Sirtuins as Players in the Signal Transduction of Citrus Flavonoids
Abstract
1. Introduction
2. Citrus Fruits and their Flavonoids
3. SIRT1
4. SIRT2
5. SIRT3
6. SIRT4
7. SIRT5
8. SIRT6
9. SIRT7
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019, 161, 48–77. [Google Scholar] [CrossRef]
- Kratz, E.M.; Kokot, I.; Dymicka-Piekarska, V.; Piwowar, A. Sirtuins-The New Important Players in Women’s Gynecological Health. Antioxidants 2021, 10, 84. [Google Scholar] [CrossRef]
- Curry, A.M.; White, D.S.; Donu, D.; Cen, Y. Human Sirtuin Regulators: The “Success” Stories. Front. Physiol. 2021, 12, 752117. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Donu, D.; Curry, A.M.; Barton, E.; Cen, Y. Multifunctional activity-based chemical probes for sirtuins. RSC Adv. 2023, 13, 11771–11781. [Google Scholar] [CrossRef] [PubMed]
- Shoba, B.; Lwin, Z.M.; Ling, L.S.; Bay, B.H.; Yip, G.W.; Kumar, S.D. Function of sirtuins in biological tissues. Anat. Rec. 2009, 292, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A.; Hirschey, M.D. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 2012, 52, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Klar, A.J.; Fogel, S.; Macleod, K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics 1979, 93, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Lin, H. Sirtuin Modulators in Cellular and Animal Models of Human Diseases. Front. Pharmacol. 2021, 12, 735044. [Google Scholar] [CrossRef] [PubMed]
- Kupis, W.; Palyga, J.; Tomal, E.; Niewiadomska, E. The role of sirtuins in cellular homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef]
- Zhang, H.N.; Dai, Y.; Zhang, C.H.; Omondi, A.M.; Ghosh, A.; Khanra, I.; Chakraborty, M.; Yu, X.B.; Liang, J. Sirtuins family as a target in endothelial cell dysfunction: Implications for vascular ageing. Biogerontology 2020, 21, 495–516. [Google Scholar] [CrossRef]
- Sacconnay, L.; Carrupt, P.A.; Nurisso, A. Human sirtuins: Structures and flexibility. J. Struct. Biol. 2016, 196, 534–542. [Google Scholar] [CrossRef]
- Fiorentino, F.; Mai, A.; Rotili, D. The role of structural biology in the design of sirtuin activators. Curr. Opin. Struct. Biol. 2023, 82, 102666. [Google Scholar] [CrossRef]
- Kratz, E.M.; Solkiewicz, K.; Kubis-Kubiak, A.; Piwowar, A. Sirtuins as Important Factors in Pathological States and the Role of Their Molecular Activity Modulators. Int. J. Mol. Sci. 2021, 22, 630. [Google Scholar] [CrossRef]
- Zietara, P.; Dziewiecka, M.; Augustyniak, M. Why Is Longevity Still a Scientific Mystery? Sirtuins-Past, Present and Future. Int. J. Mol. Sci. 2022, 24, 728. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Ogura, Y.; Kitada, M.; Koya, D. Sirtuins and Renal Oxidative Stress. Antioxidants 2021, 10, 1198. [Google Scholar] [CrossRef]
- Saiyang, X.; Deng, W.; Qizhu, T. Sirtuin 6: A potential therapeutic target for cardiovascular diseases. Pharmacol. Res. 2021, 163, 105214. [Google Scholar] [CrossRef]
- Velpuri, P.; Rai, V.; Agrawal, D.K. Role of sirtuins in attenuating plaque vulnerability in atherosclerosis. Mol. Cell Biochem. 2023, 479, 51–62. [Google Scholar] [CrossRef]
- Poniewierska-Baran, A.; Bochniak, O.; Warias, P.; Pawlik, A. Role of Sirtuins in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 1532. [Google Scholar] [CrossRef]
- Zhang, Y.; Anoopkumar-Dukie, S.; Mallik, S.B.; Davey, A.K. SIRT1 and SIRT2 modulators reduce LPS-induced inflammation in HAPI microglial cells and protect SH-SY5Y neuronal cells in vitro. J. Neural Transm. 2021, 128, 631–644. [Google Scholar] [CrossRef]
- Lin, J.; Xiong, Z.; Gu, J.; Sun, Z.; Jiang, S.; Fan, D.; Li, W. Sirtuins: Potential Therapeutic Targets for Defense against Oxidative Stress in Spinal Cord Injury. Oxid. Med. Cell Longev. 2021, 2021, 7207692. [Google Scholar] [CrossRef]
- Palmirotta, R.; Cives, M.; Della-Morte, D.; Capuani, B.; Lauro, D.; Guadagni, F.; Silvestris, F. Sirtuins and Cancer: Role in the Epithelial-Mesenchymal Transition. Oxid. Med. Cell Longev. 2016, 2016, 3031459. [Google Scholar] [CrossRef]
- Poniewierska-Baran, A.; Warias, P.; Zgutka, K. Sirtuins (SIRTs) As a Novel Target in Gastric Cancer. Int. J. Mol. Sci. 2022, 23, 15119. [Google Scholar] [CrossRef]
- Onyiba, C.I.; Scarlett, C.J.; Weidenhofer, J. The Mechanistic Roles of Sirtuins in Breast and Prostate Cancer. Cancers 2022, 14, 5118. [Google Scholar] [CrossRef]
- Cremonini, E.; Iglesias, D.E.; Kang, J.; Lombardo, G.E.; Mostofinejad, Z.; Wang, Z.; Zhu, W.; Oteiza, P.I. (−)-Epicatechin and the comorbidities of obesity. Arch. Biochem. Biophys. 2020, 690, 108505. [Google Scholar] [CrossRef]
- Montalbano, G.; Maugeri, A.; Guerrera, M.C.; Miceli, N.; Navarra, M.; Barreca, D.; Cirmi, S.; Germana, A. A White Grape Juice Extract Reduces Fat Accumulation through the Modulation of Ghrelin and Leptin Expression in an In Vivo Model of Overfed Zebrafish. Molecules 2021, 26, 1119. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Russo, C.; Musumeci, L.; Navarra, M.; Lombardo, G.E. Oleacein Attenuates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages by the Inhibition of TLR4/MyD88/NF-kappaB Pathway. Int. J. Mol. Sci. 2022, 23, 1206. [Google Scholar] [CrossRef]
- Maugeri, A.; Lombardo, G.E.; Cirmi, S.; Suntar, I.; Barreca, D.; Lagana, G.; Navarra, M. Pharmacology and toxicology of tannins. Arch. Toxicol. 2022, 96, 1257–1277. [Google Scholar] [CrossRef]
- Imran, M.; Insaf, A.; Hasan, N.; Sugandhi, V.V.; Shrestha, D.; Paudel, K.R.; Jha, S.K.; Hansbro, P.M.; Dua, K.; Devkota, H.P.; et al. Exploring the Remarkable Chemotherapeutic Potential of Polyphenolic Antioxidants in Battling Various Forms of Cancer. Molecules 2023, 28, 3475. [Google Scholar] [CrossRef]
- Darwiche, L.; Mesmar, J.; Baydoun, E.; El Kayal, W. In Vitro Evaluation of Biological and Anticancer Activities Exhibited by Five Varieties of Vitis vinifera L. Int. J. Mol. Sci. 2023, 24, 13440. [Google Scholar] [CrossRef]
- El Gaamouch, F.; Chen, F.; Ho, L.; Lin, H.Y.; Yuan, C.; Wong, J.; Wang, J. Benefits of dietary polyphenols in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1019942. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega 2022, 7, 25974–25990. [Google Scholar] [CrossRef]
- Wicinski, M.; Erdmann, J.; Nowacka, A.; Kuzminski, O.; Michalak, K.; Janowski, K.; Ohla, J.; Biernaciak, A.; Szambelan, M.; Zabrzynski, J. Natural Phytochemicals as SIRT Activators-Focus on Potential Biochemical Mechanisms. Nutrients 2023, 15, 3578. [Google Scholar] [CrossRef]
- Wei, X.Y.; Zeng, Y.F.; Guo, Q.H.; Liu, J.J.; Yin, N.; Liu, Y.; Zeng, W.J. Cardioprotective effect of epigallocatechin gallate in myocardial ischemia/reperfusion injury and myocardial infarction: A meta-analysis in preclinical animal studies. Sci. Rep. 2023, 13, 14050. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Ahmad, J.; Ahamad, J.; Kundu, S.; Goel, A.; Mishra, A. Flavonoids as promising anticancer therapeutics: Contemporary research, nanoantioxidant potential, and future scope. Phytother. Res. 2023, 37, 5159–5192. [Google Scholar] [CrossRef]
