Neuroinflammatory Biomarkers in Alzheimer’s Disease: From Pathophysiology to Clinical Implications
Abstract
1. Introduction
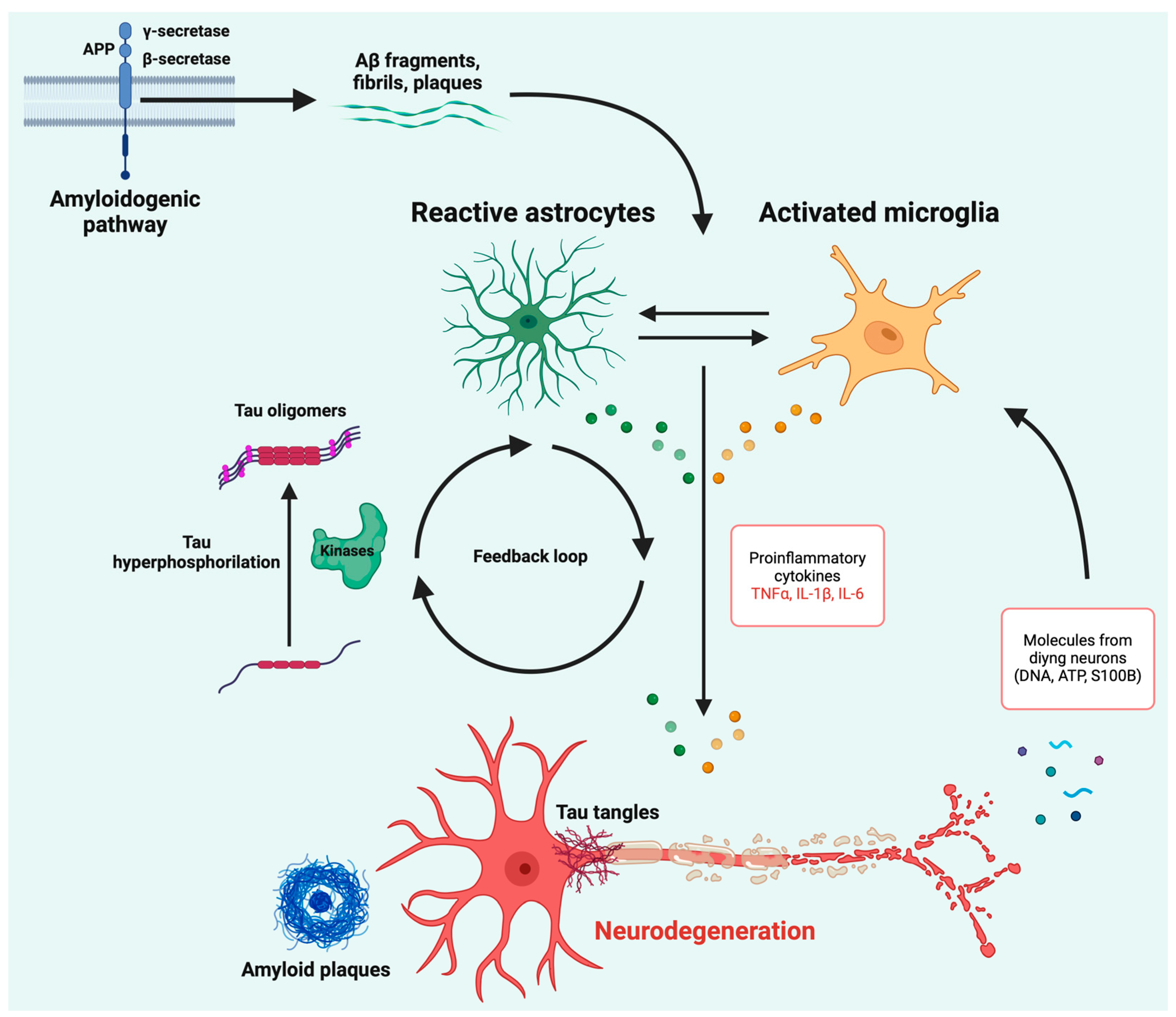
2. Neuroinflammation: A Bridge Between Aβ Aggregation and Tau Tangle Accumulation?
3. Current Evidence on Neuroinflammatory Biomarkers of Alzheimer’s Disease
3.1. Glial Fibrillary Acidic Protein (GFAP)
3.2. Soluble Triggering Receptor Expressed on Myeloid Cells 2 (sTREM2)
3.3. Chitinase-3-like Protein 1 (YKL-40)
3.4. S100B
3.5. 18 kDa Translocator Protein (TSPO)
3.6. Monoamine Oxidase B (MAO-B)
3.7. Other Neuroinflammatory Biomarkers
| Biomarker/Target | Category | Role/Mechanism | Clinical Evidence | Ref. |
|---|---|---|---|---|
| GFAP | CSF/Blood | Marker of reactive astrocytes. | Increased CSF and plasma levels in AD, linked to Aβ pathology and AD progression. Plasma GFAP is stable and predictive of conversion from MCI to AD dementia. | [14,58,63,64] |
| sTREM2 | CSF | Released during microglial activation through TREM2 shedding. | Elevated in CSF with AD progression; correlates with higher tau pathology and slower cognitive decline. Conflicting evidence regarding levels between AD patients and cognitively unimpaired individuals. | [72,73,74,75,76] |
| YKL-40 | CSF | Expressed in reactive astrocytes and microglia during neuroinflammation. | Elevated in CSF during later AD stages; associated with Aβ and tau pathology, brain atrophy, and cognitive decline. Rises earlier in familial AD. | [80,81,82] |
| S100B | CSF | Associated with neuroinflammation. | Inconsistent evidence; some studies show moderate increases in early stages, while others show no significant differences. Expression in other tissues complicates interpretation. | [88,89,90,91,92] |
| TSPO | PET | Upregulated under pathological conditions; detected via PET imaging. | Increased TSPO binding occurs in AD-affected brain regions, but lack of specificity for microglia creates limitation. Newer tracers under development. | [17,96,98] |
| MAO-B | PET | Enzyme localized in astrocytes, detectable via PET imaging. | Elevated activity in AD patients’ temporal cortex and hippocampus, particularly in early stages. Activation peaks during MCI stage. | [18,103,104] |
4. Therapeutic Strategies
5. Discussion and Conclusions
6. Search Strategy and Inclusion Criteria
Author Contributions
Funding
Conflicts of Interest
References
- Alzheimer’s Association. 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef] [PubMed]
- Lista, S.; Imbimbo, B.P. Tracking neuroinflammatory biomarkers in Alzheimer’s disease: A strategy for individualized therapeutic approaches? J. Neuroinflamm. 2024, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Botella Lucena, P.; Heneka, M.T. Inflammatory aspects of Alzheimer’s disease. Acta Neuropathol. 2024, 148, 31. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Haeberlein, S.B. The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: Perspectives from the Research Roundtable. Alzheimer’s Dement. 2018, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J. Microglia in the neuroinflammatory pathogenesis of Alzheimer’s disease and related therapeutic targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Acosta, C.; Anderson, H.D. Astrocyte dysfunction in Alzheimer disease. J. Neurosci. Res. 2017, 95, 2430–2447. [Google Scholar] [CrossRef] [PubMed]
- Jiwaji, Z.; Tiwari, S.S. Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Aß pathology. Nat. Commun. 2022, 13, 135. [Google Scholar] [CrossRef]
