Biomass-Derived Nanoporous Carbon Honeycomb Monoliths for Environmental Lipopolysaccharide Adsorption from Aqueous Media
Abstract
:1. Introduction
2. Results and Discussion
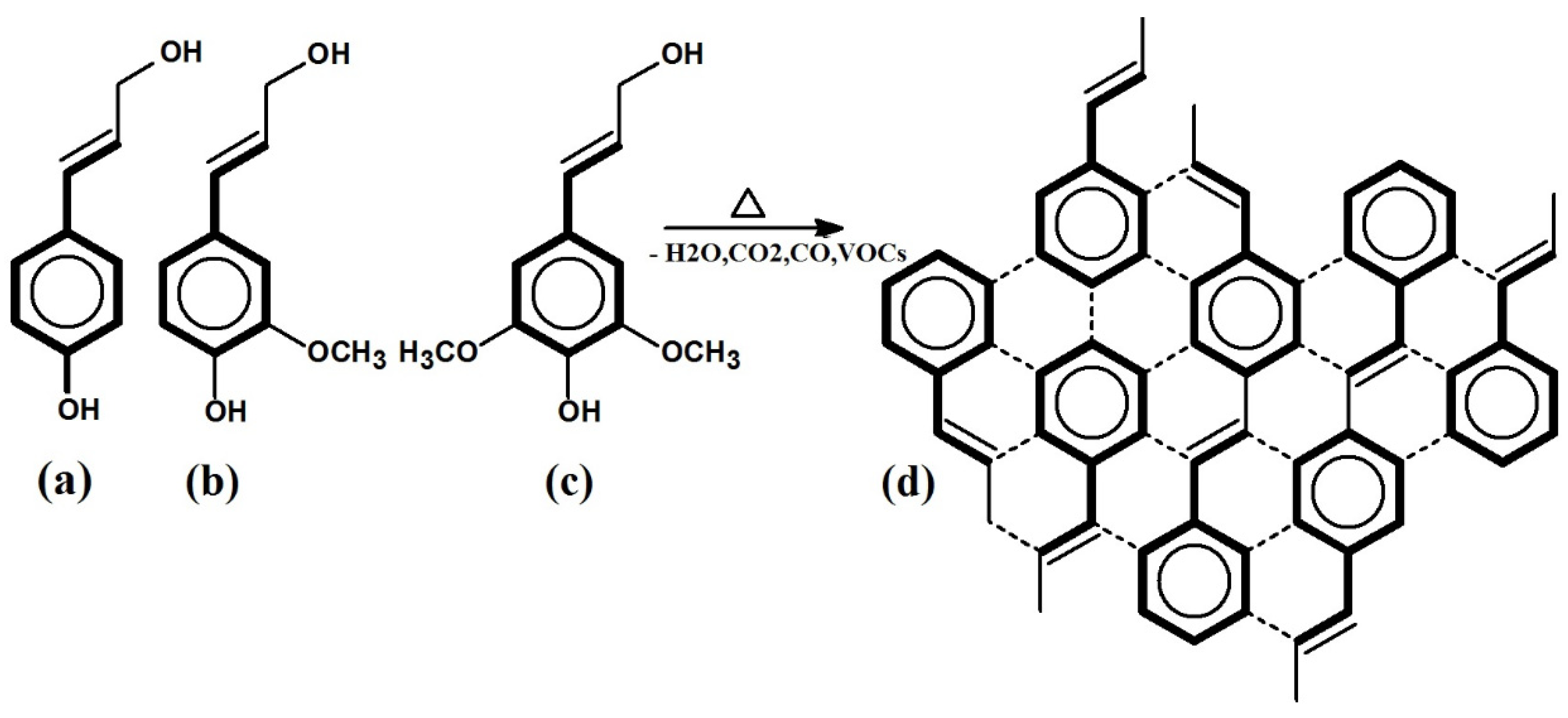
2.1. Synthesis and Structure of Rice Husk–Lignin-Derived Honeycomb Carbon Monoliths
2.2. Physicochemical Investigation of Rice Husk-Derived Honeycomb Carbon Monoliths
2.2.1. Low-Temperature Nitrogen Adsorption and Mercury Intrusion Porosimetry
2.2.2. Investigation of the Internal Morphology of the CD-Monolith by SEM Imaging
2.3. Bacterial Lipopolysaccharide Adsorption Studies
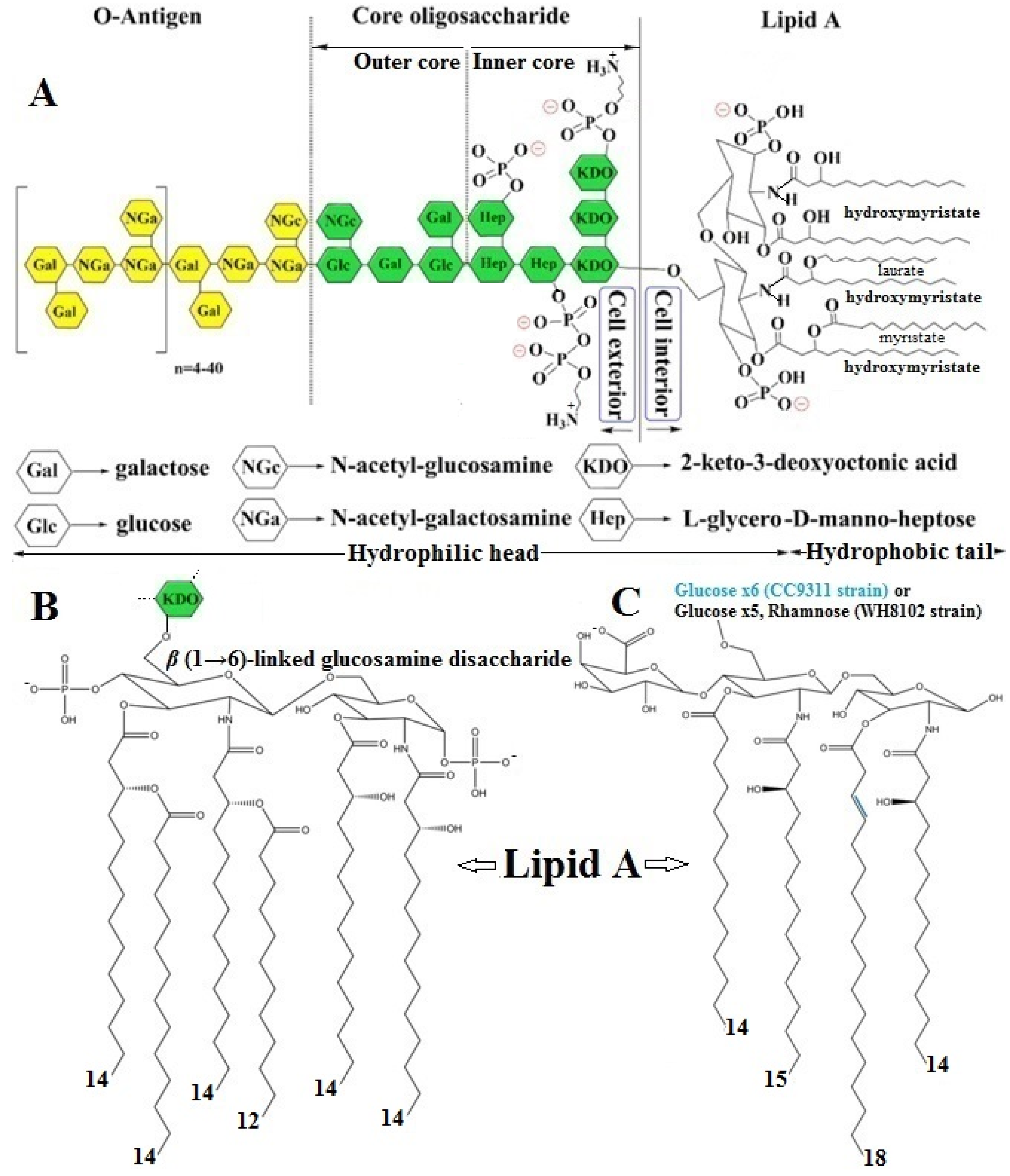
2.3.1. LPS Structure, Function, and Mode of Biochemical Action
- (1)
- A hydrophilic “core oligosaccharide” chain linkage region (core antigen or R antigen) is associated with immunogenicity and is generally composed of about 10 sugars and can be divided into an inner and outer region. The outer core region covalently binds an O-antigen moiety and contains common sugars, such as hexoses or partially N-acetylated hexosamines, whereas the inner core region, covalently bonded to the Lipid A moiety, is highly conserved across bacterial species and contains the partially phosphorylated unusual sugars, such as L-glycero-D-manno heptose and 3-deoxy-D-manno-octulosonic acid, also known as keto-deoxyoctulosonate (KDO) [47,52].
- (2)
- A periodic hydrophilic antibody-binding O-antigenic (somatic) polysaccharide side chain that extends out into the environment is highly variable depending on the bacterial serotype, e.g., species and strain. The optional “O-antigen” consists of a variety of around eight different sugar moieties, usually hexoses, or sometimes partially N-acetylated hexosamines, including a repetitive polysaccharide monomeric subunit of 3–5 sugars that can build a chain of up to 50 repeats [47,52].
- (3)
- A long, as well as bifurcated, hydrophilic carbohydrate and hydrophobic lipid moiety, termed“Lipid A”, is attached to the outer O-antigen carbohydrate chain via a“core oligosaccharide” linker and is mainly accountable for the proinflammatory endotoxic activity of the LPS molecule. The innermost Lipid A has enormous architectural diversity, but is extremely conserved across bacterial species and commonly has up to two (bi)-phosphorylated β(1→6)-linked glucosamine disaccharide backbones, which are mostly phosphorylated at position 1 and 4′ of the saccharides and acylated at positions 2 and 3 of each monosaccharide portion, connected with two to nine fatty acyl chains, typically composed of 10–16 carbon atoms each, through ester or amide linkages, anchoring the LPS molecule to the OM of the cell wall. The Lipid A glucosamine disaccharide of E. coli and Salmonella typhimurium spp. is linked to hydroxy fatty acids, such as hydroxymyristic acid, that are further acylated by nonhydroxylated fatty acids, such as myristic (tetradecanoic) and lauric (dodecanoic) acids [47,49,51] (Figure 6B).
