The Efficacy of Stem Cells in Wound Healing: A Systematic Review
Abstract
:1. Introduction
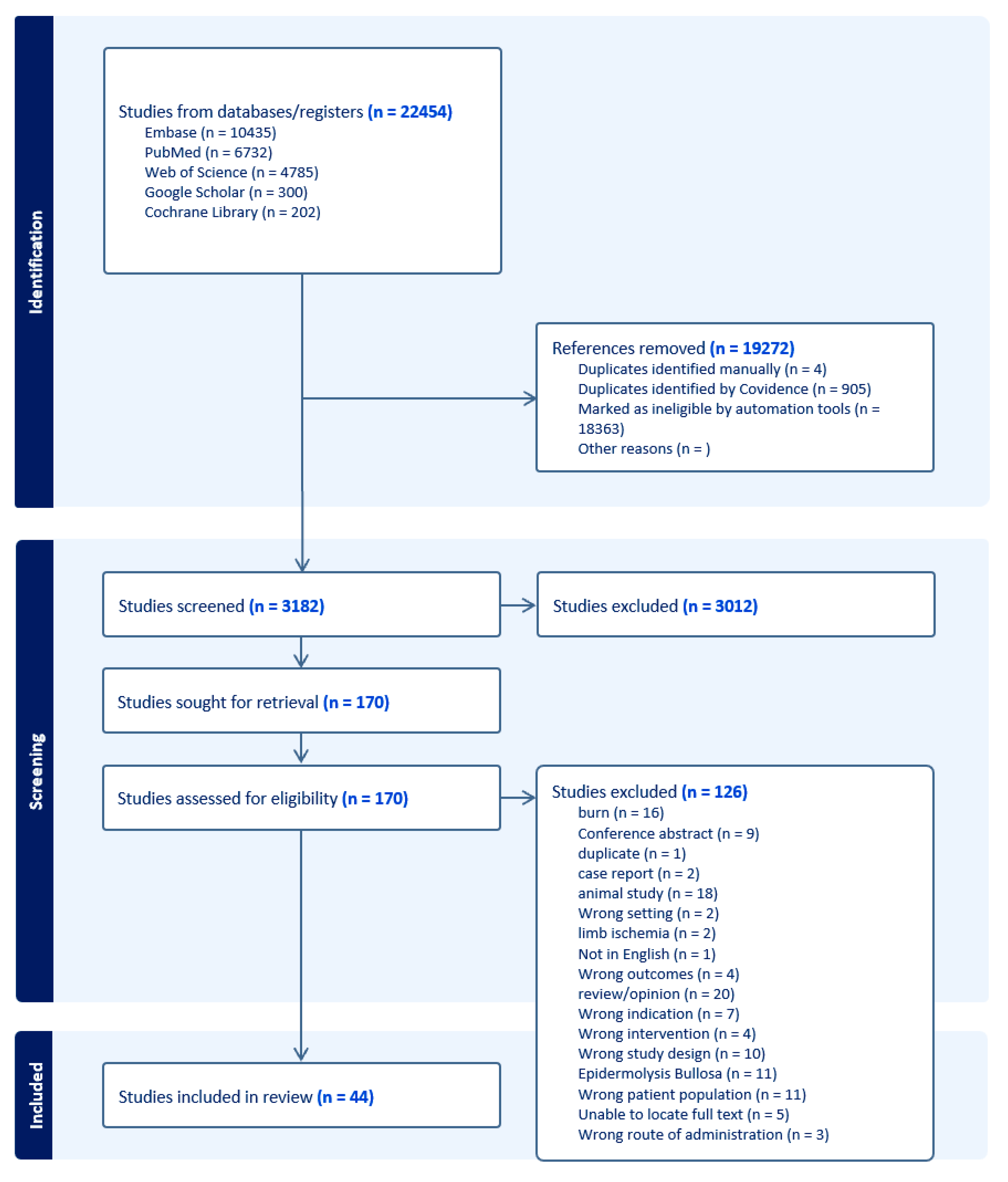
2. Materials and Methods
2.1. Information Sources
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Collection and Extraction Process
3. Results
3.1. Skin-Derived Stem Cells
3.1.1. Autologous Keratinocytes
3.1.2. Hair Follicle-Derived Stem Cells
3.2. Peripheral Blood-Derived Cells/Endothelial Progenitor Stem Cells
3.3. Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)
Bone Marrow-Derived Mononuclear Stem Cells
3.4. Adipose Tissue-Derived Mesenchymal Stem Cells
3.5. Placental/Umbilical/Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJSCs)
3.5.1. Human Embryo Fibroblast
3.5.2. Wharton’s Jelly-Derived Stem Cells
3.5.3. Human Umbilical Cord Mesenchymal Stem Cells
3.5.4. Human Placenta/Human Amniotic Membrane
3.6. Inducible Pluripotent Stem Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, W.S.; Park, B.S.; Park, S.H.; Kim, H.K.; Sung, J.H. Antiwrinkle effect of adipose-derived stem cell: Activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 2009, 53, 96–102. [Google Scholar] [CrossRef]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arter. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Kosaric, N.; Kiwanuka, H.; Gurtner, G.C. Stem cell therapies for wound healing. Expert Opin. Biol. Ther. 2019, 19, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Loretelli, C.; Ben Nasr, M.; Giatsidis, G.; Bassi, R.; Lancerotto, L.; D’Addio, F.; Valderrama-Vasquez, A.; Scherer, S.S.; Salvatore, L.; Madaghiele, M.; et al. Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetol. 2020, 57, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Huldani, H.; Kozlitina, I.A.; Alshahrani, M.; Daabo, H.M.A.; Almalki, S.G.; Oudaha, K.H.; Alawadi, A.H.; Alsalamy, A.; Joshi, S.K.; Mustafa, Y.F. Exosomes derived from adipose stem cells in combination with hyaluronic acid promote diabetic wound healing. Tissue Cell 2023, 85, 102252. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.K.; Ban, J.J.; Lee, M.; Im, W.; Kim, M. Wound healing potential of adipose tissue stem cell extract. Biochem. Biophys. Res. Commun. 2017, 485, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. J. Am. Geriatr. Soc. 2015, 63, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Dash, B.C.; Korutla, L.; Vallabhajosyula, P.; Hsia, H.C. Unlocking the Potential of Induced Pluripotent Stem Cells for Wound Healing: The Next Frontier of Regenerative Medicine. Adv. Wound Care 2022, 11, 622–638. [Google Scholar] [CrossRef]
- Gupta, G.J.; Karki, K.; Jain, P.; Saxena, A.K. Autologous Bone Marrow Aspirate Therapy for Skin Tissue Engineering and Tissue Regeneration. Adv. Wound Care 2017, 6, 135–142. [Google Scholar] [CrossRef]
