Assessment of the Salivary Concentrations of Selected Immunological Components in Adult Patients in the Late Period after Allogeneic Hematopoietic Stem Cell Transplantation—A Translational Study
Abstract
:1. Introduction
2. Results
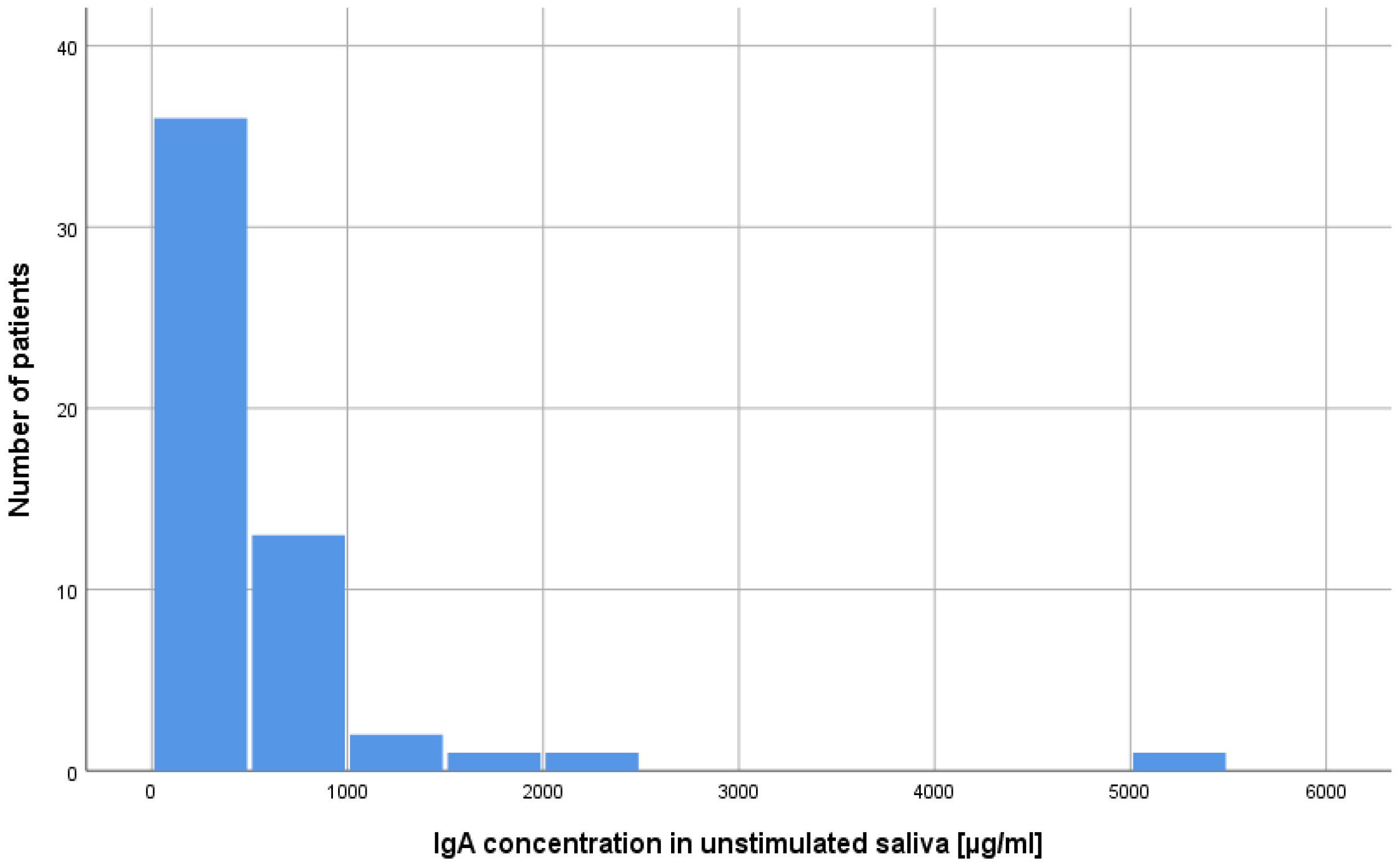
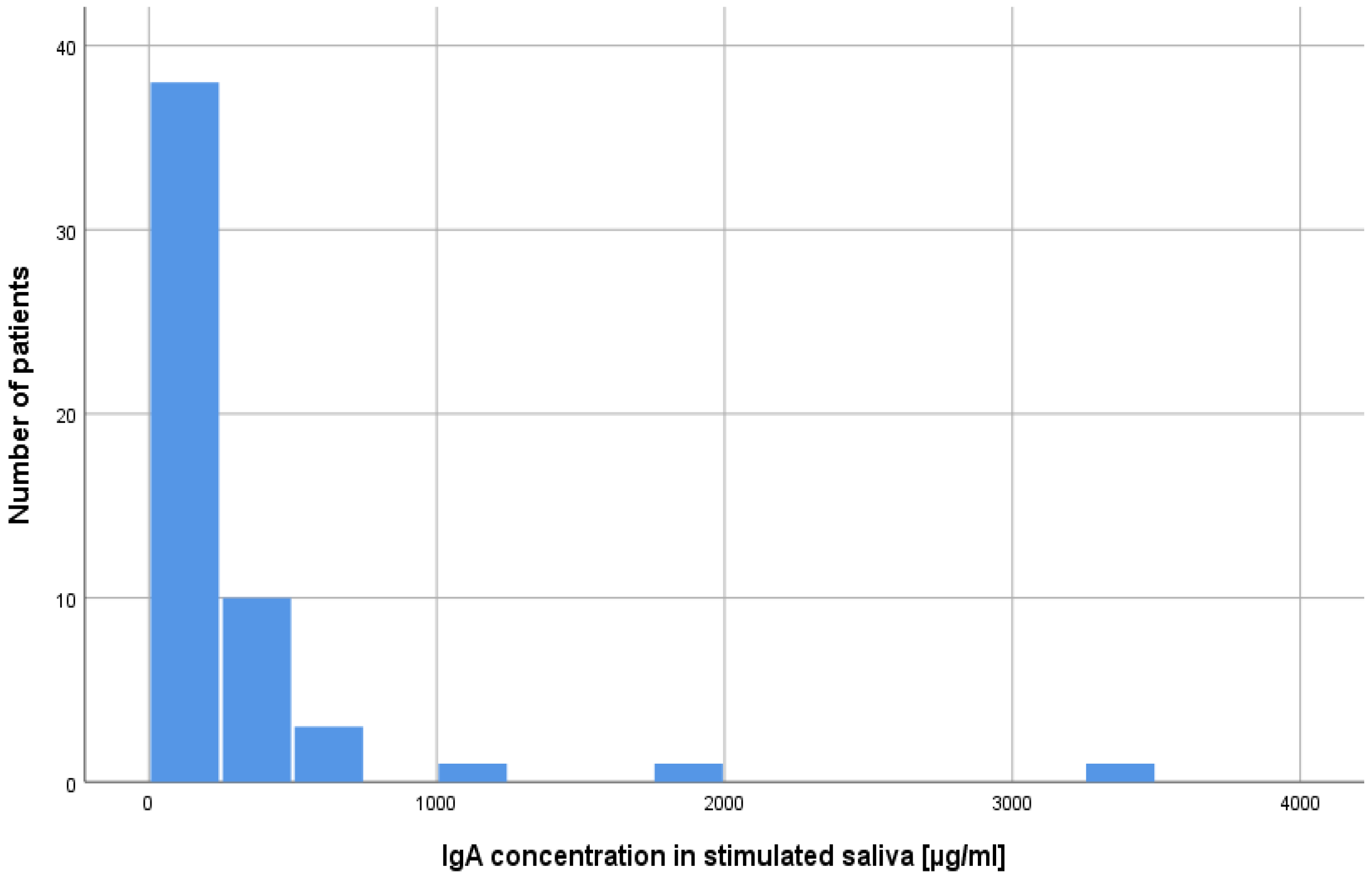
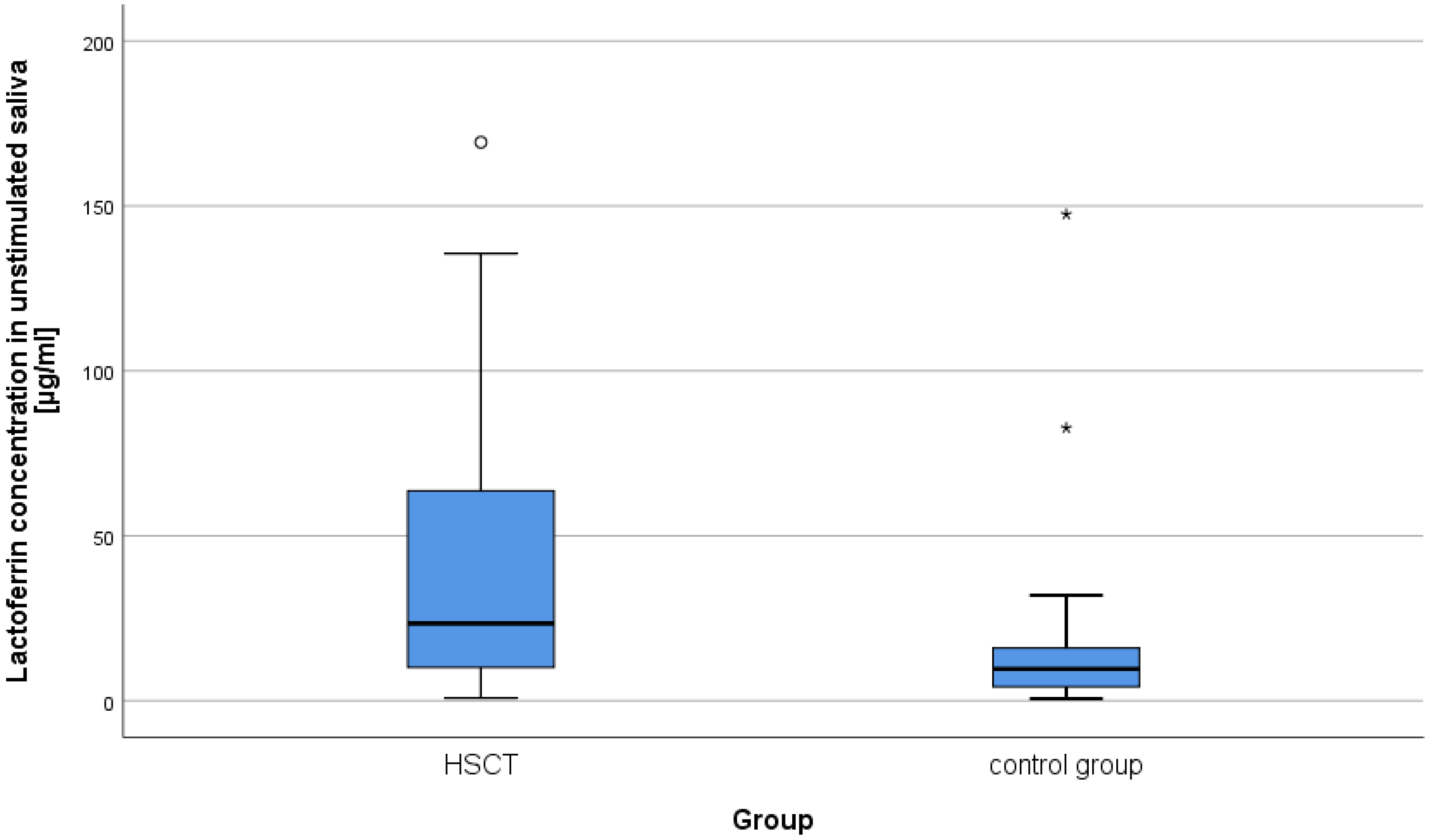
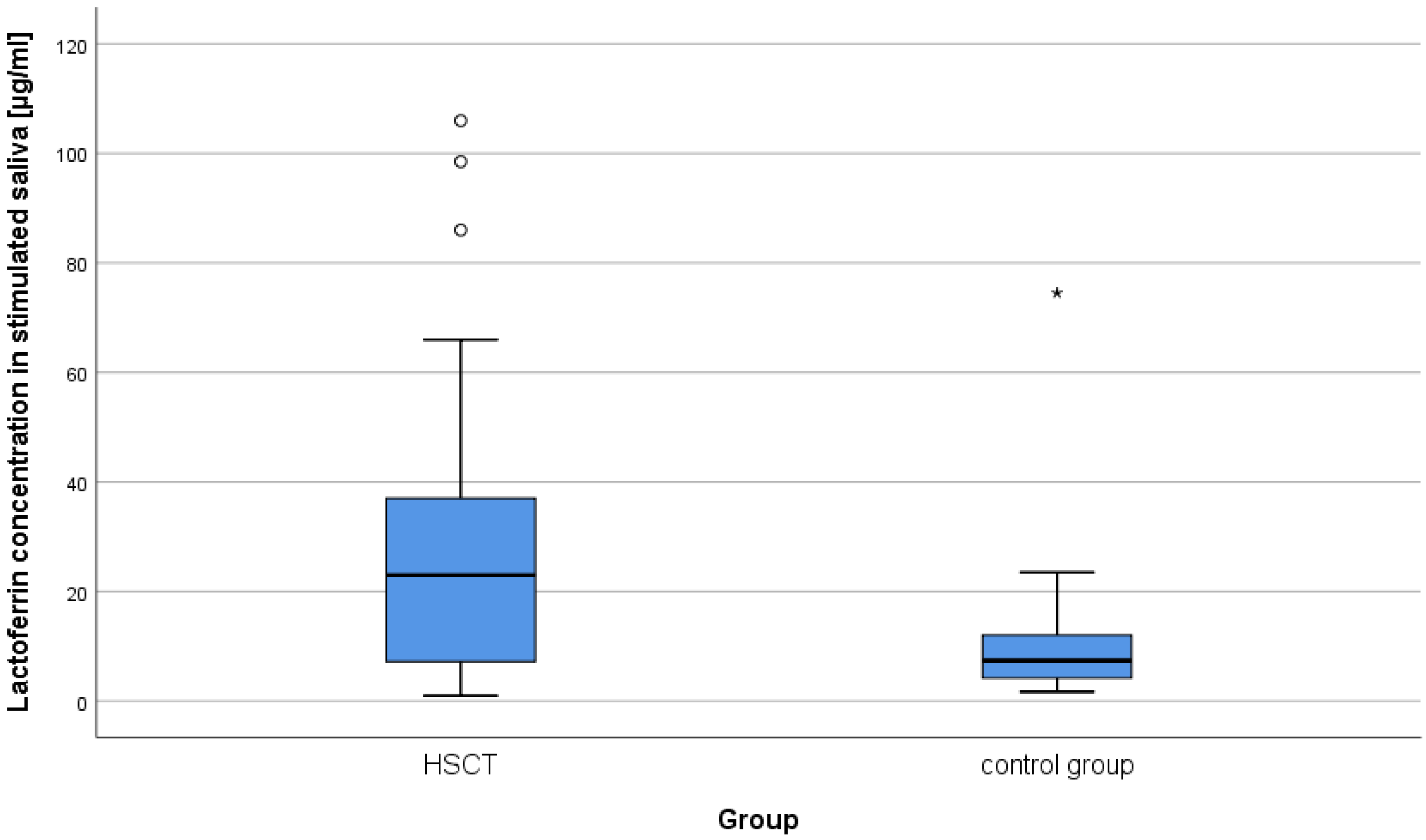
2.1. Concentration of Lysozyme, Lactoferrin, sIgA in Unstimulated and Stimulated Saliva in Patients after alloHSCT
2.2. Correlation between Lysozyme, Lactoferrin, sIgA Concentrations and the Time after alloHSCT
2.3. Correlation between Lysozyme, Lactoferrin, sIgA Concentrations, and the Unstimulated and Stimulated Saliva Flow Rate
2.4. Correlation between Lysozyme, Lactoferrin, sIgA Concentrations and S. mutans and L. acidophilus Titers
3. Discussion
4. Materials and Methods
4.1. Study Population
- -
- chemotherapy with autologous HSCT,
- -
- second alloHSCT,
- -
- any acute or chronic condition that would limit the ability of the patient to participate in the study,
- -
- smoking,
- -
- refusal to give informed consent,
- -
- failed saliva collection (severe hyposalivation, gag reflex during collection),
- -
- removable dental prostheses.
4.2. Assessment of the Rate of Salivation
4.3. Microbiological Assessment of Saliva
- (a)
- CRT Bacteria from Ivoclar-Vivadent (Lichtenstein) for culturing of S. mutans,
- (b)
- CRT Bacteria from Ivoclar-Vivadent (Lichtenstein) for culturing of L. acidophilus.
- (a)
- <105 CFU/mL of saliva: low colony count; (CFU—colony-forming units),
- (b)
- ≥105 CFU/mL of saliva: high colony count.
4.4. Determination of Concentration of Lysozyme, Lactoferrin, and sIgA in Mixed Resting and Stimulated Saliva
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752–777. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; de la Cámara, R.; Corbacioglu, S.; Dolstra, H.; Duarte, R.; Glass, B.; Greco, R.; et al. European Society for Blood and Marrow Transplantation (EBMT). Hematopoietic cell transplantation and cellular therapy survey of the EBMT: Monitoring of activities and trends over 30 years. Bone Marrow Transpl. 2021, 56, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Ogonek, J.; Kralj Juric, M.; Ghimire, S.; Varanasi, P.R.; Holler, E.; Greinix, H.; Weissinger, E. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2016, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Raber-Durlacher, J.E.; Brennan, M.T.; Saunders, D.P.; Mank, A.P.; Zadik, Y.; Quinn, B.; Epstein, J.B.; Blijlevens, N.M.A.; Waltimo, T.; et al. Basic oral care for hematology–oncology patients and hematopoietic stem cell transplantation recipients: A position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support. Care Cancer 2015, 23, 223–236. [Google Scholar] [PubMed]
