Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Meta-Analysis
3. Results
3.1. Quality Assessment of Included Studies
3.2. Characteristics of the Included Studies and Populations
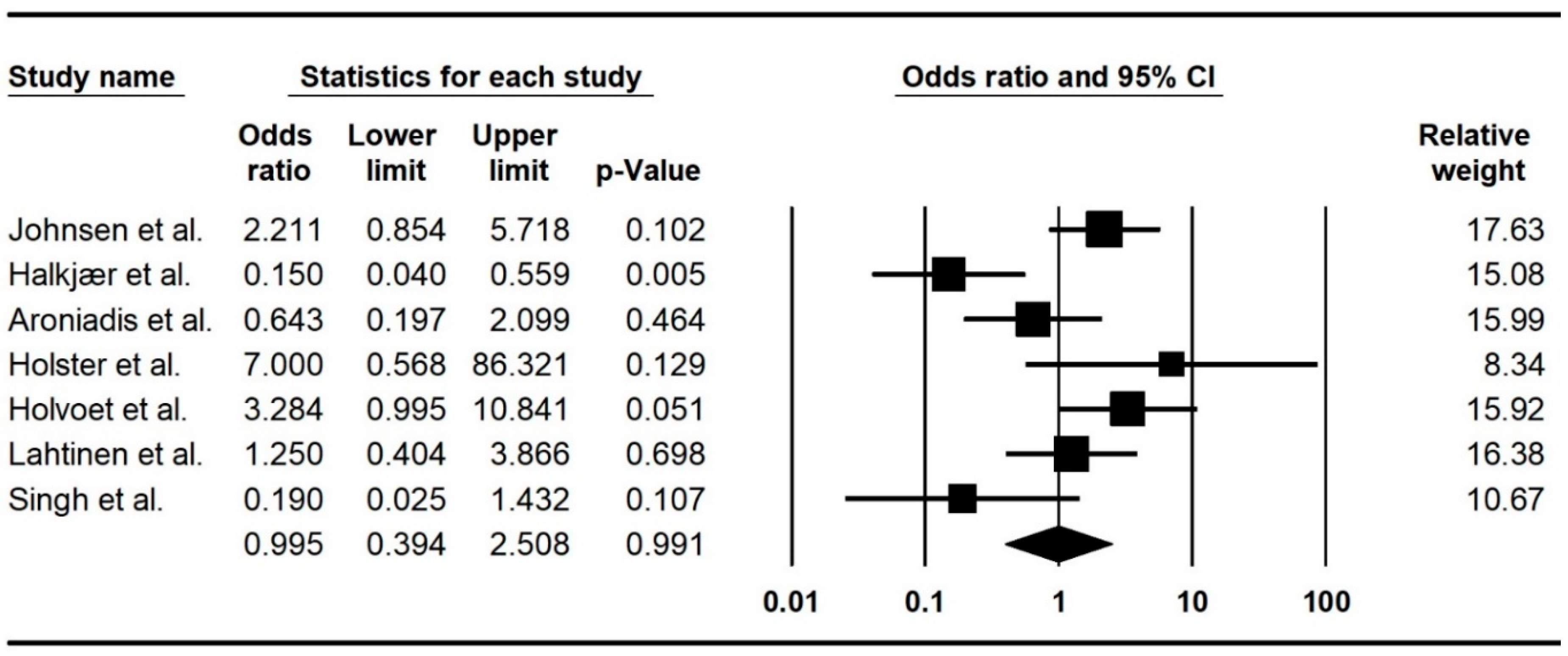
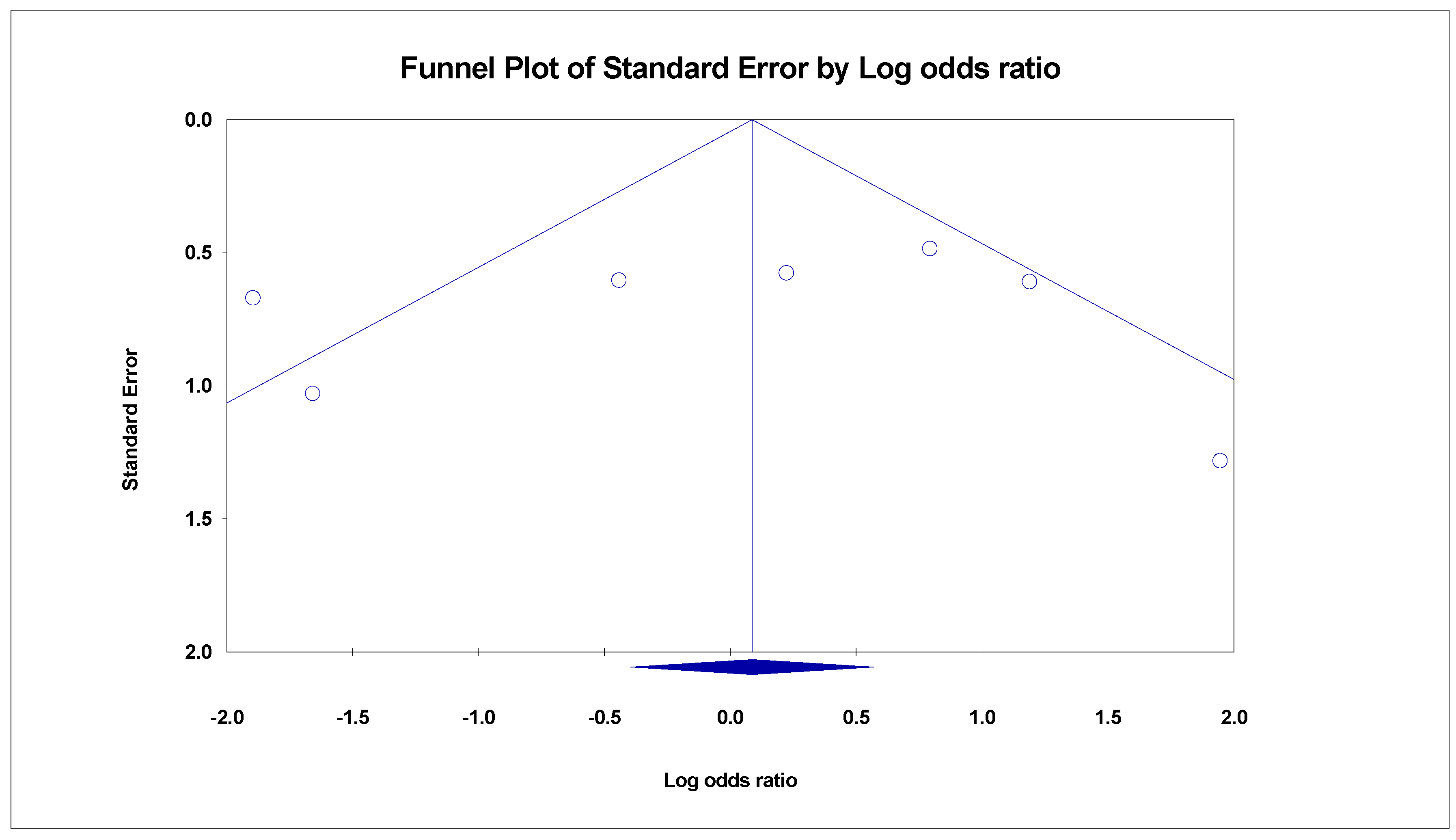
3.3. Meta-Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Masuy, I.; Pannemans, J.; Tack, J. Irritable bowel syndrome: Diagnosis and management. Minerva Gastroenterol. Dietol. 2020, 66, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Wood, E.; Ruffle, J.K. An approach to the care of patients with irritable bowel syndrome. CMAJ 2020, 192, E275–E282. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Quigley, E.M. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109 (Suppl. S1), S2–S26. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Prather, C.M. The irritable bowel syndrome: Mechanisms and a practical approach to management. Ann. Intern. Med. 1992, 116, 1001–1008. [Google Scholar] [CrossRef]
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable Bowel Syndrome: A Clinical Review. JAMA 2015, 313, 949–958. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front. Microbiol. 2022, 13, 825338. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Farsi, Y.; Tahvildari, A.; Arbabi, M.; Vazife, F.; Sechi, L.A.; Shahidi Bonjar, A.H.; Jamshidi, P.; Nasiri, M.J.; Mirsaeidi, M. Diagnostic, Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review. Front. Cell. Infect. Microbiol. 2022, 12, 182. [Google Scholar] [CrossRef]
- Jamshidi, P.; Hasanzadeh, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Mota, J.F.; Sechi, L.A.; Nasiri, M.J. Is there any association between gut microbiota and type 1 diabetes? A systematic review. Gut Pathog. 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The long-term stability of the human gut microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain–Gut Microbiome Interactions and Functional Bowel Disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Muniz Pedrogo, D.A.; Kashyap, P.C. Irritable bowel syndrome: A gut microbiota-related disorder? Am. J. Physiology. Gastrointest. Liver Physiol. 2017, 312, G52–G62. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Quigley, E.M.; Öhman, L.; Simrén, M.; O’Toole, P.W. The microbiota link to irritable bowel syndrome: An emerging story. Gut Microbes 2012, 3, 572–576. [Google Scholar] [CrossRef]
- Salonen, A.; de Vos, W.M.; Palva, A. Gastrointestinal microbiota in irritable bowel syndrome: Present state and perspectives. Microbiology 2010, 156, 3205–3215. [Google Scholar] [CrossRef]
- Hatamnejad, M.R.; Baradaran Ghavami, S.; Shirvani, M.; Asghari Ahmadabad, M.; Shahrokh, S.; Farmani, M.; Sherkat, G.; Asadzadeh Aghdaei, H.; Zali, M.R. Selective serotonin reuptake inhibitors and inflammatory bowel disease; Beneficial or malpractice. Front. Immunol. 2022, 13, 980189. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R.; Mullin, G.E. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2020, 120, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.F.; Lied, G.A. Gut microbiota and therapeutic approaches for dysbiosis in irritable bowel syndrome: Recent developments and future perspectives. Turk. J. Med. Sci. 2020, 50, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Alm, E.J.; Kelley, J.M.; Cheng, V.; Smith, M.; Kassam, Z.; Nee, J.; Iturrino, J.; Lembo, A. Effect of antibiotic pretreatment on bacterial engraftment after Fecal Microbiota Transplant (FMT) in IBS-D. Gut Microbes 2022, 14, 2020067. [Google Scholar] [CrossRef]
