Mechanism of Functional Compound Fruit Drinks in Regulating Serum Metabolism in Constipated Mice
Abstract
:1. Introduction
2. Results and Discussion
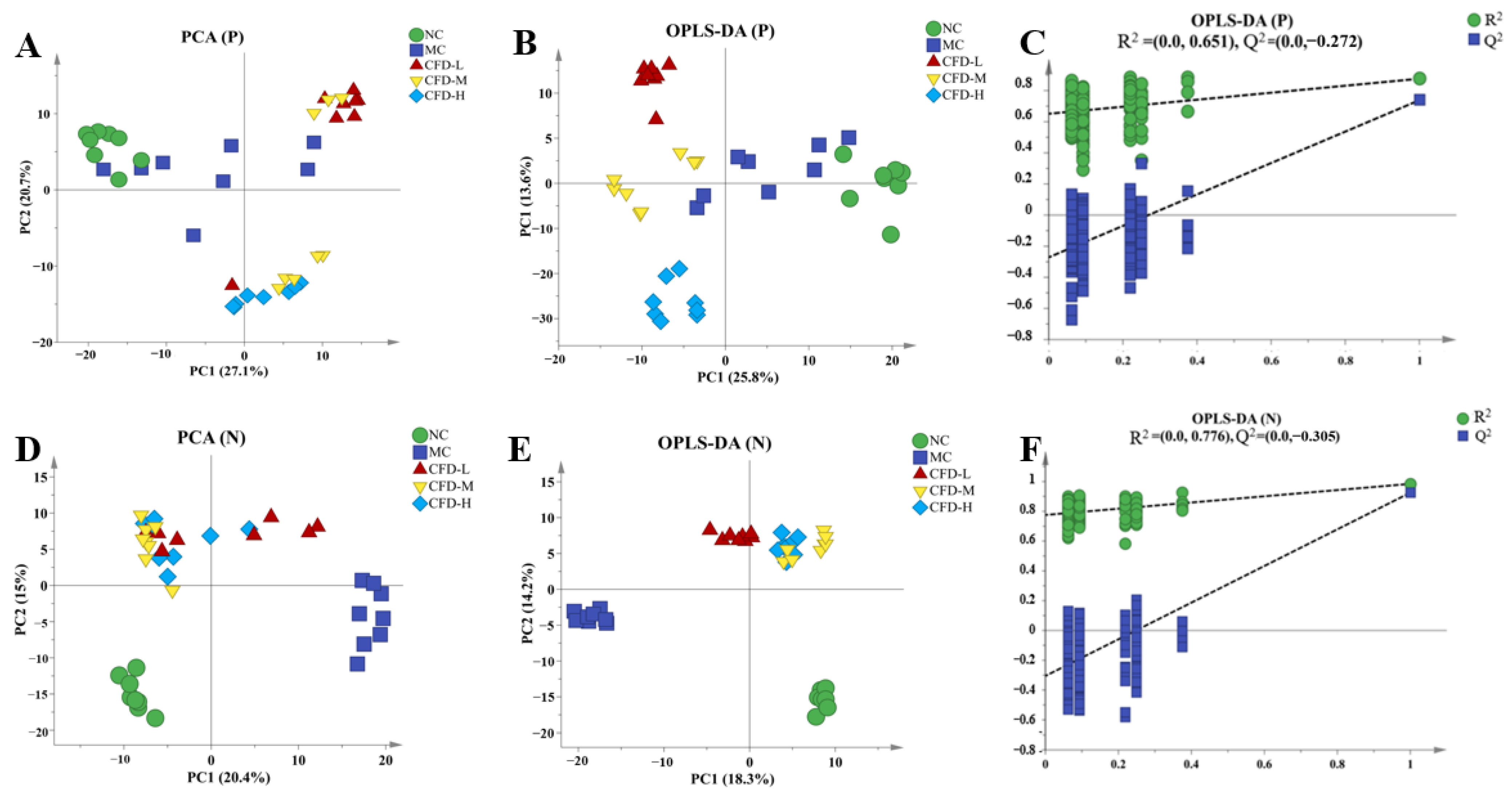
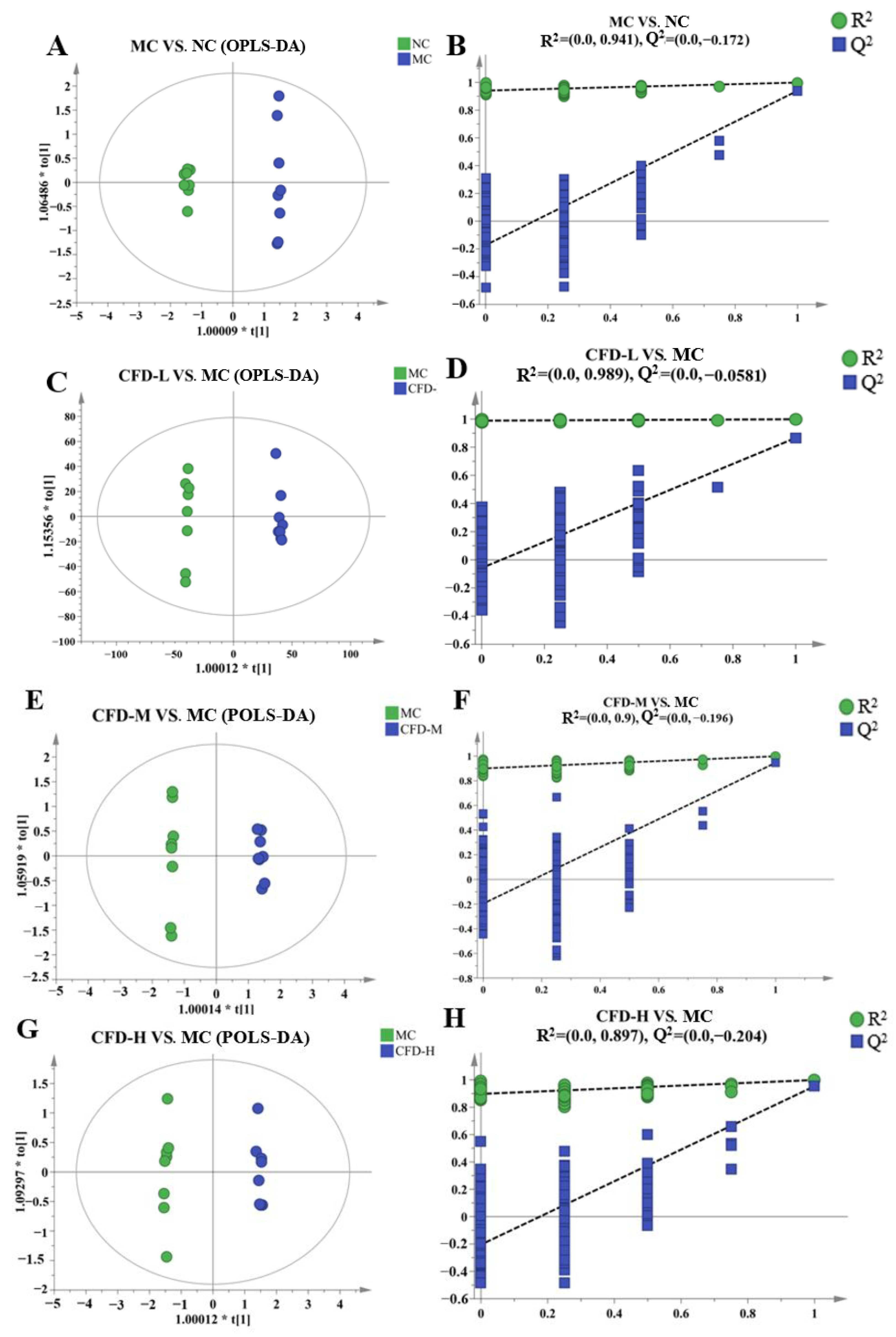
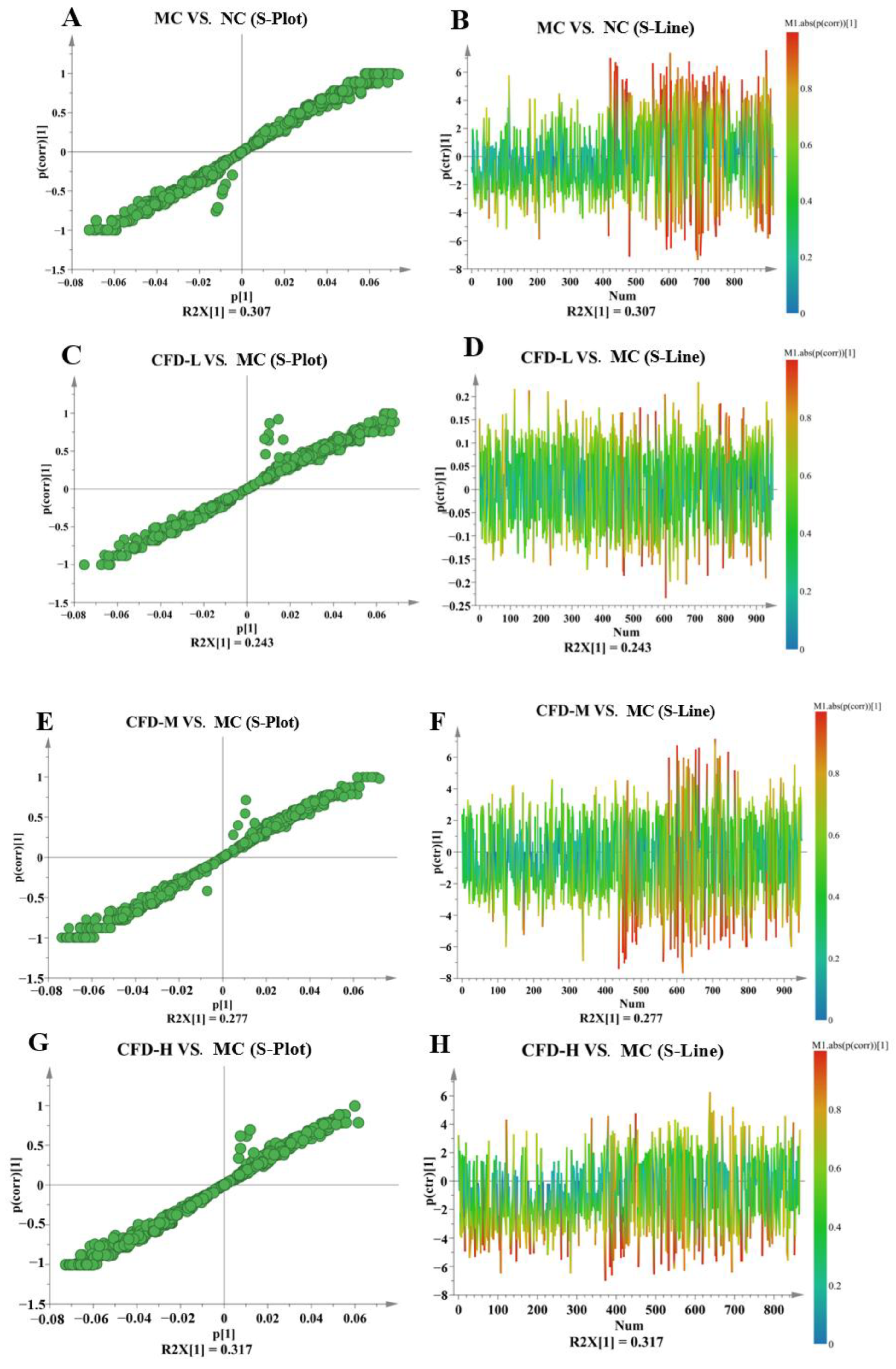
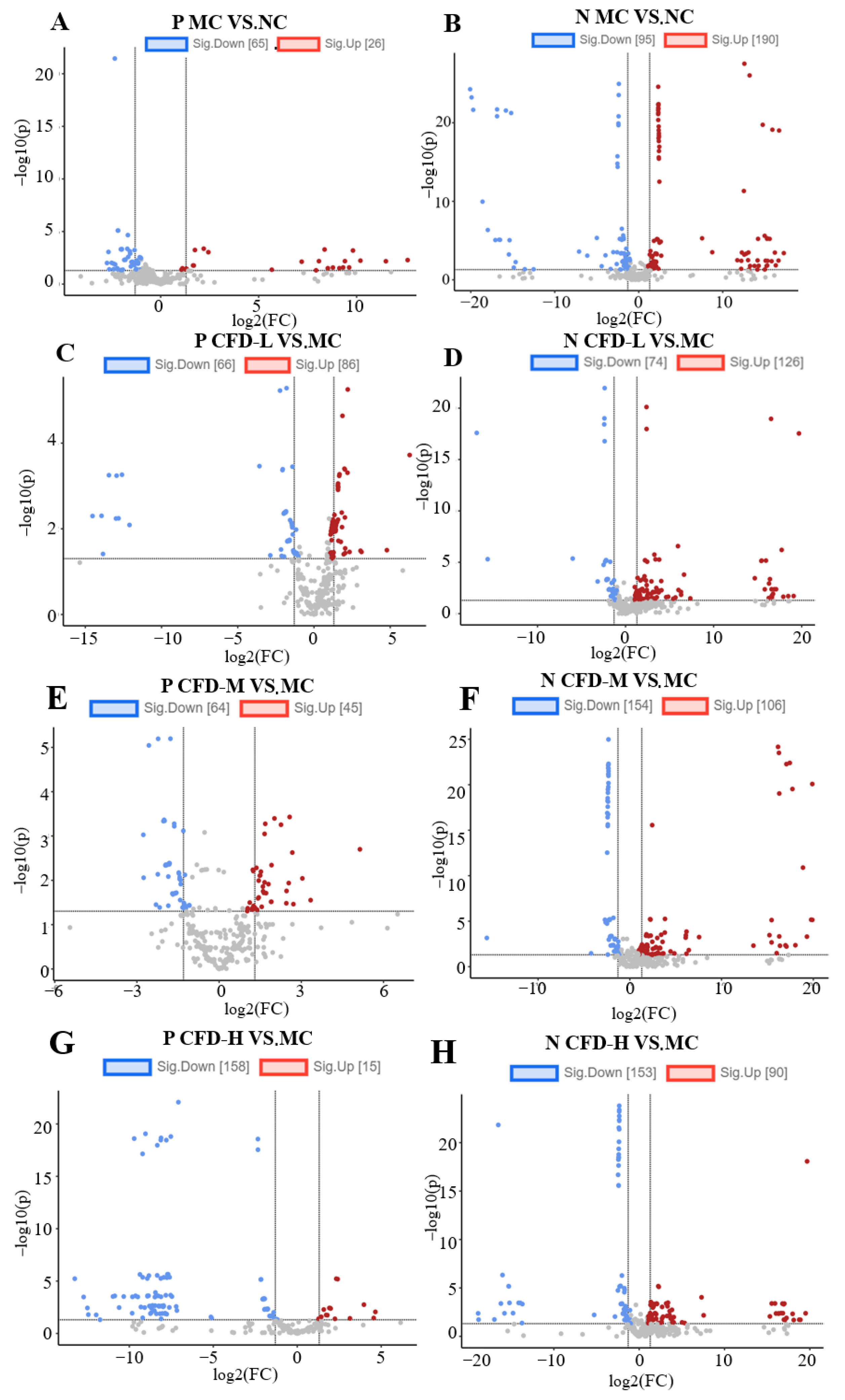
2.1. The Effect of the CFD on Serum Metabolites
2.2. The Effect of the CFD on Intergroup Metabolites
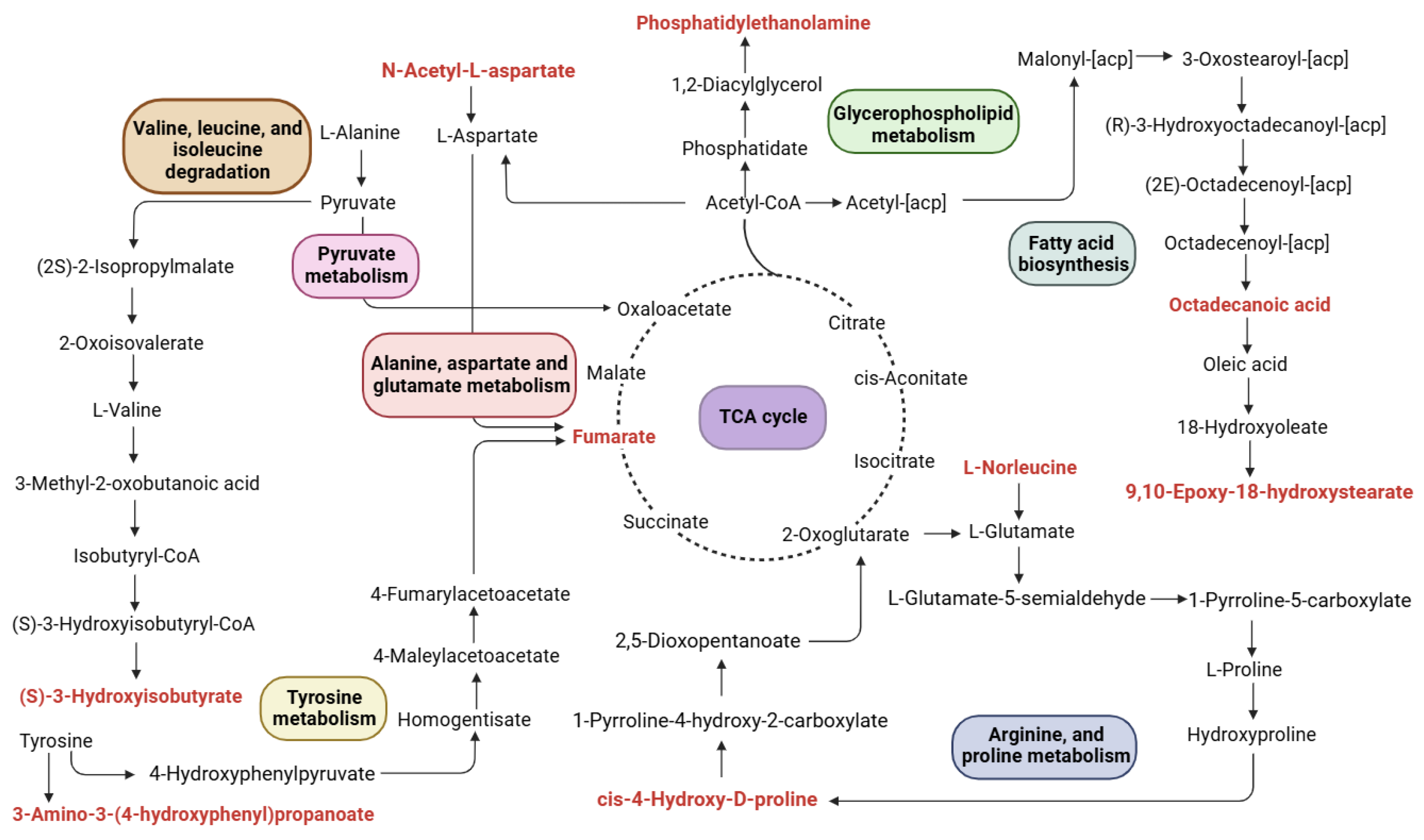
2.3. The Effect of the CFD on Metabolic Pathways in Constipated Mice
2.3.1. TCA Cycle
2.3.2. Glycerophospholipid Biosynthesis
2.3.3. Amino Acid Metabolism
3. Materials and Methods
3.1. Preparation of the CFD
3.2. Animal Experimental Design and Sample Collection
3.3. Chromatographic and Mass Spectrometry Conditions
3.4. Analysis of Biomarkers
3.5. Metabolomics and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meinds, R.J.; van Meegdenburg, M.M.; Trzpis, M.; Broens, P.M.A. On the prevalence of constipation and fecal incontinence, and their co-occurrence, in the Netherlands. Int. J. Color. Dis. 2017, 32, 475–483. [Google Scholar] [CrossRef]
- Sbahi, H.; Cash, B.D. Chronic Constipation: A Review of Current Literature. Curr. Gastroenterol. Rep. 2015, 17, 47. [Google Scholar] [CrossRef]
- Suares, N.C.; Ford, A.C. Prevalence of, and Risk Factors for, Chronic Idiopathic Constipation in the Community: Systematic Review and Meta-analysis. Off. J. Am. Coll. Gastroenterol. 2011, 106, 1582–1591. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and risk of death and cardiovascular events. Atherosclerosis 2019, 281, 114–120. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Zhou, J.; Wang, X.; Jiao, R.; Liu, Z. Efficacy of electroacupuncture compared with transcutaneous electric nerve stimulation for functional constipation: Study protocol for a randomized, controlled trial. Medicine 2018, 97, e0692. [Google Scholar] [CrossRef] [PubMed]
- Johanson, J.F.; Kralstein, J. Chronic constipation: A survey of the patient perspective. Aliment. Pharmacol. Ther. 2007, 25, 599–608. [Google Scholar] [CrossRef]