- Barrach, R.H.; de Souza, M.P.; da Silva, D.P.; Lopez, P.S.; Montovani, J.C. Oral changes in individuals undergoing hematopoietic stem cell transplantation. Braz. J. Otorhinolaryngol. 2015, 81, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bogusławska-Kapała, A.; Kochańska, B.; Piekarska, A.; Cackowska-Lass, A.; Barwińska-Płużyńska, J.; Żółtowska, A. Subjective symptoms possibly associated with disorders of saliva secretion in patients in the late period after allogeneic hematopoietic cell transplantation. J. Stomatol. 2011, 64, 553–565. [Google Scholar]
- Van Leeuwen, J.M.; Potting, C.M.J.; Huysmans, M.-C.D.N.J.M.; Blijlevens, N.M.A. Salivary Changes before and after Hematopoietic Stem Cell Transplantation: A Systematic Review. Biol. Blood Marrow Transpl. 2019, 25, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.C.; Correa, M.E.P.; Tenuta, L.M.A.; Souza, C.A.; Vigorito, A.C. Post-allogeneic Hematopoietic Stem Cell Transplantation (HSCT) changes in inorganic salivary components. Support. Care Cancer 2015, 23, 2561–2567. [Google Scholar] [CrossRef]
- Chiusolo, P.; Giammarco, S.; Fanali, C.H.; Bellesi, S.; Metafuni, E.; Sica, S.; Iavarone, F.; Cabras, T.; Messana, I.; Leone, G.; et al. Salivary Proteomic Analysis and Acute Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2013, 19, 888–892. [Google Scholar] [CrossRef]
- Van Gennip, L.L.A.; Bulthuis, M.S.; Blijlevens, N.M.A.; Huysmans, M.-C.D.N.J.M.; van Leeuwen, S.J.M.; Thomas, R.Z. Caries, periodontitis and tooth loss after haematopoietic stem cell transplantation: A systematic review. Oral Dis. 2022, 29, 2578–2591. [Google Scholar] [CrossRef]
- Almståhl, A.; Wikström, M.; Groening, J. Lactoferrin, amylase and mucin MUC5B and their relation to the oral microflora in hyposalivation of different origins. Oral Microbiol. Immunol. 2001, 16, 345–352. [Google Scholar] [CrossRef]
- Bulthuis, M.S.; van Gennip, L.L.A.; Thomas, R.Z.; Bronkhorst, E.M.; Laheij, A.M.G.A.; Raber-Durlacher, J.E.; Rozema, F.R.; Brennan, M.T.; von Bültzingslöwen, I.; Blijlevens, N.M.A.; et al. Caries Progression after Haematopoietic Stem Cell Transplantation and the Role of Hyposalivation. Caries Res. 2022, 56, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef]
