The Potential of Plant Tissue Cultures to Improve the Steviol Glycoside Profile of Stevia (Stevia rebaudiana Bertoni) Regenerants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Stevia Regenerants Obtained by Indirect Organogenesis
2.1.1. Fractionation of Steviol Glycoside Compounds by HPLC
2.1.2. Genetic Fidelity of Plants Regenerated by Indirect Organogenesis
| No of Starter | Starter Sequence 5′-3′ | Products (bp) | Polymorphism of Starter (%) | ||
|---|---|---|---|---|---|
| Monomorphic | Polymorphic | Specific | |||
| 2 | CAACAATGGCTACCACCC | 2900; 1900; 1700; 1500 | 6300; 5500; 2500; 2200; 800 | - | 55.5 |
| 4 | CAACAATGGCTACCACCT | 1900; 1400; 1000 | 2800 | - | 25.0 |
| 21 | ACGACATGGCGACCCACA | 1600; 1400; 500 | 2800; 2500; 2000; 1200; 1100; 800; 700; 300 | - | 72.8 |
| 23 | CACCATGGCTACCACCAG | 2000; 1600; 900; 400 | 3200; 3000; 2700; 1500; 1200; 700 | 7400 | 60.0 |
| 28 | CCATGGCTACCACCGCCA | 2500; 1100; 900; 800; 700; 500 | - | - | - |
| 30 | CCATGGCTACCACCGGCG | 2200; 1700; 1600; 1400; 1100 | 4300; 700 | - | 28.6 |
| 33 | CCATGGCTACCACCGCAG | 3200; 2500; 1300; 500 | 6100; 4300; 2800; 1900; 1250; 800 | - | 60.0 |
| 46 | ACAATGGCTACCACTGAG | 3800; 3100; 2700; 1600; 1400; 1200; 1100; 900 | - | 2500 | 11.1 |
| 75 | CCATGGCTACCACCGGAG | 4300; 3400; 3000; 2900; 2500; 1800; 1500; 1400; 1300; 900; 750 | 1200 | - | 8.3 |
| 83 | ACGACATGGCGACCAGCG | 2800; 2400; 1900; 1300 | 5000; 1500; 800; 700 | - | 50.0 |
| 90 | CCATGGCTACCACCGGCA | 3000; 2100; 1900; 1600; 1400; 1200; 1100; 900; 800; 730; 700; 650; 580; 520; 500; 450 | - | - | - |
| Total | 68 | 33 | 2 | 371.3 | |
| Average | 6.18 | 3 | 0.18 | 33.7 | |
2.2. Stevia Regenerants Obtained by Micropropagation
2.2.1. Fractionation of Steviol Glycoside Compounds by HPLC
2.2.2. Genetic Fidelity of Micropropagated Plants
3. Materials and Methods
3.1. Plant Material
3.2. Media Preparation
3.3. Micropropagation
3.4. Indirect Organogenesis
3.5. Genotype Analysis—SCoT Markers
3.6. Biochemical Analysis of Regenerants by HPLC
3.6.1. Sample Preparation and Extraction
3.6.2. Fractionation of Steviol Glycoside Compounds Using HPLC
3.7. Statistical Analysis of Chemical and Molecular Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J. 2021, 20, 1412–1430. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Lanestosa, A.; Moguel-Ordóñez, Y.; Segura-Campos, M. Stevia rebaudiana Bertoni: A natural alternative for treating diseases associated with metabolic syndrome. J. Med. Food 2017, 20, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.M.; Diamantino, T.; Domingues, J.; Montanari, Í., Jr.; Alves, M.N.; Gonçalves, J.C. Stevia rebaudiana germplasm characterization using microsatellite markers and steviol glycosides quantification by HPLC. Mol. Biol. Rep. 2021, 48, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Khan, I.; Blundell, R.; Azzopardi, J.; Mahomoodally, M.F. Stevia rebaudiana Bertoni.: An updated review of its health benefits, industrial applications and safety. Trends Food Sci. Technol. 2020, 100, 177–189. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Hossain, M.; Islam, R.; Kumar Saha, A.; Mandal, A. A review on natural sweetener plant–Stevia having medicinal and commercial importance. Agron. Glas. 2011, 73, 75–91. [Google Scholar]
- Gaweł-Bęben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Stevia Rebaudiana Bert. Leaf Extracts as a Multifunctional Source of Natural Antioxidants. Molecules 2015, 20, 5468–5486. [Google Scholar] [CrossRef]
- Amarakoon, S. Stevia rebaudiana—A review on agricultural, chemical and industrial applications. J. Nat. Appl. Res. 2021, 1, 14–27. [Google Scholar]
- Angelini, L.G.; Martini, A.; Passera, B.; Tavarini, S. Cultivation of Stevia rebaudiana Bertoni and Associated Challenges. In Sweeteners; Mérillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerlands, 2018. [Google Scholar] [CrossRef]
- Soejarto, D.D. Botany of Stevia and Stevia rebaudiana. In Stevia—The Genus Stevia; Kinghorn, A., Ed.; Taylor and Francis: Abingdon, UK, 2002; pp. 18–39. [Google Scholar]
- Angelini, L.; Tavarini, S. Crop productivity, steviol glycoside yield, nutrient concentration and uptake of Stevia rebaudiana Bert. under Mediterranean field conditions. Commun. Soil Sci. Plant. Anal. 2014, 45, 2577–2592. [Google Scholar] [CrossRef]
- Barbet-Massin, C.; Giuliano, S.; Alletto, L.; Daydé, J.; Berger, M. Towards a semi-perennial culture of Stevia rebaudiana (Bertoni) under temperate climate: Effects of genotype, environment and plant age on steviol glycoside content and composition. Genet. Resour. Crop. Evol. 2016, 63, 685–694. [Google Scholar] [CrossRef]
- Hastoy, C.; Cossona, P.; Cavaignac, S.; Boutié, P.; Waffo-Teguo, P.; Rolin, D.; Schurdi-Levrauda, V. Deciphering performances of fifteen genotypes of Stevia rebaudiana in southwestern France through dry biomass and steviol glycoside evaluation. Ind. Crops Prod. 2019, 128, 607–619. [Google Scholar] [CrossRef]
- Doliński, R.; Jabłońska, E. Mikrorozmnażanie stewii (Stevia rebaudiana Bert.) z eksplantatów węzłowych izolowanych z roślin wytworzonych in vitro. Ann. UMCS Sect. E. Agric. 2015, 70, 13–24. [Google Scholar] [CrossRef]