- Guo, Q.; Lin, H.; Chen, P.; Tan, S.; Wen, Z.; Lin, L.; He, J.; Wen, J.; Lu, S. Dynamic changes of intestinal flora in patients with irritable bowel syndrome combined with anxiety and depression after oral administration of enterobacteria capsules. Bioengineered 2021, 12, 11885–11897. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef]
- Waller, K.M.J.; Leong, R.W.; Paramsothy, S. An update on fecal microbiota transplantation for the treatment of gastrointestinal diseases. J. Gastroenterol. Hepatol. 2022, 37, 246–255. [Google Scholar] [CrossRef]
- Green, J.E.; Davis, J.A.; Berk, M.; Hair, C.; Loughman, A.; Castle, D.; Athan, E.; Nierenberg, A.A.; Cryan, J.F.; Jacka, F.; et al. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: A systematic review and meta-analysis. Gut Microbes 2020, 12, 1854640. [Google Scholar] [CrossRef]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar]
- Borody, T.J.; Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 88–96. [Google Scholar] [CrossRef]
- Ademe, M. Benefits of fecal microbiota transplantation: A comprehensive review. J. Infect. Dev. Ctries. 2020, 14, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Seo, G.S. Fecal Microbiota Transplantation: Is It Safe? Clin. Endosc. 2021, 54, 157–160. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients with Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J.; Oneto, C.; Feuerstadt, P.; Sherman, A.; Wolkoff, A.W.; Kassam, Z.; Sadovsky, R.G.; Elliott, R.J.; Budree, S.; et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: A double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 675–685. [Google Scholar] [CrossRef]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.; Günther, S.; Hansen, L.H.; Petersen, A.M. Fecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomized, double-blind placebo controlled study. United Eur. Gastroenterol. J. 2018, 67, 2107–2115. [Google Scholar] [CrossRef]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.J.; Tillonen, J.; Satokari, R.; et al. Randomised clinical trial: Faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef]
- Holster, S.; Lindqvist, C.M.; Repsilber, D.; Salonen, A.; De Vos, W.M.; Konig, J.; Brummer, R.J. The effect of allogenic versus autologous fecal microbiota transfer on symptoms, visceral perception and fecal and mucosal microbiota in irritable bowel syndrome: A randomized controlled study. Clin. Transl. Gastroenterol. 2019, 10, e00034. [Google Scholar] [CrossRef]
- Elhusein, A.M.; Fadlalmola, H.A. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome Patients: An Updated Systematic Review and Meta-Analysis. Gastroenterol. Nurs. 2022, 45, 11–20. [Google Scholar] [CrossRef]
- Ianiro, G.; Eusebi, L.H.; Black, C.J.; Gasbarrini, A.; Cammarota, G.; Ford, A.C. Systematic review with meta-analysis: Efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 240–248. [Google Scholar] [CrossRef]