- Werdin, F.; Tenenhaus, M.; Rennekampff, H.O. Chronic wound care. Lancet 2008, 372, 1860–1862. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Harvima, I.T.; Virnes, S.; Kauppinen, L.; Huttunen, M.; Kivinen, P.; Niskanen, L.; Horsmanheimo, M. Cultured allogeneic skin cells are effective in the treatment of chronic diabetic leg and foot ulcers. Acta Derm. Venereol. 1999, 79, 217–220. [Google Scholar] [PubMed]
- Veves, A.; Falanga, V.; Armstrong, D.G.; Sabolinski, M.L.; Apligraf Diabetic Foot Ulcer Study. Graftskin, a Human Skin Equivalent, Is Effective in the Management of Noninfected Neuropathic Diabetic Foot Ulcers: A prospective randomized multicenter clinical trial. Diabetes Care 2001, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomized trial. Diabetes Care 2003, 26, 1701–1705. [Google Scholar] [CrossRef]
- Bayram, Y.; Deveci, M.; Imirzalioglu, N.; Soysal, Y.; Sengezer, M. The cell based dressing with living allogenic keratinocytes in the treatment of foot ulcers: A case study. Br. J. Plast. Surg. 2005, 58, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.; Bullock, A.J.; Creagh, F.M.; Heller, S.; Jeffcoate, W.; Game, F.; Amery, C.; Tesfaye, S.; Ince, Z.; Haddow, D.B.; et al. Randomized, controlled, single-blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers. Regen. Med. 2007, 2, 887–902. [Google Scholar] [CrossRef]
- Vanscheidt, W.; Ukat, A.; Horak, V.; Brüning, H.; Hunyadi, J.; Pavlicek, R.; Emter, M.; Hartmann, A.; Bende, J.; Zwingers, T.; et al. Treatment of recalcitrant venous leg ulcers with autologous keratinocytes in fibrin sealant: A multinational randomized controlled clinical trial. Wound Repair Regen. 2007, 15, 308–315. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, H.S.; Kim, W.K. Efficacy and safety of fresh fibroblast allografts in the treatment of diabetic foot ulcers. Dermatol. Surg. 2009, 35, 1342–1348. [Google Scholar] [CrossRef]
- You, H.J.; Han, S.K.; Lee, J.W.; Chang, H. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes—A pilot study. Wound Repair Regen. 2012, 20, 491–499. [Google Scholar] [CrossRef]
- Andrea, M.; Marco, B.; Pier Camillo, P.; Matteo, M.; Roberto, B.; Luca, V. Allogeneic epidermal substitutes in the treatment of chronic diabetic leg and foot ulcers. Plast. Aesthetic Res. 2014, 1, 74–80. [Google Scholar]
- Hwang, Y.G.; Lee, J.W.; Park, K.H.; Han, S.H. Allogeneic keratinocyte for intractable chronic diabetic foot ulcers: A prospective observational study. Int. Wound J. 2019, 16, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Masieri, F.F.; Schneider, M.; Bartella, A.; Gaus, S.; Hahnel, S.; Zimmerer, R.; Sack, U.; Maksimovic-Ivanic, D.; Mijatovic, S.; et al. The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers. Biomolecules 2021, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Renner, R.; Harth, W.; Simon, J.C. Transplantation of chronic wounds with epidermal sheets derived from autologous hair follicles--the Leipzig experience. Int. Wound J. 2009, 6, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Shantsila, E.; Watson, T.; Lip, G.Y. Endothelial progenitor cells in cardiovascular disorders. J. Am. Coll. Cardiol. 2007, 49, 741–752. [Google Scholar] [CrossRef]