- Sivaram, L.; Mukundan, U. In vitro culture studies on Stevia rebaudiana. Vitro Cell. Dev. Biol.-Plant 2003, 39, 520–523. [Google Scholar] [CrossRef]
- Kaplan, B.; Duraklioglu, S.; Turgut, K. Sustainable micropropagation of selected Stevia rebaudiana Bertoni genotypes. Acta Sci. Pol. Hortorum Cultus 2019, 18, 47–56. [Google Scholar] [CrossRef]
- Singh, M.; Saharan, V.; Dayma, J.; Rajpurohit, D.; Sen, Y.; Sharma, A. In vitro propagation of Stevia rebaudiana (Bertoni): An overview. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1010–1022. [Google Scholar] [CrossRef]
- Al-Taweel, S.K.; Azzam, C.R.; Khaled, K.A.; Abdel-Aziz, R.M. Improvement of stevia (Stevia rebaudiana Bertoni) and steviol glycoside through traditional breeding and biotechnological approaches. Sabrao J. Breed. Genet 2021, 53, 88–111. [Google Scholar]
- Dev Gautam, R.; Kumar, R.; Kashyap, U.; Kumar, P.; Singh, S.; Singh, S.; Kumar, A. Genetic Improvement of Stevia: A Natural Non-Calorie Sweetener [Internet]. In Case Studies of Breeding Strategies in Major Plant Species; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Bugaj, B.; Leszczyńska, T.; Pysz, M.; Kopeć, A.; Pacholarz, J.; Pysz-Izdebska, K. Charakterystyka i prozdrowotne właściwości Stevia rebaudiana Bertoni. ŻNTJ 2013, 3, 27–38. [Google Scholar]
- Kolanowski, W. Glikozydy stewiolowe–właściwości i zastosowanie w żywności. Bromatol. I Chem. Toksykol. 2013, 46, 140–150. [Google Scholar]
- Wölwer-Rieck, U.; May, B.; Lankes, C.; Wüst, M. Methylerythritol and mevalonate pathway contributions to biosynthesis of mono-, sesqui-, and diterpenes in glandular trichomes and leaves of Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2014, 62, 2428–2435. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Człapka-Matyasik, M. Charakterystyka żywieniowa stewii–aktualny stan wiedzy. Bromat Chem Toksykol 2015, 47, 11–18. [Google Scholar]
- Luwańska, A.; Perz, A.; Mańkowska, G.; Wielgus, K. Application of in vitro stevia (Stevia rebaudiana Bertoni) cultures in obtaining steviol glycoside rich material. Herba Pol. 2015, 61, 50–63. [Google Scholar] [CrossRef]
- Yadav, S.K.; Guleria, P. Steviol Glycosides from Stevia: Biosynthesis Pathway Review and Their Application in Foods and Medicine. Crit. Rev. Food Sci. Nutr. 2012, 52, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Abdullah, S.N.A.; Md Hatta, M.A.; Megat Wahab, P.E. DNA Free CRISPR/DCAS9 Based Transcriptional Activation System for UGT76G1 Gene in Stevia rebaudiana Bertoni Protoplasts. Plants 2022, 11, 2393. [Google Scholar] [CrossRef]
- Dyduch-Siemińska, M.; Najda, A.; Gawroński, J.; Balant, S.; Świca, K.; Żaba, A. Stevia Rebaudiana Bertoni, a Source of High-Potency Natural Sweetener—Biochemical and Genetic Characterization. Molecules 2020, 25, 767. [Google Scholar] [CrossRef] [PubMed]