- Harris, M.A.; Ferrara, A.; Gallagher, J.; DeJesus, S.; Williamson, P.; Larach, S. Stapled Transanal Rectal Resection vs. Transvaginal Rectocele Repair for Treatment of Obstructive Defecation Syndrome. Dis. Colon Rectum 2009, 52, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Isakov, V.A.; Pilipenko, V.I.; Vlasova, A.V.; Kochetkova, A.A. Evaluation of the Efficacy of Kombucha-Based Drink Enriched with Inulin and Vitamins for the Management of Constipation-Predominant Irritable Bowel Syndrome in Females: A Randomized Pilot Study. Curr. Dev. Nutr. 2023, 7, 102037. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.G.R.; Gasga, V.M.Z.; Pescuma, M.; van Nieuwenhove, C.; Mozzi, F.; Burgos, J.A.S. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef]
- Gearry, R.; Fukudo, S.; Barbara, G.; Kuhn-Sherlock, B.; Ansell, J.; Blatchford, P.; Eady, S.; Wallace, A.; Butts, C.; Cremon, C.; et al. Consumption of 2 Green Kiwifruits Daily Improves Constipation and Abdominal Comfort—Results of an International Multicenter Randomized Controlled Trial. Off. J. Am. Coll. Gastroenterol. 2023, 118, 1058–1068. [Google Scholar] [CrossRef]
- Deng, Z.; Fu, Z.; Yan, W.; Nie, K.; Ding, L.; Ma, D.; Huang, H.; Li, T.; Xie, J.; Fu, L. The different effects of Chinese Herb Solid Drink and lactulose on gut microbiota in rats with slow transit constipation induced by compound diphenoxylate. Food Res. Int. 2021, 143, 110273. [Google Scholar] [CrossRef] [PubMed]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Liu, J.; Lyu, J.; Zhou, M.; Marszalek, K.; Trych, U. Effect of high pressure homogenization combined with juice ratio on water-soluble pectin characteristics, functional properties and bioactive compounds in mixed juices. Innov. Food Sci. Emerg. Technol. 2020, 60, 102279. [Google Scholar] [CrossRef]
- Xia, P.; Liu, X.; Hou, T.; Zhan, F.; Geng, F.; Zhang, Z.; Li, B. Evaluation of the effect of prebiotic sesame candies on loperamide-induced constipation in mice. Food Funct. 2022, 13, 5690–5700. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, Z.; Zhang, Z.; Liang, X.; Gong, P.; Yi, H.; Yang, L.; Liu, T.; Shi, H.; Zhang, L. Bifidobacterium animalis F1-7 in combination with konjac glucomannan improves constipation in mice via humoral transport. Food Funct. 2021, 12, 791–801. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Lin, J.; Shen, M.; Wang, Y.; Sarkar, A.; Wen, H.; Xie, J. Co-delivery of resveratrol and curcumin based on Mesona chinensis polysaccharides/zein nanoparticle for targeted alleviation of ulcerative colitis. Food Biosci. 2024, 59, 104060. [Google Scholar] [CrossRef]
