Application of Synthetic Microbial Communities of Kalidium schrenkianum in Enhancing Wheat Salt Stress Tolerance
Abstract
:1. Introduction
2. Results
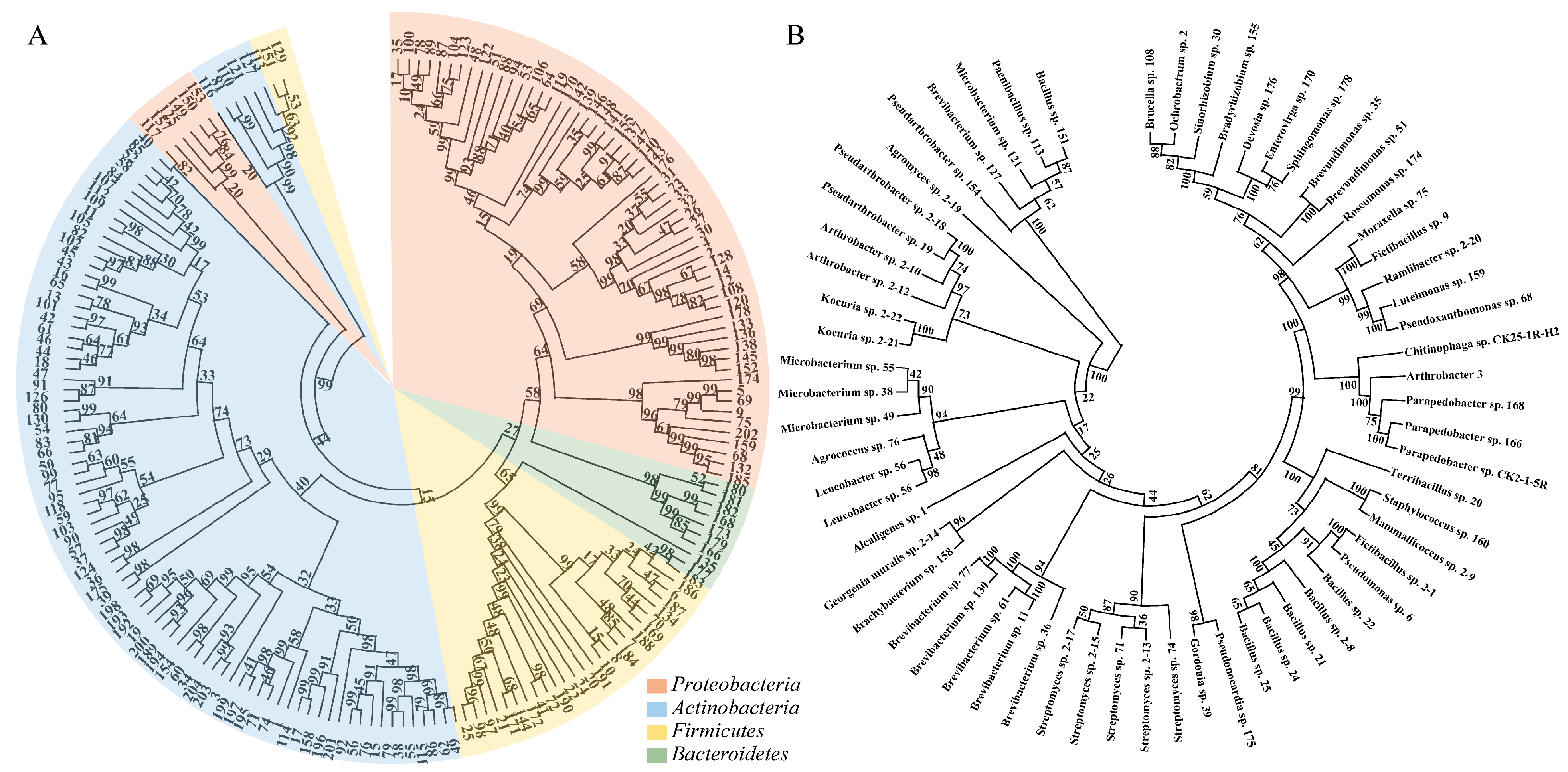
2.1. Isolation and Identification of Strains
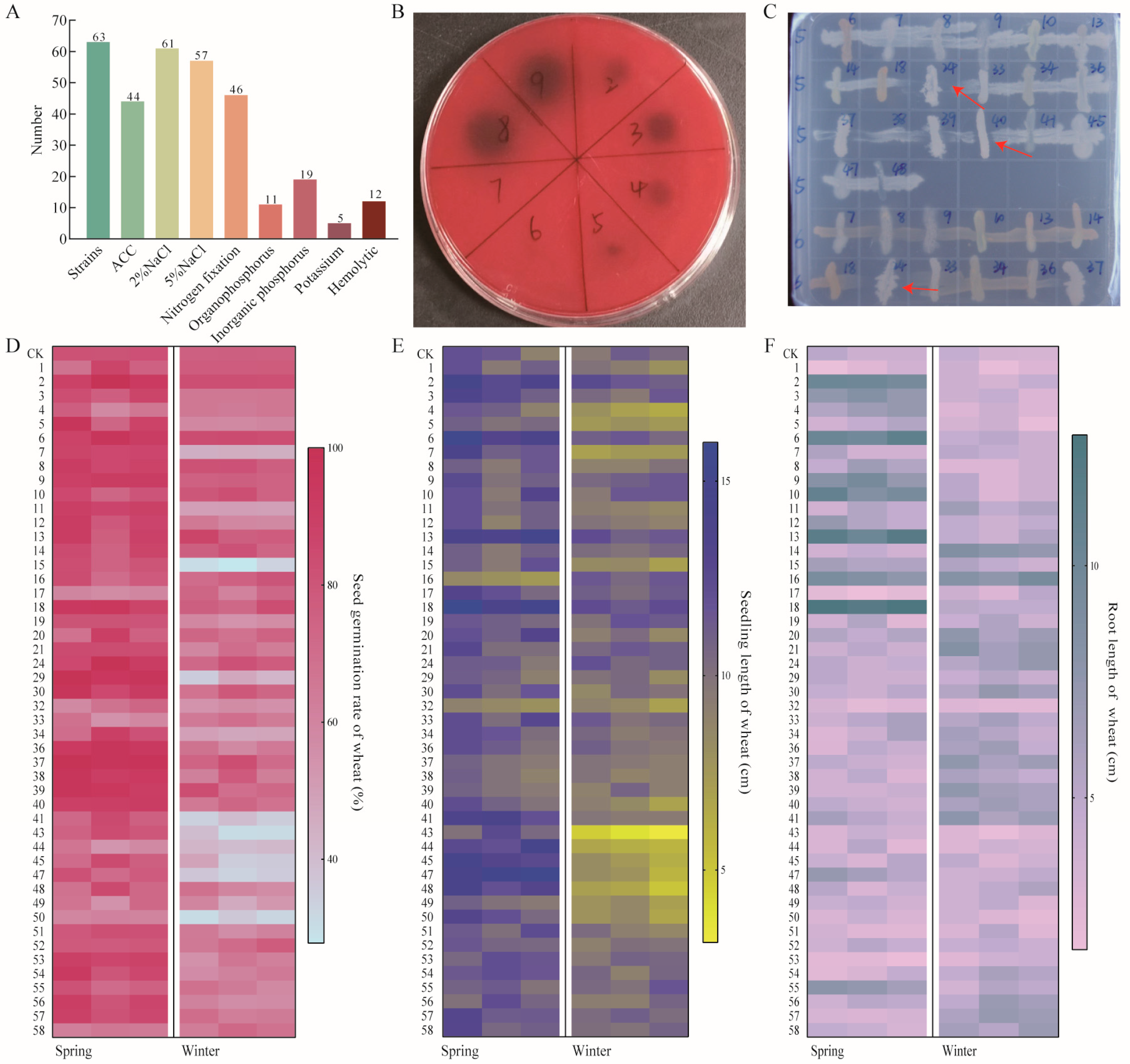
2.2. Screening of Growth-Promoting Strains
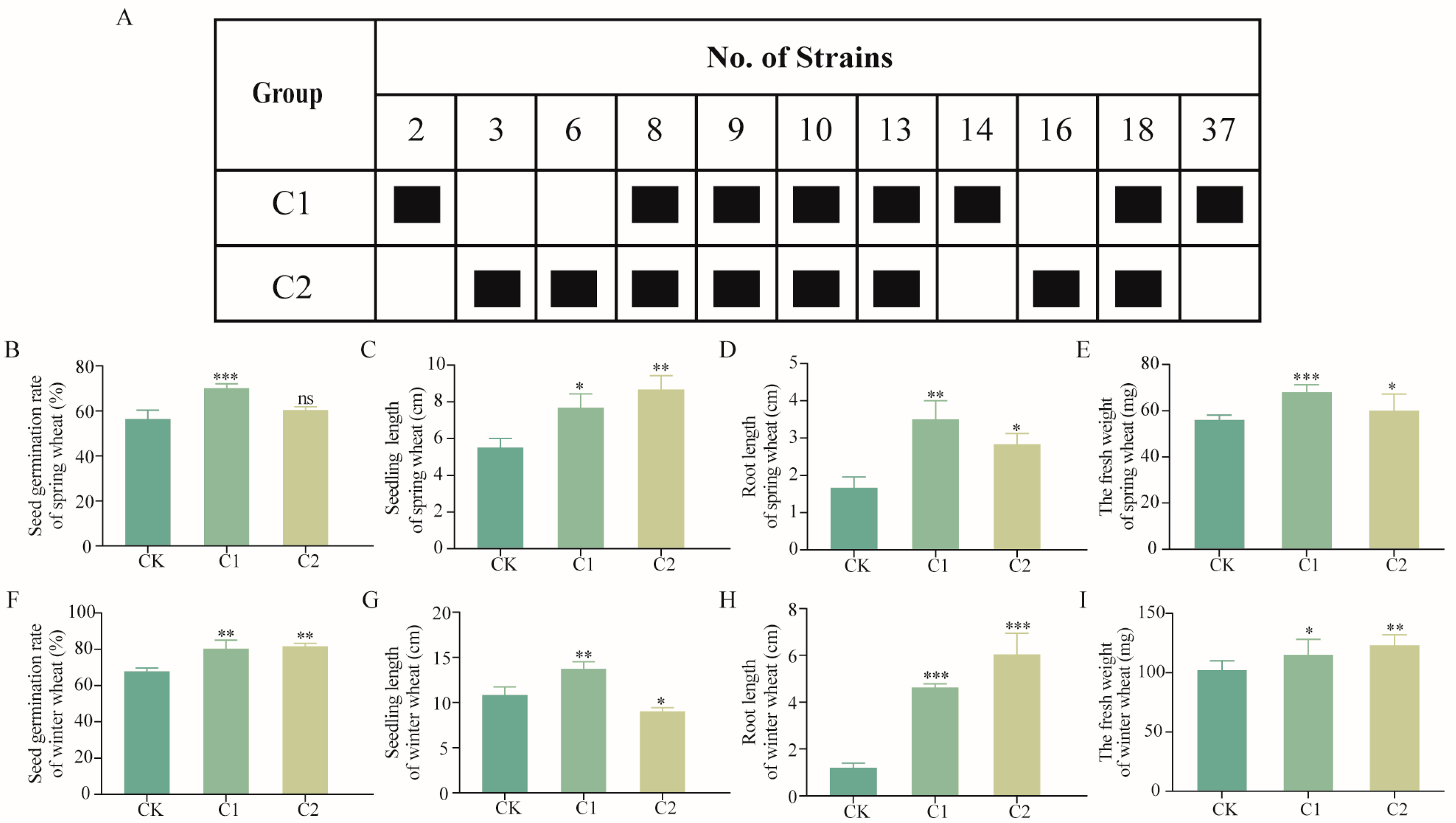
2.3. Construction of SMCs
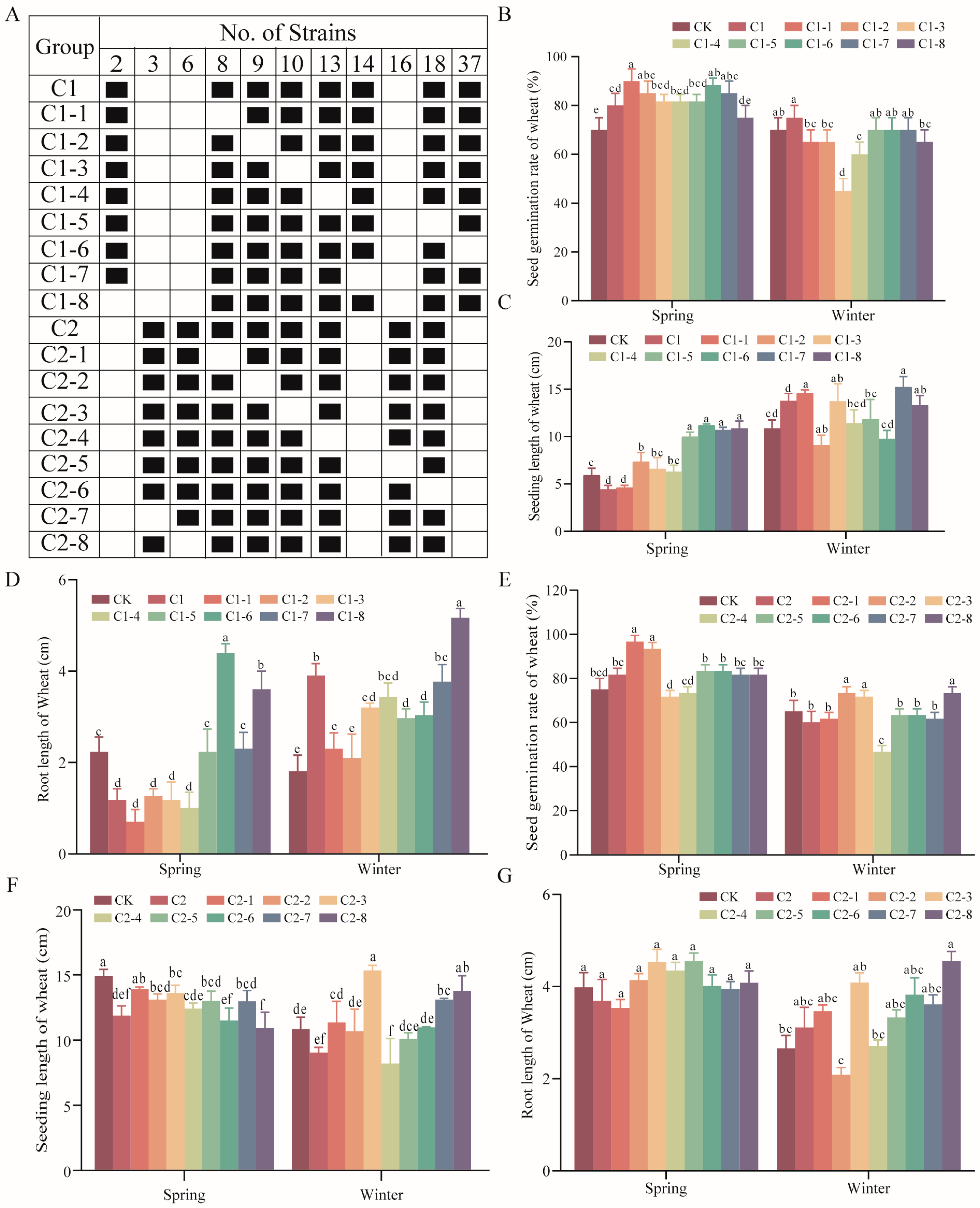
2.4. Optimization of SMCs
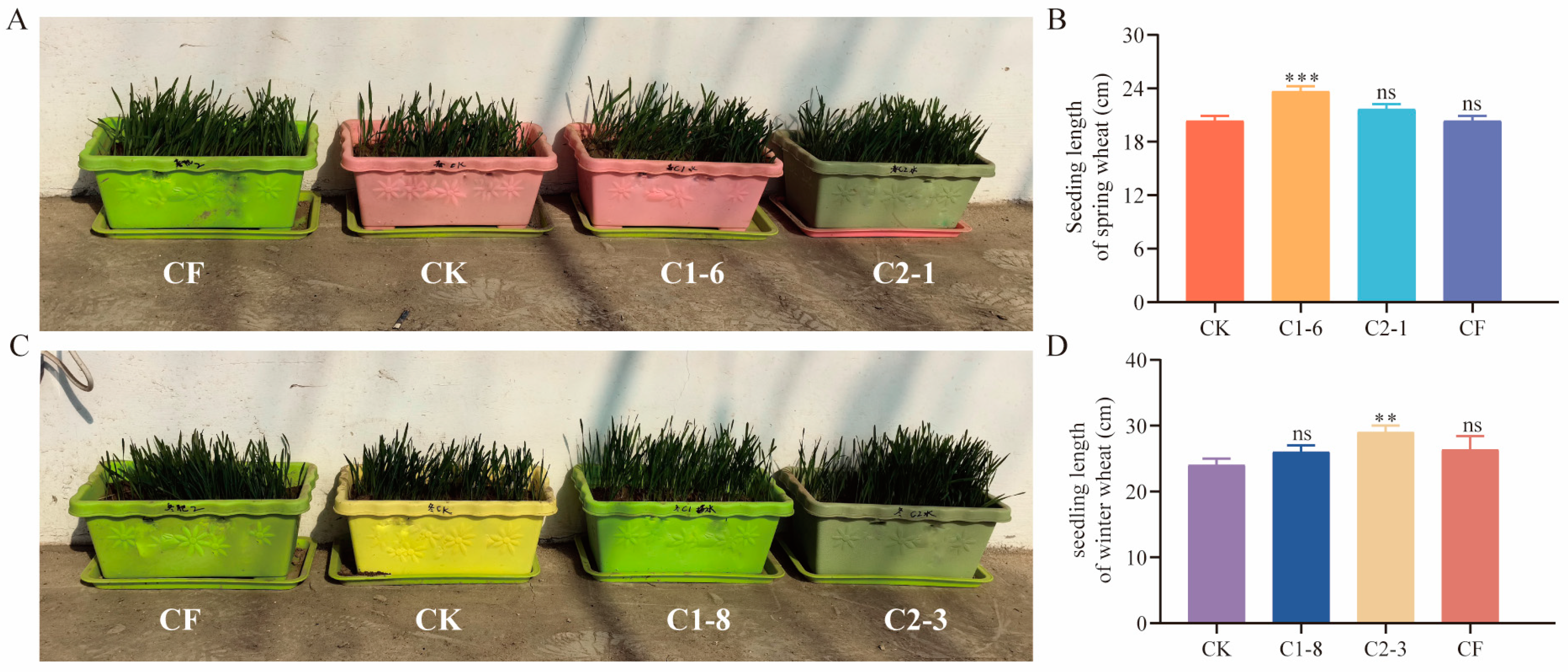
2.5. Effects of SMCs on Wheat Potted Plants
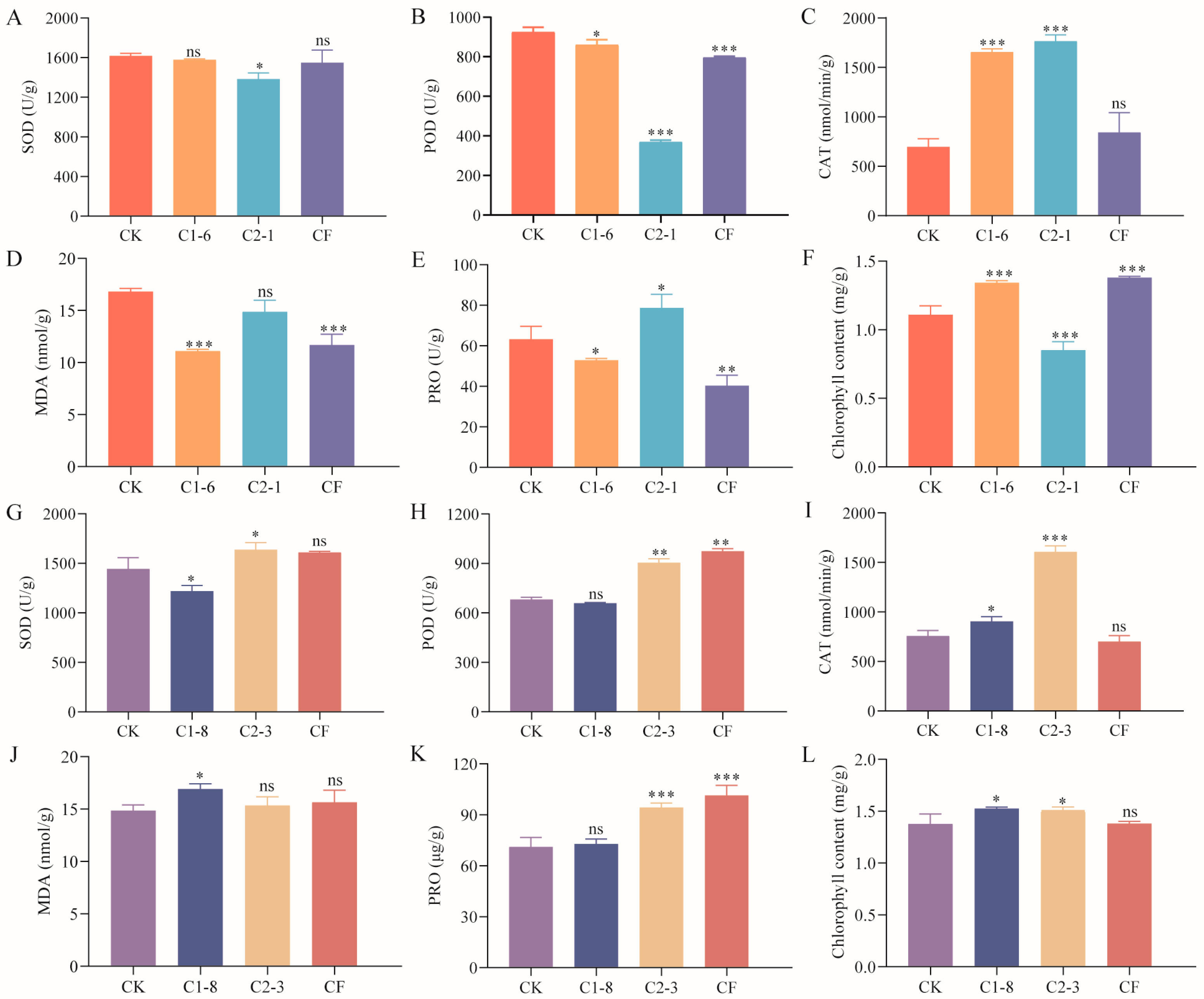
2.6. Effect of SMCs on Enzyme Activity in Potted Wheat
3. Discussion
4. Materials and Methods
4.1. Plant Samples Collection and Surface Sterilization
4.2. Isolation and Cultivation of Strains
4.3. DNA Extraction, Sequencing and Characterization
4.4. Functional Characteristics of Endophytic Bacteria
4.4.1. Salt Tolerance Assessment of Strains
4.4.2. Phosphate-Solubilizing, Potassium-Releasing, and Nitrogen-Fixing Assays
4.4.3. Screening for ACC Deaminase-Positive Bacteria
4.4.4. Hemolysis Assay
4.4.5. Antagonism Test of Strains
4.5. Study on the Plant Growth-Promoting Functions of Endophytic Bacteria
4.6. Construction of SMCs and Validation in Wheat
4.7. Wheat Pot Experiment
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.-J.; Sun, L.; Liu, Y.-H.; Wang, K.-K.; Narsing Rao, M.P.; Mohamad, O.A.A.; Fang, B.-Z.; Li, L.; Gao, L.; Li, W.-J.; et al. Two Novel Alkaliphilic Species Isolated from Saline-Alkali Soil in China: Halalkalibacter flavus sp. nov., and Halalkalibacter lacteus sp. nov. Microorganisms 2024, 12, 950. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ge, Y.; Pang, B.; Liang, W.; Ruze, T. Characteristics of soil salinity and water-salt transport in the vadose zone of salt-impacted regions with variable permeability. Environ. Geochem. Health 2024, 46, 442. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, X.; Tang, Q.-Y.; Zhang, Z.