2.3.2. Justification of LPS Concentration
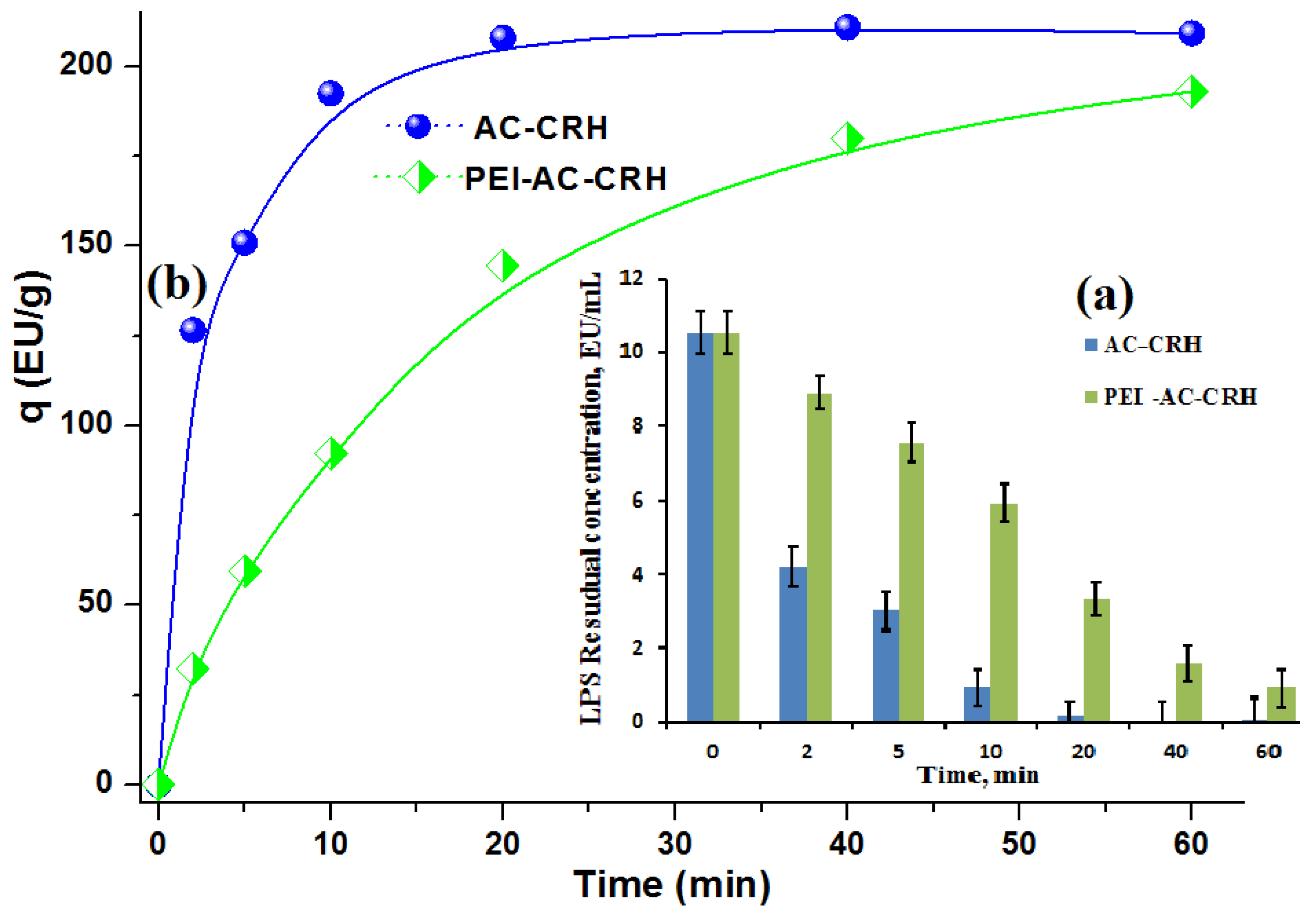
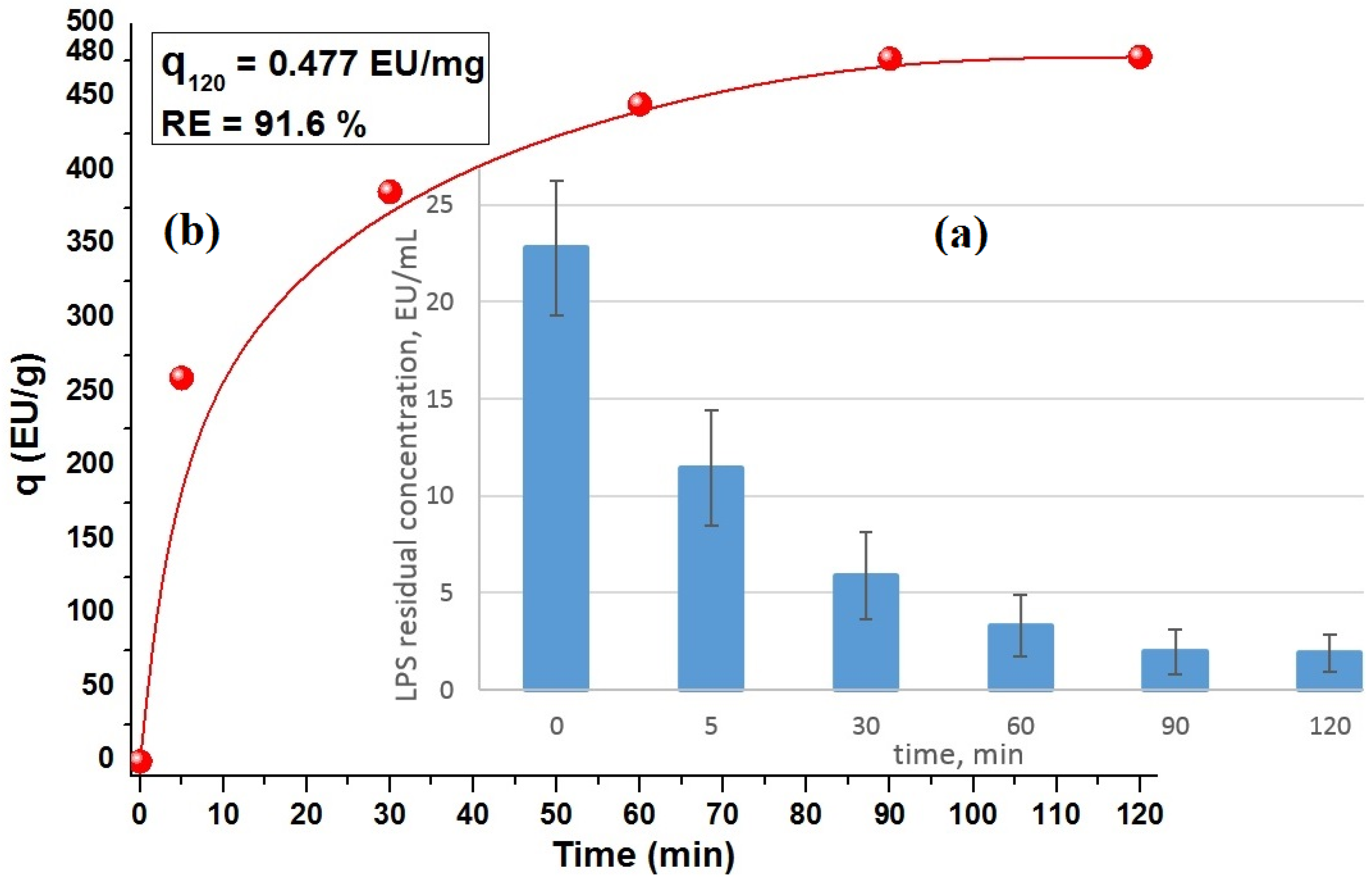
2.3.3. Adsorption of LPSs Using RH–Lignin-Derived Carbonized–Desilicated Honeycomb Monoliths
2.3.4. Relationship of LPS Adsorption with Textural Characteristics of Carbon Monoliths and Its Potential Use/Modification
3. Conclusions
4. Experimental
4.1. Materials and Methods
4.2. Preparation of AC-CRH and PEI-AC-CRH Adsorbents
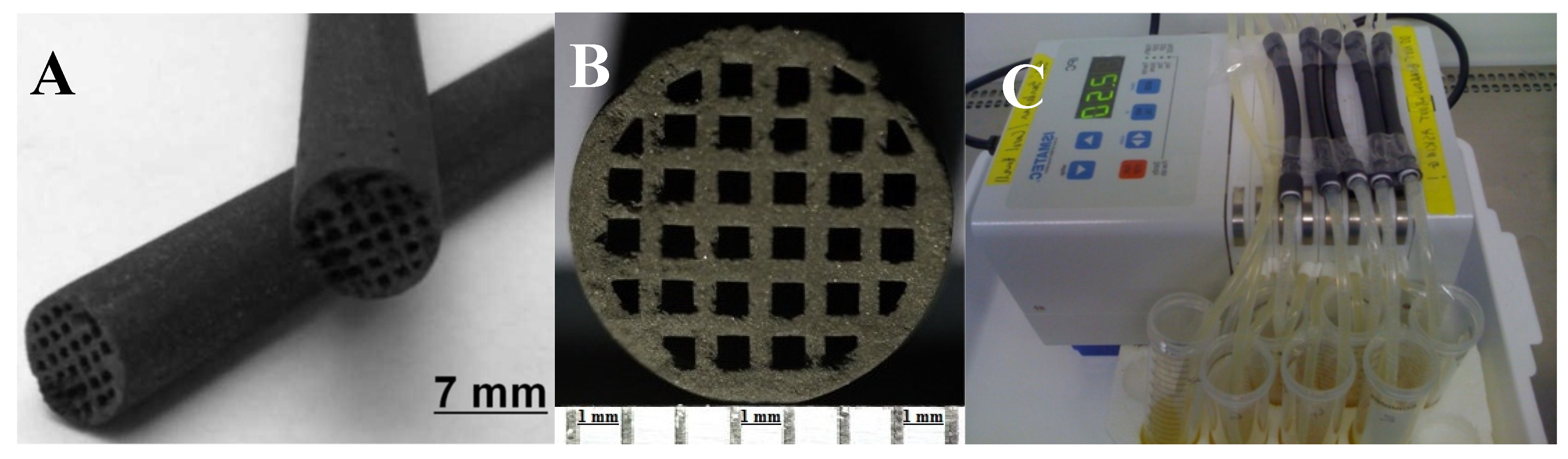
4.3. Preparation of Honeycomb Carbon Monoliths
4.4. Honeycomb Carbon Monolith Geometry Design, Parameters, and Function
4.5. Physical and Physicochemical Methods of Investigation
4.5.1. Low-Temperature Nitrogen Adsorption Studies
4.5.2. Mercury Intrusion Porosimetry
4.5.3. Scanning Electron Microscopy Imaging
4.6. In Vitro Adsorption of LPS-Endotoxins
4.6.1. Batch Adsorption of LPS by AC-CRH and PEI-AC-CRH Samples
4.6.2. In Vitro Adsorption of LPS by Carbon CD-Monoliths
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Economic and Social Affairs The 17 Goals. Available online: https://sdgs.un.org/goals (accessed on 24 December 2024).
- Water Pollution and Health. European Environment Agency, 2022. Available online: https://www.eea.europa.eu/publications/zero-pollution/health/water-pollution (accessed on 30 April 2023).
- Marshall, J.C. Lipopolysaccharide: An Endotoxin or an Exogenous Hormone? Clin. Infect. Dis. 2005, 41, S470–S480. [Google Scholar] [CrossRef] [PubMed]
- Amini Tapouk, F.; Nabizadeh, R.; Nasseri, S.; Mesdaghinia, A.; Khorsandi, H.; Mahvi, A.H.; Gholibegloo, E.; Alimohammadi, M.; Khoobi, M. Endotoxin Removal from Aqueous Solutions with Dimethylamine-Functionalized Graphene Oxide: Modeling Study and Optimization of Adsorption Parameters. J. Hazard. Mater. 2019, 368, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.O.; Lopes, A.M.; Mazzola, P.G.; Rangel-Yagui, C.; Penna, T.C.V.; Pessoa, A.J. Methods of Endotoxin Removal from Biological Preparations: A Review. J. Pharm. Sci. 2007, 10, 388–404. [Google Scholar]
- Zhang, K.; Liu, W.; Sun, W.; Zhang, M.; Qian, L.; Li, C.; Tian, F. Endotoxin Contamination and Control in Surface Water Sources and a Drinking Water Treatment Plant in Beijing, China. Water Res. 2013, 47, 3591–3599. [Google Scholar] [CrossRef]
- Anderson, W.B.; Mayfield, C.I.; Dixon, D.G.; Huck, P.M. Endotoxin Inactivation by Selected Drinking Water Treatment Oxidants. Water Res. 2003, 37, 4553–4560. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Alamri, S.; Hashem, M. Endotoxin Removal Efficiency in Conventional Drinking Water Treatment Plants, a Case Study in Egypt. Water SA 2022, 48, 180–186. [Google Scholar] [CrossRef]
- European Pharmacopoeia 10.0; Water for Injections; European Pharmacopoeia: Strasbourg, France, 2017; Volume 0169, pp. 4203–4206.