- Boer, C.C.; Correa, M.; Miranda, E.; de Souza, C.A. Taste disorders and oral evaluation in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transpl. 2010, 45, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Esser, P.; Mehnert, A.; Johansen, C.; Schwinn, A.; Schirmer, L.; Schulz-Kindermann, F.; Kruse, M.; Koch, U.; Zander, A.; et al. Depression and anxiety following hematopoietic stem cell transplantation: A prospective population-based study in Germany. Bone Marrow Transpl. 2017, 52, 1651–1657. [Google Scholar] [CrossRef]
- Inamoto, Y.; Pidala, J.; Chai, X.; Kurland, B.F.; Weisdorf, D.; Flowers, M.E.; Palmer, J.; Arai, S.; Jacobsohn, D.; Cutler, C.; et al. Chronic GVHD Consortium. Assessment of joint and fascia manifestations in chronic graft-versus-host disease. Arthritis Rheumatol. 2014, 66, 1044–1052. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Sava, G.; Benetti, A.; Ceschia, V.; Pacor, S. Lysozyme and cancer: Role of exogenous lysozyme as anticancer agent (review). Anticancer Res. 1989, 9, 583–591. [Google Scholar]
- Sun, H.; Chen, Y.; Zou, X.; Li, Q.; Li, H.; Shu, Y.; Li, X.; Li, W.; Han, L.; Ge, C. Salivary Secretory Immunoglobulin (SIgA) and Lysozyme in Malignant Tumor Patients. BioMed Res. Int. 2016, 2016, 8701423. [Google Scholar] [CrossRef]
- Bassim, C.W.; Ambatipudi, K.S.; Mays, J.W.; Edwards, D.A.; Swatkoski, S.; Fassil, H.; Baird, K.; Gucek, M.; Melvin, J.E.; Pavletic, S.Z. Quantitative Salivary Proteomic Differences in Oral Chronic Graft-versus-Host Disease. J. Clin. Immunol. 2012, 32, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Imanguli, M.M.; Atkinson, J.C.; Harveya, K.E.; Hoehn, G.T.; Hee Ryu, O.; Wud, T.; Kingmand, A.; Barrett, A.J.; Bishop, M.R.; Childse, R.W.; et al. Changes in salivary proteome following allogeneic hematopoietic stem cell transplantation. Exp. Hematol. 2007, 35, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Avivi, I.; Avraham, S.; Koren-Michowitz, M.; Zuckerman, T.; Aviv, A.; Ofran, Y.; Benyamini, N.; Nagler, A.; Rowe, J.M.; Nagler, R.M. Oral integrity and salivary profile in myeloma patients undergoing high-dose therapy followed by autologous SCT. Bone Marrow Transpl. 2009, 43, 801–806. [Google Scholar] [CrossRef]
- van Leeuwen, S.J.M.; Proctor, G.B.; Staes, A.; Laheij, A.M.G.A.; Potting, C.M.J.; Brennan, M.T.; von Bültzingslöwen, I.; Rozema, F.R.; Hazenberg, M.D.; Blijlevens, N.M.A.; et al. The salivary proteome in relation to oral mucositis in autologous hematopoietic stem cell transplantation recipients: A labelled and label-free proteomics approach. BMC Oral Health 2023, 23, 460. [Google Scholar] [CrossRef]