- Avila-Muñoz, E.; Arias, C. When astrocytes become harmful: Functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res. Rev. 2014, 18, 29–40. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Paolini Paoletti, F. CSF and blood biomarkers in neuroinflammatory and neurodegenerative diseases: Implications for treatment. Trends Pharmacol. Sci. 2020, 41, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, A.; Kamada, M. Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. J. Neurochem. 2016, 136, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.E.; El Khoury, J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Lista, S. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert. Rev. Proteomics 2017, 14, 285–299. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, K. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharm. Sin. B 2021, 11, 373–393. [Google Scholar] [CrossRef]
- Fowler, J.S.; Logan, J. Monoamine oxidase: Radiotracer chemistry and human studies. J. Labelled Comp. Radiopharm. 2015, 58, 51–64. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Ferrucci, L. Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: A systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease. Ageing Res. Rev. 2021, 68, 101339. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Giri, P.M.; Banerjee, A. Neuroinflammation in Neurodegenerative Disorders: Current Knowledge and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 3995. [Google Scholar] [CrossRef] [PubMed]
- Hippius, H.; Neundörfer, G. The discovery of Alzheimer’s disease. Dialogues Clin. Neurosci. 2003, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, X. Interactions of glial cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. Glia 2023, 71, 1383–1401. [Google Scholar] [CrossRef]
- Huffels, C.F.M.; Middeldorp, J. Aß pathology and neuron-glia interactions: A synaptocentric view. Neurochem. Res. 2023, 48, 1026–1046. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.J.; Ossenkoppele, R. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Hardy, S. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Lau, S.-F.; Fu, A.K.Y. Receptor–ligand interaction controls microglial chemotaxis and amelioration of Alzheimer’s disease pathology. J. Neurochem. 2023, 166, 891–903. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Michelucci, A.; Heurtaux, T. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 2009, 210, 3–12. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl 2), 136–153. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Dixit, R.; Ross, J.L. Differential regulation of dynein and kinesin motor proteins by tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef]
- Basheer, N.; Smolek, T. Does modulation of tau hyperphosphorylation represent a reasonable therapeutic strategy for Alzheimer’s disease? From preclinical studies to the clinical trials. Mol. Psychiatry 2023, 28, 2197–2214. [Google Scholar] [CrossRef]
- Domingues, C.; da Cruz, E. of cytokines and chemokines on alzheimer’s disease neuropathological hallmarks. Curr. Alzheimer Res. 2017, 14, 870–882. [Google Scholar] [CrossRef]
- Alonso, A.C.; Grundke-Iqbal, I. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 1996, 2, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Mudher, A.; Colin, M. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun. 2017, 5, 99. [Google Scholar] [CrossRef]
- DiPatre, P.L.; Gelman, B.B. Microglial cell activation in aging and Alzheimer disease: Partial linkage with neurofibrillary tangle burden in the hippocampus. J. Neuropathol. Exp. Neurol. 1997, 56, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, H. Tau internalization: A complex step in tau propagation. Ageing Res. Rev. 2021, 67, 101272. [Google Scholar] [CrossRef] [PubMed]