- Suh, W.; Kim, K.L.; Kim, J.M.; Shin, I.S.; Lee, Y.S.; Lee, J.Y.; Jang, H.S.; Lee, J.S.; Byun, J.; Choi, J.H.; et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells 2005, 23, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Santo, S.D.; Seiler, S.; Andres, R.; Widmer, H.R. Endothelial Progenitor Cells Conditioned Medium Supports Number of GABAergic Neurons and Exerts Neuroprotection in Cultured Striatal Neuronal Progenitor Cells. Cell Transpl. 2019, 28, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Molavi, B.; Goodarzi, A.; Alizadeh, A.; Yousefzadeh, A.; Sodeifi, N.; Arab, L.; Aghdami, N. The efficacy of platelet gel derived from umbilical cord blood on diabetic foot ulcers: A double-blind randomized clinical trial. Wound Med. 2020, 28, 100178. [Google Scholar] [CrossRef]
- Tanaka, R.; Fujimura, S.; Kado, M.; Fukuta, T.; Arita, K.; Hirano-Ito, R.; Mita, T.; Watada, H.; Kato, Y.; Miyauchi, K.; et al. Phase I/IIa Feasibility Trial of Autologous Quality- and Quantity-Cultured Peripheral Blood Mononuclear Cell Therapy for Non-Healing Extremity Ulcers. Stem Cells Transl. Med. 2022, 11, 146–158. [Google Scholar] [CrossRef]
- Johnson, J.; Law, S.Q.K.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.L.; Silva, A.; Kong, K.J.W.; Schoppet, M.; Blyth, C.; et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J. Extracell. Vesicles 2023, 12, 12332. [Google Scholar] [CrossRef]
- Abadeh, A.; Herman, S.M.; Abdalian, R. The prevalence of gastrointestinal symptoms and cobalamin deficiency in patients with chronic urticaria. Allergy Asthma Clin. Immunol. 2023, 19, 14. [Google Scholar] [CrossRef]
- Bonora, B.M.; Cappellari, R.; Mazzucato, M.; Rigato, M.; Grasso, M.; Menegolo, M.; Bruttocao, A.; Avogaro, A.; Fadini, G.P. Stem cell mobilization with plerixafor and healing of diabetic ischemic wounds: A phase IIa, randomized, double-blind, placebo-controlled trial. Stem Cells Transl. Med. 2020, 9, 965–973. [Google Scholar] [CrossRef]
- Badiavas, E.V.; Falanga, V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003, 139, 510–516. [Google Scholar] [CrossRef]
- Vojtassák, J.; Danisovic, L.; Kubes, M.; Bakos, D.; Jarábek, L.; Ulicná, M.; Blasko, M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuroendocrinol. Lett. 2006, 27 (Suppl. S2), 134–137. [Google Scholar]
- Rogers, L.C.; Bevilacqua, N.J.; Armstrong, D.G. The use of marrow-derived stem cells to accelerate healing in chronic wounds. Int. Wound J. 2008, 5, 20–25. [Google Scholar] [CrossRef]
- Jain, P.; Perakath, B.; Jesudason, M.R.; Nayak, S. The effect of autologous bone marrow-derived cells on healing chronic lower extremity wounds: Results of a randomized controlled study. Ostomy Wound Manag. 2011, 57, 38–44. [Google Scholar]