- Cron, R.Q.; Caricchio, R.; Chatham, W.W. Calming the Cytokine Storm in COVID-19. Nat. Med. 2021, 27, 1674–1675. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Duda, K.A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. A Journey from Structure to Function of Bacterial Lipopolysaccharides. Chem. Rev. 2022, 122, 15767–15821. [Google Scholar] [CrossRef]
- Vukić Lušić, D.; Maestro, N.; Cenov, A.; Lušić, D.; Smolčić, K.; Tolić, S.; Maestro, D.; Kapetanović, D.; Marinac-Pupavac, S.; Tomić Linšak, D.; et al. Occurrence of P. aeruginosa in water intended for human consumption and in swimming pool water. Environments 2021, 8, 132. [Google Scholar] [CrossRef]
- Durai, P.; Batool, M.; Choi, S. Structure and Effects of Cyanobacterial Lipopolysaccharides. Mar. Drugs 2015, 13, 4217–4230. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.-G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Crone, S. The environmental occurrence of Pseudomonas aeruginosa. J. Pathol. Microbiol. Immunol.–APMIS J. 2020, 128, 220–231. [Google Scholar] [CrossRef]
- Plaas, H.E.; Paerl, H.W. Toxic Cyanobacteria: A Growing Threat to Water and Air Quality. Environ. Sci. Technol. 2021, 55, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Bláha, L. Toxins produced in cyanobacterial water blooms–toxicity and risks. Interdisc. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Rapala, J.; Lahti, K.; Räsänen, L.A.; Esala, A.-L.; Niemelä, S.I.; Sivonen, K. Endotoxins Associated with Cyanobacteria and Their Removal during Drinking Water Treatment. Water Res. 2002, 36, 2627–2635. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, T.; Zhou, J.; Guo, H.; Qu, G.; Guo, X.; Jia, H.; Zhu, L. Intrinsic Mechanisms Underlying the Highly Efficient Removal of Bacterial Endotoxin and Related Risks in Tailwater by Dielectric Barrier Discharge Plasma. Water Res. 2022, 226, 119214. [Google Scholar] [CrossRef]
- Pérez-García, R.; Rodríguez-Benítez, P.O. Why and How to Monitor Bacterial Contamination of Dialysate? Nephrol. Dial. Transplant. 2000, 15, 760–764. [Google Scholar] [CrossRef]
- Razdan, S.; Adler, J.; Barua, D.; Barua, S. Multifunctional Biofilter to Effectively Remove Toxins. ACS Appl. Bio Mater. 2021, 4, 731–741. [Google Scholar] [CrossRef]
- Oh, B.T.; Seo, Y.S.; Sudhakar, D.; Choe, J.H.; Lee, S.M.; Park, Y.J.; Cho, M. Oxidative degradation of endotoxin by advanced oxidation process (O3/H2O2 & UV/H2O2). J. Hazard. Mater. 2014, 279, 105–110. [Google Scholar] [CrossRef]
- Zielińska, A.; Soles, B.B.; Lopes, A.R.; Vaz, B.F.; Rodrigues, C.M.; Alves, T.F.R.; Klensporf-Pawlik, D.; Durazzo, A.; Lucarini, M.; Severino, P.; et al. Nanopharmaceuticals for Eye Administration: Sterilization, Depyrogenation and Clinical Applications. Biology 2020, 9, 336. [Google Scholar] [CrossRef]