- Yücesan, B.; Büyükgöçmen, R.; Mohammed, A.; Sameeullah, M.; Altuğ, C.; Gürel, S.; Gürel, E. An efficient regeneration system and steviol glycoside analysis of Stevia rebaudiana Bertoni, a source of natural high-intensity sweetener. In Vitro Cell. Dev. Biol.-Plant 2016, 52, 330–337. [Google Scholar] [CrossRef]
- Yücesan, B.; Mohammed, A.; Büyükgoçmen, R.; Altug, C.; Kavas, O.; Gürel, S.; Gürel, E. In vitro and ex vitro propagation of Stevia rebaudiana Bertoni with high Rebaudioside-a content—A commercial scale application. Sci. Hortic. 2016, 203, 20–28. [Google Scholar] [CrossRef]
- Srivastava, V.; Chaturvedi, R. An interdisciplinary approach towards sustainable and higher steviol glycoside production from in vitro cultures of Stevia rebaudiana. J. Biotechnol. 2022, 358, 76–91. [Google Scholar] [CrossRef]
- Garro-Monge, G.; Jiménez-Quesada, K.; Alvarenga-Venutolo, S. Caracterización genética molecular de materiales procesados de Stevia rebaudiana utilizando la técnica de microssatélites. Tecnol. Marcha 2014, 27, 32–40. [Google Scholar] [CrossRef]
- Heikal, A.H.; Badawy, O.M.; Hafez, A.M. Genetic relationships among some stevia (Stevia Rebaudiana Bertoni) accessions based on ISSR analysis. Res. J. Cell Mol. Biol. 2008, 2, 1–5. [Google Scholar]
- Othman, H.S.; Zainuddin, Z.; Osman, M. Assessment of genetic diversity and hybrid identification in Stevia using Inter Simple Sequence Repeat (ISSR) markers. Trans. Persat. Genet. Malays. 2016, 3, 157–162. Available online: http://psasir.upm.edu.my/id/eprint/46530 (accessed on 12 June 2024).
- Othman, H.S.; Osman, M.; Zainuddin, Z. Genetic variabilities of Stevia rebaudiana Bertoni cultivated in Malaysia as revealed by morphological, chemical and molecular characterisations. AGRIVITA J. Agric. Sci. 2018, 40, 267–283. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, R.; Era, V. Potential of RAPD and ISSR markers for assessing genetic diversity among Stevia rebaudiana Bertoni accessions. Indian J. Biotechnol. 2016, 15, 95–100. [Google Scholar]
- Abd ElHamid, R.; Abd ElTawab, F.; Abdel Razik, A.; Allam, A.; ELDoliefy, A. Micropropagation and start codon targeted characterization of four stevia cultivars in Egypt. Arab. Univ. J. Agric. Sci. 2016, 26, 635–645. [Google Scholar] [CrossRef]
- Luz, G.C.; Strioto, D.K.; Mangolin, C.A.; Machado, M.F.P.S. ISSR markers to assess genetic diversity of cultivated populations from artificial selection of Stevia rebaudiana (Bert.) Bertoni. Breed. Sci. 2020, 70, 508–514. [Google Scholar] [CrossRef]
