Abstract
Primary familial brain calcification (PFBC), also known as Fahr’s disease, is a rare inherited disorder characterized by bilateral calcification in the basal ganglia according to neuroimaging. Other brain regions, such as the thalamus, cerebellum, and subcortical white matter, can also be affected. Among the diverse clinical phenotypes, the most common manifestations are movement disorders, cognitive deficits, and psychiatric disturbances. Although patients with PFBC always exhibit brain calcification, nearly one-third of cases remain clinically asymptomatic. Due to advances in the genetics of PFBC, the diagnostic criteria of PFBC may need to be modified. Hitherto, seven genes have been associated with PFBC, including four dominant inherited genes (SLC20A2, PDGFRB, PDGFB, and XPR1) and three recessive inherited genes (MYORG, JAM2, and CMPK2). Nevertheless, around 50% of patients with PFBC do not have pathogenic variants in these genes, and further PFBC-associated genes are waiting to be identified. The function of currently known genes suggests that PFBC could be caused by the dysfunction of the neurovascular unit, the dysregulation of phosphate homeostasis, or mitochondrial dysfunction. An improved understanding of the underlying pathogenic mechanisms for PFBC may facilitate the development of novel therapies.
1. Introduction
Primary familial brain calcification (PFBC), previously known as Fahr’s disease or idiopathic basal ganglia calcification, is a rare inherited disorder characterized by bilateral calcification in the basal ganglia according to neuroimaging. Calcification may also affect other brain regions, such as the thalamus, cerebellum, and subcortical white matter. The clinical phenotypes are quite diverse, but the most common manifestations are movement disorders, cognitive deficits, and psychiatric disturbances. Although PFBC has nearly complete penetrance regarding neuroradiological findings, it has reduced penetrance with regard to clinical manifestations, with nearly one-third of cases being clinically asymptomatic [1,2,3]. The prevalence of PFBC is estimated to be 2.1–6.6 per 1000 persons [4,5], although it may be underestimated due to incomplete clinical penetrance and heterogenous presentations.
To date, two inheritance patterns have been found in PFBC patients. Heterozygous variants in SLC20A2, PDGFRB, PDGFB, and XPR1 are responsible for autosomal dominant PFBC [6,7,8,9], while biallelic changes in MYORG, JAM2, and CMPK2 are associated with autosomal recessive forms of the disease [10,11,12,13]. At present, around 50% of patients with PFBC do not have a pathogenic variant in the seven currently known genes [2], which indicates a more diverse genetic heterogeneity. The clinical findings and pathogenesis of PFBC have been described in details in recent reviews [14,15]. Therefore, this article focuses on the most pertinent knowledge of PFBC, emphasizing the recent advances in clinical genetics and summarizing the underlying mechanisms.
2. Clinical Presentations
Although PFBC can be found in all age groups, the onset usually occur between 40 and 60 years [3]. The age at onset among patients with PDGFB variants seems to be lower than that of those with SLC20A2, XPR1, and MYORG variants [3]. The clinical presentations of PFBC are quite diverse [16], with movement disorders, cognitive deficits, and psychiatric disturbances being most common ones [3]. Various types of movement disorders, including parkinsonism, tremors, dystonia, chorea, myoclonus, tics, and ataxia, have been reported, of which parkinsonism is the most frequent symptom [3,16]. In addition, paroxysmal kinesigenic dyskinesia has also been reported [17,18,19]. The cognitive deficits range from mild cognitive decline to dementia with frontal subcortical dysfunction [20], and memory and executive functions are the most commonly affected domains [21]. With regard to psychiatric disturbance, depression is the most commonly seen symptom, followed by psychosis and anxiety [3].
A wide range of speech disturbances, including dystonic, spastic, scanned, or slurred speech, can be presented in PFBC [16]; especially, a speech disturbance is the cardinal presentation of patients with MYORG variants [3,22]. The other neurological symptoms include various types of seizures, headaches/migraines, pyramidal signs, and stroke [21,23,24,25,26,27].
Interestingly, due to advances in neuroimaging and sequencing technology, around one-third of patients with brain calcification and pathogenic variants in PFBC genes remain clinically asymptomatic during their lifetime [1,2,3]. The penetrance varies among different PFBC genes; PDGFB, MYORG, and JAM2 have the highest clinical penetrance (more than 85%), followed by XPR1 and SLC20A2 (70% and 60%, respectively). In contrast, PDGFRB has the lowest clinical penetrance (46%) [3]. The clinical variability and reduced penetrance may be influenced by other yet unidentified genetic modifiers [28].