- Metzger, K.F.J.; Voloshin, A.; Schillinger, H.; Kühnel, H.; Maurer, M. Adsorptive Filtration: A Case Study for Early Impurity Reduction in an Escherichia Coli Production Process. Biotechnol. Prog. 2020, 36, e2948. [Google Scholar] [CrossRef] [PubMed]
- Ismagilov, Z.; Shikina, N.; Andrievskaya, I.; Rudina, N.; Mansurov, Z.; Burkitbaev, M.; Biisenbaev, M.; Kurmanbekov, A. Preparation of Carbonized Rice Husk Monoliths and Modification of the Porous Structure by SiO2 Leaching. Catal. Today 2014, 147, S58–S65. [Google Scholar] [CrossRef]
- Li, Y.; Ding, X.; Guo, Y.; Rong, C.; Wang, L.; Qu, Y.; Ma, X.; Wang, Z. A New Method of Comprehensive Utilization of Rice Husk. J. Hazard. Mater. 2011, 186, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, E.; Nordin, A.; Rao, A.N. Overview of Combustion and Gasification of Rice Husk in Fluidized Bed Reactors. Biomass Bioenergy 1998, 14, 533–546. [Google Scholar] [CrossRef]
- Jandosov, J.; Mansurov, Z.A.; Bijsenbayev, M.A.; Tulepov, M.I.; Ismagilov, Z.R.; Shikina, N.V.; Ismagilov, I.Z.; Andrievskaya, I.P. Synthesis of Microporous-Mesoporous Carbons from Rice Husk via H3PO4-Activation. Adv. Mater. Res. 2013, 602, 85–89. [Google Scholar] [CrossRef]
- Dias, D.; Don, D.; Jandosov, J.; Bernardo, M.; Pinto, F.; Fonseca, I.; Sanches, A.; Sá Caetano, P.; Lyubchyk, S.; Lapa, N. Highly efficient porous carbons for the removal of W (VI) oxyanion from wastewaters. J. Hazard. Mater. 2021, 412, 125201. [Google Scholar] [CrossRef]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Thomas Choong, S.Y. Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Neethirajan, S.; Gordon, R.; Wang, L. Potential of Silica Bodies (Phytoliths) for Nanotechnology. Trends Biotechnol. 2009, 27, 461–467. [Google Scholar] [CrossRef]
- Merkel, A.; Satayeva, A.; Cannon, F.; Howell, C.; Meikle, S.; László, K.; Inglezakis, V.; Jandosov, J.; Ray, S.; Mansurov, Z.; et al. Characterisation of activated carbons obtained from rice husk. Eurasian Chem.-Technol. J. 2016, 18, 299–304. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Biogenic Silica-Metal Phosphate (Metal = Ca, Fe or Zn) Nanocomposites: Fabrication from Rice Husk and Their Biomedical Applications. J. Mater. Sci. Mater. Med. 2014, 25, 1637–1644. [Google Scholar] [CrossRef]
- Malik, P.K. Use of Activated Carbons Prepared from Sawdust and Rice-Husk for Adsorption of Acid Dyes: A Case Study of Acid Yellow 36. Dye. Pigment. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Jandosov, J.M.; Mansurov, Z.A.; Biisenbayev, M.A.; Ismagilov, Z.R.; Shikina, N.V.; Ismagilov, I.Z.; Konuspayev, S.R.; Shaymardan, M. Mesoporous carbon-based rhodium catalysts for benzene hydrogenation. Eurasian Chem.-Technol. J. 2012, 14, 37–40. [Google Scholar] [CrossRef]