- Joel, T.J.; Suguna, S.S.; Steffi, S.R. Antimicrobial Activity of Lysozyme Against Oral Pathogens. Asian J. Pharm. Res. Health Care 2016, 8, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-K.; Dodds, M.W.J.; Zuo, P.; Johnson, D.A. A population-based study of salivary lysozyme concentrations and candidal count. Arch. Oral Biol. 1997, 42, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, M.; Sattari, M.; Kooshki, F.; Fotuhi, F.; Modarresi, M.; Sadrabad, Z.K.; Shadkar, M.S. Relationship of salivary lactoferrin and lysozyme concentrations with early childhood caries. J. Dent. Res. Dent. Clin. Dent. Prospect. 2015, 9, 109–114. [Google Scholar] [CrossRef]
- Felizardo, K.R.; Gonçalves, R.B.; Schwarcz, W.D.; Poli-Frederico, R.C.; Maciel, S.M.; de Andrade, F.B. An evaluation of the expression profiles of salivary proteins lactoferrin and lysozyme and their association with caries experience and activity. Rev. Odonto Ciênc 2010, 25, 344–349. [Google Scholar] [CrossRef]
- Tenovuo, J. Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme and lactoferrin in xerostomia: Efficacy and safety. Oral Dis. 2002, 8, 23–29. [Google Scholar] [CrossRef]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary Defense Proteins: Their Network and Role in Innate and Acquired Oral Immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Piątkowski, J.; Kościelniak, D.; Gregorczyk-Maga, I.; Kołodziej, I.; Papież, M.A.; Olczak-Kowalczyk, D. Effect of histatin-5 and lysozyme on the ability of Streptococcus mutans to form biofilms in in vitro conditions. Postep. Hig. Med. Dosw. 2015, 20, 1056–1066. [Google Scholar]
- Koch, A.L. Bacterial wall as target for attack: Past, present, and future research. Clin. Microbiol. Rev. 2003, 16, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Catic, T.; Catic, M.; Mehic, A.; Lokvančić, A.; Kapo, B. Lysozyme—Enzybiotic as a potential solution of the global problem of antibiotic resistance. Farm. Glas. 2017, 3, 273–288. [Google Scholar]
- Eminagić, D.; Lokvančić, A.; Hasanbegović, B.; Mekić-Abazović, A.; Avidičević, A.; Marijanović, I.; Janković, S.M.; Kapo, B. Efficacy and safety of local lysozyme treatment in patientswith oral mucositis after chemotherapy and radiotherapy. Acta Pharm. 2019, 69, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Farnaud, S.; Evans, R.W. Lactoferrin—A multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer effects of lactoferrin: Underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef]