- Gao, B.; Lu, Y.; Sheng, Y.; Chen, P.; Yu, L. Differentiating Organic and Conventional Sage by Chromatographic and Mass Spectrometry Flow Injection Fingerprints Combined with Principal Component Analysis. J. Agric. Food Chem. 2013, 61, 2957–2963. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, W.; Han, L.; Ding, J.; Chang, Y. Metabolomics analysis of sea cucumber (Apostichopus japonicus) in different geographical origins using UPLC–Q-TOF/MS. Food Chem. 2020, 333, 127453. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xing, L. Effects of ultrasound on the taste components from aqueous extract of unsmoked bacon. Food Chem. 2021, 365, 130411. [Google Scholar] [CrossRef]
- Song, G.; Chen, K.; Wang, H.; Zhang, M.; Yu, X.; Wang, J.; Shen, Q. In situ and real-time authentication of Thunnus species by iKnife rapid evaporative ionization mass spectrometry based lipidomics without sample pretreatment. Food Chem. 2020, 318, 126504. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, K.; Cao, J.; Lin, X.; Shen, X.; Duan, Z.; Li, C. Relationship between Micromolecules and Quality Changes of Tilapia Fillets after Partial Freezing Treatment with Polyphenols. J. Agric. Food Chem. 2021, 69, 8213–8226. [Google Scholar] [CrossRef]
- Zhao, X.; Qian, Y.; Li, G.; Yi, R.; Park, K.-Y.; Song, J.-L. Lactobacillus plantarum YS2 (yak yogurt Lactobacillus) exhibited an activity to attenuate activated carbon-induced constipation in male Kunming mice. J. Dairy Sci. 2019, 102, 26–36. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.; Chen, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on metabolite profiles of tilapia (Oreochromis niloticus) fillets during storage. Food Chem. 2019, 293, 418–428. [Google Scholar] [CrossRef]
- Li, R.; Sun, Z.; Zhao, Y.; Li, L.; Yang, X.; Cen, J.; Chen, S.; Li, C.; Wang, Y. Application of UHPLC-Q-TOF-MS/MS metabolomics approach to investigate the taste and nutrition changes in tilapia fillets treated with different thermal processing methods. Food Chem. 2021, 356, 129737. [Google Scholar] [CrossRef] [PubMed]