- Mohan, B.P.; Loganathan, P.; Khan, S.R.; Garg, G.; Muthusamy, A.; Ponnada, S.; Pasam, R.T.; Chandan, S.; Tuteja, A. Fecal microbiota transplant delivered via invasive routes in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Indian J. Gastroenterol. 2023, 42, 315–323. [Google Scholar] [CrossRef]
- Myneedu, K.; Deoker, A.; Schmulson, M.J.; Bashashati, M. Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Rodrigues Fialho, S.; Araújo, J.R.; Rocha, R.; Moreira-Rosário, A. Procedures in Fecal Microbiota Transplantation for Treating Irritable Bowel Syndrome: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1725. [Google Scholar] [CrossRef]
- Wu, J.; Lv, L.; Wang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2022, 12, 827395. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, V.L.; Steiner, C.A.; Berinstein, J.A.; Eswaran, S.; Waljee, A.K.; Higgins, P.D.R.; Owyang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Barberio, B.; Houghton, L.A.; Yiannakou, Y.; Savarino, E.V.; Black, C.J.; Ford, A.C. Symptom Stability in Rome IV vs. Rome III Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 362–371. [Google Scholar] [CrossRef]
- Goodoory, V.C.; Houghton, L.A.; Yiannakou, Y.; Black, C.J.; Ford, A.C. Natural History and Disease Impact of Rome IV Vs Rome III Irritable Bowel Syndrome: A Longitudinal Follow-Up Study. Clin. Gastroenterol. Hepatol. 2022, 20, 569–577. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hausken, T.; Hatlebakk, J.G. Increasing the dose and/or repeating faecal microbiota transplantation (FMT) increases the response in patients with irritable bowel syndrome (IBS). Nutrients 2019, 11, 1415. [Google Scholar] [CrossRef]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef]
- Goll, R.; Johnsen, P.H.; Hjerde, E.; Diab, J.; Valle, P.C.; Hilpusch, F.; Cavanagh, J.P. Effects of fecal microbiota transplantation in subjects with irritable bowel syndrome are mirrored by changes in gut microbiome. Gut Microbes 2020, 12, 1794263. [Google Scholar] [CrossRef]
- Ghaffari, P.; Shoaie, S.; Nielsen, L.K. Irritable bowel syndrome and microbiome; Switching from conventional diagnosis and therapies to personalized interventions. J. Transl. Med. 2022, 20, 173. [Google Scholar] [CrossRef]
- Singh, P.; Lembo, A. Emerging Role of the Gut Microbiome in Irritable Bowel Syndrome. Gastroenterol. Clin. N. Am. 2021, 50, 523–545. [Google Scholar] [CrossRef]
- Ivashkin, V.; Poluektov, Y.; Kogan, E.; Shifrin, O.; Sheptulin, A.; Kovaleva, A.; Kurbatova, A.; Krasnov, G.; Poluektova, E. Disruption of the pro-inflammatory, anti-inflammatory cytokines and tight junction proteins expression, associated with changes of the composition of the gut microbiota in patients with irritable bowel syndrome. PLoS ONE 2021, 16, e0252930. [Google Scholar] [CrossRef]
- Chlebicz-Wójcik, A.; Śliżewska, K. Probiotics, Prebiotics, and Synbiotics in the Irritable Bowel Syndrome Treatment: A Review. Biomolecules 2021, 11, 1154. [Google Scholar] [CrossRef]
- Xie, C.R.; Tang, B.; Shi, Y.Z.; Peng, W.Y.; Ye, K.; Tao, Q.F.; Yu, S.G.; Zheng, H.; Chen, M. Low FODMAP Diet and Probiotics in Irritable Bowel Syndrome: A Systematic Review with Network Meta-analysis. Front. Pharmacol. 2022, 13, 853011. [Google Scholar] [CrossRef]