- Askø Andersen, J.; Rasmussen, A.; Frimodt-Møller, M.; Engberg, S.; Steeneveld, E.; Kirketerp-Møller, K.; O’Brien, T.; Rossing, P. Novel topical allogeneic bone-marrow-derived mesenchymal stem cell treatment of hard-to-heal diabetic foot ulcers: A proof of concept study. Stem Cell Res. Ther. 2022, 13, 280. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Guo, M.; Bian, C.; Sun, Z.; Yang, Z.; Zeng, Y.; Ai, H.; Zhao, R.C. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: Clinical report. Biol. Blood Marrow Transpl. 2010, 16, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Boberg, E.; von Bahr, L.; Afram, G.; Lindström, C.; Ljungman, P.; Heldring, N.; Petzelbauer, P.; Garming Legert, K.; Kadri, N.; Le Blanc, K. Treatment of chronic GvHD with mesenchymal stromal cells induces durable responses: A phase II study. Stem Cells Transl. Med. 2020, 9, 1190–1202. [Google Scholar] [CrossRef]
- Amato, B.; Compagna, R.; Amato, M.; Butrico, L.; Fugetto, F.; Chibireva, M.D.; Barbetta, A.; Cannistrà, M.; de Franciscis, S.; Serra, R. The role of adult tissue-derived stem cells in chronic leg ulcers: A systematic review focused on tissue regeneration medicine. Int. Wound J. 2016, 13, 1289–1298. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kubo, T.; Murakami, T.; Takahashi, M.; Hakamata, Y.; Kobayashi, E.; Yoshida, S.; Hosokawa, K.; Yoshikawa, K.; Itami, S. Bone marrow cells differentiate into wound myofibroblasts and accelerate the healing of wounds with exposed bones when combined with an occlusive dressing. Br. J. Dermatol. 2005, 152, 616–622. [Google Scholar] [CrossRef]
- Wettstein, R.; Savic, M.; Pierer, G.; Scheufler, O.; Haug, M.; Halter, J.; Gratwohl, A.; Baumberger, M.; Schaefer, D.J.; Kalbermatten, D.F. Progenitor cell therapy for sacral pressure sore: A pilot study with a novel human chronic wound model. Stem Cell Res. Ther. 2014, 5, 18. [Google Scholar] [CrossRef]
- Sarasúa, J.G.; López, S.P.; Viejo, M.A.; Basterrechea, M.P.; Rodríguez, A.F.; Gutiérrez, A.F.; Gala, J.G.; Menéndez, Y.M.; Augusto, D.E.; Arias, A.P.; et al. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J. Spinal Cord Med. 2011, 34, 301–307. [Google Scholar] [CrossRef]
- Dicker, A.; Le Blanc, K.; Aström, G.; van Harmelen, V.; Götherström, C.; Blomqvist, L.; Arner, P.; Rydén, M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp. Cell Res. 2005, 308, 283–290. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Grieco, M.P.; Raposio, E. The science behind autologous fat grafting. Ann. Med. Surg. 2017, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chen, Y. The role of adipose-derived stem cells-derived extracellular vesicles in the treatment of diabetic foot ulcer: Trends and prospects. Front. Endocrinol. 2022, 13, 902130. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Nambu, M.; Kishimoto, S.; Nakamura, S.; Mizuno, H.; Yanagibayashi, S.; Yamamoto, N.; Azuma, R.; Nakamura, S.; Kiyosawa, T.; Ishihara, M.; et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann. Plast. Surg. 2009, 62, 317–321. [Google Scholar] [CrossRef]