- Pintus, M.C.; Lussu, M.; Dessi, A.; Pintus, R.; Noto, A.; Masile, V.; Marcialis, M.A.; Puddu, M.; Fanos, V.; Atzori, L. Urinary 1H-NMR Metabolomics in the First Week of Life Can Anticipate BPD Diagnosis. Oxidative Med. Cell. Longev. 2018, 2018, 7620671. [Google Scholar] [CrossRef]
- Glasgow, I.; Mattar, K.; Krantis, A. Rat gastroduodenal motility in vivo: Involvement of NO and ATP in spontaneous motor activity. Am. J. Physiol. 1998, 275, G889–G896. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, B.; Sun, G.; Zheng, J.; Hu, H.; Yang, H.; Cheng, X.; Lin, A.; Liu, H. Plasma metabolomic profiles reveal regulatory effect of chitosan oligosaccharides on loperamide-induced constipation in mice. J. Pharm. Biomed. Anal. 2022, 211, 114590. [Google Scholar] [CrossRef] [PubMed]
- Halama, A.; Guerrouahen, B.S.; Pasquier, J.; Diboun, I.; Karoly, E.D.; Suhre, K.; Rafii, A. Metabolic signatures differentiate ovarian from colon cancer cell lines. J. Transl. Med. 2015, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Meirer, K.; Steinhilber, D.; Proschak, E. Inhibitors of the Arachidonic Acid Cascade: Interfering with Multiple Pathways. Basic Clin. Pharmacol. Toxicol. 2014, 114, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Bhujwalla, Z.M.; Glunde, K. Targeting Phospholipid Metabolism in Cancer. Front. Oncol. 2016, 6, 266. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. Biomed. Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, F.; Wang, Z.; Cao, J.; Li, C. Effect and mechanism of functional compound fruit drink on gut microbiota in constipation mice. Food Chem. 2023, 401, 134210. [Google Scholar] [CrossRef]

| NO. | Compound | Ionization Model | Formula | HMDB ID | MC/NC | CFD-H/MC | ||
|---|---|---|---|---|---|---|---|---|
| Log FC | Change | Log FC | Change | |||||
| 1 | 3,3-Difluoro-17-methyl-5alpha-androstan 17beta-ol | + | C20H32F2O | 4.4505 | up | −3.218392 | down | |
| 2 | 4’-O-Methylneobavaisoflavone 7-O-(2’’-p coumaroylglucoside) | + | C36H36O11 | 2.7664113 | up | −2.766411 | down | |
| 3 | 6-Hydroxydelphinidin 3-(6 malonylglucoside) | + | C24H23O16 | 2.4042294 | up | −14.65394 | down | |
| 4 | Ala Arg | + | C9H19N5O3 | 3.0752976 | up | −3.075298 | down | |