- Keskin, M.; Kompuinen, J.; Harmankaya, I.; Karaçetin, D.; Nissilä, V.; Gürsoy, M.; Sorsa, T.; Gürsoy, K. Oral Cavity Calprotectin and Lactoferrin Levels in Relation to Radiotherapy. Curr. Issues Mol. Biol. 2022, 44, 4439–4446. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral properties of lactoferrin—A natural immunity molecule. Molecules 2011, 16, 6992–7012. [Google Scholar] [CrossRef]
- Arnold, R.R.; Brewer, M.; Gauthier, J.J. Bactericidal activity of human lactoferrin: Sensitivity of a variety of microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [CrossRef]
- Sikorska, M.; Mielnik-Błaszczak, M.; Kapeć, E. The relationship between the levels of SigA, lactoferrin and α1 proteinase isnhibitor in saliva and permanent dentition caries in 15-years-olds. Oral Microbiol. Immunol. 2002, 17, 272–276. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Ann. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Van’T Hof, W.; Veerman, E.C.I.; Nieuw-Amerongen, A.V.; Ligtenberg, A.J.M. Antimicrobial defense systems in saliva. Monogr. Oral Sci. 2014, 24, 40–51. [Google Scholar] [PubMed]
- Corthésy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Seemann, R.; Hägewald, S.J.; Sztankay, V.; Drews, J.; Bizhang, M.; Kage, A. Levels of parotid and submandibular/sublingual salivary immunoglobulin A in response to experimental gingivitis in humans. Clin Oral Investig. 2004, 8, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Childers, N.K.; Greenleaf, C.; Li, F.; Dasanayake, A.P.; Powell, W.D.; Michalek, S.M. Effect of age on immunoglobulin A subclass distribution in human parotid saliva. Oral Microbiol. Immunol. 2003, 18, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Abey, S.K.; Yuana, Y.; Joseph, P.V.; Kenea, N.D.; Fourie, N.H.; Sherwin, L.B.; Gonye, G.E.; Smyser, P.A.; Stempinski, E.S.; Boulineaux, C.M.; et al. Lysozyme association with circulating RNA, extracellular vesicles, and chronic stress. BBA Clin. 2017, 7, 23–35. [Google Scholar] [CrossRef]
- Rathnayake, N.; Akerman, S.; Klinge, B.; Lundegren, N.; Jansson, H.; Tryselius, Y.; Sorsa, T.; Gustafsson, A. Salivary biomarkers for detection of systemic diseases. PLoS ONE 2013, 8, e61356. [Google Scholar] [CrossRef]
- Main, B.E.; Calman, K.C.; Ferguson, M.M. The effects of cytotoxic therapy on saliva and oral flora. Oral Surg. Oral Med. Oral Pathol. 1984, 58, 545–548. [Google Scholar] [CrossRef]
- Tonguc Altin, K.; Topcuoglu, N.; Duman, G.; Unsal, M.; Celik, A.; Senem Selvi, K.; Ezgi, K.; Fikrettin, S.; Guven, K. Antibacterial effects of saliva substitutes containing lysozyme or lactoferrin against Streptococcus mutans. Arch. Oral Biol. 2021, 129, 105183. [Google Scholar] [CrossRef]
