374. https://doi.org/10.3390/ijms26031374
Chicago/Turabian StyleMartinez-Fierro, Margarita L., Idalia Garza-Veloz, Sidere Monserrath Zorrilla-Alfaro, Andrés Eduardo Campuzano-Garcia, and Monica Rodriguez-Borroel. 2025. "Peripheral Blood Mononuclear Cells Cytokine Profile in a Patient with Toxic Epidermal Necrolysis Triggered by Lamotrigine and COVID-19: A Case Study" International Journal of Molecular Sciences 26, no. 3: 1374. https://doi.org/10.3390/ijms26031374
APA StyleMartinez-Fierro, M. L., Garza-Veloz, I., Zorrilla-Alfaro, S. M., Campuzano-Garcia, A. E., & Rodriguez-Borroel, M. (2025). Peripheral Blood Mononuclear Cells Cytokine Profile in a Patient with Toxic Epidermal Necrolysis Triggered by Lamotrigine and COVID-19: A Case Study. International Journal of Molecular Sciences, 26(3), 1374. https://doi.org/10.3390/ijms26031374