| 5 | Ammothamnidin | + | C25H28O5 | 3.5802338 | up | −1.706038 | down | |
| 6 | Anabasine | + | C10H14N2 | HMDB04350 | −8.89981 | down | 2.7541528 | up |
| 7 | Arg Lys Asp | + | C16H31N7O6 | −3.9354963 | down | 1.4209375 | up | |
| 8 | Asp Tyr Gln | + | C18H24N4O8 | 3.1950102 | up | −1.556805 | down | |
| 9 | Benzo[ghi]fluoranthene | + | C18H10 | 3.30509 | up | −3.30509 | down | |
| 10 | Boschniakine | + | C10H11NO | −6.325095 | down | 3.3662586 | up | |
| 11 | C.I Orange G | + | C16H12N2O7S2 | −7.0338855 | down | 1.3723431 | up | |
| 12 | Carbamorph | + | C8H16N2OS2 | 3.253957 | up | −1.591061 | down | |
| 13 | Cyclic adenosine diphosphate ribose | + | C15H21N5O13P2 | −4.64132 | down | 4.572323 | up | |
| 14 | DG(17:0/22:1(13Z)/0:0) | + | C42H80O5 | −3.099382 | down | 6.0656075 | up | |
| 15 | Dimethylenetriurea | + | C5H12N6O3 | 4.5084496 | up | −2.203515 | down | |
| 16 | FTY720 phenoxy-biotin | + | C27H44N4O5S | 1.5744886 | up | −1.574489 | down | |
| 17 | Lys Lys Lys | + | C18H38N6O4 | −5.022828 | down | 4.394418 | up | |
| 18 | Mephobarbital | + | C13H14N2O3 | 1.2537704 | up | −1.25377 | down | |
| 19 | Myricanene A 5-[arabinosyl-(1-6) glucoside] | + | C32H42O13 | HMDB39351 | 1.4820877 | up | −1.482088 | down |
| 20 | Oxolucidine B | + | C30H49N3O2 | 2.976674 | up | −13.87485 | down | |
| 21 | Pachymic acid | + | C33H52O5 | −3.5755281 | down | 1.7724607 | up | |
| 22 | PS(20:4(5Z,8Z,11Z,14Z)/21:0) | + | C47H84NO10P | −3.8085504 | down | 6.5558763 | up | |
| 23 | Quercetagetin 4’-methyl ether 7-(6-(E) caffeylglucoside) | + | C31H28O16 | 2.6961927 | up | −1.310424 | down | |
| 24 | Ssioriside | + | C27H38O12 | HMDB38934 | 1.4357603 | up | −1.404921 | down |
| 25 | Tetradecanoylcarnitine | + | C21H42NO4 | HMDB05066 | 2.4656892 | up | −3.78558 | down |
| 26 | Tyr Val | + | C14H20N2O4 | −1.6964296 | down | 4.730801 | up | |
| 27 | Val Ile Leu | + | C17H33N3O4 | 5.294032 | up | −10.76906 | down | |
| 28 | (S)-3-Hydroxyisobutyrate | − | C4H8O3 | −1.8594704 | down | 4.038164 | up | |
| 29 | (+)-trans-alpha-Irone | − | C14H22O | −1.5312119 | down | 1.5506911 | up | |
| 30 | (9S,13S)-1a,1b-dihomo-jasmonic acid | − | C14H22O3 | −5.3799334 | down | 8.763475 | up | |
| 31 | (3a,5b)-24-oxo-24-[(2 sulfoethyl)amino]cholan-3-yl-b-D Glucopyranosiduronic acid | − | C32H53NO11S | HMDB02429 | 11.074536 | up | −11.07454 | down |