- Eliasson, L.; Almståhl, A.; Lingström, P.; Wikström, M.; Carlen, A. Minor gland saliva flow rate and proteins in subjects with hyposalivation due to Sjögren’s syndrome and radiation therapy. Arch. Oral Biol. 2005, 50, 293–299. [Google Scholar] [CrossRef]
- Groennik, J.; Walgreen-Weterings, E.; Nami, K.; Bolsher, J.G.M.; Veerman, E.C.I.; van Vlkenhof, A.J.; Nieuw-Amerongen, A.V. Salivary lactoferrin and low-Mr, mucin MG2 in Actinobacillus actinomycetemcomitans- associated peiodontitis. J. Clin. Periodontol. 1996, 26, 269–275. [Google Scholar] [CrossRef]
- Dodds, M.; Johnson, D.A.; Yeh, C.K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Chrustowicz, Ł.; Brzezińska-Błaszczyk, E. The role of salivary IgA in oral conditions—Review of literature. J. Stomatol. 2006, 7, 518–524. [Google Scholar]
- Eliasson, L.; Carlen, A. An update on minor salivary gland secretions. Eur. J. Oral Sci. 2010, 118, 435–442. [Google Scholar] [CrossRef]
- Van Nieuw Amerongen, A.; Veerman, E.C.I. Saliva—The defender of the oral cavity. Oral Dis. 2002, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Ueki, S.; Shimanuki, H.; Kasai, T.; Takato, J.; Ozaki, H.; Kawakami, Y.; Haga, H. Effects of low—Intensity physical exercise on acute changes in resting saliva secretory IgA levels in the eldery. Geriatr. Gerontol. Int. 2005, 5, 202–206. [Google Scholar] [CrossRef]
- Sistig, S.; Vučićević-Boras, V.; Lukač, J.; Kusić, Z. Salivary IgA and IgG subclasses in oral mucosal diseases. Oral Dis. 2002, 8, 282–286. [Google Scholar] [CrossRef]
- Bachanek, T.; Drop, B.; Jarmolińska, K.; Wolańska, E. The saliva immunoglobulin level in patients with diseases of leukocytic system (a preliminary report). Ann. Univ. Lub. 2006, 28, 161–165. [Google Scholar]
- Nikifrarjam, J.; Pourpak, Z.; Shahrabi, M.; Kouhkan, A.; Moazeni, M.; Aghamohammadi, A. Oral manifestations in selective IgA deficiency. Int. J. Dent. Hig. 2004, 2, 19–25. [Google Scholar] [CrossRef]
- Aufricht, C.; Tenner, W.; Salzer, H.R.; Hoss, A.E.; Wurst, E.; Herkner, K. Salivary IgA Concentration is Influenced by the Saliva Collection Method. Eur. J. Clin. Chem. Clin. Biochem. 1992, 30, 81–83. [Google Scholar]
- Funegård, U.; Franzén, L.; Ericson, T.; Henriksson, R. Parotid saliva composition during and after irradiation of head and neck cancer. Eur. J. Cancer B Oral Oncol. 1994, 30, 230–233. [Google Scholar] [CrossRef]