- Rai, M.K. Start codon targeted (SCoT) polymorphism marker in plant genome analysis: Current status and prospects. Planta 2023, 257, 34. [Google Scholar] [CrossRef]
- Collard, B.C.; Mackill, D.J. Start codon targeted (SCoT) polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Mesa-Valle, C.; Manzano-Agugliaro, F. Trends in plant research using molecular markers. Planta 2018, 247, 543–557. [Google Scholar] [CrossRef]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; Rabaiolli, S.M.D.S.; Stefanel, C.M. Determining the Polymorphism Information Content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef]
- Amiteye, S. Basic concepts and methodologies of DNA marker systems in plant molecular breeding. Heliyon 2021, 7, e08093. [Google Scholar] [CrossRef]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
- Taha, M.A.; Tawfik, A.A.; Mahmoud, A.M.; Mohamed, M.F. Assessment of Somaclonal Variants of Salvia splendens at Different Subcultures Using Molecular Markers. Assiut J. Agric. Sci. 2024, 55, 78–96. [Google Scholar] [CrossRef]
- Sharma, N.; Gauchan, D.P.; Dhakal, A.; Luitel, A.; Shakya, S.; Shakya, R. Establishment of regenerative callus, cell suspension system and molecular characterization of Stevia rebaudiana Bertoni for the production of stevioside in in vitro. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 133–148. [Google Scholar]
- Khan, M.A.; Nicolls, M.R.; Surguladze, B.; Saadoun, I. Complement components as potential therapeutic targets for asthma treatment. Respir. Med. 2014, 108, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; He, X.H.; Chen, H.; Ou, S.J.; Gao, M.P. Analysis of diversity and relationships among mango cultivars using Start Codon Targeted (SCoT) markers. Biochem. Syst. Ecol. 2010, 38, 1176–1184. [Google Scholar] [CrossRef]
- Qin, G.-X.; Liang, G.-L.; Lei, J.-J. Studies on the Genetic Relationship of Chinese Diploid Strawberry Species Based on Scot Analysis. Acta Hortic. 2014, 1049, 301–304. [Google Scholar] [CrossRef]
- Soliman, H.I.A.; Metwali, E.M.R.; Almaghrabi, O.A.H. Micropropagation of Stevia rebaudiana Betroni and assessment of genetic stability of in vitro regenerated plants using inter simple sequence repeat (ISSR) marker. Plant Biotechnol. 2014, 31, 249–256. [Google Scholar] [CrossRef]
- Kamińska, M.; Tretyn, A.; Trejgell, A. Genetic stability assessment of Taraxacum pieninicum plantlets after long-term slow growth storage using ISSR and SCoT markers. Biologia 2020, 75, 599–604. [Google Scholar] [CrossRef]