| 32 | L-Norleucine | − | C6H13NO2 | HMDB01645 | −1.8873906 | down | 4.8549323 | up |
| 33 | (R)-Pantolactone | − | C6H10O3 | −4.170607 | down | 9.963216 | up | |
| 34 | 1,8-Naphthyridine-3-carboxylic acid, 1 ethyl-1,4-dihydro-7-hydroxy-4-oxo- | − | C11H10N2O4 | −2.956582 | down | 6.1091447 | up | |
| 35 | cis-4-Hydroxy-D-proline | − | C5H9NO3 | −2.724896 | down | 5.8799667 | up | |
| 36 | 10-Deoxygeniposide tetraacetate | − | C25H32O13 | 10.379653 | up | −10.37965 | down | |
| 37 | 11-Hydroxyprogesterone 11-glucuronide | − | C27H38O9 | 16.0126 | up | −16.0126 | down | |
| 38 | 1-O-[(6’-O-hexadecanoyl)-a-D glucopyranosyl]-(2-hexadecanoyloxy) eicosan-1-ol | − | C58H112O9 | 12.955203 | up | −8.445993 | down | |
| 39 | 1-Octen-3-yl glucoside | − | C14H26O6 | HMDB32959 | −3.689945 | down | 4.358061 | up |
| 40 | 2-[[(3a,5b,7b)-7-hydroxy-24-oxo-3 (sulfooxy)cholan-24-yl]amino] Ethanesulfonic acid | − | C26H45NO9S2 | HMDB02449 | 16.910694 | up | −14.94277 | down |
| 41 | 2-Chloro-1,1,2-trifluoroethyl ethyl ether | − | C4H6ClF3O | −4.176031 | down | 3.1676066 | up | |
| 42 | 2-oxo-tetradecanoic acid | − | C14H26O3 | 1.2939825 | up | −11.21112 | down | |
| 43 | 3-Amino-3-(4-hydroxyphenyl)propanoate | − | C9H11NO3 | HMDB03831 | 3.940454 | up | −5.338254 | down |
| 44 | 3-Isochromanone | − | C9H8O2 | −2.7620492 | down | 5.775576 | up | |
| 45 | 5-Hydroxydantrolene | − | C14H10N4O6 | HMDB60776 | −2.713782 | down | 7.952751 | up |
| 46 | 9,10-Epoxy-18-hydroxystearate | − | C18H34O4 | −6.57066 | down | 5.5488477 | up | |
| 47 | Anhwiedelphinine | − | C35H44N2O10 | 12.642545 | up | −12.64255 | down | |
| 48 | Arg Phe Arg | − | C21H35N9O4 | 16.213037 | up | −14.42369 | down | |
| 49 | Armillatin | − | C38H58O6 | HMDB38743 | −6.374333 | down | 8.174591 | up |
| 50 | Aromatized deshydroxy-C-1027 chromophore | − | C43H44ClN3O12 | 10.24949 | up | −8.825224 | down | |
| 51 | Auriculoside | − | C22H26O10 | 12.162231 | up | −12.16223 | down | |
| 52 | Bambuterol | − | C18H29N3O5 | HMDB15478 | −1.3087604 | down | 2.2535157 | up |
| 53 | beta-D-Mannosylphosphodecaprenol | − | C56H93O9P | −16.54321 | down | 4.239726 | up | |