-D. Seasonal Dynamics and Persistency of Endophyte Communities in Kalidium schrenkianum Shifts Under Radiation Stress. Front. Microbiol. 2021, 12, 778327. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Olowe, O.M.; Ayilara, M.S.; Fasusi, O.A.; Omotayo, O.P.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Biosynthesis of nanoparticles using microorganisms: A focus on endophytic fungi. Heliyon 2024, 10, e39636. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Dakora, F.D. Microbes in Agriculture: Prospects and Constraints to Their Wider Adoption and Utilization in Nutrient-Poor Environments. Microorganisms 2024, 12, 2225. [Google Scholar] [CrossRef] [PubMed]
- Mim, M.F.; Chowdhury, M.Z.H.; Rohman, M.M.; Naz, A.; Bhuiyan, A.-U.-A.; Mohi-Ud-Din, M.; Haque, M.A.; Islam, S.M.N. Metarhizium anisopliae (MetA1) seed priming improves photosynthesis, growth, plant defense and yield of wheat under drought stress. Plant Physiol. Biochem. 2024, 217, 109239. [Google Scholar] [CrossRef]
- Nashat, L.H.; Haleem, R.A.; Ali, S.H. Molecular identification and antimicrobial potential of endophytic fungi against some grapevine pathogens. PLoS ONE 2024, 19, e0309041. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Dufossé, L.; Deshmukh, S.K.; Chhipa, H.; Gupta, M.K. Endophytic Fungi: A Treasure Trove of Antifungal Metabolites. Microorganisms 2024, 12, 1903. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Gupta, S.; Stirk, W.A.; Finnie, J.F.; van Staden, J. Endophyte Inoculation Enhances Growth, Secondary Metabolites and Biological Activity of Endostemon obtusifolius Grown Under Drought Stress. J. Plant Growth Regul. 2024, 43, 1103–1117. [Google Scholar] [CrossRef]
- Al-Hawamdeh, F.; Ayad, J.Y.; Alananbeh, K.M.; Akash, M.W. Bacterial Endophytes and Their Contributions to Alleviating Drought and Salinity Stresses in Wheat: A Systematic Review of Physiological Mechanisms. Agriculture 2024, 14, 769. [Google Scholar] [CrossRef]