- Kumari, M.; Chandra, S. Stevioside glycosides from in vitro cultures of Stevia rebaudiana and antimicrobial assay. Rev. Bras. Bot. 2015, 38, 761–770. [Google Scholar] [CrossRef]
- Clapa, D.; Radomir, A.M.; Peticilă, A.G.; Hârța, M. Evaluation of Biomass Production of Stevia Rebaudiana Bertoni Using Classical In Vitro Culture and Temporary Immersion Bioreactor System. Sci. Papers. Ser. B Hortic. 2023, 67, 558–565. [Google Scholar]
- Thiyagarajan, M.; Venkatachalam, P. Evaluation of the genetic fidelity of in vitro propagated natural sweetener plant (Stevia rebaudiana Bert.) using DNA-based markers. Plant Cell Biotech. Mol. Biol. 2012, 13, 99–104. [Google Scholar]
- Singh, P.; Dwivedi, P.; Atri, N. In Vitro Shoot Multiplication of Stevia and Assessment of Stevioside Content and Genetic Fidelity of the Regenerants. Sugar Tech. 2014, 16, 430–439. [Google Scholar] [CrossRef]
- Dyduch-Siemińska, M. A fast and effective protocol forobtaining genetically diverse stevia (Stevia rebaudiana Bertoni) regenerants through indirect organogenesis. Ann. UMCS Sect. E Agric. 2021, LXXVI, 47–62. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Elect. 2001, 4, 9. [Google Scholar]


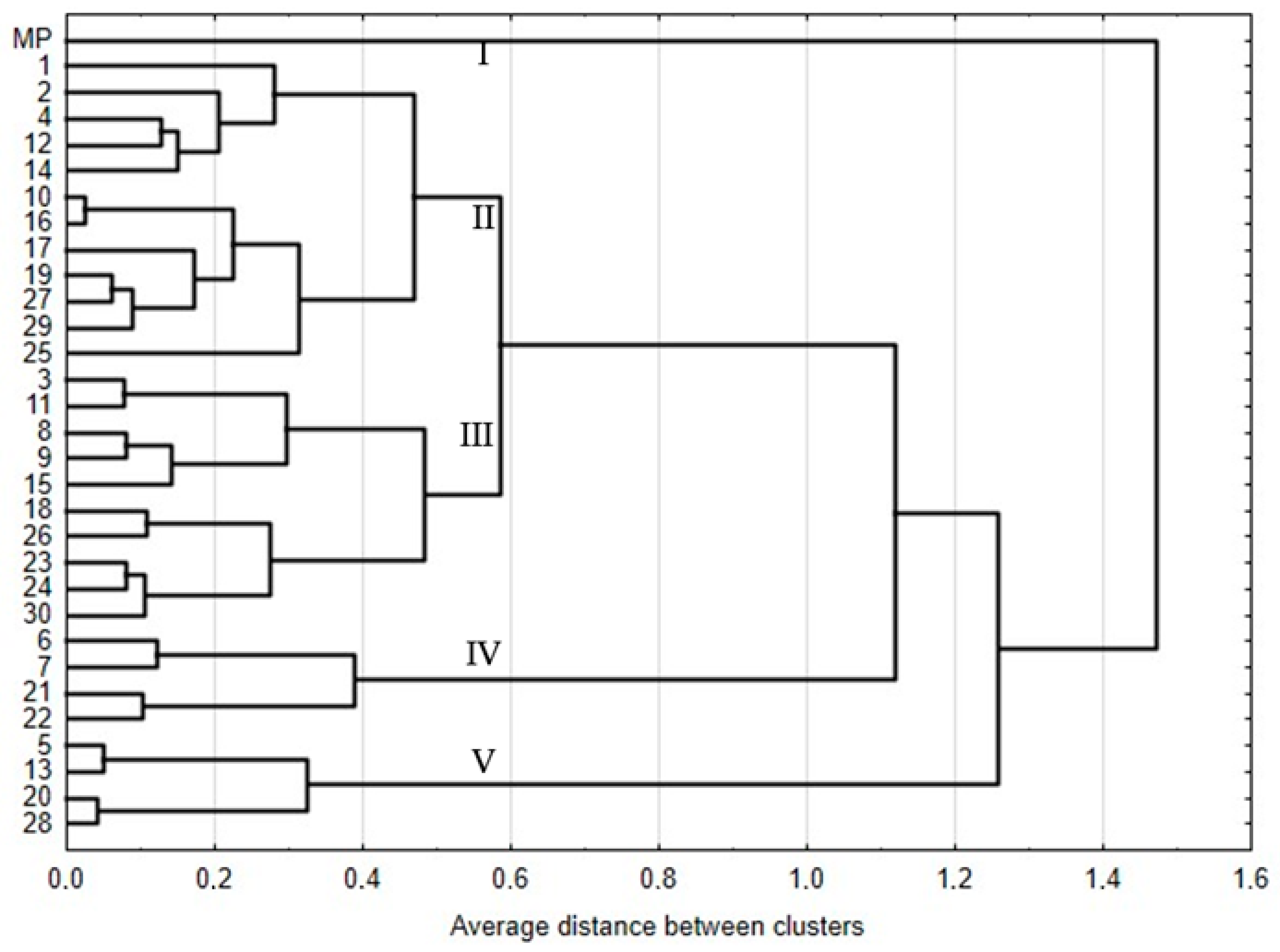
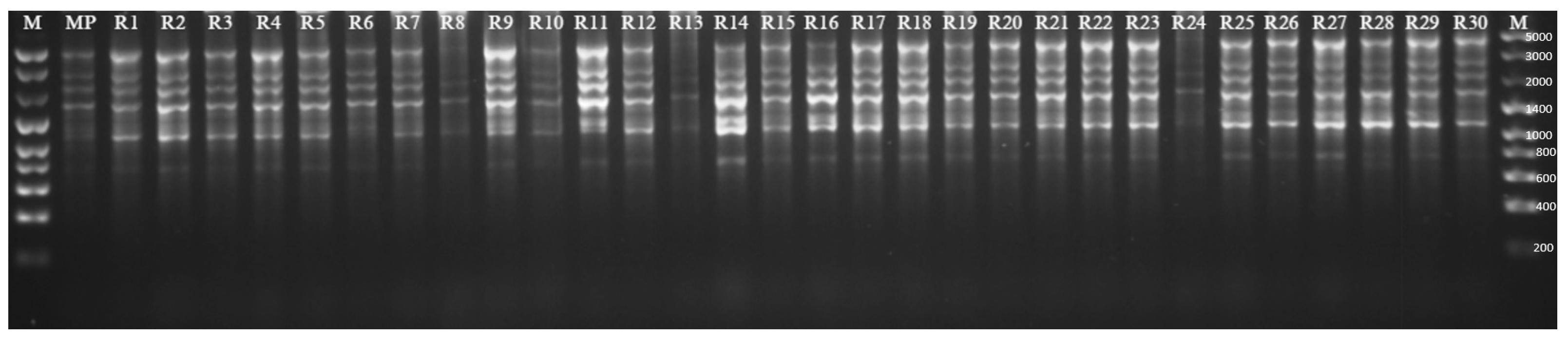
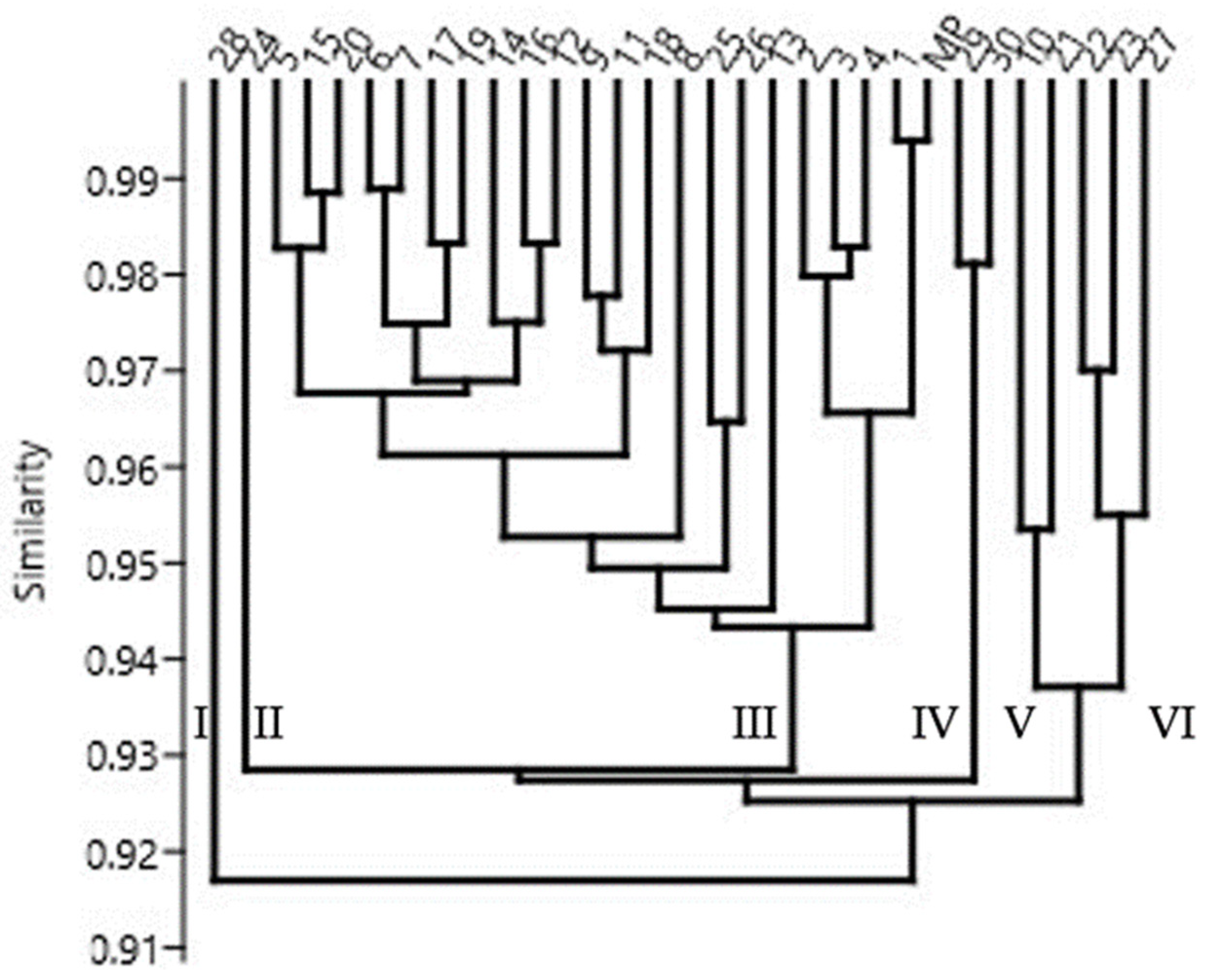
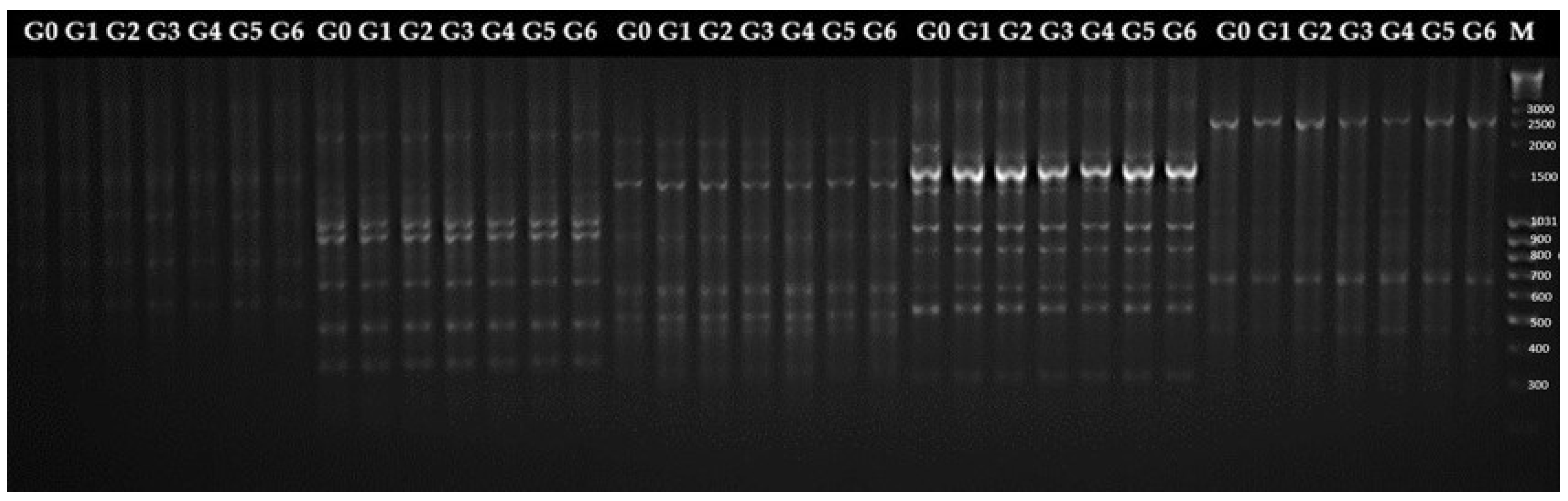
| Genotypes | Stevioside | Rebaudioside A | RebaudiosideA/ Stevioside Ratio | Rebaudioside C | Rebaudioside D | Total |
|---|---|---|---|---|---|---|
| MP | 5.46 ± 0.76 a1 | 1.55 ± 0.21 j–l | 0.28 | 0.96 ± 0.10 a | 0.21 ± 0.02 a–c | 8.18 ± 0.94 a |
| 1 | 4.94 ± 0.70 b | 1.67 ± 0.24 i–k | 0.34 | 0.48 ± 0.05 b | 0.11 ± 0.01 d–f | 7.20 ± 0.81 b–d |
| 2 | 4.72 ± 0.60 c | 1.57 ± 0.19 jk | 0.33 | 0.41 ± 0.06 b–d | 0.11 ± 0.01 d–f | 6.81 ± 0.72 e–h |
| 3 | 4.68 ± 0.55 c | 1.97 ± 0.31 d–i | 0.42 | 0.39 ± 0.04 b–e | 0.12 ± 0.01 c–f | 7.16 ± 0.80 b–e |
| 4 | 4.70 ± 0.52 c | 1.72 ± 0.20 h–k | 0.37 | 0.45 ± 0.05 cbc | 0.18 ± 0.02 a–d | 7.05 ± 0.76 c–f |
| 5 | 3.61 ± 0.41 g | 1.18 ± 0.13 mn | 0.33 | 0.25 ± 0.03 f–j | 0.11 ± 0.01 d–f | 5.15 ± 0.60 o |
| 6 | 3.73 ± 0.38 g | 2.48 ± 0.29 a | 0.66 | 0.26 ± 0.03 f–j | 0.15 ± 0.01 a–f | 6.62 ± 0.63 g–j |
| 7 | 3.74 ± 0.40 g | 2.39 ± 0.33 ab | 0.64 | 0.25 ± 0.03 f–j | 0.23 ± 0.02 ab | 6.61 ± 0.65 g–j |
| 8 | 4.73 ± 0.42 c | 2.24 ± 0.21 a–d | 0.47 | 0.31 ± 0.03 d–i | 0.13 ± 0.01 c–f | 7.41 ± 0.69 bc |
| 9 | 4.71 ± 0.60 c | 2.31 ± 0.25 a–d | 0.49 | 0.28 ± 0.03 e–j | 0.12 ± 0.01 c–f | 7.42 ± 0.76 bc |
| 10 | 4.51 ± 0.59 d | 1.53 ± 0.16 j–l | 0.34 | 0.36 ± 0.04 b–f | 0.11 ± 0.01 d–f | 6.51 ± 0.59 h–k |
| 11 | 4.67 ± 0.58 c | 2.03 ± 0.28 c–h | 0.43 | 0.35 ± 0.04 c–g | 0.09 ± 0.01 d–f | 7.14 ± 0.80 b–e |