- El-Salhy, M.; Mazzawi, T. Fecal microbiota transplantation for managing irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 439–445. [Google Scholar] [CrossRef]
- Asha, M.Z.; Khalil, S.F.H. Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ. Med. J. 2020, 20, e13–e24. [Google Scholar] [CrossRef] [PubMed]
- Wollny, T.; Daniluk, T.; Piktel, E.; Wnorowska, U.; Bukłaha, A.; Głuszek, K.; Durnaś, B.; Bucki, R. Targeting the gut microbiota to relieve the symptoms of irritable bowel syndrome. Pathogens 2021, 10, 1545. [Google Scholar] [CrossRef]
- Xu, H.; Ma, C.; Zhao, F.; Chen, P.; Liu, Y.; Sun, Z.; Cui, L.; Kwok, L.Y.; Zhang, H. Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Eur. J. Nutr. 2021, 60, 2553–2565. [Google Scholar] [CrossRef] [PubMed]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kanarek, E.; Sowińska, A.; Cukrowska, B. The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients 2021, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Chen, H.T.; Luo, Q.L.; Xu, H.M.; He, J.; Li, Y.Q.; Zhou, Y.L.; Yao, F.; Nie, Y.Q.; Zhou, Y.J. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota. J. Dig. Dis. 2019, 20, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Lin, Z.; Tian, H.; Yang, B.; Zhao, D.; Ye, C.; Li, N.; Qin, H.; Chen, Q. Long-Term Follow-Up Results of Fecal Microbiota Transplantation for Irritable Bowel Syndrome: A Single-Center, Retrospective Study. Front. Med. 2021, 8, 710452. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; El-Salhy, M. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand. J. Gastroenterol. 2022, 57, 792–796. [Google Scholar] [CrossRef]
- Gulati, M.; Singh, S.K.; Corrie, L.; Kaur, I.P.; Chandwani, L. Delivery routes for faecal microbiota transplants: Available, anticipated and aspired. Pharmacol. Res. 2020, 159, 104954. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.-J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule–vs. Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Surawicz, C.M. Fecal Microbiota Transplantation. Gastroenterol. Clin. N. Am. 2017, 46, 171–185. [Google Scholar] [CrossRef]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment. Pharmacol. Ther. 2021, 53, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.; Wilson, D.J.; Feil, E.; Eyre-Walker, A. The distribution of bacterial doubling times in the wild. Proceedings. Biol. Sci. 2018, 285, 20180789. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium difficile Infection with Fecal Microbiota Transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Kuo, C.-H.; Kuo, F.-C.; Wang, Y.-K.; Hsu, W.-H.; Yu, F.-J.; Hu, H.-M.; Hsu, P.-I.; Wang, J.-Y.; Wu, D.-C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef]

| Author | Random SequenceGeneration | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete OutcomeData | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| Johnsen et al. [34] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Halkjær et al. [37] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Aroniadis et al. [36] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Holster et al. [39] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Holvoet et al. [35] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lahtinen et al. [38] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Singh et al. [24] | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk |
| First Author | Year of Publication | Type of Study | Country | Study Population | Gender Male/Female | Mean Age (Years) | Comorbidities | Co-Medications | Diet Modification | IBS Criteria | IBS Subtype | IBS Severity * | Donor Selection Criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Johnsen et al. [34] | 2017 | Randomized, double-blinded, controlled clinical study | Norway | 83 | 28/55 | 44.33 | Fibromyalgia, chronic fatigue syndrome, jaw pain, pelvic pain, anxiety, and depression | NA | Low FODMAPs | Rome III | IBS-D, IBS-M | ≥175 | Exclusion criteria: Use of antibiotics in the past 3 months; new tattoos or piercings in the past 3 months; high-risk sexual behaviors; former imprisonment; or history of any of the following conditions: chronic diarrhea, constipation, inflammatory bowel disease, IBS, colorectal polyps or cancer, immunosuppression, obesity, metabolic syndrome, atopic skin disease, or chronic fatigue. Tests for parasites, ova, and cysts; Salmonella spp., Shigella spp., Campylobacter spp., Yersinia spp., and toxin-producing C-difficile; fecal tests for Helicobacter pylori antigen, viruses (Norovirus, Rotavirus, Sapovirus, Adenovirus), calprotectin, and occult blood; blood samples for glycated hemoglobin; and serology for HIV, Treponema pallidum, and hepatitis A, B, and C. |
| Halkjær et al. [37] | 2018 | Randomized, double-blinded, controlled clinical study | Denmark | 46 | 16/30 | 36.39 | Asthma, allergies | PPI | NA | Rome III | IBS-D, IBS-M, IBS-C | ≥175 | Inclusion criteria: Aged between 18 and 45 years; previously and currently healthy; normal weight (body mass index (BMI) between 18.5 and 24.9 kg/m2); normal bowel movements (defined as 1–2 per day and type 3–4 at Bristol Stool Form Scale); no medication consumption. Exclusion criteria: Known or high risk of infectious diseases such as HIV, HAV, HBV or HCV; positive stool sample for C. difficile toxin, parasites or other enteropathogens; antibiotic treatment in the past 6 months; abuse of alcohol or drugs; smoking; tattoo or body piercing within the last 6 months; allergy, asthma or eczema; family history of GI diseases, cancer, diabetes, obesity, autoimmune diseases, allergy, asthma, eczema, cardiovascular diseases, neurologic or mental illnesses; participation in high-risk sexual behaviors; born by caesarean section. |
| Aroniadis et al. [36] | 2019 | Randomized, double-blinded, controlled clinical study | USA | 48 | 30/18 | 37.3 | NA | NA | NA | Rome III | IBS-D | ≥175 | NA (A non-profit stool bank (OpenBiome, Somerville, MA, USA)) |