- Brelstaff, J.H.; Mason, M. Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Sci. Adv. 2021, 7, eabg4980. [Google Scholar] [CrossRef] [PubMed]
- Mothes, T.; Portal, B. Astrocytic uptake of neuronal corpses promotes cell-to-cell spreading of tau pathology. Acta Neuropathol. Commun. 2023, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Richetin, K.; Steullet, P. Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Eltom, K.; Mothes, T. Astrocytic accumulation of tau fibrils isolated from Alzheimer’s disease brains induces inflammation, cell-to-cell propagation and neuronal impairment. Acta Neuropathol. Commun. 2024, 12, 34. [Google Scholar] [CrossRef]
- Giusti, V.; Kaur, G. Brain clearance of protein aggregates: A close-up on astrocytes. Mol. Neurodegener. 2024, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, R.M.; Proctor, E.A. Astrocytic propagation of tau in the context of Alzheimer’s disease. Front. Cell. Neurosci. 2021, 15, 645233. [Google Scholar] [CrossRef]
- Miners, J.S.; Kehoe, P.G. CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology. Alzheimer’s Res. Ther. 2019, 11, 1–6. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Theotokis, P. CNS border-associated macrophages: Ontogeny and potential implication in disease. Curr. Issues Mol. Biol. 2023, 45, 4285–4300. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Yap, J.K.Y.; Pickard, B.S. The role of neuronal NLRP1 inflammasome in Alzheimer’s disease: Bringing neurons into the neuroinflammation game. Mol. Neurobiol. 2019, 56, 7741–7753. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Victor, M.B. Human microglial state dynamics in Alzheimer’s disease progression. Cell 2023, 186, 4386–4403.e29. [Google Scholar] [CrossRef] [PubMed]
- Ekonomou, A.; Savva, G.M. Stage-specific changes in neurogenic and glial markers in Alzheimer’s disease. Biol. Psychiatry 2015, 77, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, A.; Afridi, R. Bidirectional communication between microglia and astrocytes in neuroinflammation. Curr. Neuropharmacol. 2023, 21, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Opland, C.K.; Bryan, M.R. Activity-dependent tau cleavage by caspase-3 promotes neuronal dysfunction and synaptotoxicity. iScience 2023, 26, 106905. [Google Scholar] [CrossRef] [PubMed]
- Bieger, A.; Rocha, A. Neuroinflammation Biomarkers in the AT(N) Framework Across the Alzheimer’s Disease Continuum. J. Prev. Alzheimer’s Dis. 2023, 10, 401–417. [Google Scholar] [CrossRef]
- Garwood, C.J.; Ratcliffe, L.E. Review: Astrocytes in Alzheimer’s disease and other age-associated dementias: A supporting player with a central role. Neuropathol. Appl. Neurobiol. 2017, 43, 281–298. [Google Scholar] [CrossRef]
- Elahi, F.M.; Casaletto, K.B. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, 681–695. [Google Scholar] [CrossRef]
- Muramori, F.; Kobayashi, K. A quantitative study of neurofibrillary tangles, senile plaques and astrocytes in the hippocampal subdivisions and entorhinal cortex in Alzheimer’s disease, normal controls and non-Alzheimer neuropsychiatric diseases. Psychiatry Clin. Neurosci. 1998, 52, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Halbgebauer, S. Glial fibrillary acidic protein in serum is increased in Alzheimer’s disease and correlates with cognitive impairment. J. Alzheimer’s Dis. 2019, 67, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Verberk, I.M.W.; Thijssen, E. Combination of plasma amyloid beta((1-42/1-40)) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimer’s Res. Ther. 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; An, Y. Longitudinal progression of blood biomarkers reveals a key role of astrocyte reactivity in preclinical Alzheimer’s disease. medRxiv 2024. [Google Scholar] [CrossRef]