| 12 | 4.72 ± 0.53 c | 1.73 ± 0.20 h–k | 0.37 | 0.34 ± 0.04 c–g | 0.12 ± 0.01 c–f | 6.91 ± 0.71 d–g |
| 13 | 3.61 ± 0.49 g | 1.22 ± 0.14 l–n | 0.34 | 0.28 ± 0.03 e–j | 0.12 ± 0.01 c–f | 5.23 ± 0.56 o |
| 14 | 4.69 ± 0.61 c | 1.78 ± 0.15 g–j | 0.38 | 0.31 ± 0.03 d–i | 0.24 ± 0.03 a | 7.02 ± 0.68 c–f |
| 15 | 4.63 ± 0.59 c–d | 2.29 ± 0.24 a–d | 0.49 | 0.35 ± 0.04 c–g | 0.21 ± 0.02 a–c | 7.48 ± 0.82 b |
| 16 | 4.53 ± 0.52 d | 1.53 ± 0.15 j–l | 0.34 | 0.35 ± 0.04 c–g | 0.12 ± 0.01 c–f | 6.53 ± 0.70 h–j |
| 17 | 4.33 ± 0.60 e | 1.44 ± 0.16 k–m | 0.33 | 0.30 ± 0.03 d–j | 0.08 ± 0.01 ef | 6.15 ± 0.69 k–n |
| 18 | 4.29 ± 0.59 e | 1.81 ± 0.10 f–j | 0.42 | 0.28 ± 0.03 e–j | 0.15 ± 0.02 a–f | 6.53 ± 0.71 h–j |
| 19 | 4.31 ± 0.46 e | 1.58 ± 0.21 j–k | 0.37 | 0.33 ± 0.03 c–h | 0.12 ± 0.01 c–f | 6.34 ± 0.69 i–l |
| 20 | 3.31 ± 0.38 h | 1.09 ± 0.11 n | 0.33 | 0.18 ± 0.02 j | 0.08 ± 0.01 ef | 4.66 ± 0.50 p |
| 21 | 3.42 ± 0.40 h | 2.28 ± 0.26 a–d | 0.67 | 0.19 ± 0.02 i–j | 0.10 ± 0.01 df | 5.99 ± 0.60 l–n |
| 22 | 3.43 ± 0.29 h | 2.19 ± 0.29 a–e | 0.64 | 0.18 ± 0.02 j | 0.15 ± 0.02 a–f | 5.95 ± 0.62 mn |
| 23 | 4.33 ± 0.60 e | 2.06 ± 0.22 c–h | 0.48 | 0.23 ± 0.02 g–j | 0.09 ± 0.01 d–f | 6.71 ± 0.73 f–i |
| 24 | 4.32 ± 0.58 e | 2.12 ± 0.24 b–f | 0.49 | 0.21 ± 0.02 h–j | 0.14 ± 0.01 b–f | 6.79 ± 0.75 e–h |
| 25 | 4.13 ± 0.57 f | 1.41 ± 0.18 k–n | 0.34 | 0.26 ± 0.03 f–j | 0.08 ± 0.01 ef | 5.88 ± 0.57 n |
| 26 | 4.28 ± 0.57 e | 1.86 ± 0.19 e–j | 0.43 | 0.25 ± 0.02 f–j | 0.06 ± 0.01 f | 6.45 ± 0.68 h–k |
| 27 | 4.33 ± 0.60 e | 1.59 ± 0.21 jk | 0.37 | 0.29 ± 0.03 d–j | 0.08 ± 0.01 ef | 6.29 ± 0.70 j–n |
| 28 | 3.31 ± 0.46 h | 1.12 ± 0.13 mn | 0.34 | 0.21 ± 0.02 h–j | 0.08 ± 0.01 l | 4.72 ± 0.53 p |
| 29 | 4.30 ± 0.55 e | 1.64 ± 0.18 ik | 0.38 | 0.31 ± 0.03 d–i | 0.16 ± 0.02 a–e | 6.41 ± 0.71 i–k |
| 30 | 4.24 ± 0.59 e–f | 2.10 ± 0.23 b–g | 0.50 | 0.18 ± 0.02 j | 0.14 ± 0.01 b–f | 6.66 ± 0.80 g–j |
| Average | 4.28 | 1.79 | 0.42 | 0.32 | 0.13 | 6.51 |
| Genotypes | Stevioside | Rebaudioside A | RebaudiosideA/ Stevioside Ratio | Rebaudioside C | Rebaudioside D | Total |
|---|---|---|---|---|---|---|
| MP | 5.46 ± 0.76 a1 | 1.55 ± 0.21 a | 0.28 | 0.96 ± 0.10 a | 0.21 ± 0.02 a | 8.18 ± 0.94 a |
| G1 | 5.28 ± 0.69 a | 1.51 ± 0.20 a | 0.28 | 0.98 ± 0.12 a | 0.23 ± 0.02 a | 8.00 ± 1.05 a |
| G2 | 5.71 ± 0.63 a | 1.58 ± 0.20 a | 0.27 | 0.96 ± 0.12 a | 0.22 ± 0.02 a | 8.47 ± 1.10 a |
| G3 | 5.56 ± 0.68 a | 1.60 ± 0.22 a | 0.29 | 0.99 ± 0.16 a | 0.24 ± 0.03 a | 8.39 ± 1.08 a |
| G4 | 5.40 ± 0.66 a | 1.53 ± 0.21 a | 0.28 | 0.95 ± 0.12 a | 0.200 ± 0.02 a | 8.08 ± 0.99 a |