| Holster et al. [39] | 2019 | Randomized, double-blinded, controlled clinical study | Sweden | 16 | 8/8 | 36.5 | NA | Participants were asked to keep their medication stable | Participants were asked to keep their diet stable | Rome III | IBS-D, IBS-M, IBS-C | ≥175 | Exclusion criteria: Current communicable diseases; known organic gastrointestinal disease; gastrointestinal malignancy or polyposis, history of major gastrointestinal surgery; eosinophilic disorders of the gastrointestinal tract; known or high risk of infectious diseases such as HIV or hepatitis; non-gastrointestinal malignancy; dementia, severe depression or major psychiatric disorder; metabolic syndrome; autoimmune diseases; allergies; chronic pain syndromes; severe or morbid obesity; pregnancy or breast-feeding; use of immunosuppressive or chemotherapy agents; antimicrobial treatment within last 6 months; abuse of alcohol or drugs; tattoo or body piercing obtained within the 6 months before screening; high-risk sexual behaviors; travelling to areas with endemic diarrhea during 3 months before screening; positive stool tests for Clostridium difficile toxin, enteral pathogens (Salmonella, Shigella, enteroinvasive E. coli, Campylobacter, enterohaemorrhagic E. coli, enterotoxigenic E. coli, Yersinia enterocolitica, Vibrio, and Plesiomonas shigelloides), ova, parasites, Giardia antigen, Cryptosporidium antigen; positive blood tests for HIV, Hepatitis A, B, or C. |
| Holvoet et al. [35] | 2020 | Randomized, double-blinded, controlled clinical study | Belgium | 62 | 24/38 | 38.7 | NA | NA | NA | Rome III | IBS-D, IBS-M | ≥175 | Inclusion criteria: Being in good overall condition, between 18 and 65 years of age, with normal, regular bowel movements and no gastrointestinal symptoms. Exclusion criteria: Body mass index (BMI) > 30, antibiotic use in the past 6 months, chronic disease or abnormal screening results. Donors were subjected to a clinical examination at the start of the trial and were screened for various transmittable diseases at six-month intervals. Screening tests included testing for hepatitis A, B, C and E, HIV-1 and 2 and Treponema pallidum; stool culture for the presence of Salmonella spp., Shigella spp., Yersinia enterocolitica, Yersinia pseudotuberculosis, Campylobacter spp., Clostridioides difficile and Aeromonas spp. Additionally, specific screening for antibiotic-resistant strains was performed using the active detection of carbapenemase-producing Enterobacterales (CPE) and extended spectrum beta-lactamase (ESBL) producing organisms. Microscopic examination was performed to confirm the absence of eggs, cysts and/or larvae of parasites, and the presence of Clostridioides difficile toxins A and B was screened using an enzyme immuno-assay |
| Lahtinen et al. [38] | 2020 | Randomized, double-blinded, controlled clinical study | Finland | 49 | 20/29 | 46.76 | NA | NA | NA | Rome III | IBS-D, IBS-M | ≥175 | Inclusion criteria: Being in good general health and normal weight; delivered through vaginal childbirth; not having antibiotics during the previous year; not being a health care worker. Exclusion criteria: Having any long-term diagnoses; using any permanent medications; a history of high-risk sexual behavior, use of illicit drugs or recently travelled to areas with a high incidence of infectious diarrhea. The donors were screened with the following diagnostic tests: HIV; hepatitis A, B and C; culture of fecal bacterial pathogens (Salmonella, Yersinia and Campylobacter) and antibiotic-resistant bacteria (MRSA, ESBL); detection of Clostridioides difficile toxin; Helicobacter pylori and fecal parasites (ova and protozoa) |