| 54 | Broussoflavonol D | − | C30H32O7 | 13.831339 | up | −13.83134 | down | |
| 55 | Bufotalin | − | C26H36O6 | −10.536349 | down | 10.325614 | up | |
| 56 | Chlorpromazine sulfone | − | C17H19ClN2O2S | −5.340466 | down | 10.195431 | up | |
| 57 | Citranaxanthin | − | C33H44O | HMDB36883 | 13.942801 | up | −13.9428 | down |
| 58 | Cromakalim | − | C16H18N2O3 | −3.9542727 | down | 10.889442 | up | |
| 59 | Cypridina luciferin | − | C22H27N7O | 15.838913 | up | −8.986485 | down | |
| 60 | decanamide | − | C10H21NO | 1.2328752 | up | −8.027856 | down | |
| 61 | Fenoterol sulfate | − | C17H21NO7S | 2.8744664 | up | −3.97058 | down | |
| 62 | Fexaramine | − | C32H36N2O3 | 9.810449 | up | −9.810449 | down | |
| 63 | Fumaric acid | − | C4H4O4 | HMDB00134 | −4.888547 | down | 3.24939 | up |
| 64 | Ganglioside GA2 (d18:1/12:0) | − | C50H92N2O18 | HMDB04888 | 13.875375 | up | −13.87538 | down |
| 65 | Gingerglycolipid A | − | C33H56O14 | HMDB41093 | −1.4199212 | down | 4.305419 | up |
| 66 | Gingerol | − | C17H26O4 | HMDB05783 | 9.376551 | up | −9.376551 | down |
| 67 | Ginkgolide J | − | C20H24O10 | 9.069026 | up | −6.129434 | down | |
| 68 | Gliadin | − | C29H41N7O9 | HMDB34486 | 12.318045 | up | −13.5362 | down |
| 69 | Glu Trp Ala | − | C19H24N4O6 | 9.855809 | up | −11.23999 | down | |
| 70 | Helilupolone | − | C30H38O4 | −9.420137 | down | 5.3089356 | up | |
| 71 | His-Phe-OH | − | C21H20N4O6 | −1.8027265 | down | 4.804309 | up | |
| 72 | Kobusone | − | C14H22O2 | −1.4395556 | down | 3.3383055 | up | |
| 73 | Lauroyl diethanolamide | − | C16H33NO3 | HMDB32358 | −5.614107 | down | 5.2017584 | up |
| 74 | Leu Leu Phe | − | C21H33N3O4 | 12.384389 | up | −10.56313 | down | |
| 75 | Maraviroc | − | C29H41F2N5O | HMDB15584 | −1.3437376 | down | 4.8805656 | up |
| 76 | Menthol propylene glycol carbonate | − | C14H26O4 | HMDB39785 | 16.521048 | up | −1.216217 | down |
| 77 | Notoginsenoside I | − | C54H92O22 | HMDB31371 | 7.2977753 | up | −1.388841 | down |
| 78 | Octadecanoic acid | − | C19H36O2 | 11.138865 | up | −9.72685 | down | |
| 79 | Patuletin 3-rhamnoside-7-(4” acetylrhamnoside) | − | C30H34O17 | 12.763924 | up | −2.99884 | down | |
| 80 | PE(18:4(6Z,9Z,12Z,15Z)/20:2(11Z,14Z)) | − | C43H74NO8P | HMDB09198 | −1.393867 | down | 10.985636 | up |