- Sandeman, S.R.; Zheng, Y.; Ingavle, G.C.; Howell, C.A.; Mikhalovsky, S.V.; Basnayake, K.; Boyd, O.; Davenport, A.; Beaton, N.; Davies, N.A. Haemocompatible and Scalable Nanoporous Adsorbent Monolith Synthesised Using a Novel Lignin Binder Route to Augment the Adsorption of Poorly Removed Uraemic Toxins in Haemodialysis. Biomed. Mater. 2017, 12, 35001. [Google Scholar] [CrossRef] [PubMed]
- Śmiechowicz, J. The Rationale and Current Status of Endotoxin Adsorption in the Treatment of Septic Shock. J. Clin. Med. 2022, 11, 619. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Betz, W.R.; Patel, S.; Murphy, M.C.; Mikhalovsky, S. V Adsorption of Lipopolysaccharide on Carbon Sieves. Carbon 2006, 44, 1258–1262. [Google Scholar] [CrossRef]
- Mansurov, Z.; Jandosov, J.; Kerimkulova, A.; Azat, S.; Zhubanova, A.; Digel, I.; Savistkaya, I.; Akimbekov, N.; Kistaubaeva, A.; Kistaubaeva, A. Nanostructured carbon materials for biomedical use. Eurasian Chem.-Technol. J. 2013, 15, 209–217. [Google Scholar] [CrossRef]
- Jandosov, J.; Mikhalovska, L.; Howell, C.; Chenchik, D.; Kosher, B.; Lyubchik, S.; Silvestre-Albero, J.; Ablaikhanova, N.; Srailova, G.; Tuleukhanov, S.; et al. Synthesis, Morphostructure, Surface Chemistry and Preclinical Studies of Nanoporous Rice Husk-Derived Biochars for Gastrointestinal Detoxification. Eurasian Chem. J. 2017, 19, 303–313. [Google Scholar] [CrossRef]
- Suhas, C.P.J.; Ribeiro, C.M.M. Lignin-from natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Rezaei, F.; Behrooz, R.; Arbab, S.; Sabet, E.N. Bacterial cellulose as a carbon nano-fiber precursor: Enhancement of thermal stability and electrical conductivity. BioResources 2020, 15, 3408–3426. [Google Scholar] [CrossRef]
- Jandosov, J.; Shikina, N.; Bijsenbayev, M.; Shamalov, M.; Ismagilov, Z.; Mansurov, Z. Evaluation of Synthetic conditions for H3PO4 chemically activated rice husk and preparation of honeycomb monoliths. Eurasian Chem.-Technol. J. 2009, 11, 245–252. [Google Scholar] [CrossRef]
- Haul, R.S.J.; Gregg, K.S.W. Sing: Adsorption, Surface Area and Porosity. 2. Auflage, Academic Press, London 1982. 303 Seiten, Preis: $ 49.50. Berichte Bunsenges. Phys. Chem. 1982, 86, 957. [Google Scholar] [CrossRef]
- Yushin, G.; Hoffman, E.N.; Barsoum, M.W.; Gogotsi, Y.; Howell, C.A.; Sandeman, S.R.; Phillips, G.J.; Lloyd, A.W.; Mikhalovsky, S.V. Mesoporous Carbide-Derived Carbon with Porosity Tuned for Efficient Adsorption of Cytokines. Biomaterials 2006, 27, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Fux, A.C.; Melo, C.; Michelini, S.; Swartzwelter, B.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Oyler, B.; Goodlett, D.; Ernst, R. Lipid A Structural Modifications in Extreme Conditions and Identification of Unique Modifying Enzymes to Define the Tolllike Receptor 4 Structure-Activity Relationship. Biochim. Biophys. Acta 2017, 1862, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Steimle, A.; Autenrieth, I.; Frick, J. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Sondhi, P.; Maruf, M.H.U.; Stine, K.J. Nanomaterials for Biosensing Lipopolysaccharide. Biosensors 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, function and bacterial identification. OCL 2020, 27, 31. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and biogenesis of lipopolysaccharides. EcoSal Plus 2018, 8, 10–1128. [Google Scholar] [CrossRef]