- Jena, R.; Mukherjee, A.K.; Khandual, A.; Swain, H. Mechanism of betterment towards growth and induction of defense in rice (Oryza sativa L.) by biopriming with bacterial endophytes isolated from wild rice. Microb. Pathog. 2024, 197, 106966. [Google Scholar] [CrossRef]
- Fracchia, F.; Guinet, F.; Engle, N.L.; Tschaplinski, T.J.; Veneault-Fourrey, C.; Deveau, A. Microbial colonisation rewires the composition and content of poplar root exudates, root and shoot metabolomes. Microbiome 2024, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Yang, B.; Jingmei, Q.; Jianmin, Z.; Wei, Q. Crop Microbiome: Breakthrough technology for agriculture. Bull. Chin. Acad. Sci. 2017, 32, 260–265. (In Chinese) [Google Scholar]
- Martins, S.J.; Pasche, J.; Silva, H.A.O.; Selten, G.; Savastano, N.; Abreu, L.M.; Bais, H.P.; Garrett, K.A.; Kraisitudomsook, N.; Pieterse, C.M.J.; et al. The Use of Synthetic Microbial Communities to Improve Plant Health. Phytopathology 2023, 113, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Rafieenia, R.; Klemm, C.; Hapeta, P.; Fu, J.; García, M.G.; Ledesma-Amaro, R. Designing synthetic microbial communities with the capacity to upcycle fermentation byproducts to increase production yields. Trends Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fan, Z.; Hu, Z.; Li, Z.; Fu, Y.; Wang, Q.; Lin, X.; Feng, Y. Synthetic communities derived from the core endophytic microbiome of hyperaccumulators and their role in cadmium phytoremediation. Microbiome 2024, 12, 236. [Google Scholar] [CrossRef]
- Kumar, S.; Sindhu, S.S. Drought stress mitigation through bioengineering of microbes and crop varieties for sustainable agriculture and food security. Curr. Res. Microb. Sci. 2024, 7, 100285. [Google Scholar] [CrossRef] [PubMed]
- Northen, T.R.; Kleiner, M.; Torres, M.; Kovács, Á.T.; Nicolaisen, M.H.; Krzyżanowska, D.M.; Sharma, S.; Lund, G.; Jelsbak, L.; Baars, O.; et al. Community standards and future opportunities for synthetic communities in plant-microbiota research. Nat. Microbiol. 2024, 9, 2774–2784. [Google Scholar] [CrossRef]