- Cicognola, C.; Janelidze, S. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimer’s Res. Ther. 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Ebenau, J.L.; Pelkmans, W. Association of CSF, Plasma, and Imaging Markers of Neurodegeneration With Clinical Progression in People With Subjective Cognitive Decline. Neurology 2022, 98, e1315–e1326. [Google Scholar] [CrossRef]
- Stocker, H.; Beyer, L. Association of plasma biomarkers, p-tau181, glial fibrillary acidic protein, and neurofilament light, with intermediate and long-term clinical Alzheimer’s disease risk: Results from a prospective cohort followed over 17 years. Alzheimer’s Dement. 2022, 19, 25–35. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl. Psychiatry 2021, 11, 27. [Google Scholar] [CrossRef]
- Klesney-Tait, J.; Turnbull, I.R. The TREM receptor family and signal integration. Nat. Immunol. 2006, 7, 1266–1273. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron 2018, 97, 1023–1031.e1027. [Google Scholar] [CrossRef]
- Ulland, T.K.; Wilbur, M. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 2017, 170, 649–663.e13. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Schlepckow, K.; Monroe, K.M. Enhancing protective microglial activities with a dual function TREM2 antibody to the stalk region. 60. EMBO Mol. Med. 2020, 12, e11227. [Google Scholar] [CrossRef] [PubMed]
- Heslegrave, A.; Heywood, W. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Kleinberger, G. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 2016, 8, 466–476. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Araque Caballero, M. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci. Transl. Med. 2016, 8, 369ra178. [Google Scholar] [CrossRef]
- Ma, L.Z.; Tan, L. Dynamic changes of CSF sTREM2 in preclinical Alzheimer’s disease: The CABLE study. Mol. Neurodegener. 2020, 15, 25. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Morenas-Rodríguez, E. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related neurodegeneration but not with amyloid-β pathology. Mol. Neurodegener. 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Thüne, K. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol. Neurodegener. 2017, 12, 83. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Wang, G. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neuro- logical diseases. J. Neuroinflamm. 2010, 7, 34. [Google Scholar] [CrossRef]
- Craig-Schapiro, R.; Perrin, R. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry 2010, 68, 903–912. [Google Scholar] [CrossRef]
- Baldacci, F.; Lista, S. The neuroinflammatory biomarker YKL-40 for neurodegenerative diseases: Advances in development. Expert Rev. Proteom. 2019, 16, 593–600. [Google Scholar] [CrossRef]
- Kester, M.I.; Teunissen, C.E. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimer’s Res. Ther. 2015, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, H.W. Plasma level of chitinase 3-like 1 protein increases in patients with early Alzheimer’s disease. J. Neurol. 2011, 258, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, A.; Lista, S. Association of plasma YKL-40 with brain amyloid-β levels, memory performance, and sex in subjective memory complainers. Neurobiol. Aging 2020, 96, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, I.O.C.; Nicholas, J.M. Cerebrospinal Fluid YKL-40 and Chitotriosidase Levels in Frontotemporal Dementia Vary by Clinical, Genetic and Pathological Subtype. Dement. Geriatr. Cogn. Disord. 2020, 49, 56–76. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 57–172. [Google Scholar] [CrossRef]