- Jakubowska, N.; Szeląg-Wasielewska, E. Toxic picoplanktonic cyanobacteria–review. Mar. Drugs 2015, 18, 1497–1518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keleti, G.; Sykora, J.L. Production and Properties of Cyanobacterial Endotoxins. Appl. Environ. Microbiol. 1982, 43, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Keleti, G.; Sykora, J.L.; Lippy, E.C.; Shapiro, M.A. Composition and biological properties of lipopolysaccharides isolated from Schizothrix calcicola (Ag.) Gomont (Cyanobacteria). Appl. Environ. Microbiol. 1979, 38, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Skočková, V.; Vašíček, O.; Sychrová, E.; Sovadinová, I.; Babica, P.; Šindlerová, L. Cyanobacterial Harmful Bloom Lipopolysaccharides Induce Pro-nflammatory Effects in Immune and Intestinal Epithelial Cells In Vitro. Toxins 2023, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Klemm, L.C.; Czerwonka, E.; Hall, M.L.; Williams, P.G.; Mayer, A.M. Cyanobacteria Scytonema javanicum and Scytonema ocellatum Lipopolysaccharides Elicit Release of Superoxide Anion, Matrix- Metalloproteinase-9, Cytokines and Chemokines by Rat Microglia In Vitro. Toxins 2018, 10, 130. [Google Scholar] [CrossRef]
- Simazaki, D.; Hirose, M.; Hashimoto, H.; Yamanaka, S.; Takamura, M.; Watanabe, J.; Akiba, M. Occurrence and Fate of Endotoxin Activity at Drinking Water Purification Plants and Healthcare Facilities in Japan. Water Res. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Moon, K.-W.; Kim, Y.-W.; Shon, J.-R. Relationships between Levels of Heterotrophic Plate Count Bacteria and Endotoxin in Point-of-Use Water Treatment Systems. Korean Environ. Health Soc. Conf. 2003, 2003, 132–135. [Google Scholar]
- Zong, W.; Chen, J.; Han, W.; Cheng, G.; Chen, J.; Wang, Y.; Wang, W.; Ou, L.; Yu, Y.; Shen, J. Preparation of PVA/amino multi-walled carbon nanotubes nanocomposite microspheres for endotoxin adsorption. Artif. Cells Nanomed. Biotechnol. 2018, 46, 185–191. [Google Scholar] [CrossRef]
- Hou, G.; Liu, T.; Wang, H.; Fu, G.; Yuan, Z.; Liu, B.; Sun, L.; Fang, J.; Li, D.; Qiu, Q. Preparation of Adsorbents for the Removal of Endotoxin. Artif. Cells Blood Substit. Biotechnol. 2005, 33, 227–237. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, W.; Li, J.; Hou, G.; Fang, J.; Yuan, Z. Studies on Endotoxin Removal Mechanism of Adsorbents with Amino Acid Ligands. J. Chromatogr. B. 2007, 852, 288–292. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, B.; Zhang, X.; Dong, H. Polymyxin B Immobilized on Cross-Linked Cellulose Microspheres for Endotoxin Adsorption. Carbohydr. Polym. 