- Altman, A.M.; Yan, Y.; Matthias, N.; Bai, X.; Rios, C.; Mathur, A.B.; Song, Y.H.; Alt, E.U. IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells 2009, 27, 250–258. [Google Scholar] [CrossRef]
- Blanton, M.W.; Hadad, I.; Johnstone, B.H.; Mund, J.A.; Rogers, P.I.; Eppley, B.L.; March, K.L. Adipose stromal cells and platelet-rich plasma therapies synergistically increase revascularization during wound healing. Plast. Reconstr. Surg. 2009, 123, 56s–64s. [Google Scholar] [CrossRef]
- Chopinaud, M.; Labbé, D.; Creveuil, C.; Marc, M.; Bénateau, H.; Mourgeon, B.; Chopinaud, E.; Veyssière, A.; Dompmartin, A. Autologous Adipose Tissue Graft to Treat Hypertensive Leg Ulcer: A Pilot Study. Dermatology 2017, 233, 234–241. [Google Scholar] [CrossRef]
- Moon, K.C.; Suh, H.S.; Kim, K.B.; Han, S.K.; Young, K.W.; Lee, J.W.; Kim, M.H. Potential of Allogeneic Adipose-Derived Stem Cell-Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Kim, H.R.; Kim, W.K. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: A pilot study. Wound Repair Regen. 2010, 18, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; An, S.G.; Lee, H.W.; Park, J.S.; Cha, K.S.; Hong, T.J.; Park, J.H.; Lee, S.Y.; Kim, S.P.; Kim, Y.D.; et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 2012, 76, 1750–1760. [Google Scholar] [CrossRef]
- Masłowski, L.; Paprocka, M.; Czyżewska-Buczyńska, A.; Bielawska-Pohl, A.; Duś, D.; Grendziak, R.; Witkiewicz, W.; Czarnecka, A. Autotransplantation of the Adipose Tissue-Derived Mesenchymal Stromal Cells in Therapy of Venous Stasis Ulcers. Arch. Immunol. Ther. Exp. 2020, 68, 5. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; De Sena, G.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef]
- Doi, K.; Tanaka, S.; Iida, H.; Eto, H.; Kato, H.; Aoi, N.; Kuno, S.; Hirohi, T.; Yoshimura, K. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: Bench and bed analysis. J. Tissue Eng. Regen. Med. 2013, 7, 864–870. [Google Scholar] [CrossRef]
- Fraser, J.K.; Hicok, K.C.; Shanahan, R.; Zhu, M.; Miller, S.; Arm, D.M. The Celution(®) System: Automated Processing of Adipose-Derived Regenerative Cells in a Functionally Closed System. Adv. Wound Care 2014, 3, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Alinda, M.D.; Christopher, P.M.; Listiawan, M.Y.; Endaryanto, A.; Suroto, H.; Rantam, F.A.; Hendradi, E.; Notobroto, H.B.; Prakoeswa, C.R.S. The efficacy of topical adipose mesenchymal stem cell-conditioned medium versus framycetin gauze dressing in chronic plantar ulcer of leprosy: A randomized controlled trial. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Del Papa, N.; Di Luca, G.; Sambataro, D.; Zaccara, E.; Maglione, W.; Gabrielli, A.; Fraticelli, P.; Moroncini, G.; Beretta, L.; Santaniello, A.; et al. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with systemic sclerosis. Cell Transpl. 2015, 24, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Monreal, J. Safety and Efficacy of Stromal Vascular Fraction Enriched Fat Grafting Therapy for Vulvar Lichen Sclerosus. Cureus 2020, 12, e7096. [Google Scholar] [CrossRef] [PubMed]