- Jagmann, N.; Philipp, B. Design of synthetic microbial communities for biotechnological production processes. J. Biotechnol. 2014, 184, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, K.; Han, Y.; Yan, L.; Zheng, Y.; Bi, Z.; Zhang, X.; Zhang, X.; Min, D. Genome-wide analysis of the VQ motif-containing gene family and expression profiles during phytohormones and abiotic stresses in wheat (Triticum aestivum L.). BMC Genom. 2022, 23, 292. [Google Scholar] [CrossRef] [PubMed]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Ashraful Alam, M.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P. Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Sun, Z. Success to iron biofortification of wheat grain by combining both plant and microbial genetics. Rhizosphere 2020, 15, 100218. [Google Scholar] [CrossRef]
- Mao, H.; Jiang, C.; Tang, C.; Nie, X.; Du, L.; Liu, Y.; Cheng, P.; Wu, Y.; Liu, H.; Kang, Z.; et al. Wheat adaptation to environmental stresses under climate change: Molecular basis and genetic improvement. Mol. Plant 2023, 16, 1564–1589. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Zhao, Y.; Tang, G.; Nan, R.; Zhang, Y.; Sun, F.; Xi, Y.; Zhang, C. Evaluation of wheat drought resistance using hyperspectral and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2024, 219, 109415. [Google Scholar] [CrossRef] [PubMed]
- Lamba, K.; Kumar, M.; Singh, V.; Chaudhary, L.; Sharma, R.; Yashveer, S.; Dalal, M.S. Heat stress tolerance indices for identification of the heat tolerant wheat genotypes. Sci. Rep. 2023, 13, 10842. [Google Scholar] [CrossRef]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress. Front. Plant Sci. 2020, 11, 614971. [Google Scholar] [CrossRef]