- Sutphen, C.L.; Mateusz, S. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 2015, 72, 1029–1042. [Google Scholar] [CrossRef]
- Piazza, O.; Leggiero, E. S100B induces the release of pro-inflammatory cytokines in alveolar type I-like cells. Int. J. Immunopathol. Pharmacol. 2013, 26, 383–391. [Google Scholar] [CrossRef]
- Schulz, I.; Kruse, N. Systematic Assessment of 10 Biomarker Candidates Focusing on α-Synuclein-Related Disorders. Mov. Disord. 2021, 36, 2874–2887. [Google Scholar] [CrossRef]
- Green, A.J.; Harvey, R.J. Increased S100beta in the cerebrospinal fluid of patients with frontotemporal dementia. Neurosci. Lett. 1997, 235, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Peskind, E.R.; Griffin, W.S. Cerebrospinal fluid S100B is elevated in the earlier stages of Alzheimer’s disease. Neurochem. Int. 2001, 39, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Maetzler, W.; Berg, D. Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of Lewy body-associated dementias. J. Alzheimer’s Dis. 2011, 26, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.; Gottfries, J. Evaluation of CSF biomarkers for axonal and neuronal degeneration, gliosis, and beta-amyloid metabolism in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 71, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Teitsdottir, U.D.; Jonsdottir, M.K. Association of glial and neuronal degeneration markers with Alzheimer’s disease cerebrospinal fluid profile and cognitive functions. Alzheimer’s Res. Ther. 2020, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Milà-Alomà, M.; Shekari, M. Cognitively unimpaired individuals with a low burden of Aβ pathology have a distinct CSF biomarker profile. Alzheimer’s Res. Ther. 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Edison, P.; Brooks, D.J. Role of neuroinflammation in the trajectory of Alzheimer’s disease and in vivo quantification using PET. J. Alzheimer’s Dis. 2018, 64, S339–S351. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Ninan, S. Does Microglial Activation Influence Hippocampal Volume and Neuronal Function in Alzheimer’s Disease and Parkinson’s Disease Dementia? J. Alzheimer’s Dis. 2016, 51, 1275–1289. [Google Scholar] [CrossRef]
- Yokokura, M.; Terada, T. Depiction of microglial activation in aging and dementia: Positron emission tomography with [11C]DPA713 versus [11C]( R)PK11195. J. Cereb. Blood Flow. Metab. 2017, 37, 877–889. [Google Scholar] [CrossRef]
- Mizrahi, R.; Rusjan, P.M. Translocator protein (18kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J. Cereb. Blood Flow Metab. 2012, 32, 968–972. [Google Scholar] [CrossRef]
- Xiang, X.; Wind, K. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci. Transl. Med. 2021, 13, eabe5640. [Google Scholar] [CrossRef]
- Levitt, P.; Pintar, J.E. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc. Natl. Acad. Sci. USA 1982, 79, 6385–6389. [Google Scholar] [CrossRef]