- Sedov, V.M.; Andreev, D.; Smirnova, T.D.; Paramonov, B.A.; En’kina, T.N.; Sominina, A.A.; Kiselev, O.I.; Suissi, I.; Lebedev, L.V. Cell therapy in treatment of trophic ulcers of lower extremities. Vestn. Khir. Im. I. I. Grek. 2006, 165, 90–94. [Google Scholar] [PubMed]
- Hegazy, A.A. Anatomy and embryology of umbilicus in newborns: A review and clinical correlations. Front. Med. 2016, 10, 271–277. [Google Scholar] [CrossRef]
- Heil, J.R.; Bordoni, B. Embryology, Umbilical Cord. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hashemi, S.S.; Mohammadi, A.A.; Kabiri, H.; Hashempoor, M.R.; Mahmoodi, M.; Amini, M.; Mehrabani, D. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. J. Cosmet. Dermatol. 2019, 18, 1961–1967. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, S.; Liu, X.; Song, B.; Shi, L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn’s Disease: A Randomized Controlled Clinical Trial. Gut Liver 2018, 12, 73–78. [Google Scholar] [CrossRef]
- Tan, S.T.; Aisyah, P.B.; Firmansyah, Y.; Nathasia, N.; Budi, E.; Hendrawan, S. Effectiveness of Secretome from Human Umbilical Cord Mesenchymal Stem Cells in Gel (10% SM-hUCMSC Gel) for Chronic Wounds (Diabetic and Trophic Ulcer)—Phase 2 Clinical Trial. J. Multidiscip. Healthc. 2023, 16, 1763–1777. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Lu, Z.; Chen, J.; Zhang, H.; Sun, L. Umbilical cord mesenchymal stem cell transplantation in drug-induced Stevens-Johnson syndrome. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 659–661. [Google Scholar] [CrossRef]
- Dehghani, M.; Azarpira, N.; Mohammad Karimi, V.; Mossayebi, H.; Esfandiari, E. Grafting with Cryopreserved Amniotic Membrane versus Conservative Wound Care in Treatment of Pressure Ulcers: A Randomized Clinical Trial. Bull. Emerg. Trauma 2017, 5, 249–258. [Google Scholar] [CrossRef]
- Farivar, B.S.; Toursavadkohi, S.; Monahan, T.S.; Sharma, J.; Ucuzian, A.A.; Kundi, R.; Sarkar, R.; Lal, B.K. Prospective study of cryopreserved placental tissue wound matrix in the management of chronic venous leg ulcers. J. Vasc. Surg Venous Lymphat. Disord. 2019, 7, 228–233. [Google Scholar] [CrossRef]
- Suzdaltseva, Y.; Zhidkih, S.; Kiselev, S.L.; Stupin, V. Locally Delivered Umbilical Cord Mesenchymal Stromal Cells Reduce Chronic Inflammation in Long-Term Nonhealing Wounds: A Randomized Study. Stem Cells Int. 2020, 2020, 5308609. [Google Scholar] [CrossRef] [PubMed]