- Shekhawat, K.; Saad, M.M.; Sheikh, A.; Mariappan, K.; Al-Mahmoudi, H.; Abdulhakim, F.; Eida, A.A.; Jalal, R.; Masmoudi, K.; Hirt, H. Root endophyte induced plant thermotolerance by constitutive chromatin modification at heat stress memory gene loci. EMBO Rep. 2021, 22, e51049. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, Y.; Wang, G.; Amombo, E.; Li, X.; Xue, Y.; Fu, J. Mitigation of salt stress on low temperature in bermudagrass: Resistance and forage quality. Front. Plant Sci. 2022, 13, 1042855. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Zeyad, M.T.; Syed, A.; Singh, U.B.; Mohamed, A.; Bahkali, A.H.; Elgorban, A.M.; Pichtel, J. Stress-Tolerant Endophytic Isolate Priestia aryabhattai BPR-9 Modulates Physio-Biochemical Mechanisms in Wheat (Triticum aestivum L.) for Enhanced Salt Tolerance. Int. J. Environ. Res. Public Health 2022, 19, 10883. [Google Scholar] [CrossRef]
- Mauceri, A.; Puccio, G.; Faddetta, T.; Abbate, L.; Polito, G.; Caldiero, C.; Renzone, G.; Lo Pinto, M.; Alibrandi, P.; Vaccaro, E.; et al. Integrated omics approach reveals the molecular pathways activated in tomato by Kocuria rhizophila, a soil plant growth-promoting bacterium. Plant Physiol. Biochem. 2024, 210, 108609. [Google Scholar] [CrossRef] [PubMed]
- Soumya, P.; Jayachandran, K.; Radhakrishnan, E.K. Endophytic Microbiome: An Insight into the Hidden World of Microorganisms Within Plants. In Plant-Microbe Interaction and Stress Management; Singh Chauhan, P., Tewari, S.K., Misra, S., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 265–287. [Google Scholar]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Sierra, B.E.G.; et al. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Ouhdouch, Y.; Hafidi, M.; Kouisni, L. Multifunctional role of Actinobacteria in agricultural production sustainability: A review. Microbiol. Res. 2022, 261, 127059. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Raaijmakers, J.M.; Carrión, V.J. Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, A.; Anandham, R.; Johnson, I.; Krishnamoorthy, R.; Senthilkumar, M.; Raghu, R.; Gopal, N.O.; Mukherjee, P.K. Bacillus and Streptomyces for Management of Biotic Stresses in Plants for Sustainable Agriculture. In Plant Microbiome for Plant Productivity and Sustainable Agriculture; Chhabra, S., Prasad, R., Maddela, N.R., Tuteja, N., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 263–288. [Google Scholar]