- Maugeri, A.; Calderaro, A.; Patane, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef]
- Siracusa, R.; Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Navarra, M.; Cuzzocrea, S.; Esposito, E. The Association of Palmitoylethanolamide with Luteolin Decreases Neuroinflammation and Stimulates Autophagy in Parkinson’s Disease Model. CNS Neurol. Disord. Drug Targets 2015, 14, 1350–1365. [Google Scholar] [CrossRef]
- Abbate, F.; Maugeri, A.; Laura, R.; Levanti, M.; Navarra, M.; Cirmi, S.; Germana, A. Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids. Antioxidants 2021, 10, 668. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Miao, P.; Xu, F.; Chen, J.; Jiang, X.; Hui, Z.; Wang, L.; Bai, R. Discovery of anti-inflammatory natural flavonoids: Diverse scaffolds and promising leads for drug discovery. Eur. J. Med. Chem. 2023, 260, 115791. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Musumeci, L.; De Sarro, G.; Cirmi, S.; Navarra, M. Inflammation and Obesity: The Pharmacological Role of Flavonoids in the Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 2899. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef]
- Qiu, M.; Wei, W.; Zhang, J.; Wang, H.; Bai, Y.; Guo, D.A. A Scientometric Study to a Critical Review on Promising Anticancer and Neuroprotective Compounds: Citrus Flavonoids. Antioxidants 2023, 12, 669. [Google Scholar] [CrossRef]
- Atoki, A.V.; Aja, P.M.; Shinkafi, T.S.; Ondari, E.N.; Awuchi, C.G. Naringenin: Its chemistry and roles in neuroprotection. Nutr. Neurosci. 2023, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.K.; Babaei, E.; Al-Khafaji, A.S.K. Hesperetin effect on MLH1 and MSH2 expression on breast cancer cells BT-549. J. Adv. Pharm. Technol. Res. 2023, 14, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Kuang, Y.; Wu, W.; Peng, B.; Fu, Q. Quercetin inhibits the metabolism of arachidonic acid by inhibiting the activity of CYP3A4, thereby inhibiting the progression of breast cancer. Mol. Med. 2023, 29, 127. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Dai, S.; Wang, C.; Fu, K.; Wu, R.; Zhao, X.; Yao, Y.; Li, Y. Luteolin as a potential hepatoprotective drug: Molecular mechanisms and treatment strategies. Biomed. Pharmacother. 2023, 167, 115464. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Navarra, M.; Woodside, J.V.; Cantwell, M.M. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol. Res. 2018, 133, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Cirmi, S.; Minciullo, P.L.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Citrus fruits and inflammaging: A systematic review. Phytochem. Rev. 2019, 18, 1025–1049. [Google Scholar] [CrossRef]
- Musumeci, L.; Maugeri, A.; Russo, C.; Lombardo, G.E.; Cirmi, S.; Navarra, M. Citrus Flavonoids and Autoimmune Diseases: A Systematic Review of Clinical Studies. Curr. Med. Chem. 2023, 30, 2191–2204. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of Citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, C.; Calapai, F.; Cardia, L.; Inferrera, G.; D’Arena, G.; Di Pietro, M.; Navarra, M.; Gangemi, S.; Ventura Spagnolo, E.; Calapai, G. Clinical Pharmacology of Citrus aurantium and Citrus sinensis for the Treatment of Anxiety. Evid. Based Complement. Alternat Med. 2018, 2018, 3624094. [Google Scholar] [CrossRef]
- Santos, K.G.D.; Yoshinaga, M.Y.; Glezer, I.; Chaves-Filho, A.B.; Santana, A.A.; Kovacs, C.; Magnoni, C.D.; Lajolo, F.M.; Miyamoto, S.; Aymoto Hassimotto, N.M. Orange juice intake by obese and insulin-resistant subjects lowers specific plasma triglycerides: A randomized clinical trial. Clin. Nutr. ESPEN 2022, 51, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Randazzo, B.; Russo, C.; Musumeci, L.; Maugeri, A.; Montalbano, G.; Guerrera, M.C.; Lombardo, G.E.; Levanti, M. Anti-inflammatory effect of a flavonoid-rich extract of orange juice in adult zebrafish subjected to Vibrio anguillarum-induced enteritis. Nat. Prod. Res. 2021, 35, 5350–5353. [Google Scholar] [CrossRef]
- Citraro, R.; Navarra, M.; Leo, A.; Donato Di Paola, E.; Santangelo, E.; Lippiello, P.; Aiello, R.; Russo, E.; De Sarro, G. The Anticonvulsant Activity of a Flavonoid-Rich Extract from Orange Juice Involves both NMDA and GABA-Benzodiazepine Receptor Complexes. Molecules 2016, 21, 1261. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon-A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Urzi, O.; Cafora, M.; Ganji, N.R.; Tinnirello, V.; Gasparro, R.; Raccosta, S.; Manno, M.; Corsale, A.M.; Conigliaro, A.; Pistocchi, A.; et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience 2023, 26, 107041. [Google Scholar] [CrossRef]
- Testai, L.; De Leo, M.; Flori, L.; Polini, B.; Braca, A.; Nieri, P.; Pistelli, L.; Calderone, V. Contribution of irisin pathway in protective effects of mandarin juice (Citrus reticulata Blanco) on metabolic syndrome in rats fed with high fat diet. Phytother. Res. 2021, 35, 4324–4333. [Google Scholar] [CrossRef]
- Cilla, A.; Rodrigo, M.J.; De Ancos, B.; Sanchez-Moreno, C.; Cano, M.P.; Zacarias, L.; Barbera, R.; Alegria, A. Impact of high-pressure processing on the stability and bioaccessibility of bioactive compounds in Clementine mandarin juice and its cytoprotective effect on Caco-2 cells. Food Funct. 2020, 11, 8951–8962. [Google Scholar] [CrossRef]
- Celano, M.; Maggisano, V.; De Rose, R.F.; Bulotta, S.; Maiuolo, J.; Navarra, M.; Russo, D. Flavonoid Fraction of Citrus reticulata Juice Reduces Proliferation and Migration of Anaplastic Thyroid Carcinoma Cells. Nutr. Cancer 2015, 67, 1183–1190. [Google Scholar] [CrossRef]
- Chen, M.L.; Chen, Y.E.; Lee, H.F. The Effect of Bergamot Essential Oil Aromatherapy on Improving Depressive Mood and Sleep Quality in Postpartum Women: A Randomized Controlled Trial. J. Nurs. Res. 2022, 30, e201. [Google Scholar] [CrossRef]
- Lombardo, G.E.; Cirmi, S.; Musumeci, L.; Pergolizzi, S.; Maugeri, A.; Russo, C.; Mannucci, C.; Calapai, G.; Navarra, M. Mechanisms Underlying the Anti-Inflammatory Activity of Bergamot Essential Oil and Its Antinociceptive Effects. Plants 2020, 9, 704. [Google Scholar] [CrossRef]
- Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Russo, C.; Gangemi, S.; Calapai, G.; Cirmi, S.; Navarra, M. Bergamottin and 5-Geranyloxy-7-methoxycoumarin Cooperate in the Cytotoxic Effect of Citrus bergamia (Bergamot) Essential Oil in Human Neuroblastoma SH-SY5Y Cell Line. Toxins 2021, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Peditto, M.; Oteri, G.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Treatment With a Flavonoid-Rich Fraction of Bergamot Juice Improved Lipopolysaccharide-Induced Periodontitis in Rats. Front. Pharmacol. 2018, 9, 1563. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Russo, M.; Trapasso, E.; Ursino, M.R.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Navarra, M. NF-kappaB mediates the antiproliferative and proapoptotic effects of bergamot juice in HepG2 cells. Life Sci. 2016, 146, 81–91. [Google Scholar] [CrossRef]