- Steinbrenner, M.; Hafer, R.; Gruhn, B.; Muller, A.; Fuchs, D.; Hermann, J.; Zintl, F. T-cell independent production of salivary secretory IgA after hematopoietic stem cell transplantation in children. Oral Microbilol Immunol. 2005, 20, 282–288. [Google Scholar] [CrossRef]
- Wu, Z.; Gong, Y.; Wang, C.; Lin, J.; Zhao, J. Association between salivary s-IgA concentration and dental caries: An updated meta-analysis. Biosci. Rep. 2020, 40, BSR20203208. [Google Scholar] [CrossRef]
- Zalewska, A.; Waszkiel, D.; Błahuszewska, K.; Marciniak, J.; Kowalczyk, A. Buffer capacity, pH, and immunoglobulin A concentration in whole resting saliva in caries resistant or susceptible young patients. Czas. Stomatol. 2006, 4, 221–226. [Google Scholar]
- Cole, M.F.; Arnold, R.R.; Rhodes, M.J. Immune Disfunction and Dental Caries: A Preliminary Report. J. Dent. Res. 1977, 56, 198–204. [Google Scholar] [CrossRef]
- Navazesh, M.; Kumar, S.K.S. Measuring salivary flow: Challenges and opportunities. JADA 2008, 139, 35–40. [Google Scholar]
- Field, E.A.; Longmann, L.P.; Bucknall, R.; Kaye, S.B.; Higham, S.; Edgar, W.M. The establishment of xerostomia clinic: A prospective study. Br. J. Oral Maxiofacial Surg. 1997, 35, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed]
| Selected Components of Saliva | Type of Saliva | alloHSCT (n = 54) | Control Group (n = 30) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Me | M | SD | Me | U | Z | p | r | ||
| Lysozyme | unstimulated | 13.85 | 17.03 | 9.28 | 13.46 | 10.24 | 11.65 | 757.5 | −0.49 | 0.624 | 0.05 |
| stimulated | 18.32 | 26.59 | 10.05 | 11.55 | 8.24 | 9.03 | 726.0 | −0.78 | 0.433 | 0.09 | |
| Lactoferrin | unstimulated | 40.18 | 39.83 | 23.45 | 17.58 | 28.99 | 9.65 | 417.5 | −3.66 | <0.001 | 0.40 |
| stimulated | 27.33 | 24.70 | 23 | 10.69 | 13.20 | 7.4 | 431.5 | −3.53 | <0.001 | 0.39 | |
| sIgA | unstimulated | 512.53 | 808.54 | 256.05 | 286.21 | 172.36 | 261.5 | 735.0 | −0.70 | 0.484 | 0.08 |
| stimulated | 282.66 | 521.69 | 142.49 | 165.20 | 94.27 | 155 | 800.0 | −0.09 | 0.926 | 0.01 | |
| Selected Components of Saliva | Type of Saliva | Time after alloHSCT | |
|---|---|---|---|
| Lysozyme | unstimulated | Spearman’s ρ | −0.12 |
| significance | −0.388 | ||
| stimulated | Spearman’s ρ | −0.02 | |
| significance | 0.888 | ||
| Lactoferrin | unstimulated | Spearman’s ρ | 0.01 |
| significance | 0.957 | ||
| stimulated | Spearman’s ρ | −0.01 | |
| significance | 0.923 | ||
| sIgA | unstimulated | Spearman’s ρ | 0.10 |
| significance | 0.455 | ||
| stimulated | Spearman’s ρ | 0.09 | |
| significance | 0.502 |