- Giannelli, G.; Potestio, S.; Visioli, G. The Contribution of PGPR in Salt Stress Tolerance in Crops: Unravelling the Molecular Mechanisms of Cross-Talk between Plant and Bacteria. Plants 2023, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wei, Z.; Friman, V.-P.; Gu, S.-H.; Wang, X.-F.; Eisenhauer, N.; Yang, T.-J.; Ma, J.; Shen, Q.-R.; Xu, Y.-C.; et al. Probiotic Diversity Enhances Rhizosphere Microbiome Function and Plant Disease Suppression. mBio 2016, 7, e01790-16. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Paulson, J.N.; Zheng, X.; Kolter, R. Simplified and representative bacterial community of maize roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial Interkingdom Interactions in Roots Promote Arabidopsis Survival. Cell 2018, 175, 973–983.e14. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Li, Y.; Wang, Z.; Yu, Y.; Zhang, N.; Yang, C.; Zeng, Q.; Wang, Q. Synthetic community with six Pseudomonas strains screened from garlic rhizosphere microbiome promotes plant growth. Microb. Biotechnol. 2021, 14, 488–502. [Google Scholar] [CrossRef]
- Castrillo, G.; Teixeira, P.J.P.L.; Paredes, S.H.; Law, T.F.; de Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, X.; Jiao, S.; Li, Y.; Li, P.; Yang, Y.; Zhang, H.; Wei, G. A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance. Microbiome 2021, 9, 217. [Google Scholar] [CrossRef]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; de Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; van Velzen, R.; et al. Synthetic bacterial community derived from a desert rhizosphere confers salt stress resilience to tomato in the presence of a soil microbiome. ISME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) With Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effects of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Plant-microbe interactions in adaptation of agricultural crops to abiotic stress conditions. In Probiotics and Plant Health; Springer: Singapore, 2017; pp. 163–200. [Google Scholar]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]