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apc(am1137)). Eur. J. Nutr. 2020, 59, 885–894. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In vitro effect of bergamot (Citrus bergamia) juice against cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Complement. Altern. Med. 2015, 15, 256. [Google Scholar] [CrossRef]
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia Risso et Poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016, 30, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macri, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Nandave, M.; Acharjee, R.; Bhaduri, K.; Upadhyay, J.; Rupanagunta, G.P.; Ansari, M.N. A pharmacological review on SIRT 1 and SIRT 2 proteins, activators, and inhibitors: Call for further research. Int. J. Biol. Macromol. 2023, 242, 124581. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Pan, L. SIRT1 and gynecological malignancies (Review). Oncol. Rep. 2021, 45, 43. [Google Scholar] [CrossRef]
- Alqrad, M.A.I.; El-Agamy, D.S.; Ibrahim, S.R.M.; Sirwi, A.; Abdallah, H.M.; Abdel-Sattar, E.; El-Halawany, A.M.; Elsaed, W.M.; Mohamed, G.A. SIRT1/Nrf2/NF-kappaB Signaling Mediates Anti-Inflammatory and Anti-Apoptotic Activities of Oleanolic Acid in a Mouse Model of Acute Hepatorenal Damage. Medicina 2023, 59, 1351. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Eid, A.H.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.K.; Darwish, H.W.; Georgy, G.S. Neuroprotective Impact of Linagliptin against Cadmium-Induced Cognitive Impairment and Neuropathological Aberrations: Targeting SIRT1/Nrf2 Axis, Apoptosis, and Autophagy. Pharmaceuticals 2023, 16, 1065. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, F.; Yang, C.; Hou, H.; Xing, Z.; Chen, J.; Liu, L.; Peng, C.; Li, D. SIRT1 activation by 2,3,5,6-tetramethylpyrazine alleviates neuroinflammation via inhibiting M1 microglia polarization. Front. Immunol. 2023, 14, 1206513. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Z.; Wang, J. Tetrahydropalmatine ameliorates hepatic steatosis in nonalcoholic fatty liver disease by switching lipid metabolism via AMPK-SREBP-1c-Sirt1 signaling axis. Phytomedicine 2023, 119, 155005. [Google Scholar] [CrossRef] [PubMed]
- Helmy, H.S.; Senousy, M.A.; El-Sahar, A.E.; Sayed, R.H.; Saad, M.A.; Elbaz, E.M. Aberrations of miR-126-3p, miR-181a and sirtuin1 network mediate Di-(2-ethylhexyl) phthalate-induced testicular damage in rats: The protective role of hesperidin. Toxicology 2020, 433–434, 152406. [Google Scholar] [CrossRef] [PubMed]
- Rizk, F.H.; Soliman, N.A.; Abo-Elnasr, S.E.; Mahmoud, H.A.; Abdel Ghafar, M.T.; Elkholy, R.A.; OA, E.L.; Mariah, R.A.; Amin Mashal, S.S.; El Saadany, A.A. Fisetin ameliorates oxidative glutamate testicular toxicity in rats via central and peripheral mechanisms involving SIRT1 activation. Redox Rep. 2022, 27, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Cao, Z.; Li, W.; Liu, R.; Chen, Y.; Li, C.; Song, Y.; Liu, G.; Hu, J.; et al. Naringenin ameliorates homocysteine induced endothelial damage via the AMPKalpha/Sirt1 pathway. J. Adv. Res. 2021, 34, 137–147. [Google Scholar] [CrossRef]
- Arora, S.; Venugopalan, A.; Dharavath, R.N.; Bishnoi, M.; Kondepudi, K.K.; Chopra, K. Naringenin Ameliorates Chronic Sleep Deprivation-Induced Pain via Sirtuin1 Inhibition. Neurochem. Res. 2021, 46, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhu, C.; Xuan, A.; Zhang, J.; Zhu, Z.; Tang, L.; Ruan, D. Fisetin regulates the biological effects of rat nucleus pulposus mesenchymal stem cells under oxidative stress by sirtuin-1 pathway. Immun. Inflamm. Dis. 2023, 11, e865. [Google Scholar] [CrossRef] [PubMed]
- Na, J.Y.; Song, K.; Kim, S.; Kwon, J. Rutin protects rat articular chondrocytes against oxidative stress induced by hydrogen peroxide through SIRT1 activation. Biochem. Biophys. Res. Commun. 2016, 473, 1301–1308. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Z.; Huang, D.; Sun, C.; Xie, J.; Chen, T.; Zhao, X.; Huang, Y.; Li, D.; Wu, B.; et al. Myricetin inhibits TNF-alpha-induced inflammation in A549 cells via the SIRT1/NF-kappaB pathway. Pulm. Pharmacol. Ther. 2020, 65, 102000. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Y.; Wu, W.; Zhu, C.; Zhang, R.; Chen, J.; Zeng, J. Metabolomics Analysis of the Peels of Different Colored Citrus Fruits (Citrus reticulata cv. ‘Shatangju’) During the Maturation Period Based on UHPLC-QQQ-MS. Molecules 2020, 25, 396. [Google Scholar] [CrossRef]
- Munoz, A.L.; Cuellar, A.F.; Arevalo, G.; Santamaria, B.D.; Rodriguez, A.K.; Buendia-Atencio, C.; Reyes Chaparro, A.; Tenorio Barajas, A.Y.; Segura, N.A.; Bello, F.; et al. Antiviral activity of myricetin glycosylated compounds isolated from Marcetia taxifolia against chikungunya virus. EXCLI J. 2023, 22, 716–731. [Google Scholar] [CrossRef]
- Kumar Sethiya, N.; Ghiloria, N.; Srivastav, A.; Bisht, D.; Kumar Chaudhary, S.; Walia, V.; Sabir Alam, M. Therapeutic Potential of Myricetin in the Treatment of Neurological, Neuropsychiatric, and Neurodegenerative Disorders. CNS Neurol. Disord. Drug Targets 2023. [Google Scholar] [CrossRef]
- Felice, M.R.; Maugeri, A.; De Sarro, G.; Navarra, M.; Barreca, D. Molecular Pathways Involved in the Anti-Cancer Activity of Flavonols: A Focus on Myricetin and Kaempferol. Int. J. Mol. Sci. 2022, 23, 4411. [Google Scholar] [CrossRef]
- Wang, S.; He, N.; Xing, H.; Sun, Y.; Ding, J.; Liu, L. Function of hesperidin alleviating inflammation and oxidative stress responses in COPD mice might be related to SIRT1/PGC-1alpha/NF-kappaB signaling axis. J. Recept. Signal Transduct. Res. 2020, 40, 388–394. [Google Scholar] [CrossRef]
- Wu, Y.X.; Yang, X.Y.; Han, B.S.; Hu, Y.Y.; An, T.; Lv, B.H.; Lian, J.; Wang, T.Y.; Bao, X.L.; Gao, L.; et al. Naringenin regulates gut microbiota and SIRT1/ PGC-1a signaling pathway in rats with letrozole-induced polycystic ovary syndrome. Biomed. Pharmacother. 2022, 153, 113286. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Magd, N.F.; El-Kashef, D.H.; El-Sherbiny, M.; Eraky, S.M. Hepatoprotective and cognitive-enhancing effects of hesperidin against thioacetamide-induced hepatic encephalopathy in rats. Life Sci. 2023, 313, 121280. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, R.; Huang, M.; Chen, J.; Li, P.; Ma, Y.; Chen, X. Luteolin can ameliorate renal interstitial fibrosis-induced renal anaemia through the SIRT1/FOXO3 pathway. Food Funct. 2022, 13, 11896–11914. [Google Scholar] [CrossRef] [PubMed]