3. Imaging Features
Brain calcification is essential for the diagnosis of PFBC, and the best imaging tool to detect brain calcification is non-contrast computed tomography (CT). The bilateral basal ganglia, especially the internal globus pallidus, are preferentially affected [29]. The other commonly involved brain areas include the thalamus, dentate nucleus, and subcortical white matter. Less frequently, calcifications can also be found in the internal capsule, cerebral and cerebellar cortex, and brainstem [3,16].
The pattern of brain calcification varies among different PFBC genes. Patients with biallelic variants in MYORG and JAM2 tend to have more extensive calcified areas than patients with autosomal dominant PFBC genes do [3]. The distinctive features of MYORG related PFBC are calcification in the brainstem, especially in the pons, plus various degrees of cerebellar atrophy [22]. In contrast to MYORG, patients with JAM2 have more severe and confluent bilateral parietal, temporal, and occipital cortical calcifications [12].
Of note, more restricted brain calcification, predominantly limited to the basal ganglia, can be found in monoallelic carriers of MYORG, JAM2, and CMPK2 [13,30,31]. This suggests that the neuroradiological phenotype may be transmitted as a semi-dominant trait via heterozygous variants in autosomal recessive PFBC genes [15,30].
The magnetic resonance imaging (MRI) of the brain can provide better anatomic information than CT can; however, in PFBC, routine brain MRI is less sensitive and may lead to underdiagnosis [29]. New MRI techniques, such as susceptibility-weighted imaging (SWI), can exploit the magnetic susceptibility of various compounds, including intracranial calcification [32,33]. However, whether these new MRI technologies can replace CT as the first line diagnosis of PFBC remains to be thoroughly investigated.
4. Diagnostic Criteria
The current diagnostic criteria for PFBC were adapted from Moskowitz et al. [34], Ellie et al. [35], and Manyam et al. [36]. The diagnosis of PFBC is based on the presence of all four of the following criteria:
- Progressive neurological dysfunction, usually including movement and neuropsychiatric manifestations;
- The bilateral calcification of basal ganglia according to neuroimaging, with or without the involvement of other brain regions;
- The exclusion of other causes of calcification, such as metabolic problems, mitochondrial diseases, infectious, toxic, or traumatic causes;
- A positive family history of PFBC [29].
However, due to advances in the genetic research on PFBC in the past decade, some of the diagnostic criteria are no longer valid and may need to be modified. For example, not all patients present with clinical symptoms throughout their lifetime [3], and this may lead to the exclusion of some genetic and/or imaging-positive asymptomatic cases based on the current criteria. In addition, PFBC can be diagnosed in patients without a positive family history because affected relatives have unrecognized symptoms or are asymptomatic. Moreover, patients with de novo mutations or autosomal recessive inheritance can present with a negative family history [37]. Hence, the absence of a family history cannot completely rule out the possibility of PFBC, and the current diagnostic criteria may be suboptimal and may miss asymptomatic or non-familial cases. The future modification of the criteria to include genetic information may, thus, be required.
5. Differential Diagnosis
The calcification of the basal ganglia has also been identified in about 0.3–20% of individuals, especially among elderly people. Usually, age-related physiological calcification is not associated with clinical symptoms or a family history, and it may be misdiagnosed as PFBC [38,39,40]. To differentiate pathological basal ganglion calcification from frequently seen physiological calcification in normal aging, Nicolas et al. developed a visual grading system based on CT scans, called the total calcification score (TCS). Age-specific thresholds were determined according to the value of the 99th percentile of the TCS in different age categories among controls [21]. The authors concluded that the TCS is rarely affected by cerebral atrophy in the elderly, and therefore, it is a useful tool for the diagnosis of PFBC, with high inter-rater reliability [21]. However, the development of TCS predates the recent discovery of most of the PFBC genes. As a result, the use of the TCS for different forms of genetic PFBC requires further validation.
6. Genetics and Disease Mechanism
PFBC is genetically heterogeneous. To date, seven genes have been associated with PFBC, including four dominant genes and three recessive genes (Table 1).

Table 1.
Summary of PFBC-causative genes.