- Meamar, R.; Ghasemi-Mobarakeh, L.; Norouzi, M.R.; Siavash, M.; Hamblin, M.R.; Fesharaki, M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int. Immunopharmacol. 2021, 101, 108282. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Nejad, A.; Amirkhani, M.A.; Ebrahimi, A.; Ghorani, S.M.; Alamoutifard, E.; Nilforoushzadeh, M.A.; Mollapour-Sisakht, M. The Therapeutic Efficacy of Freeze-Dried Human Amniotic Membrane Allograft Gel for Diabetic Foot Ulcers: A Phase-1 Clinical Trial. Int. J. Low. Extrem. Wounds 2023. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Kalsan, M.; Kumar, N.; Saini, A.; Chandra, R. Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol. 2015, 3, 2. [Google Scholar] [CrossRef]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Sebastiano, V.; Zhen, H.H.; Haddad, B.; Bashkirova, E.; Melo, S.P.; Wang, P.; Leung, T.L.; Siprashvili, Z.; Tichy, A.; Li, J.; et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci. Transl. Med. 2014, 6, 264ra163. [Google Scholar] [CrossRef]
- Clayton, Z.E.; Tan, R.P.; Miravet, M.M.; Lennartsson, K.; Cooke, J.P.; Bursill, C.A.; Wise, S.G.; Patel, S. Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model. Biosci. Rep. 2018, 38, BSR20180563. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the role of stem cells in cutaneous wound healing. Exp. Dermatol. 2009, 18, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.E.; Yoo, D.; LeRoux, M.A.; Danilkovitch-Miagkova, A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm. Allergy Drug Targets 2009, 8, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; O’Sullivan, C.; Gergely, P. Sphingosine 1-Phosphate Signaling and Its Pharmacological Modulation in Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2017, 18, 2027. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Duarte, A.C.; Costa, E.C.; Filipe, H.A.L.; Saraiva, S.M.; Jacinto, T.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Animal-derived products in science and current alternatives. Biomater. Adv. 2023, 151, 213428. [Google Scholar] [CrossRef]
- Díez, J.M.; Bauman, E.; Gajardo, R.; Jorquera, J.I. Culture of human mesenchymal stem cells using a candidate pharmaceutical grade xeno-free cell culture supplement derived from industrial human plasma pools. Stem Cell Res. Ther. 2015, 6, 28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farabi, B.; Roster, K.; Hirani, R.; Tepper, K.; Atak, M.F.; Safai, B. The Efficacy of Stem Cells in Wound Healing: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3006. https://doi.org/10.3390/ijms25053006
Farabi B, Roster K, Hirani R, Tepper K, Atak MF, Safai B. The Efficacy of Stem Cells in Wound Healing: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(5):3006. https://doi.org/10.3390/ijms25053006
Chicago/Turabian StyleFarabi, Banu, Katie Roster, Rahim Hirani, Katharine Tepper, Mehmet Fatih Atak, and Bijan Safai. 2024. "The Efficacy of Stem Cells in Wound Healing: A Systematic Review" International Journal of Molecular Sciences 25, no. 5: 3006. https://doi.org/10.3390/ijms25053006