- Risitano, R.; Curro, M.; Cirmi, S.; Ferlazzo, N.; Campiglia, P.; Caccamo, D.; Ientile, R.; Navarra, M. Flavonoid fraction of Bergamot juice reduces LPS-induced inflammatory response through SIRT1-mediated NF-kappaB inhibition in THP-1 monocytes. PLoS ONE 2014, 9, e107431. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Ferlazzo, N.; De Luca, L.; Gitto, R.; Navarra, M. The link between the AMPK/SIRT1 axis and a flavonoid-rich extract of Citrus bergamia juice: A cell-free, in silico, and in vitro study. Phytother. Res. 2019, 33, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Gao, S.; Guan, H.; Zhang, X.; Gao, Y.; Su, Y.; Song, Y.; Jiang, Y.; Li, N. Naringin protects human nucleus pulposus cells against TNF-alpha-induced inflammation, oxidative stress, and loss of cellular homeostasis by enhancing autophagic flux via AMPK/SIRT1 activation. Oxid. Med. Cell Longev. 2022, 2022, 7655142. [Google Scholar] [CrossRef]
- Shokri Afra, H.; Zangooei, M.; Meshkani, R.; Ghahremani, M.H.; Ilbeigi, D.; Khedri, A.; Shahmohamadnejad, S.; Khaghani, S.; Nourbakhsh, M. Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. J. Physiol. Biochem. 2019, 75, 125–133. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.Z.; Ren, Y.L.; Zhu, J.J.; Cao, J.N.; Zhang, J.; Pan, L.L. Luteolin decreases atherosclerosis in LDL receptor-deficient mice via a mechanism including decreasing AMPK-SIRT1 signaling in macrophages. Exp. Ther. Med. 2018, 16, 2593–2599. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Teng, C.; Wang, J.; Yu, J.; Jin, C.; Wang, L.; Wu, L.; Lin, Z.; Yu, Z.; et al. Orientin downregulating oxidative stress-mediated endoplasmic reticulum stress and mitochondrial dysfunction through AMPK/SIRT1 pathway in rat nucleus pulposus cells in vitro and attenuated intervertebral disc degeneration in vivo. Apoptosis 2022, 27, 1031–1048. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Lee, E.J.; Park, J.S.; Jang, S.E.; Kim, D.H.; Kim, H.S. Anti-Inflammatory and Antioxidant Mechanism of Tangeretin in Activated Microglia. J. Neuroimmune Pharmacol. 2016, 11, 294–305. [Google Scholar] [CrossRef]
- Hao, R.; Song, X.; Li, F.; Tan, X.; Sun-Waterhouse, D.; Li, D. Caffeic acid phenethyl ester reversed cadmium-induced cell death in hippocampus and cortex and subsequent cognitive disorders in mice: Involvements of AMPK/SIRT1 pathway and amyloid-tau-neuroinflammation axis. Food Chem. Toxicol. 2020, 144, 111636. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.S.; Dos Santos, N.A.G.; Bernardes, C.P.; Sisti, F.M.; Amaral, L.; Fontana, A.C.K.; Dos Santos, A.C. Caffeic Acid Phenethyl Ester (CAPE) Protects PC12 Cells Against Cisplatin-Induced Neurotoxicity by Activating the AMPK/SIRT1, MAPK/Erk, and PI3k/Akt Signaling Pathways. Neurotox. Res. 2019, 36, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, B.; Xie, S.; Yang, W.; Lin, J.; Li, Z.; Zhan, Y.; Gui, S.; Lin, B. Naringenin protects RPE cells from NaIO3-induced oxidative damage in vivo and in vitro through up-regulation of SIRT1. Phytomedicine 2021, 80, 153375. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Reza, M.I.; Yadav, H.; Gayen, J.R. Hesperidin inhibits NOX4 mediated oxidative stress and inflammation by upregulating SIRT1 in experimental diabetic neuropathy. Exp. Gerontol. 2023, 172, 112064. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, A.; Ali, W.; Jo, M.H.; Park, J.; Ikram, M.; Kim, M.O. Fisetin Rescues the Mice Brains Against D-Galactose-Induced Oxidative Stress, Neuroinflammation and Memory Impairment. Front. Pharmacol. 2021, 12, 612078. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.A.A.; Yassen, N.N.; Mansour, H.M. Naringin protects mice from D-galactose-induced lung aging and mitochondrial dysfunction: Implication of SIRT1 pathways. Life Sci. 2023, 324, 121471. [Google Scholar] [CrossRef]
- Peng, Z.; Li, X.; Xing, D.; Du, X.; Wang, Z.; Liu, G.; Li, X. Nobiletin alleviates palmitic acid-induced NLRP3 inflammasome activation in a sirtuin 1-dependent manner in AML-12 cells. Mol. Med. Rep. 2018, 18, 5815–5822. [Google Scholar] [CrossRef]
- Hosawi, S. Current Update on Role of Hesperidin in Inflammatory Lung Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches. Life 2023, 13, 937. [Google Scholar] [CrossRef]
- Li, S.; Shao, L.; Fang, J.; Zhang, J.; Chen, Y.; Yeo, A.J.; Lavin, M.F.; Yu, G.; Shao, H. Hesperetin attenuates silica-induced lung injury by reducing oxidative damage and inflammatory response. Exp. Ther. Med. 2021, 21, 297. [Google Scholar] [CrossRef]
- Yang, A.Y.; Choi, H.J.; Kim, K.; Leem, J. Antioxidant, Antiapoptotic, and Anti-Inflammatory Effects of Hesperetin in a Mouse Model of Lipopolysaccharide-Induced Acute Kidney Injury. Molecules 2023, 28, 2759. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, W.; Sheng, H.; Bai, Y.F.; Weng, Y.Y.; Fan, X.Y.; Zheng, F.; Zhu, X.T.; Xu, Z.C.; Zhang, F. Hesperetin, a SIRT1 activator, inhibits hepatic inflammation via AMPK/CREB pathway. Int. Immunopharmacol. 2020, 89, 107036. [Google Scholar] [CrossRef]
- Hua, Y.Q.; Zeng, Y.; Xu, J.; Xu, X.L. Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoe−/−mice: Role of SIRT1. Phytomedicine 2021, 81, 153412. [Google Scholar] [CrossRef]
- Du, Y.; Ma, J.; Fan, Y.; Wang, X.; Zheng, S.; Feng, J.; Li, J.; Fan, Z.; Li, G.; Ye, Q. Naringenin: A Promising Therapeutic Agent against Organ Fibrosis. Oxid. Med. Cell Longev. 2021, 2021, 1210675. [Google Scholar] [CrossRef]
- Li, H.L.; Wei, Y.Y.; Li, X.H.; Zhang, S.S.; Zhang, R.T.; Li, J.H.; Ma, B.W.; Shao, S.B.; Lv, Z.W.; Ruan, H.; et al. Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol. Sin. 2022, 43, 919–932. [Google Scholar] [CrossRef]
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The effect of hesperidin and quercetin on oxidative stress, NF-kappaB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed. Pharmacother. 2017, 90, 500–508. [Google Scholar] [CrossRef]
- Rostami, A.; Baluchnejadmojarad, T.; Roghani, M. Hepatoprotective Effect of Myricetin following Lipopolysaccharide/DGalactosamine: Involvement of Autophagy and Sirtuin 1. Curr. Mol. Pharmacol. 2023, 16, 419–433. [Google Scholar] [CrossRef]
- Khajevand-Khazaei, M.R.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018, 833, 307–313. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Zhang, L.; Lv, J.; Ni, H. Rutin alleviates hypoxia/reoxygenation-induced injury in myocardial cells by up-regulating SIRT1 expression. Chem. Biol. Interact. 2019, 297, 44–49. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Z.; Zhu, C.; Fu, Z.; Yu, D. Luteolin alleviates myocardial ischemia reperfusion injury in rats via Siti1/NLRP3/NF-kappaB pathway. Int. Immunopharmacol. 2020, 85, 106680. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2021, 12, 808480. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Zhang, T.; Yuan, X.; Ge, A.; Wang, S.; Xu, H.; Zeng, L.; Ge, J. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 949746. [Google Scholar] [CrossRef]