- Ekblom, J.; Jossan, S.S. Monoamine oxidase-B in astrocytes. Glia 1993, 8, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, J.; Jossan, S.S. Reactive gliosis and monoamine oxidase B. J. Neural Transm. Suppl. 1994, 41, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Jossan, S.S.; Gillberg, P.G. Monoamine oxidase B in brains from patients with Alzheimer’s disease: A biochemical and autoradiographical study. Neuroscience 1991, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.F.; Scholl, M. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: A multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J. Nucl. Med. 2012, 53, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Scholl, M.; Carter, S.F. Early astrocytosis in autosomal dominant Alzheimer’s disease measured in vivo by multi-tracer positron emission tomography. Sci. Rep. 2015, 5, 16404. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Harada, R. First-in-Humans Evaluation of (18)F-SMBT-1, a Novel (18)F-Labeled Monoamine Oxidase-B PET Tracer for Imaging Reactive Astrogliosis. J. Nucl. Med. 2022, 63, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhou, X.-W. The dual roles of cytokines in Alzheimer’s disease: Update on interleukins, TNF-α, TGF-β and IFN-γ. Transl. Neurodegener. 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Brosseron, F.; Maass, A. Soluble TAM receptors sAXL and sTyro3 predict structural and functional pro- tection in Alzheimer’s disease. Neuron 2022, 110, 1009–1022.e1004. [Google Scholar] [CrossRef] [PubMed]
- Horti, A.G.; Naik, R. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc. Natl. Acad. Sci. USA 2019, 116, 1686–1691. [Google Scholar] [CrossRef]
- Walker, D.G.; Tang, T.M. Studies on Colony Stimulating Factor Receptor-1 and Ligands Colony Stimulating Factor-1 and Interleukin-34 in Alzheimer’s Disease Brains and Human Microglia. Front. Aging Neurosci. 2017, 9, 244. [Google Scholar] [CrossRef]
- Ruiz de Martín Esteban, S.; Benito-Cuesta, I. Cannabinoid CB2 Receptors Modulate Microglia Function and Amyloid Dynamics in a Mouse Model of Alzheimer’s Disease. Front. Pharmacol. 2022, 13, 841766. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Koistinen, N.A. Astroglial tracer BU99008 detects multiple binding sites in Alzheimer’s disease brain. Mol. Psychiatry 2021, 26, 5833–5847. [Google Scholar] [CrossRef]
- Calsolaro, V.; Matthews, P.M. Astrocyte reactivity with late-onset cognitive impairment assessed in vivo using (11)C-BU99008 PET and its relationship with amyloid load. Mol. Psychiatry 2021, 26, 5848–5855. [Google Scholar] [CrossRef] [PubMed]
- Livingston, N.R.; Calsolaro, V. Relationship between astrocyte reactivity, using novel (11)C-BU99008 PET, and glucose metabolism, grey matter volume and amyloid load in cognitively impaired individuals. Mol. Psychiatry 2022, 27, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Chen, Y.J. Astrocyte activation imaging with 11C-acetate and amyloid PET in mild cognitive impairment due to Alzheimer pathology. Nucl. Med. Commun. 2021, 42, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.-H.; Ko, H.Y. Visualization of reactive astrocytes in living brain of Alzheimer’s disease patient. bioRxiv 2021, 439744. [Google Scholar] [CrossRef]
- Etxeberria, A.; Shen, Y.A. Neutral or Detrimental Effects of TREM2 Agonist Antibodies in Preclinical Models of Alzheimer’s Disease and Multiple Sclerosis. J. Neurosci. 2024, 44, e2347232024. [Google Scholar] [CrossRef]
- Wang, S.; Mustafa, M. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef]
- Olmos-Alonso, A.; Schetters, S.T.T.; Sri, S.; Askew, K.; Mancuso, R.; Vargas-Caballero, M.; Holscher, C.; Perry, V.H.; Gomez-Nicola, D. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 2016, 139, 891–907. [Google Scholar] [CrossRef]
- Hagan, N.; Kane, J.L.; Grover, D.; Woodworth, L.; Madore, C.; Saleh, J.; Sancho, J.; Liu, J.; Li, Y.; Proto, J.; et al. CSF1R signaling is a regulator of pathogenesis in progressive MS. Cell Death Dis. 2020, 11, 904. [Google Scholar] [CrossRef]