| G5 | 5.42 ± 0.69 a | 1.55 ± 0.20 a | 0.28 | 0.98 ± 0.16 a | 0.21 ± 0.02 a | 8.16 ± 1.03 a |
| G6 | 5.50 ± 0.69 a | 1.57 ± 0.20 a | 0.28 | 0.96 ± 0.14 a | 0.22 ± 0.03 a | 8.25 ± 1.10 a |
| Average | 5.47 | 1.55 | 0.28 | 0.97 | 0.22 | 8.22 |
| No of Starter | Starter Sequence 5′-3′ | No of Monomorphic Bands | Range of Size (bp) |
|---|---|---|---|
| Starter 2 | CAACAATGGCTACCACCC | 5 | 600–3100 |
| Starter 4 | CAACAATGGCTACCACCT | 5 | 1050–2350 |
| Starter 14 | ACGACATGGCGACCACGC | 8 | 460–2200 |
| Starter 15 | ACGACATGGCGACCGCGA | 7 | 380–2200 |
| Starter 16 | ACCATGGCTACCACCGAC | 7 | 450–2900 |
| Starter 18 | ACCATGGCTACCACCGCC | 8 | 550–2050 |
| Starter 19 | ACCATGGCTACCACCGGC | 9 | 350–3100 |
| Starter 21 | ACGACATGGCGACCCACA | 3 | 500–2500 |
| Starter 23 | CACCATGGCTACCACCAG | 4 | 350–1850 |
| Starter 24 | CACCATGGCTACCACCAT | 6 | 550–2100 |
| Starter 26 | ACCATGGCTACCACCGTC | 5 | 550–1700 |
| Starter 27 | ACCATGGCTACCACCGTG | 4 | 700–2300 |
| Starter 28 | CCATGGCTACCACCGCCA | 7 | 600–1900 |
| Starter 30 | CCATGGCTACCACCGGCG | 4 | 650–1400 |
| Starter 33 | CCATGGCTACCACCGCAG | 5 | 450–2200 |
| Starter 46 | ACAATGGCTACCACTGAG | 6 | 650–6000 |
| Starter 75 | CCATGGCTACCACCGGAG | 9 | 450–2400 |
| Starter 83 | ACGACATGGCGACCAGCG | 4 | 250–1800 |
| Starter 84 | ACGACATGGCGACCACGT | 5 | 750–4200 |
| Starter 90 | CCATGGCTACCACCGGCA | 5 | 600–1700 |
| Total | 116 | 260–6000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyduch-Siemińska, M.; Wawerska, K.; Gawroński, J. The Potential of Plant Tissue Cultures to Improve the Steviol Glycoside Profile of Stevia (Stevia rebaudiana Bertoni) Regenerants. Int. J. Mol. Sci. 2024, 25, 13584. https://doi.org/10.3390/ijms252413584
Dyduch-Siemińska M, Wawerska K, Gawroński J. The Potential of Plant Tissue Cultures to Improve the Steviol Glycoside Profile of Stevia (Stevia rebaudiana Bertoni) Regenerants. International Journal of Molecular Sciences. 2024; 25(24):13584. https://doi.org/10.3390/ijms252413584
Chicago/Turabian StyleDyduch-Siemińska, Magdalena, Karolina Wawerska, and Jacek Gawroński. 2024. "The Potential of Plant Tissue Cultures to Improve the Steviol Glycoside Profile of Stevia (Stevia rebaudiana Bertoni) Regenerants" International Journal of Molecular Sciences 25, no. 24: 13584. https://doi.org/10.3390/ijms252413584
APA StyleDyduch-Siemińska, M., Wawerska, K., & Gawroński, J. (2024). The Potential of Plant Tissue Cultures to Improve the Steviol Glycoside Profile of Stevia (Stevia rebaudiana Bertoni) Regenerants. International Journal of Molecular Sciences, 25(24), 13584. https://doi.org/10.3390/ijms252413584