| Singh et al. [24] | 2021 | Randomized, placebo-controlled, single-center study | USA | 23 | 12/11 | 37.4 | NA | IBS medications | NA | Rome III | IBS-D | ≥150 | NA (stool bank (OpenBiome, Somerville, MA, USA)) |
| First Author | FMT Preparation | Placebo Preparation | FMT Route of Administration | FMT Frequency & Dosing | FMT Duration | Follow-Up Duration | Follow-Up Technique | Primary Outcome | Secondary Outcome | Adverse Effects | Efficacy of FMT (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Johnsen et al. [34] | Multiple donors’ feces (frozen and fresh) | Autologous | Colonoscope | 50–80 g of feces, single dose | One day | 12 months | Self-assessment questionnaires (IBS SSS, fatigue, and quality of life) | Symptom relief of more than 75 points assessed by IBS-SSS | The primary outcome was reassessed at 12 months after FMT for the secondary endpoint. | Abdominal pain, nausea | Yes |
| Halkjær et al. [37] | Multiple donors’ frozen feces | Saline, glycerol, and food coloring E150, 30% glycerol | Oral capsules | 300 g of feces daily (25 capsules daily) | 12 days | Six months | Change in IBS-QoL | Reduction in IBS-SSS in the treatment group compared with the placebo group | Change in IBS-QoL scores at three months and changes in microbiota diversity before and after FMT. | Abdominal pain, nausea, diarrhea, constipation, bloating, vomiting, fatigue, fever | No |
| Aroniadis et al. [36] | Donor whole fresh stool | Non-toxic brown pigment | Oral capsules | 28 g, 75 FMT capsules (25 capsules everyday) | Three days | Six months | Questionnaire, sequencing 16S rRNA gene with Illumina Miseq technology | Differences in IBS-SSS between the groups | The IBS-QOL questionnaire, Hospital Anxiety and Depression Scale (HADS), Bristol Stool Form Scale (BSFS), microbiome profiles as assessed by 16S rRNA sequencing, and adverse events. | Abdominal pain, nausea, diarrhea, constipation, bloating, vomiting, fatigue, belching, loss of appetite | No |
| Holster et al. [39] | Single-donor feces (frozen) | Autologous | Colonoscope | 30 g, single dose | One day | Six months | GSRS-Ib, IBS-SSS questionnaire | Effect of FMT on IBS symptoms | IBS symptoms using the IBS-SSS, their general health and quality of life, and IBS-QoL, anxiety, and depression status | Abdominal pain, nausea, diarrhea, constipation, bloating | No |
| Holvoet et al. [35] | Two donors’ fresh feces | Autologous | Nasojejunal probe | 300 mL of the donor solution, single dose | One day | One year | IBS-related symptoms were assessed using a daily symptom diary, IBS-QoL questionnaire, sequencing 16S rRNA gene with Illumina Miseq technology | Relief of general IBS symptoms and abdominal bloating | Changes in daily assessed IBS symptoms, quality of life, changes of fecal microbiota composition, 4 IBS-related symptoms one year following FMT. | NA | Yes |