6.1. SLC20A2
The first PFBC-causative gene, SLC20A2, was identified in 2012 [6]. It is located on chromosome 8 and encodes for type III sodium-dependent inorganic phosphate (Pi) transporter 2 (PiT2). PiT2 has 12 transmembrane domains, and this protein is responsible for the uptake of Pi into cells [2,6,16]. SLC20A2 is expressed ubiquitously, but at a higher level in the brain, especially in the globus pallidus, thalamus, and cerebellum [41]. SLC20A2 is the most common PFBC gene; heterozygous variants have been identified in more than 60% of genetically confirmed PFBC patients [3]. A missense change is the most common variant type, followed by frameshift, nonsense, and splice site variations, without obvious hotspots for pathogenic variants (Figure 1a) [3,6,7,17,18,21,23,37,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. Functionally, both haploinsufficiency and dominant negative effects have been described; the loss of normal PiT2 function results in extracellular Pi accumulation and subsequent calcium phosphate formation [6,42]. Calcification around the blood vessels or within the vessel walls of involved brain regions has been demonstrated in Slc20a2 homozygous knockout mice (Slc20a2−/− mice) and in an autopsied SLC20A2-PFBC patient [86,87,88]. Calcification in Slc20a2−/− mice was also found inside cells, mainly in the pericytes and astrocytes, which suggested the intracellular cytosolic initiation of calcification [87]. Moreover, increasing T-cell infiltration in the brain parenchyma was found in Slc20a2−/− mice, which is positively associated with brain calcification and aging. Impaired blood–brain barrier (BBB) permeability with the enhancement of endocytosis and transcytosis was also demonstrated, which may be explained by the dysfunction of pericytes and astrocytes due to intracellular calcification [89]. In addition, PiT2 is known to be expressed in the apical membrane of choroid plexus epithelial cells in spiny dogfish and rats, suggesting that PiT2 plays an important role in actively transporting Pi from the cerebrospinal fluid (CSF) to the blood to maintain phosphate homeostasis in the CSF [90]. The level of Pi in CSF is significantly elevated in both Slc20a2 homozygous knockout mice and PFBC patients with SLC20A2 pathogenic variants [87,91,92]. In summary, PiT2 dysfunction can leads to a local increase in the extracellular and CSF Pi concentrations, which then trigger cell-mediated mineralization progression, ensuing calcification [86,87].
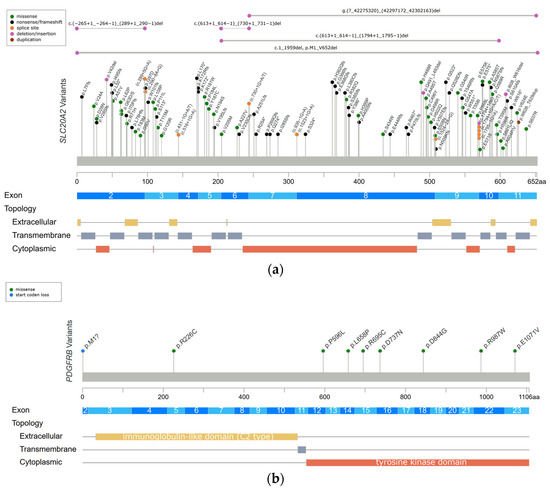
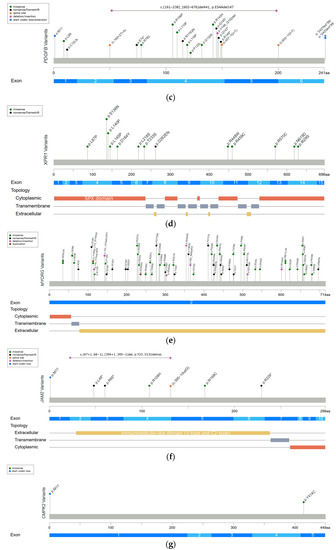
Figure 1.
Reported variants in seven genes linked to PFBC along the protein sequence and their topologic protein models. (a) The reported variants in the SLC20A2 gene and topology protein model of PiT2. (b) PDGFRB gene and PDGF-Rβ. (c) PDGFB gene. (d) XPR1 gene and XPR1. (e) MYORG gene and MYORG. (f) JAM2 gene and JAM2. (g) CMPK2 gene. There is no obvious hotspot for SLC20A2, MYORG, and JAM2 genes. The variants tend to cluster in the tyrosine kinase domain of the PDGFRB gene, the mature protein product between positions 82 and 190 of the PDGFB gene, and in the SPX domain of the XPR1 gene. Meaning of symbols: *, stop codon; ?, unknown (a variant affecting the initiation codon cannot be predicted).