- Qi, G.; Guo, R.; Tian, H.; Li, L.; Liu, H.; Mi, Y.; Liu, X. Nobiletin protects against insulin resistance and disorders of lipid metabolism by reprogramming of circadian clock in hepatocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 549–562. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Chen, X.; Zhu, D.; Ma, J.; Yan, Y.; Si, M.; Li, X.; Sun, C.; Yang, B.; et al. Neohesperidin Exerts Lipid-Regulating Effects in vitro and in vivo via Fibroblast Growth Factor 21 and AMP-Activated Protein Kinase/Sirtuin Type 1/Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1alpha Signaling Axis. Pharmacology 2017, 100, 115–126. [Google Scholar] [CrossRef]
- Paraiso, A.F.; Sousa, J.N.; Andrade, J.M.O.; Mangabeira, E.S.; Lelis, D.F.; de Paula, A.M.B.; Martins, A.; Lima, W.J.N.; Guimaraes, A.L.S.; Melo, G.A.; et al. Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue: A molecular and bioinformatic approach. Life Sci. 2019, 237, 116914. [Google Scholar] [CrossRef]
- Kashyap, B.; Saikia, K.; Samanta, S.K.; Thakur, D.; Banerjee, S.K.; Borah, J.C.; Talukdar, N.C. Kaempferol 3-O-rutinoside from Antidesma acidum Retz. Stimulates glucose uptake through SIRT1 induction followed by GLUT4 translocation in skeletal muscle L6 cells. J. Ethnopharmacol. 2023, 301, 115788. [Google Scholar] [CrossRef]
- Li, N.; Yin, L.; Shang, J.; Liang, M.; Liu, Z.; Yang, H.; Qiang, G.; Du, G.; Yang, X. Kaempferol attenuates nonalcoholic fatty liver disease in type 2 diabetic mice via the Sirt1/AMPK signaling pathway. Biomed. Pharmacother. 2023, 165, 115113. [Google Scholar] [CrossRef]
- Sayed, A.M.; Hassanein, E.H.M.; Salem, S.H.; Hussein, O.E.; Mahmoud, A.M. Flavonoids-mediated SIRT1 signaling activation in hepatic disorders. Life Sci. 2020, 259, 118173. [Google Scholar] [CrossRef]
- Xi, Y.; Chi, Z.; Tao, X.; Zhai, X.; Zhao, Z.; Ren, J.; Yang, S.; Dong, D. Naringin against doxorubicin-induced hepatotoxicity in mice through reducing oxidative stress, inflammation, and apoptosis via the up-regulation of SIRT1. Environ. Toxicol. 2023, 38, 1153–1161. [Google Scholar] [CrossRef]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front. Physiol. 2021, 12, 733696. [Google Scholar] [CrossRef]
- Chen, S.; Sun, T.; Li, X. Nobiletin alleviates the hypoxia/reoxygenation-induced damage in myocardial cells by modulating the miR-433/SIRT1 axis. J. Food Biochem. 2021, 45, e13844. [Google Scholar] [CrossRef]
- Dusabimana, T.; Kim, S.R.; Kim, H.J.; Park, S.W.; Kim, H. Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, L.; Li, X.; Zhao, L.; Hu, X.; Ma, Z. Short-term pretreatment of naringin isolated from Citrus wilsonii Tanaka attenuates rat myocardial ischemia/reperfusion injury. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1047–1059. [Google Scholar] [CrossRef]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Di Cesare Mannelli, L.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxid. Med. Cell Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef]
- Zheng, G.D.; Hu, P.J.; Chao, Y.X.; Zhou, Y.; Yang, X.J.; Chen, B.Z.; Yu, X.Y.; Cai, Y. Nobiletin induces growth inhibition and apoptosis in human nasopharyngeal carcinoma C666-1 cells through regulating PARP-2/SIRT1/AMPK signaling pathway. Food Sci. Nutr. 2019, 7, 1104–1112. [Google Scholar] [CrossRef]
- Moghadam, D.; Zarei, R.; Vakili, S.; Ghojoghi, R.; Zarezade, V.; Veisi, A.; Sabaghan, M.; Azadbakht, O.; Behrouj, H. The effect of natural polyphenols Resveratrol, Gallic acid, and Kuromanin chloride on human telomerase reverse transcriptase (hTERT) expression in HepG2 hepatocellular carcinoma: Role of SIRT1/Nrf2 signaling pathway and oxidative stress. Mol. Biol. Rep. 2023, 50, 77–84. [Google Scholar] [CrossRef]
- Finnin, M.S.; Donigian, J.R.; Pavletich, N.P. Structure of the histone deacetylase SIRT2. Nat. Struct. Biol. 2001, 8, 621–625. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet. Res. 2022, 2022, 9282484. [Google Scholar] [CrossRef]
- Lu, W.; Ji, H.; Wu, D. SIRT2 plays complex roles in neuroinflammation neuroimmunology-associated disorders. Front. Immunol. 2023, 14, 1174180. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhu, K.; Chi, S.; Wang, C.; Xie, A. Emerging Role of Sirtuin 2 in Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, P.; Hu, C. The role of SIRT2 in cancer: A novel therapeutic target. Int. J. Cancer 2020, 147, 3297–3304. [Google Scholar] [CrossRef]
- Taneja, A.; Ravi, V.; Hong, J.Y.; Lin, H.; Sundaresan, N.R. Emerging roles of Sirtuin 2 in cardiovascular diseases. FASEB J. 2021, 35, e21841. [Google Scholar] [CrossRef]
- Heger, V.; Tyni, J.; Hunyadi, A.; Horakova, L.; Lahtela-Kakkonen, M.; Rahnasto-Rilla, M. Quercetin based derivatives as sirtuin inhibitors. Biomed. Pharmacother. 2019, 111, 1326–1333. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.K.; Garg, G.; Rizvi, S.I. Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sci. 2018, 193, 171–179. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Rong, H.; Zhang, X.; Dong, M. Ferulic Acid Ameliorates MPP+/MPTP-Induced Oxidative Stress via ERK1/2-Dependent Nrf2 Activation: Translational Implications for Parkinson Disease Treatment. Mol. Neurobiol. 2020, 57, 2981–2995. [Google Scholar] [CrossRef]
- Maugeri, A.; Russo, C.; Musumeci, L.; Lombardo, G.E.; De Sarro, G.; Barreca, D.; Cirmi, S.; Navarra, M. The Anticancer Effect of a Flavonoid-Rich Extract of Bergamot Juice in THP-1 Cells Engages the SIRT2/AKT/p53 Pathway. Pharmaceutics 2022, 14, 2168. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; De Luca, L.; Gitto, R.; Lombardo, G.E.; Musumeci, L.; De Sarro, G.; Cirmi, S.; Navarra, M. The SIRT2 Pathway Is Involved in the Antiproliferative Effect of Flavanones in Human Leukemia Monocytic THP-1 Cells. Biomedicines 2022, 10, 2383. [Google Scholar] [CrossRef]
- Deng, J.; Huang, M.; Wu, H. Protective effect of limonin against doxorubicin-induced cardiotoxicity via activating nuclear factor—Like 2 and Sirtuin 2 signaling pathways. Bioengineered 2021, 12, 7975–7984. [Google Scholar] [CrossRef]
- Zhou, L.; Pinho, R.; Gu, Y.; Radak, Z. The Role of SIRT3 in Exercise and Aging. Cells 2022, 11, 2596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, S.; Yang, Y.; Wei, J.; Li, X.; Luo, P.; Jiang, X. Role of Sirtuin 3 in Degenerative Diseases of the Central Nervous System. Biomolecules 2023, 13, 735. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Z.; Liu, Z.; Chen, J.; Wang, Y. Sirtuin-3: A potential target for treating several types of brain injury. Front. Cell Dev. Biol. 2023, 11, 1154831. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, S.; Zhang, B.; Liu, J. SIRT3 as a potential therapeutic target for heart failure. Pharmacol. Res. 2021, 165, 105432. [Google Scholar] [CrossRef]
- Yapryntseva, M.A.; Maximchik, P.V.; Zhivotovsky, B.; Gogvadze, V. Mitochondrial sirtuin 3 and various cell death modalities. Front. Cell Dev. Biol. 2022, 10, 947357. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.E.; Arcidiacono, B.; De Rose, R.F.; Lepore, S.M.; Costa, N.; Montalcini, T.; Brunetti, A.; Russo, D.; De Sarro, G.; Celano, M. Normocaloric Diet Restores Weight Gain and Insulin Sensitivity in Obese Mice. Front. Endocrinol. 2016, 7, 49. [Google Scholar] [CrossRef]