| Selected Components of Saliva | Unstimulated Saliva Flow Rate | Stimulated Saliva Flow Rate | |||
|---|---|---|---|---|---|
| alloHSCT (n = 54) | Control Group (n = 30) | alloHSCT (n = 54) | Control Group (n = 30) | ||
| Lysozyme | Spearman’s ρ | 0.05 | 0.09 | −0.04 | −0.11 |
| significance | 0.708 | 0.628 | 0.771 | 0.580 | |
| Lactoferrin | Spearman’s ρ | −0.41 | −0.38 | −0.43 | −0.21 |
| significance | 0.002 | 0.036 | 0.001 | 0.272 | |
| sIgA | Spearman’s ρ | −0.42 | −0.41 | −0.50 | −0.08 |
| significance | 0.001 | 0.025 | <0.001 | 0.656 | |
| Selected Components of Saliva | S. mutans Count | L. acidophilus Count | |||
|---|---|---|---|---|---|
| alloHSCT (n = 54) | Control Group (n = 30) | alloHSCT (n = 54) | Control Group (n = 30) | ||
| Lysozyme | Spearman’s ρ | 0.32 | 0.22 | −0.10 | −0.09 |
| significance | 0.017 | 0.245 | 0.479 | 0.649 | |
| Lactoferrin | Spearman’s ρ | 0.05 | 0.35 | 0.18 | 0.20 |
| significance | 0.729 | 0.060 | 0.200 | 0.291 | |
| sIgA | Spearman’s ρ | 0.31 | 0.46 | 0.01 | 0.04 |
| significance | 0.021 | 0.011 | 0.940 | 0.848 | |
| Characteristics | Number |
|---|---|
| Patient age, median (range) | 39.5 (19–67) |
| Women | 24 |
| Men | 30 |
| Time after alloHSCT day 0 (months) | 3–96 |
| Patients with cGvHD | 36 |
| cGvHD duration (months) | 9.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodzikowska, A.; Kochańska, B.; Bogusławska-Kapała, A.; Strużycka, I.; Górski, B.; Miskiewicz, A. Assessment of the Salivary Concentrations of Selected Immunological Components in Adult Patients in the Late Period after Allogeneic Hematopoietic Stem Cell Transplantation—A Translational Study. Int. J. Mol. Sci. 2024, 25, 1457. https://doi.org/10.3390/ijms25031457
Brodzikowska A, Kochańska B, Bogusławska-Kapała A, Strużycka I, Górski B, Miskiewicz A. Assessment of the Salivary Concentrations of Selected Immunological Components in Adult Patients in the Late Period after Allogeneic Hematopoietic Stem Cell Transplantation—A Translational Study. International Journal of Molecular Sciences. 2024; 25(3):1457. https://doi.org/10.3390/ijms25031457
Chicago/Turabian StyleBrodzikowska, Aniela, Barbara Kochańska, Agnieszka Bogusławska-Kapała, Izabela Strużycka, Bartłomiej Górski, and Andrzej Miskiewicz. 2024. "Assessment of the Salivary Concentrations of Selected Immunological Components in Adult Patients in the Late Period after Allogeneic Hematopoietic Stem Cell Transplantation—A Translational Study" International Journal of Molecular Sciences 25, no. 3: 1457. https://doi.org/10.3390/ijms25031457