| Lahtinen et al. [38] | One donor’s fresh feces | Autologous | Colonoscope | 30 g, single dose | One day | One year | IBS-related symptoms were assessed using a daily symptom diary, IBS-QoL questionnaire, sequencing 16S rRNA gene with Illumina Miseq technology | Decline in the IBS-SSS score of 50 points or more | Changes in quality of life, depression, anxiety, gut microbiota composition and stool consistency. | Abdominal pain, diarrhea, bloating | No |
| Singh et al. [24] | Single-donor capsules; six separate donors (frozen) | 19 capsules containing glycerol with a brown coloring agent | Oral capsules | 14.25 g (single dose of 19 capsules with each pill consisting of 0.75 g) | One day | Ten weeks | IBS-SSS, IBS-QoL, IBS-GIS | Decrease in IBS-SSS ≥ 50 points | NA | NA | No (improvement in IBS symptoms but not statistically significant) |
| First Author | Intestinal Microbiota Modifications |
|---|---|
| Johnsen et al. [34] | Alistipes spp. ↑, Bacteroides spp. ↑, Prevotella spp. ↑, Firmicutes spp. ↑, Akkermansia muciniphila ↑ Eubacterium hallii group ↓, Dorea spp.↓ |
| Halkjær et al. [37] | Clostridiales ↑, Bacteroidales ↑, biodiversity ↑ |
| Aroniadis et al. [36] | Bacteroidetes to Firmicutes ratio ↑, Prevotella spp.↑ |
| Holster et al. [39] | Butyrate-producing bacteria did not change. |
| Holvoet et al. [35] | NA |
| Lahtinen et al. [38] | Microbial richness ↑ |
| Singh et al. [24] | None |
| Subgroups | Number of Studies | RR (CI 95%) | p-Value | I2 | Publication Bias (p-Value) | |
|---|---|---|---|---|---|---|
| FMT preparation | ||||||
| Frozen vs. non-FMT placebo | 3 | 0.30 (0.13–0.68) | 0.004 | 29.78 | 1.00 | |
| One donor vs. autologous FMT | 2 | 1.67 (0.59–4.67) | 0.32 | 33.46 | - | |
| Multiple donors vs. autologous FMT | 2 | 2.54 (1.20–5.37) | 0.01 | 0.00 | - | |
| Multiple donors vs. non-FMT placebo | 2 | 0.16 (0.05–0.48) | 0.00 | 0.00 | - | |
| FMT frequency | ||||||
| Single dose vs. autologous FMT | 4 | 2.20 (1.20–4.03) | 0.010 | 0.00 | 0.30 | |
| Multiple doses vs. non-FMT placebo | 2 | 0.32 (0.07–1.32) | 0.11 | 61.46 | - | |
| FMT route | ||||||
| Lower GI vs. autologous FMT * | 4 | 2.20 (1.20–4.03) | 0.010 | 0.00 | 0.30 | |
| Upper GI vs. non-FMT placebo ** | 3 | 0.30 (0.13–0.68) | 0.004 | 29.78 | 1.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamshidi, P.; Farsi, Y.; Nariman, Z.; Hatamnejad, M.R.; Mohammadzadeh, B.; Akbarialiabad, H.; Nasiri, M.J.; Sechi, L.A. Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2023, 24, 14562. https://doi.org/10.3390/ijms241914562
Jamshidi P, Farsi Y, Nariman Z, Hatamnejad MR, Mohammadzadeh B, Akbarialiabad H, Nasiri MJ, Sechi LA. Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Molecular Sciences. 2023; 24(19):14562. https://doi.org/10.3390/ijms241914562
Chicago/Turabian StyleJamshidi, Parnian, Yeganeh Farsi, Zahra Nariman, Mohammad Reza Hatamnejad, Benyamin Mohammadzadeh, Hossein Akbarialiabad, Mohammad Javad Nasiri, and Leonardo A. Sechi. 2023. "Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Molecular Sciences 24, no. 19: 14562. https://doi.org/10.3390/ijms241914562
APA StyleJamshidi, P., Farsi, Y., Nariman, Z., Hatamnejad, M. R., Mohammadzadeh, B., Akbarialiabad, H., Nasiri, M. J., & Sechi, L. A. (2023). Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Molecular Sciences, 24(19), 14562. https://doi.org/10.3390/ijms241914562