- Akindehin, S.; Jung, Y.S.; Kim, S.N.; Son, Y.H.; Lee, I.; Seong, J.K.; Jeong, H.W.; Lee, Y.H. Myricetin Exerts Anti-Obesity Effects through Upregulation of SIRT3 in Adipose Tissue. Nutrients 2018, 10, 1962. [Google Scholar] [CrossRef]
- Lee, A.; Gu, H.; Gwon, M.H.; Yun, J.M. Hesperetin suppresses LPS/high glucose-induced inflammatory responses via TLR/MyD88/NF-kappaB signaling pathways in THP-1 cells. Nutr. Res. Pract. 2021, 15, 591–603. [Google Scholar] [CrossRef]
- Kim, A.; Lee, W.; Yun, J.M. Luteolin and fisetin suppress oxidative stress by modulating sirtuins and forkhead box O3a expression under in vitro diabetic conditions. Nutr. Res. Pract. 2017, 11, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Kitada, M.; Xu, J.; Monno, I.; Koya, D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging 2020, 12, 11325–11336. [Google Scholar] [CrossRef]
- Li, J.J.; Jiang, H.C.; Wang, A.; Bu, F.T.; Jia, P.C.; Zhu, S.; Zhu, L.; Huang, C.; Li, J. Hesperetin derivative-16 attenuates CCl4-induced inflammation and liver fibrosis by activating AMPK/SIRT3 pathway. Eur. J. Pharmacol. 2022, 915, 174530. [Google Scholar] [CrossRef] [PubMed]
- Ji-Hong, Y.; Yu, M.; Ling-Hong, Y.; Jing-Jing, G.; Ling-Li, X.; Lv, W.; Yong-Mei, J. Baicalein attenuates bleomycin-induced lung fibroblast senescence and lung fibrosis through restoration of Sirt3 expression. Pharm. Biol. 2023, 61, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M.; Dong, X.; Xue, X.D.; Zhang, J.; Li, Z.; Wu, H.J.; Yang, Z.L.; Yang, Y.; Wang, H.S. Naringenin improves mitochondrial function and reduces cardiac damage following ischemia-reperfusion injury: The role of the AMPK-SIRT3 signaling pathway. Food Funct. 2019, 10, 2752–2765. [Google Scholar] [CrossRef]
- Ahmedy, O.A.; Abdelghany, T.M.; El-Shamarka, M.E.A.; Khattab, M.A.; El-Tanbouly, D.M. Apigenin attenuates LPS-induced neurotoxicity and cognitive impairment in mice via promoting mitochondrial fusion/mitophagy: Role of SIRT3/PINK1/Parkin pathway. Psychopharmacology 2022, 239, 3903–3917. [Google Scholar] [CrossRef]
- Mu, J.; Ma, H.; Chen, H.; Zhang, X.; Ye, M. Luteolin Prevents UVB-Induced Skin Photoaging Damage by Modulating SIRT3/ROS/MAPK Signaling: An in vitro and in vivo Studies. Front. Pharmacol. 2021, 12, 728261. [Google Scholar] [CrossRef]
- Mu, J.; Chen, H.; Ye, M.; Zhang, X.; Ma, H. Acacetin resists UVA photoaging by mediating the SIRT3/ROS/MAPKs pathway. J. Cell Mol. Med. 2022, 26, 4624–4628. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.J.; Wang, Y.Z.; Feng, S.; Jing, G.; Fu, X.L. Myricetin nanoliposomes induced SIRT3-mediated glycolytic metabolism leading to glioblastoma cell death. Artif. Cells Nanomed. Biotechnol. 2018, 46, S180–S191. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, F.; Chen, X.; Wang, C.; Wang, J.; Liu, T.; Li, Y.; He, B. SIRT4 is the last puzzle of mitochondrial sirtuins. Bioorg Med. Chem. 2018, 26, 3861–3865. [Google Scholar] [CrossRef]
- Tomaselli, D.; Steegborn, C.; Mai, A.; Rotili, D. Sirt4: A Multifaceted Enzyme at the Crossroads of Mitochondrial Metabolism and Cancer. Front. Oncol. 2020, 10, 474. [Google Scholar] [CrossRef]
- Pannek, M.; Simic, Z.; Fuszard, M.; Meleshin, M.; Rotili, D.; Mai, A.; Schutkowski, M.; Steegborn, C. Crystal structures of the mitochondrial deacylase Sirtuin 4 reveal isoform-specific acyl recognition and regulation features. Nat. Commun. 2017, 8, 1513. [Google Scholar] [CrossRef]
- Park, E.S.; Kang, J.C.; Jang, Y.C.; Park, J.S.; Jang, S.Y.; Kim, D.E.; Kim, B.; Shin, H.S. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J. Ethnopharmacol. 2014, 153, 552–560. [Google Scholar] [CrossRef]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zha, X. Overview of SIRT5 as a potential therapeutic target: Structure, function and inhibitors. Eur. J. Med. Chem. 2022, 236, 114363. [Google Scholar] [CrossRef] [PubMed]
- Curry, A.M.; Rymarchyk, S.; Herrington, N.B.; Donu, D.; Kellogg, G.E.; Cen, Y. Nicotinamide riboside activates SIRT5 deacetylation. FEBS J. 2023, 290, 4762–4776. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Hu, H.; Qu, S.; Wang, J.; Hua, C.; Zhang, J.; Wei, P.; He, X.; Hao, J.; Liu, P.; et al. SIRT5 deacylates metabolism-related proteins and attenuates hepatic steatosis in ob/ob mice. EBioMedicine 2018, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizi, E.; Fiorentino, F.; Carafa, V.; Altucci, L.; Mai, A.; Rotili, D. Emerging Roles of SIRT5 in Metabolism, Cancer, and SARS-CoV-2 Infection. Cells 2023, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Wang, J.; Liu, Y.; Yan, P.; Meng, Q.; Yin, Y.; Wang, S. SIRT5-Related Desuccinylation Modification Contributes to Quercetin-Induced Protection against Heart Failure and High-Glucose-Prompted Cardiomyocytes Injured through Regulation of Mitochondrial Quality Surveillance. Oxid. Med. Cell Longev. 2021, 2021, 5876841. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yang, Y.; Pang, X.; Shi, J.; Jiang, T.; Zheng, X. Quercetin inhibits DNA damage responses to induce apoptosis via SIRT5/PI3K/AKT pathway in non-small cell lung cancer. Biomed. Pharmacother. 2023, 165, 115071. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.; Liu, H.; Zhang, W.; Zhou, J. Emerging roles of SIRT6 in human diseases and its modulators. Med. Res. Rev. 2021, 41, 1089–1137. [Google Scholar] [CrossRef] [PubMed]
- Gertler, A.A.; Cohen, H.Y. SIRT6, a protein with many faces. Biogerontology 2013, 14, 629–639. [Google Scholar] [CrossRef]
- Fiorentino, F.; Mai, A.; Rotili, D. Emerging Therapeutic Potential of SIRT6 Modulators. J. Med. Chem. 2021, 64, 9732–9758. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal 2018, 28, 711–732. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Y.; Liu, R.; Wang, H.; Rui, T. The role of mammalian Sirtuin 6 in cardiovascular diseases and diabetes mellitus. Front. Physiol. 2023, 14, 1207133. [Google Scholar] [CrossRef]
- Smirnov, D.; Eremenko, E.; Stein, D.; Kaluski, S.; Jasinska, W.; Cosentino, C.; Martinez-Pastor, B.; Brotman, Y.; Mostoslavsky, R.; Khrameeva, E.; et al. SIRT6 is a key regulator of mitochondrial function in the brain. Cell Death Dis. 2023, 14, 35. [Google Scholar] [CrossRef]