2016, 136, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yuan, Z.; Zhao, D.; Wang, F.; Zhang, K.; Li, Y.; Wen, Y.; Wang, C. Polymyxin B immobilized nanofiber sponge for endotoxin adsorption. Eur. Polym. J. 2019, 110, 69–75. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Shi, Z.; Wang, Y.; Song, X.; Wang, L.; Han, M.; Du, H.; He, C.; Zhao, W.; et al. Anticoagulant Chitosan-Kappa-Carrageenan Composite Hydrogel Sorbent for Simultaneous Endotoxin and Bacteria Cleansing in Septic Blood. Carbohydr. Polym. 2020, 243, 116470. [Google Scholar] [CrossRef] [PubMed]
- Du, X.N.; Niu, Z.; Zhou, G.Z.; Li, Z.M. Effect of activated charcoal on endotoxin adsorption. Part I. An in vitro study. Biomaterials. Artif. Cells Artif. Organs 1987, 15, 229–235. [Google Scholar] [CrossRef]
- Rezaee, A.; Ghanizadeh, G.; Behzadiyannejad, G.; Yazdanbakhsh, A.; Siyadat, S.D. Adsorption of Endotoxin from Aqueous Solution Using Bone Char. Bull. Environ. Contam. Toxicol. 2009, 82, 732–737. [Google Scholar] [CrossRef]
- Richter, W.; Vogel, V.; Howe, J.; Steiniger, F.; Brauser, A.; Koch, M.H.; Roessle, M.; Gutsmann, T.; Garidel, P.; Mäntele, W.; et al. Morphology, Size Distribution, and Aggregate Structure of Lipopolysaccharide and Lipid A Dispersions from Enterobacterial Origin. Innate Immun. 2011, 17, 427–438. [Google Scholar] [CrossRef]
- Sali, W.; Patoli, D.; Pais de Barros, J.P.; Labbé, J.; Deckert, V.; Duhéron, V.; Le Guern, N.; Blache, D.; Chaumont, D.; Lesniewska, E.; et al. Polysaccharide Chain Length of Lipopolysaccharides From Salmonella Minnesota Is a Determinant of Aggregate Stability, Plasma Residence Time and Proinflammatory Propensity in vivo. Front. Microbiol. 2019, 10, 1774. [Google Scholar] [CrossRef]
- Harm, S.; Schildböck, C.; Strobl, K.; Hartmann, J. An in vitro study on factors affecting endotoxin neutralization in human plasma using the Limulus amebocyte lysate test. Sci. Rep. 2021, 11, 4192. [Google Scholar] [CrossRef]
- Gorbet, M.; Sefton, M. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef]
- Rice, A.; Rooney, M.T.; Greenwood, A.I.; Cotton, M.L.; Wereszczynski, J. Lipopolysaccharide Simulations Are Sensitive to Phosphate Charge and Ion Parameterization. J. Chem. Theory Comput. 2020, 16, 1806–1815. [Google Scholar] [CrossRef]
- Jandosov, J.; Mansurov, Z.; Biisenbayev, M.; Kerimkulova, A.; Ismagilov, Z.; Shikina, N.; Ismagilov, I.; Andrievskaya, I. Mesoporous composite materials from Acivated rice husk carbon and montmorillonite. Eurasian Chem.-Technol. J. 2011, 13, 105–113. [Google Scholar] [CrossRef]

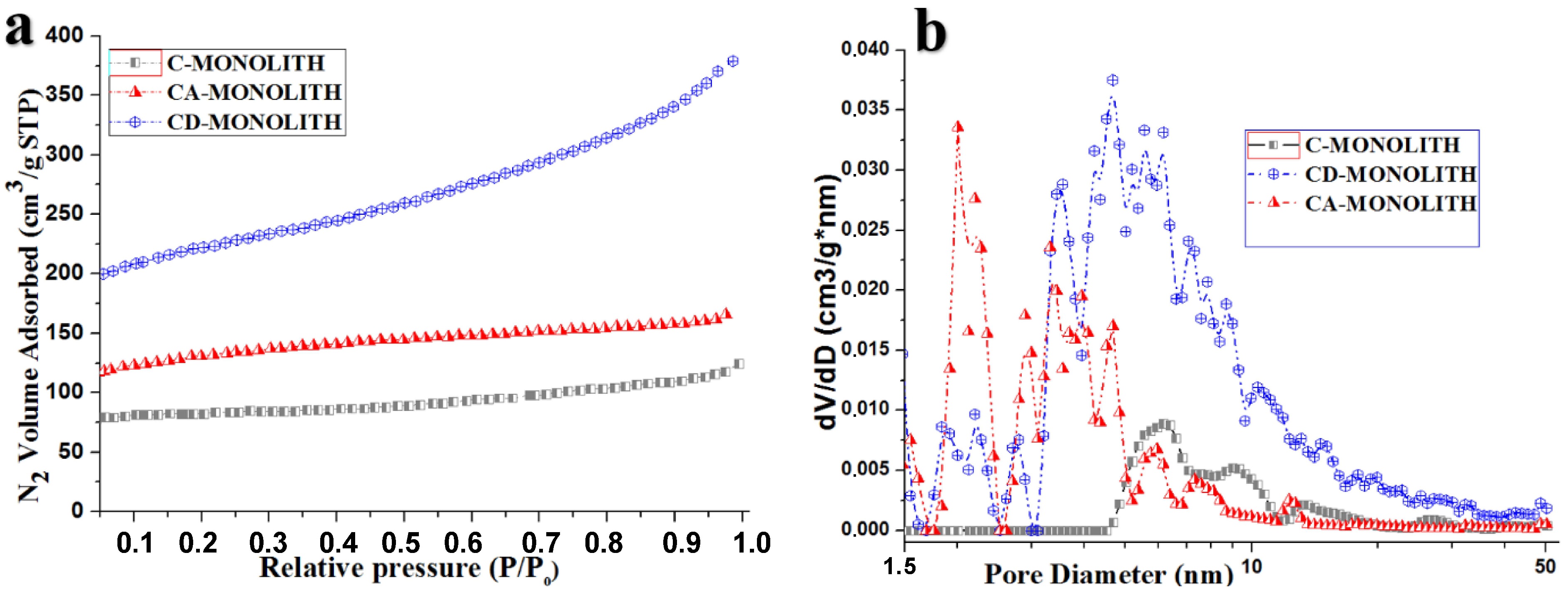
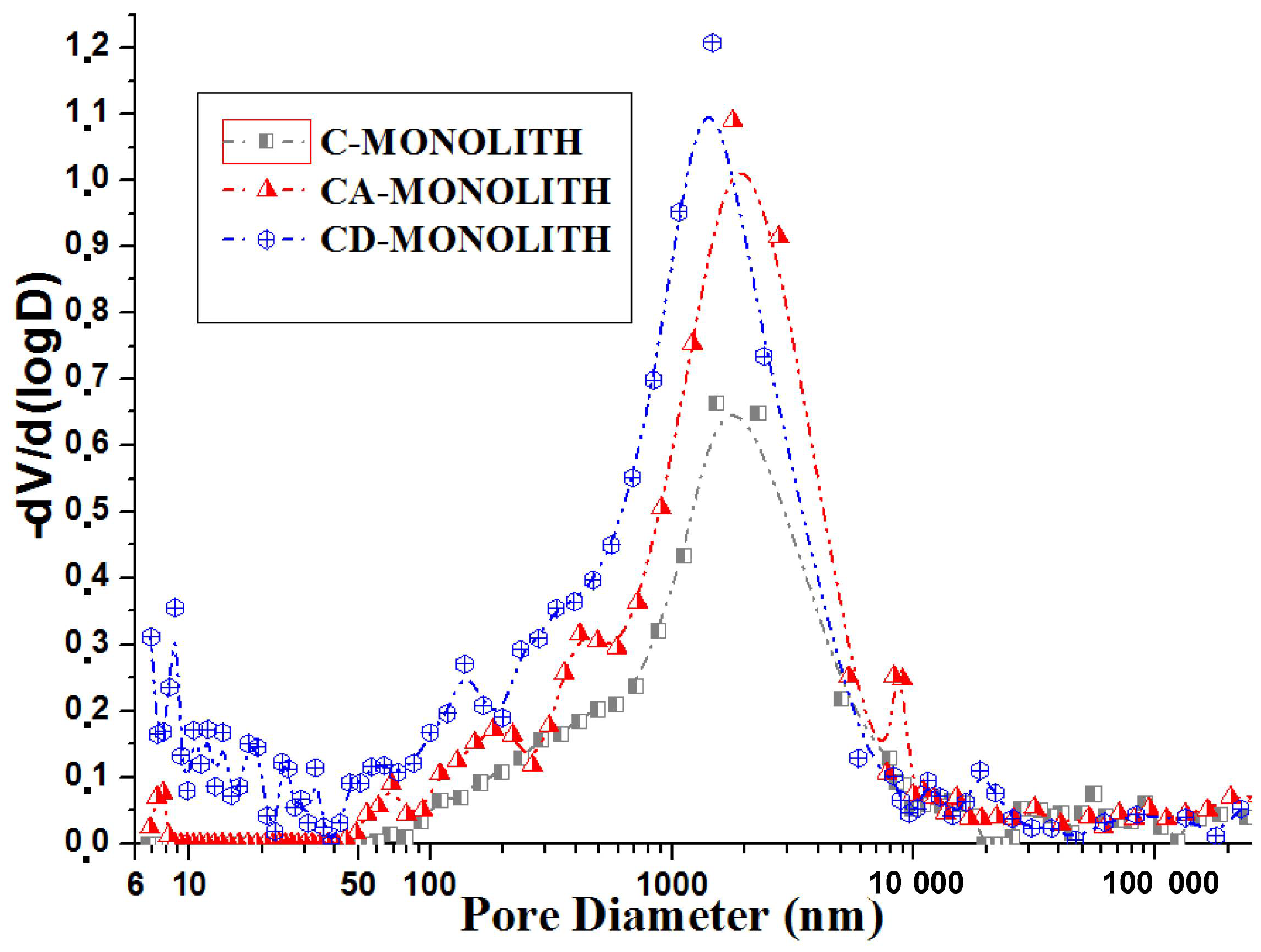


| Sample Code | SBET,m2/g | SDFT,m2/g | VDFT,m2/g | DDFT, nm | VDR, cm3/g | VBJH, cm3/g | VMIP≤100 nm, cm3/g | VMIP≤300nm, cm3/g |