| 81 | Perindopril lactam | − | C19H30N2O4 | 2.6985683 | up | −2.698568 | down | |
| 82 | Phe Lys Trp | − | C26H33N5O4 | −11.943593 | down | 7.563178 | up | |
| 83 | PI(22:0/20:0) | − | C51H99O13P | −12.499487 | down | 4.0756774 | up | |
| 84 | PI(22:4(7Z,10Z,13Z,16Z)/21:0) | − | C52H93O13P | 4.5378876 | up | −4.537888 | down | |
| 85 | PI(P-20:0/15:0) | − | C44H85O12P | 15.159404 | up | −15.1594 | down | |
| 86 | PI-Cer(d18:0/16:0) | − | C40H80NO11P | 15.341745 | up | −15.34175 | down | |
| 87 | Prieuranin acetate | − | C40H52O17 | −9.76871 | down | 8.086171 | up | |
| 88 | Rofecoxib | − | C17H14O4S | −1.2988684 | down | 12.447737 | up | |
| 89 | Saphenamycin | − | C23H18N2O5 | 11.704618 | up | −1.794679 | down | |
| 90 | Tamsulosin | − | C20H28N2O5S | HMDB14844 | 10.714832 | up | −15.24225 | down |
| 91 | TG(17:2(9Z,12Z)/20:1(11Z)/22:1(11Z)) | − | C62H112O6 | −15.996837 | down | 4.5640464 | up | |
| 92 | TG(20:4(5Z,8Z,11Z,14Z)/20:5(5Z,8Z,11Z,1 4Z,17Z)/22:2(13Z,16Z)) | − | C65H104O6 | 12.925481 | up | −4.356386 | down | |
| 93 | Trabectedin | − | C39H43N3O11S | 9.429379 | up | −6.670994 | down | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Shi, Y.; Wen, X.; Zhu, L.; Zhang, L.; Zhu, K.; Cao, J.; Li, C. Mechanism of Functional Compound Fruit Drinks in Regulating Serum Metabolism in Constipated Mice. Int. J. Mol. Sci. 2025, 26, 702. https://doi.org/10.3390/ijms26020702
Lu Q, Shi Y, Wen X, Zhu L, Zhang L, Zhu K, Cao J, Li C. Mechanism of Functional Compound Fruit Drinks in Regulating Serum Metabolism in Constipated Mice. International Journal of Molecular Sciences. 2025; 26(2):702. https://doi.org/10.3390/ijms26020702
Chicago/Turabian StyleLu, Quanhong, Yali Shi, Xin Wen, Lulu Zhu, Longteng Zhang, Kexue Zhu, Jun Cao, and Chuan Li. 2025. "Mechanism of Functional Compound Fruit Drinks in Regulating Serum Metabolism in Constipated Mice" International Journal of Molecular Sciences 26, no. 2: 702. https://doi.org/10.3390/ijms26020702
APA StyleLu, Q., Shi, Y., Wen, X., Zhu, L., Zhang, L., Zhu, K., Cao, J., & Li, C. (2025). Mechanism of Functional Compound Fruit Drinks in Regulating Serum Metabolism in Constipated Mice. International Journal of Molecular Sciences, 26(2), 702. https://doi.org/10.3390/ijms26020702