- Li, D.; Cao, C. The correlation of serum sirt6 with clinical outcome and prognosis in patients with gastric cancer. Medicine 2022, 101, e31568. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Wilson, D.R.; Fugmann, S.D.; Moaddel, R. Synthesis and characterization of SIRT6 protein coated magnetic beads: Identification of a novel inhibitor of SIRT6 deacetylase from medicinal plant extracts. Anal. Chem. 2011, 83, 7400–7407. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Zheng, W.; Weiss, S.; Chua, K.F.; Steegborn, C. Structural basis for the activation and inhibition of Sirtuin 6 by quercetin and its derivatives. Sci. Rep. 2019, 9, 19176. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Deng, Y.; Ren, L.; Jin, Z.; Yang, J.; Yao, F.; Liu, Y.; Zheng, Z.; Chen, D.; Wang, B.; et al. Isoliquiritigenin from licorice flavonoids attenuates NLRP3-mediated pyroptosis by SIRT6 in vascular endothelial cells. J. Ethnopharmacol. 2023, 303, 115952. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Yuan, J.; Mei, L.; Li, P.; Pan, R. Luteolin suppresses TNF-alpha-induced inflammatory injury and senescence of nucleus pulposus cells via the Sirt6/NF-kappaB pathway. Exp. Ther. Med. 2022, 24, 469. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Wang, X.; Zhang, Z.; Cao, D.; Huang, K.; Wang, Y.; Liu, Z.; Su, S.; Wang, Q. Hesperetin attenuates cognitive dysfunction via SIRT6/NLRP3 pathway in scopolamine-induced mice. Metab. Brain Dis. 2023, 38, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, Z.; Zhan, G.; Li, X.; Li, S.; Wang, X.; Li, S.; Luo, A. Caffeic Acid Phenethyl Ester Suppresses Oxidative Stress and Regulates M1/M2 Microglia Polarization via Sirt6/Nrf2 Pathway to Mitigate Cognitive Impairment in Aged Mice following Anesthesia and Surgery. Antioxidants 2023, 12, 714. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Qiao, H. Downregulation of hippocampal SIRT6 activates AKT/CRMP2 signaling and ameliorates chronic stress-induced depression-like behavior in mice. Acta Pharmacol. Sin. 2020, 41, 1557–1567. [Google Scholar] [CrossRef]
- Tang, M.; Tang, H.; Tu, B.; Zhu, W.G. SIRT7: A sentinel of genome stability. Open Biol. 2021, 11, 210047. [Google Scholar] [CrossRef]
- Mitra, N.; Dey, S. Biochemical characterization of mono ADP ribosyl transferase activity of human sirtuin SIRT7 and its regulation. Arch. Biochem. Biophys. 2020, 680, 108226. [Google Scholar] [CrossRef]
- Barber, M.F.; Michishita-Kioi, E.; Xi, Y.; Tasselli, L.; Kioi, M.; Moqtaderi, Z.; Tennen, R.I.; Paredes, S.; Young, N.L.; Chen, K.; et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 2012, 487, 114–118. [Google Scholar] [CrossRef]
- Mendes, K.L.; Lelis, D.F.; Santos, S.H.S. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor. Rev. 2017, 38, 98–105. [Google Scholar] [CrossRef]
- Huo, Q.; Chen, S.; Zhuang, J.; Quan, C.; Wang, Y.; Xie, N. SIRT7 Downregulation Promotes Breast Cancer Metastasis Via LAP2alpha-Induced Chromosomal Instability. Int. J. Biol. Sci. 2023, 19, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, H.; Yang, Y.; Wang, H.; Zhang, H.; Guo, S.; Chen, J.; Du, J.; Tian, Y.; Ma, J.; et al. SIRT7 orchestrates melanoma progression by simultaneously promoting cell survival and immune evasion via UPR activation. Signal Transduct. Target. Ther. 2023, 8, 107. [Google Scholar] [CrossRef]
- Li, G.; Xu, W.; Li, X.; Chen, M.; Shi, Y.; Wei, M.; Peng, D. Oncogenic SIRT7 inhibits GATA4 transcriptional activity and activates the Wnt signaling pathway in ovarian cancer. Gynecol. Oncol. 2023, 171, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xian, G.; Zeng, J.; Zhong, G.; An, D.; Peng, Y.; Hu, D.; Lin, Y.; Li, J.; Su, S.; et al. Hesperetin, a Promising Dietary Supplement for Preventing the Development of Calcific Aortic Valve Disease. Antioxidants 2022, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, F.H.; Mesbah-Ardakani, M.; Nasr-Esfahani, M.H. Ferulic Acid exerts concentration-dependent anti-apoptotic and neuronal differentiation-inducing effects in PC12 and mouse neural stem cells. Eur. J. Pharmacol. 2018, 841, 104–112. [Google Scholar] [CrossRef] [PubMed]
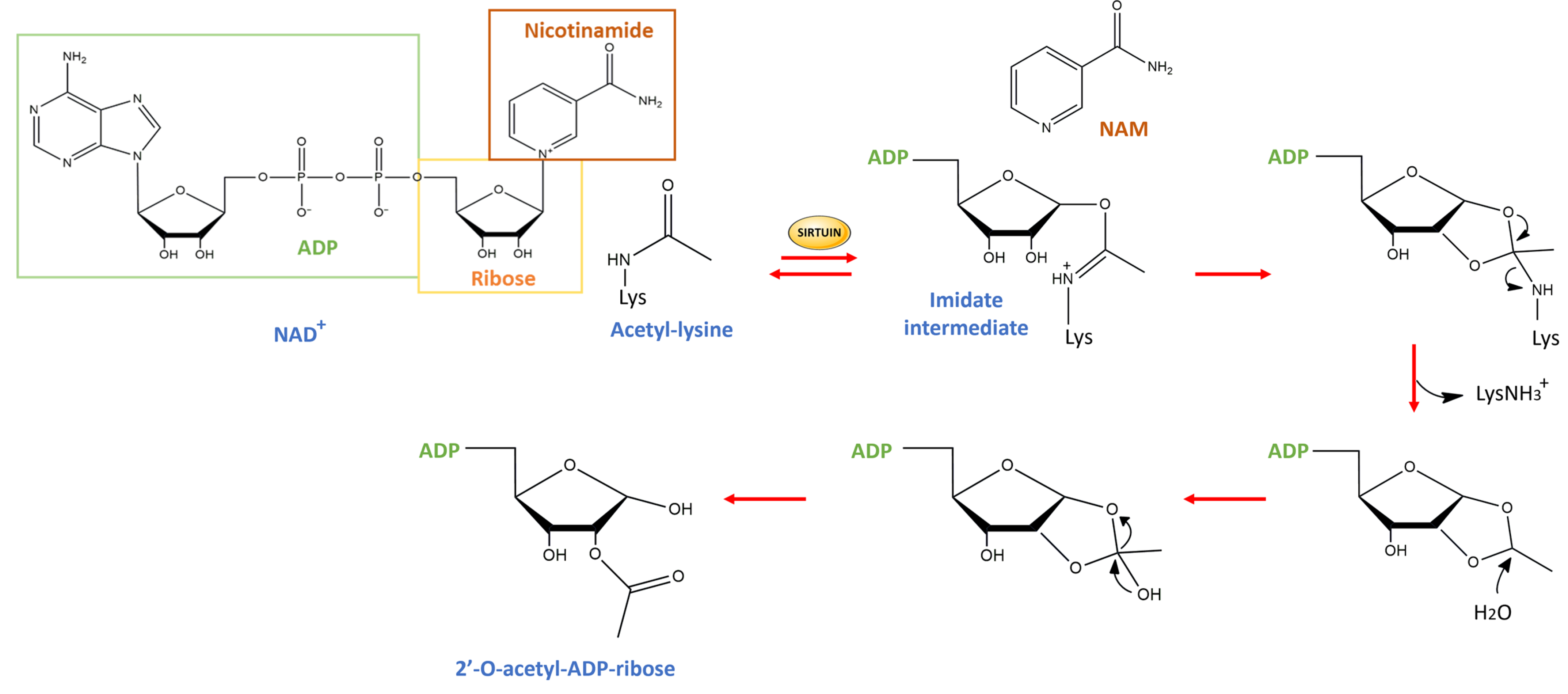
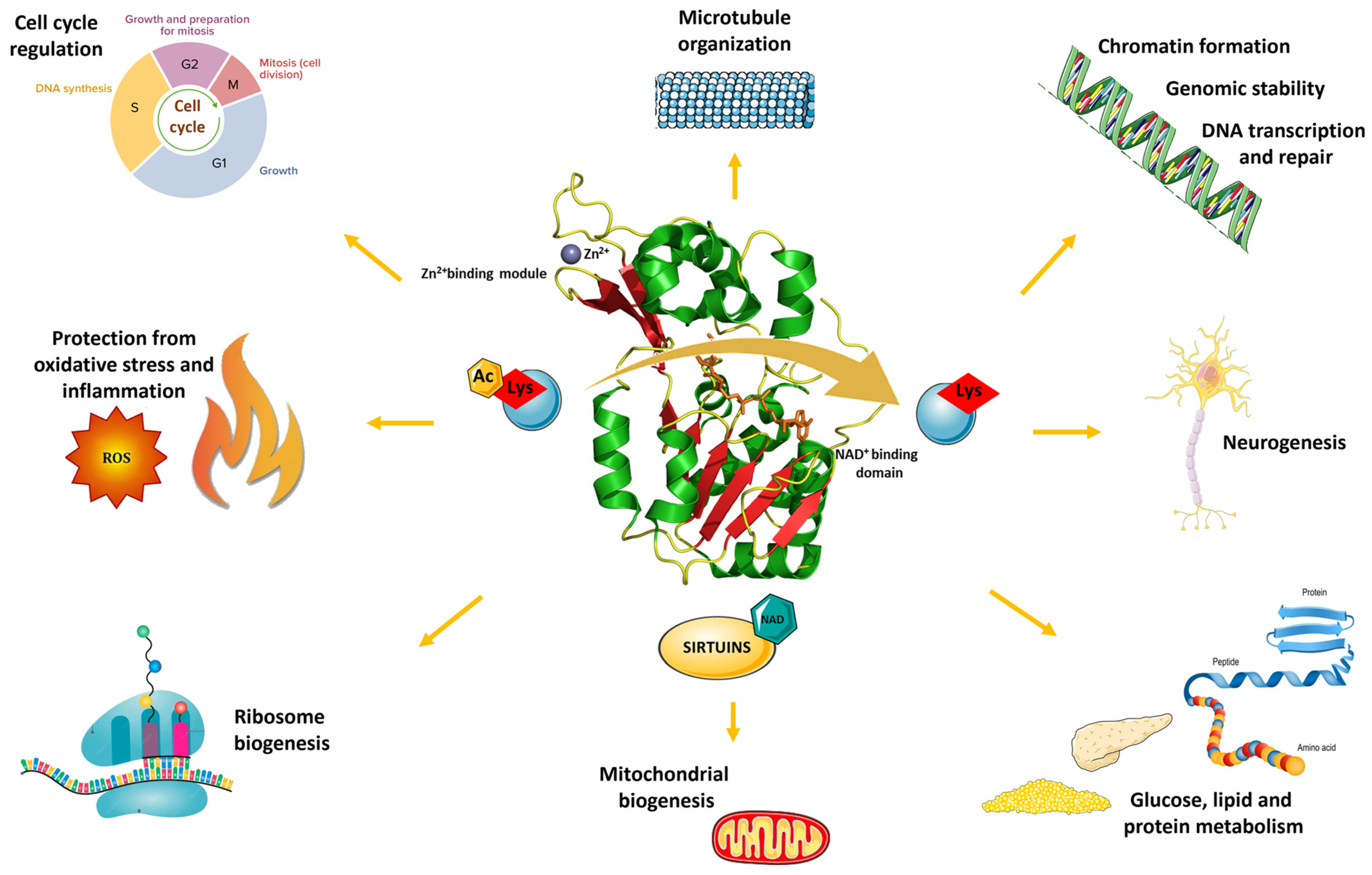

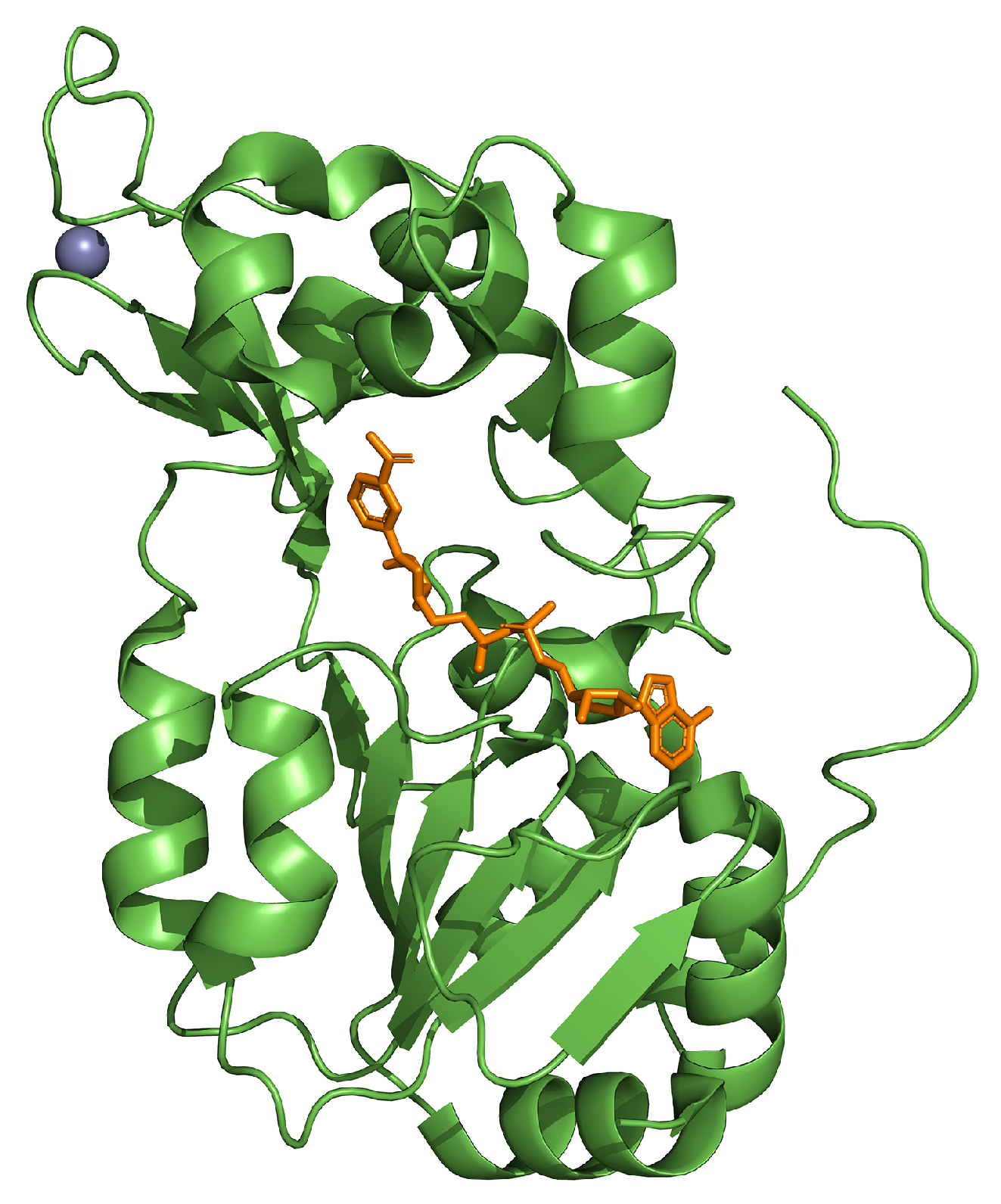
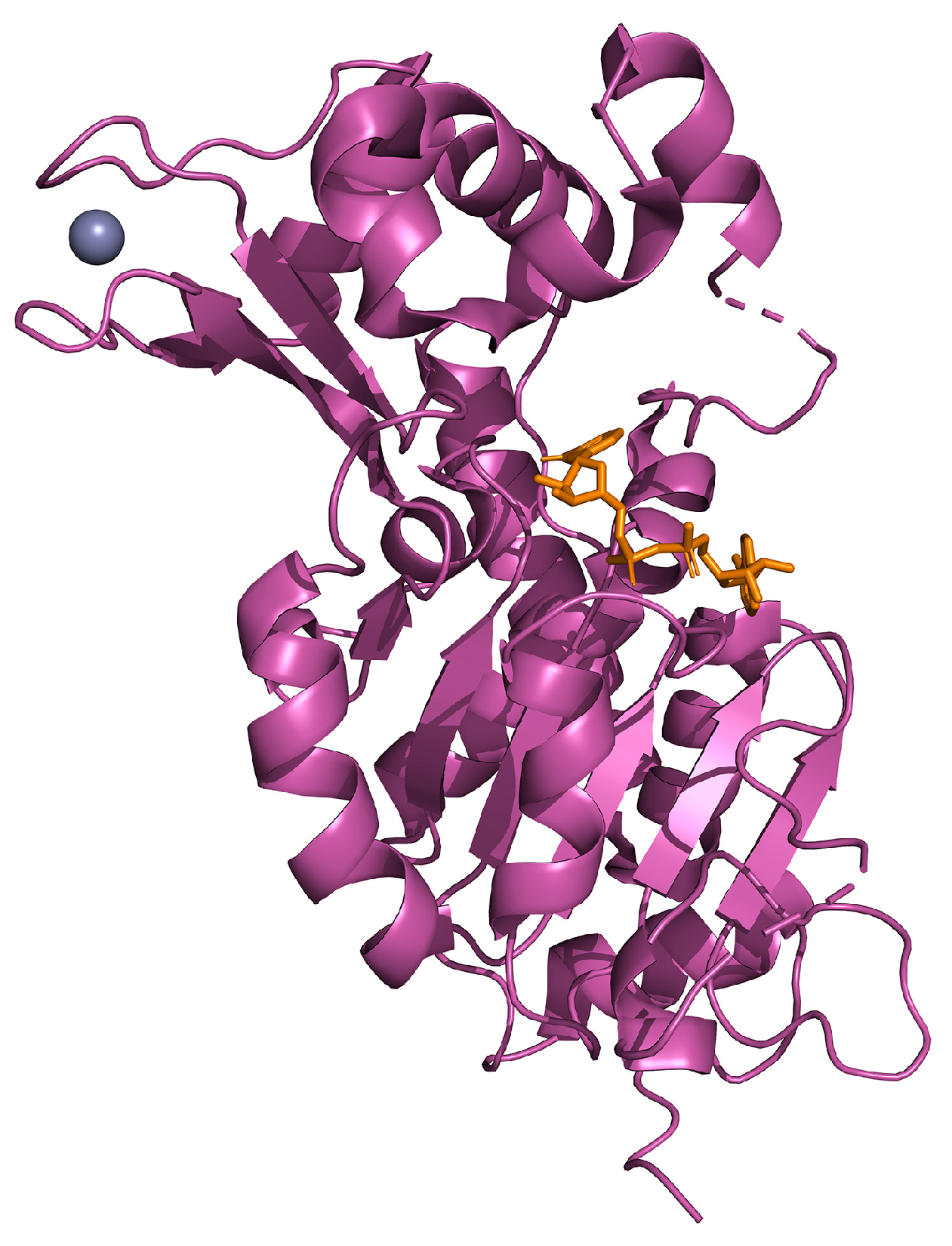

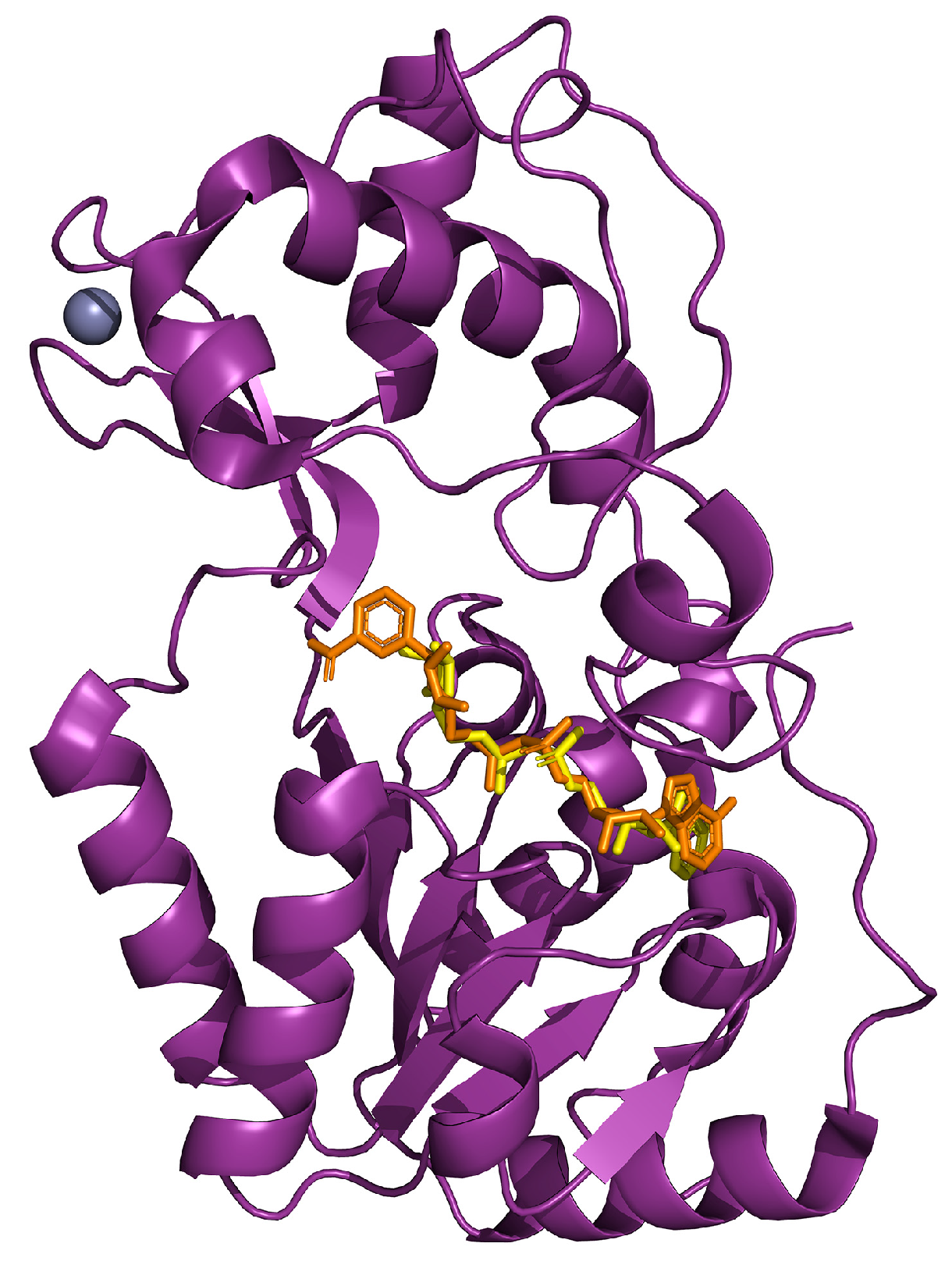

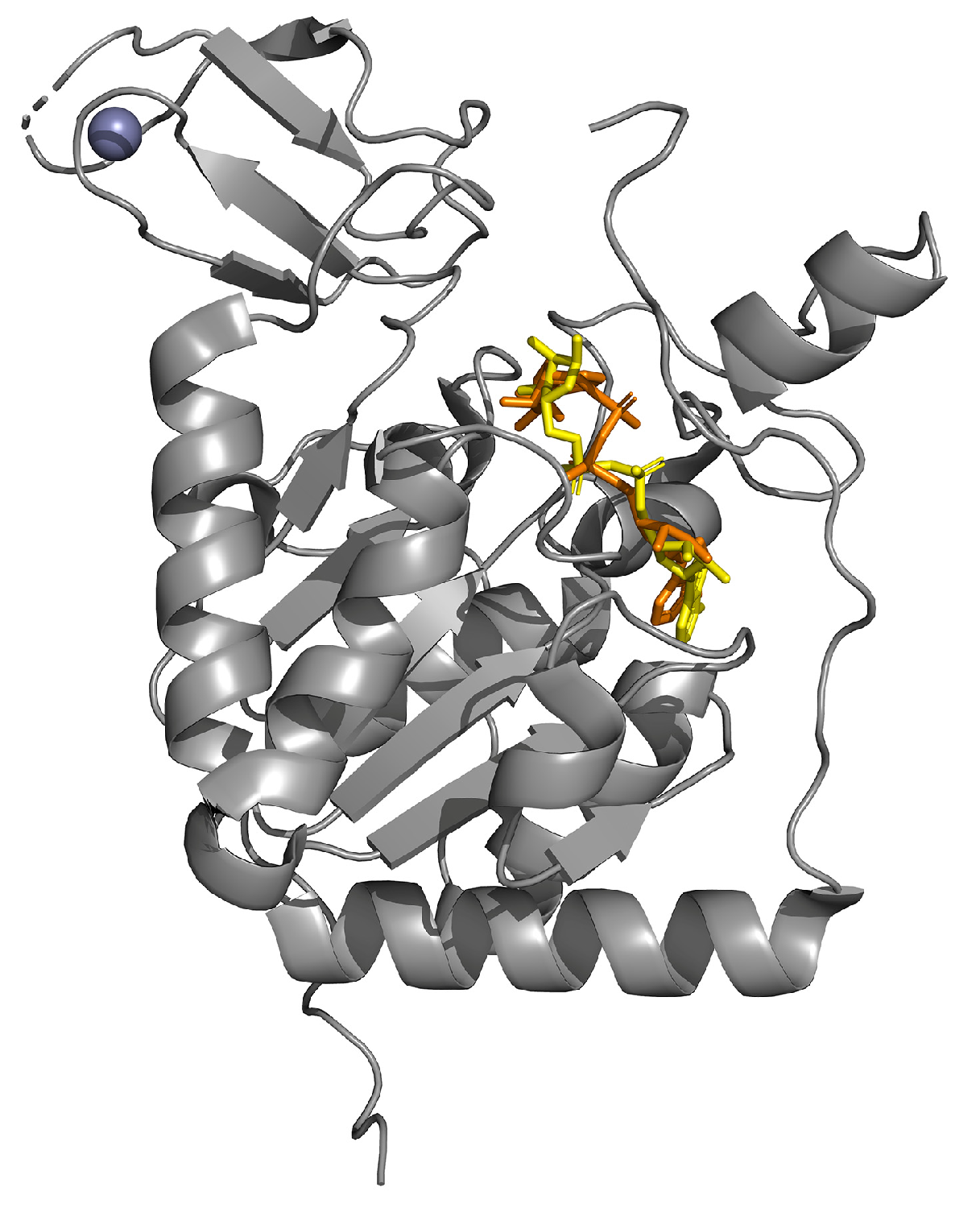
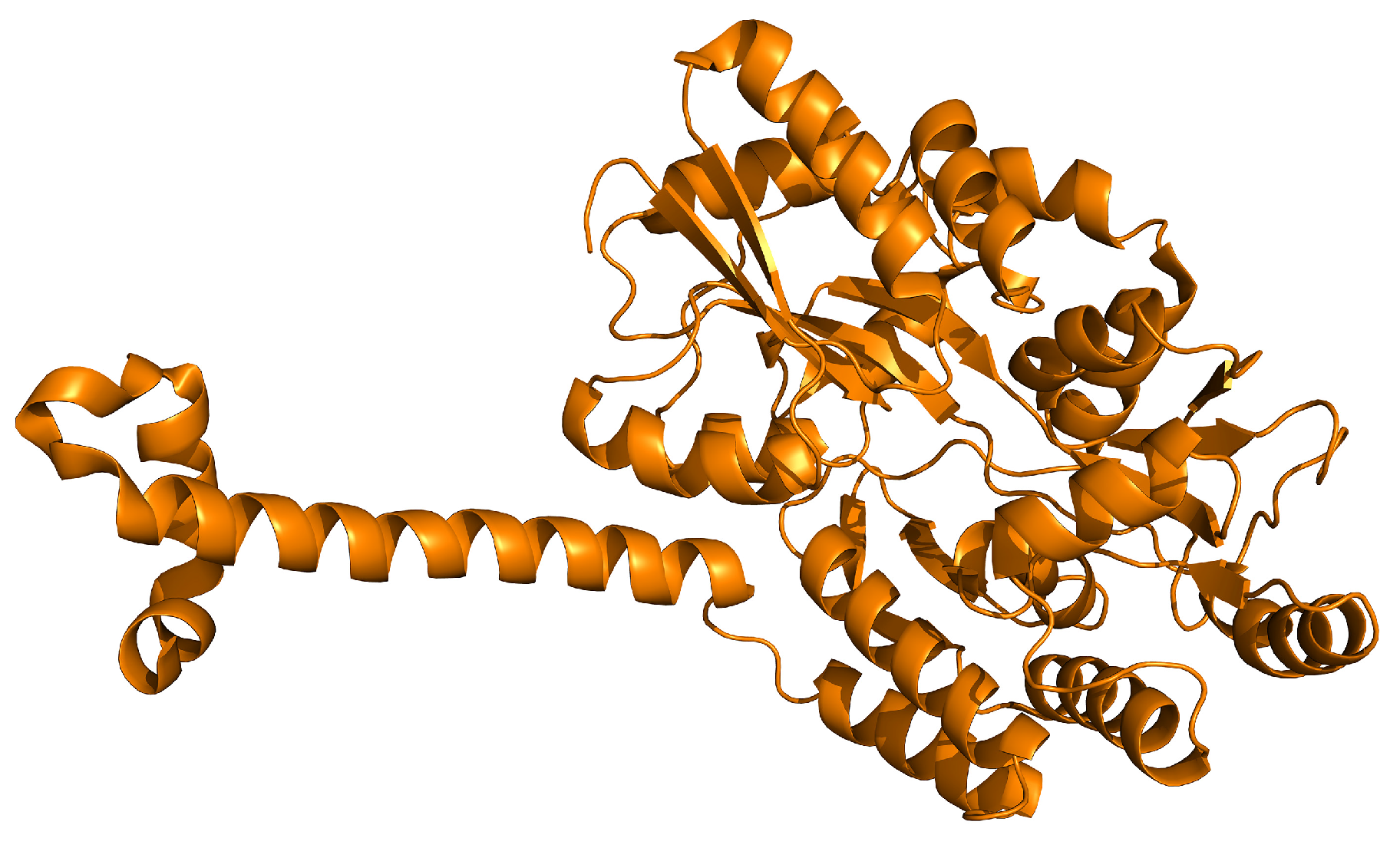

| Experimental Models | Citrus Flavonoid | Effect | Reference |
|---|---|---|---|
| Wistar rats | HES | Antioxidant effect by activating miR-126-3p/miR-181a-SIRT1 network | [79] |
| Wistar albino rats | FIS | Reduction in testicular toxicity via SIRT1 activation | [80] |
| SD rats HUVEC cells | NGN | Antioxidant effect against endothelial damage by activating AMPKα/SIRT1 | [81] |
| Swiss albino mice | NGN | Amelioration of chronic sleep deprivation-induced pain via SIRT1 activation | [82] |
| Primary rat NPMSCs | FIS | Anti-inflammatory and antioxidant effects mitigating intervertebral disc degeneration through the activation of SIRT1 pathway | [83] |
| Rat chondrocytes | RU | Mitigation of osteoarthritis pathogenesis through the activation of SIRT1 | [84] |
| A549 cells | MYR | Anti-inflammatory effect via SIRT1/NF-κB pathway | [85] |
| C57BL/6 mice | HES | Anti-inflammatory and antioxidant effects by the upregulation of SIRT1/NF-κB | [90] |
| SD rats | NGN | Mitigation of polycystic ovary syndrome by upregulating the gut microbiota and SIRT-1/PGC-1α | [91] |
| SD rats | HES | Antioxidant and anti-inflammatory effects via SIRT1/FOXO activation in hepatic encephalopathy | [92] |
| NRK49F cells/in vivo | LU | Attenuation of renal anemia caused by renal fibrosis through the SIRT1/FOXO3 pathway | [93] |
| THP-1 cells | NGN, NAR, HSP, NEO | Anti-inflammatory effect through the modulation of AMPK/SIRT1 axis | [94,95] |
| Human NPCs | NGN | Anti-inflammatory effect through the activation of AMPK/SIRT1 axis | [96] |
| HepG2 cells | HSP | Anti-inflammatory effect by activating SIRT1-AMPK pathway | [97] |
| THP-1 cells/LDLR−/− knockout mice | LU | Counteraction of atherosclerosis via the AMPK/SIRT1 signaling pathway | [98] |
| Rat NPCs | ORI | Antioxidant effect against mitochondrial dysfunction through AMPK/SIRT1 axis | [99] |
| BV2 cells/primary microglia | TAN | Anti-inflammatory effect via upregulation of SIRT1 in microglia | [100] |
| Kunming mice | CA | Neuroprotective effect via the activation of AMPK/SIRT1 | [101] |
| PC12 cells | CAPE | Neuroprotective effect via the activation of AMPK/SIRT1 | [102] |
| SD rats | QUE/NGN | Anti-inflammaging effect by increasing SIRT1 level in hippocampus | [103] |
| Kunming mice ARPE-19 cells | NGN | Antioxidant effect by upregulation of SIRT1 | [104] |
| SD rats Rat glial C6 cells | HES | Antioxidant and anti-inflammatory effects by SIRT1/NOX4 activation | [105] |
| C57BL/6N mice | FIS | Protection from neuroinflammation by activation of SIRT1/Nrf2 axis | [106] |
| Swiss mice | NAR | Protection from mitochondrial dysfunction in lung by activation of SIRT1 | [107] |
| AML-12 cells | NOB | Suppression of NLRP3 inflammasome by activating SIRT1 | [108] |
| RAW264.7/AML-12—BALB/C mice | HSP | Protection from hepatic inflammation via AMPK/CREB through SIRT1 activation | [112] |
| ApoE−/− mice AML-12 cells | NGN | Anti-inflammatory, antioxidant, antifibrotic effects in NAFLD/NASH by activating hepatic SIRT1 | [113] |
| C57BL/6 mice/Caco-2 and IEC-6 cells | DIO | Amelioration of colon inflammation and oxidative stress via the circ-SIRT1/SIRT1 axis | [115] |