|---|---|---|---|---|---|---|---|---|
| C-Monolith | 327 | 390 | 0.168 | 6.16 | 0.131 | 0.083 | 0.005 | 0.062 |
| CA-Monolith | 492 | 472 | 0.233 | 1.19 | 0.206 | 0.064 | 0.022 | 0.090 |
| CD-Monolith | 837 | 802 | 0.540 | 1.10 | 0.348 | 0.294 | 0.129 | 0.256 |
| Adsorbent Matrix | q* (EU×g−1) | q* (μg×g−1) | *RE (%) | Ref. |
|---|---|---|---|---|
| Dimethylamine-functionalized graphene oxide (GO-ECH-DMA) | 12,147 | 1.215 × 101 | >98 | [4] |
| PVA/amino multi-walled carbon nanotubes nanocomposite microsphere (PVA-AMWCNT) | 114 | 1.14 × 10−2 | >90 | [61] |
| Aminoalkylagarose-hexamethylenediamine-L-lysine | 152.2 | 1.522 × 10−2 | 73 | [62] |
| Aminoalkylagarose-L-phenylalanine (His) | 292.4 | 2.924 × 10−2 | 37.1 | [63] |
| Cross-linked cellulose microspheres (CL-CMs) | 3605 | 3.605 × 10−1 | >70 | [64] |
| Polymyxin B immobilized polyacrylonitrile/SiO2 nanofiber sponge | 17.9 | 1.789× 101 | 90 | [65] |
| Carrageenan-immobilized genipin-cross-linked chitosan hydrogel | 202.8 | 2.03 × 10−2 | 63 | [66] |
| Cross-linked agarose-coated activated charcoal (CAAC-II) | 58.2 | 5.82 × 10−3 | 69 | [67] |
| Bone Char (BC) | 28.8 | 2.88 × 10−3 | 98 | [68] |
| AC CRH | 209.3 | 2.09 × 10−2 | >99 | [39] |
| PEI-AC-CRH | 193.1 | 1.931 × 10−2 | 91.5 | Present work |
| Monolith-CD | 477.3 | 4.77 × 10−2 | 91.6 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jandosov, J.; Berillo, D.; Misra, A.; Alavijeh, M.; Chenchik, D.; Baimenov, A.; Bernardo, M.; Azat, S.; Mansurov, Z.; Silvestre-Albero, J.; et al. Biomass-Derived Nanoporous Carbon Honeycomb Monoliths for Environmental Lipopolysaccharide Adsorption from Aqueous Media. Int. J. Mol. Sci. 2025, 26, 952. https://doi.org/10.3390/ijms26030952
Jandosov J, Berillo D, Misra A, Alavijeh M, Chenchik D, Baimenov A, Bernardo M, Azat S, Mansurov Z, Silvestre-Albero J, et al. Biomass-Derived Nanoporous Carbon Honeycomb Monoliths for Environmental Lipopolysaccharide Adsorption from Aqueous Media. International Journal of Molecular Sciences. 2025; 26(3):952. https://doi.org/10.3390/ijms26030952
Chicago/Turabian StyleJandosov, Jakpar, Dmitriy Berillo, Anil Misra, Mo Alavijeh, Dmitriy Chenchik, Alzhan Baimenov, Maria Bernardo, Seitkhan Azat, Zulkhair Mansurov, Joaquin Silvestre-Albero, and et al. 2025. "Biomass-Derived Nanoporous Carbon Honeycomb Monoliths for Environmental Lipopolysaccharide Adsorption from Aqueous Media" International Journal of Molecular Sciences 26, no. 3: 952. https://doi.org/10.3390/ijms26030952
APA StyleJandosov, J., Berillo, D., Misra, A., Alavijeh, M., Chenchik, D., Baimenov, A., Bernardo, M., Azat, S., Mansurov, Z., Silvestre-Albero, J., & Mikhalovsky, S. (2025). Biomass-Derived Nanoporous Carbon Honeycomb Monoliths for Environmental Lipopolysaccharide Adsorption from Aqueous Media. International Journal of Molecular Sciences, 26(3), 952. https://doi.org/10.3390/ijms26030952