- Chang, Y.F.; Zhang, D. Semaglutide-mediated protection against Aβ correlated with enhancement of autophagy and inhibition of apotosis. J. Clin. Neurosci. 2020, 81, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Chuang, Y.C. Alternative role of glucagon-like Peptide-1 receptor agonists in neurodegenerative diseases. Eur. J. Phamcol 2023, 938, 175439. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.R.; Yousefi, K. Metformin in Alzheimer’s disease: An overview of potential mechanisms, preclinical and clinical findings. Biochem. Pharmacol. 2022, 197, 114945. [Google Scholar] [CrossRef]
- Khaleghi-Mehr, M.; Delshad, A.A. Metformin mitigates amyloid β1-40-induced cognitive decline via attenuation of oxidative/nitrosative stress and neuroinflammation. Metab. Brain Dis. 2023, 38, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Paladugu, L.; Gharaibeh, A. Liraglutide Has Anti-Inflammatory and Anti-Amyloid Properties in Streptozotocin-Induced and 5xFAD Mouse Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 860. [Google Scholar] [CrossRef]
- Nørgaard, C.H.; Friedrich, S. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement 2022, 8, e12268. [Google Scholar] [CrossRef]
- Wium-Andersen, I.K.; Osler, M. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: A nested case-control study. Eur. J. Endocrinol. 2019, 181, 499–507. [Google Scholar] [CrossRef]
- Scheltens, P.; Atri, A. Baseline Characteristics from Evoke and Evoke+: Two Phase 3 Randomized Placebo-controlled Trials of Oral Semaglutide in Patients with Early Alzheimer’s Disease (P11-9013). Neurology 2024, 102, 3350. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef] [PubMed]
- Thu Thuy Nguyen, V.; Endres, K. Targeting gut microbiota to alleviate neuroinflammation in Alzheimer’s disease. Adv. Drug Deliv. Rev. 2022, 188, 114418. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Liu, S.; Qiao, X.; Xing, Y.; Zhou, Q.; Zhang, Z. Epigenetic Regulation of Neuroinflammation in Alzheimer’s Disease. Cells 2024, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Perkovic, M.; Videtic Paska, A.; Konjevod, M.; Kouter, K.; Svob Strac, D.; Nedic Erjavec, G.; Pivac, N. Epigenetics of Alzheimer’s Disease. Biomolecules 2021, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, Q.; Yao, H.; Tan, J.; Liu, Z.; Zhou, Y.; Zou, Z. Epigenetics in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 911635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roveta, F.; Bonino, L.; Piella, E.M.; Rainero, I.; Rubino, E. Neuroinflammatory Biomarkers in Alzheimer’s Disease: From Pathophysiology to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 11941. https://doi.org/10.3390/ijms252211941
Roveta F, Bonino L, Piella EM, Rainero I, Rubino E. Neuroinflammatory Biomarkers in Alzheimer’s Disease: From Pathophysiology to Clinical Implications. International Journal of Molecular Sciences. 2024; 25(22):11941. https://doi.org/10.3390/ijms252211941
Chicago/Turabian StyleRoveta, Fausto, Lucrezia Bonino, Elisa Maria Piella, Innocenzo Rainero, and Elisa Rubino. 2024. "Neuroinflammatory Biomarkers in Alzheimer’s Disease: From Pathophysiology to Clinical Implications" International Journal of Molecular Sciences 25, no. 22: 11941. https://doi.org/10.3390/ijms252211941
APA StyleRoveta, F., Bonino, L., Piella, E. M., Rainero, I., & Rubino, E. (2024). Neuroinflammatory Biomarkers in Alzheimer’s Disease: From Pathophysiology to Clinical Implications. International Journal of Molecular Sciences, 25(22), 11941. https://doi.org/10.3390/ijms252211941