| Wistar rats | HES/QUE | Antioxidant effect by upregulating SIRT1, hampering NF-κB activation | [116] |
| C57BL/6 mice | MYR | Antioxidant and anti-inflammatory effects through the upregulation of hepatic SIRT1 | [117] |
| C57BL/6 mice | RU | Alleviation of acute endotoxemic kidney injury by upregulating SIRT1 | [118] |
| H9c2 cells | RU | Mitigation of H/R in myocardial injury increasing SIRT1 expression | [119] |
| SD rats | LU | Mitigation of myocardial ischemia reperfusion injury via SIRT1/NLRP3/NF-κB | [120] |
| Kunming mice | HSP | Antioxidant, anti-inflammatory effects in myocardial ischemia by activation of SIRT1/Nrf2 | [121] |
| HepG2 cells/Primary hepatocytes from C57BL/6 | NOB | Mitigation of lipid metabolism by upregulating SIRT1 in hepatocytes and circadian rhythms | [124] |
| HepG 2 cells/ Homozygous C57BL/6 (C57) mice | NEO | Reduction in lipid metabolism through AMPK/SIRT1/PGC-1α axis | [125] |
| L6 cells | KOR | Stimulation of glucose uptake through SIRT1 induction | [127] |
| HepG2 cells/db/db mice | KMF | Counteraction of NAFLD through the activation of SIRT1/AMPK axis | [128] |
| AML-12 cells | NAR | Protection from liver damage by upregulation of SIRT1 | [130] |
| H9c2 myocardial cells | NOB | Protection against myocardial I/R injury via the modulation of the miR-433/SIRT1 axis | [132] |
| C57BL/6 mice | NOB | Protection against hepatic I/R via SIRT1/FOXO3a activation | [133] |
| SD rats | NAR | Attenuation of myocardial I/R by reducing oxidative stress and inflammation, through SIRT1 activation | [134] |
| Enzymatic assay, computational study, C57BL/6J, H9c2 cells | NGN | Anti-senescence effect by reducing inflammation and ROS enhancing the expression of SIRT1 | [135] |
| C666-1 nasopharyngeal carcinoma cells | NOB | Antiproliferative effect by the upregulation of PARP-2/SIRT1/AMPK pathways | [136] |
| Experimental Models | Citrus Flavonoid | Effect | Reference |
|---|---|---|---|
| Enzymatic assay | 2-Chloro-1,4-naphtoquinone-quercetin | Potent inhibition of SIRT2 enzymatic activity | [144] |
| Wistar rat Primary neuronal cells | FIS | Neuroprotective effect against aging-induced oxidative stress by the downregulation of SIRT2 gene | [145] |
| Enzymatic assay SH-SY5Y cells | FA | Antioxidant effect via the inhibition of SIRT2 activity | [146] |
| THP-1 cells | NGN/HSP | Anti-leukemic effect via reduction in SIRT2 activity | [148] |
| Experimental Models | Citrus Flavonoid | Effect | Reference |
|---|---|---|---|
| C3H10T1/2 cells/C57BL6/J mice | MYR | Anti-obesity effect through the upregulation of SIRT3 ex-pression in adipose tissue | [157] |
| THP-1 cells | HSP | Suppression of inflammation in diabetes via TLR/MyD88/NF-κB increasing SIRT3 | [158] |
| THP-1 cells | FIS/LU | Suppression of oxidative stress in hyperglycemic condition through the upregulation of SIRT1, SIRT3, SIRT6 | [159] |
| ZLRs and ZDFRs rats | API | Mitigates mitochondrial oxidative stress through the upregulation of SIRT3 | [160] |
| LX-2 cells/C57BL/6J mice | HSP | Hepatoprotective effect by activating the AMPK/SIRT3 pathway | [161] |
| Mice | BAI | Counteraction of lung fibrosis by restoring SIRT3 expression | [162] |
| SD rats/H9c2 cells | NAG | Antioxidant effect in myocardial I/R through the activation of AMPK/SIRT3 axis | [163] |
| Swiss albino mice | API | Attenuation of neurotoxicity via promoting mitochondrial homeostasis by activating SIRT3 | [164] |
| SD rats/Human dermal fibroblasts cells | LU | Protection from skin photoaging by upregulating the SIRT3/MAPKs axis | [165] |
| SD rats | ACA | Protection from skin photoaging by upregulating the SIRT3/MAPKs axis | [166] |
| DBTRG-05MG cells | MYR | Antiproliferative effects in glioblastoma cells by reducing SIRT3 levels | [167] |
| Experimental Models | Citrus Flavonoid | Effect | Reference |
|---|---|---|---|
| H9c2 cardiomyoblast cells | RHM | Cardioprotective and antioxidant effects due to an increase in both SIRT3 and SIRT4 expression | [171] |
| Experimental Models | Citrus Flavonoid | Effect | Reference |
|---|---|---|---|
| HL-1 cells/C57BL/6J mice | QUE | Antioxidant and anti-inflammatory effects increasing SIRT5 expression | [177] |
| BEAS-2B, Human NSCLC, A559 and H1299 cells | QUE | Inhibition of DNA damage and induction of apoptosis via the direct binding and upregulation of SIRT5, along with the modulation of PI3K/AKT pathway | [178] |
| Experimental Models | Citrus Flavonoid | Effect | Reference |
|---|---|---|---|
| THP-1 cells | HSP | Mitigation of diabetic inflammation through the modulation of TLR/MyD88/NF-κB signaling pathways, increasing SIRT6 expression | [158] |
| THP-1 cells | LU/FIS | Inhibition of ROS production through the elevation of SIRT6 and FOXO3a expression | [159] |
| Enzymatic assay/U2OS cells | QUE, LU | Modulation of SIRT6 activity | [187] |
| HUVEC cells | ISL | Decrease in vascular endothelial cell pyroptosis via the upregulation of SIRT6 | [188] |
| HNPC cells | LU | Anti-inflammatory effect by upregulating SIRT6 and hindering the downstream activation of NF-κB pathway | [189] |
| C57BL/6J mice | HSP | Counteraction of neuroinflammation and oxidative stress by increasing SIRT6 levels | [190] |
| C57BL/6J mice/BV2 cells | CAPE | Mitigation of post-operative cognitive dysfunction, hindering oxidative stress by enhancing the SIRT6/Nrf2 pathway | [191] |
| C57BL/6 mice/Human HEK-293T and mouse HT-22 cells | FA | Reduction in depression-like behaviors by suppressing AKT/CRMP2 and acting as downregulator of SIRT6 | [192] |
| Experimental Models | Citrus Flavonoid | Effect | Reference |
|---|---|---|---|
| C57BL/6 mice/ Docking studies/Human VICs | HSP | Protective effect in the aortic valve, increasing the SIRT7-mediated activation of the Nrf2–ARE axis | [200] |
| PC12 cells | FA | Neuroprotective effect through the upregulation of SIRT1 and SIRT7 | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, G.E.; Russo, C.; Maugeri, A.; Navarra, M. Sirtuins as Players in the Signal Transduction of Citrus Flavonoids. Int. J. Mol. Sci. 2024, 25, 1956. https://doi.org/10.3390/ijms25041956
Lombardo GE, Russo C, Maugeri A, Navarra M. Sirtuins as Players in the Signal Transduction of Citrus Flavonoids. International Journal of Molecular Sciences. 2024; 25(4):1956. https://doi.org/10.3390/ijms25041956
Chicago/Turabian StyleLombardo, Giovanni Enrico, Caterina Russo, Alessandro Maugeri, and Michele Navarra. 2024. "Sirtuins as Players in the Signal Transduction of Citrus Flavonoids" International Journal of Molecular Sciences 25, no. 4: 1956. https://doi.org/10.3390/ijms25041956
APA StyleLombardo, G. E., Russo, C., Maugeri, A., & Navarra, M. (2024). Sirtuins as Players in the Signal Transduction of Citrus Flavonoids. International Journal of Molecular Sciences, 25(4), 1956. https://doi.org/10.3390/ijms25041956