- Sun, X.; Ding, Q.; Hyde, K.; Guo, L. Community structure and preference of endophytic fungi of three woody plants in a mixed forest. Fungal Ecol. 2012, 5, 624–632. [Google Scholar] [CrossRef]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000, 147, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Mengesha, A.S.; Legesse, N.H. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of lentil (Lens culinaris M.) collected from Hagere Mariam district, Central Ethiopia. PLoS ONE 2024, 19, e0308915. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Sandhya, V.; Venkateswar Rao, L. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann. Microbiol. 2014, 64, 493–502. [Google Scholar] [CrossRef]
- Sum, R.; Swaminathan, M.; Rastogi, S.K.; Piloto, O.; Cheong, I. Beta-Hemolytic Bacteria Selectively Trigger Liposome Lysis, Enabling Rapid and Accurate Pathogen Detection. ACS Sens. 2017, 2, 1441–1451. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, X.; Zhang, Z.-D.; Tang, Q.-Y.; Gu, M.-Y.; Zhang, L.-J.; Hou, M.; Sharon, A.; Yuan, H.-L. Effect of Ionizing Radiation on the Bacterial and Fungal Endophytes of the Halophytic Plant Kalidium schrenkianum. Microorganisms 2021, 9, 1050. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Y.; Sun, S.; Lu, X.; Li, Q.; Li, L.; Wang, K.; Liu, J. Effects of Salt Stress on the Antioxidant Activity and Malondialdehyde, Solution Protein, Proline, and Chlorophyll Contents of Three Malus Species. Life 2022, 12, 1929. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Han, W.; Jiang, Y.; Yang, X.; He, J.; Kong, M.; Huo, Q.; Lv, G. Physiological and transcriptomic analysis reveals the coating of microcapsules embedded with bacteria can enhance wheat salt tolerance. BMC Plant Biol. 2024, 24, 1004. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Lu, S.; Nai, G.; Ma, W.; Ren, J.; Guo, L.; Chen, B.; Mao, J. The role of gibberellin synthase gene VvGA2ox7 acts as a positive regulator to salt stress in Arabidopsis thaliana. BMC Plant Biol. 2024, 24, 1051. [Google Scholar] [CrossRef] [PubMed]
| Number of Strains | The Taxonomic Generic (Species) Name of the Strains |
|---|---|
| 2 | Gordonia hydrophobica |
| 3 | Streptomyces monticola |
| 6 | Planomicrobium okeanokoites |
| 8 | Microbacterium amylolyticum |
| 9 | Pseudomonas stutzeri |
| 10 | Brachybacterium alimentarium |
| 13 | Luteimonas huabeiensis |
| 14 | Bacillus australimaris |
| 16 | Microbacterium thalassium |
| 18 | Brevibacterium epidermidis |
| 37 | Bacillus cabrialesii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Jia, Q.; Tang, Q.-Y.; Osman, G.; Gu, M.-Y.; Wang, N.; Zhang, Z.-D. Application of Synthetic Microbial Communities of Kalidium schrenkianum in Enhancing Wheat Salt Stress Tolerance. Int. J. Mol. Sci. 2025, 26, 860. https://doi.org/10.3390/ijms26020860
Zhu J, Jia Q, Tang Q-Y, Osman G, Gu M-Y, Wang N, Zhang Z-D. Application of Synthetic Microbial Communities of Kalidium schrenkianum in Enhancing Wheat Salt Stress Tolerance. International Journal of Molecular Sciences. 2025; 26(2):860. https://doi.org/10.3390/ijms26020860
Chicago/Turabian StyleZhu, Jing, Qiong Jia, Qi-Yong Tang, Ghenijan Osman, Mei-Ying Gu, Ning Wang, and Zhi-Dong Zhang. 2025. "Application of Synthetic Microbial Communities of Kalidium schrenkianum in Enhancing Wheat Salt Stress Tolerance" International Journal of Molecular Sciences 26, no. 2: 860. https://doi.org/10.3390/ijms26020860
APA StyleZhu, J., Jia, Q., Tang, Q.-Y., Osman, G., Gu, M.-Y., Wang, N., & Zhang, Z.-D. (2025). Application of Synthetic Microbial Communities of Kalidium schrenkianum in Enhancing Wheat Salt Stress Tolerance. International Journal of Molecular Sciences, 26(2), 860. https://doi.org/10.3390/ijms26020860