6.2. PDGFRB
The PDGFRB gene was identified in PFBC patients in 2013 [7], and it is located on chromosome 5 and encodes for platelet-derived growth factor receptor-β (PDGF-Rβ). The structure of PDGF-Rβ includes five extracellular immunoglobulin-like C2 type domains, a transmembrane domain, and a tyrosine kinase domain. PDGF-Rβ is a cell-surface tyrosine kinase receptor for members of the platelet-derived growth factor (PDGF) family, with a high affinity for homodimeric PDGF-B and PDGF-D. At the tissue level, the PDGFRB gene is highly expressed in the brain, especially in the basal ganglia and dentate nucleus of the cerebellum. At the cellular level, the PDGFRB gene is expressed in neurons, vascular smooth muscle cells (SCMs), and pericytes. The signal transduction of PDGF-Rβ and its ligand is essential for the proliferation and migration of vascular SCMs and pericytes, and subsequently, the angiogenesis and formation of the BBB [2,7,16]. Heterozygous variants in the PDGFRB gene have been identified in 5% of genetically confirmed PFBC patients [3]. The missense change is currently the only variant type to be identified in the PDGFRB gene. The variants tend to cluster in the tyrosine kinase domain (Figure 1b) [3,7,21,56,93,94], and they are likely to cause haploinsufficiency and affect the kinase function of the protein [43]. The functional loss of PDGF-Rβ may lead to pericytes dysfunction, which would impact BBB integrity and secondarily induce calcium depositions in the vessel walls or perivascular space. Several studies have shown that homodimeric PDGF-B can directly induce vascular SCM calcification via enhancing the expression of inorganic phosphate transporter 1 (PiT1) [95,96]. Hence, Nicolas et al. hypothesized that activating variants in PDGFRB may impact the PDGF-PiT1 pathway and cause vascular calcification [7]. Interestingly, PiT1 is encoded by the SLC20A1 gene, which is one of two type III sodium-dependent Pi transporters. The other is PiT2 encoded by SLC20A2 [7,94]. However, there is no further evidence supporting this hypothesis [14].
6.3. PDGFB
The PDGFB gene was also reported in 2013 [8], and it is located on chromosome 22 and encodes for the PDGF-B precursor protein. This precursor protein is cleaved at positions 81 and 191, and subsequently forms a homodimer through disulfide bonds, which is the main ligand of PDGF-Rβ [16]. The PDGFB gene is expressed in neurons and endothelial cells in the brain [8]. PDGF-B is a growth factor for mesenchymal cells and plays a crucial role in the proliferation and recruitment of pericytes and vascular SMCs. [44,45]. Heterozygous variants in the PDGFB gene have been identified in 12% of genetically confirmed PFBC patients [3]. The missense change is the most common variant type in the PDGFB gene, followed by nonsense, splice site, and extension variants. The variants cluster between protein positions 82 and 190, which are retained in the mature PDGF-B protein (Figure 1c) [3,8,17,56,59,69,82,97,98,99,100,101,102,103,104,105]. The same as PDGFRB does, PDGFB variants also cause haploinsufficiency, either by deleting critical parts of protein or disrupting normal protein function. The loss of normal PDGF-B function leads to BBB impairment via PDGF-Rβ, and then triggers the process of calcification [2,8].
6.4. XPR1
The XPR1 gene was identified in 2015 [9], and it is located on chromosome 1 and encodes for xenotropic and polytropic retrovirus receptor 1 (XPR1). XPR contains eight transmembrane domains and an amino-terminal SPX domain [16]. This protein mediates Pi efflux from cells [2,9]. XPR1 is expressed universally, and a high XPR1 mRNA level has been demonstrated in mouse brains, especially in the cerebellum and striatum [46]. Heterozygous variants in the XPR1 gene have been identified in 6% of genetically confirmed PFBC patients [3]. The missense change is the most common variant in XPR1-related PFBC patients, which tends to cluster in the SPX domain (Figure 1d) [3,9,19,53,56,106,107]. XPR1 variants may cause haploinsufficiency, leading to the intracellular accumulation of Pi and formation of calcium phosphate [9]. Interestingly, mutual interactions between XPR1 and PDGFRB were found in a recent immunoprecipitation study [46]. It is hypothesized that these two proteins may form a complex on the cell membrane, further suggesting that PDGFRB may be the upstream regulator of XPR1 [46].
6.5. MYORG
In 2018, the first and most common autosomal recessive PFBC-causative gene, MYORG, was identified. MYORG is located on chromosome 9 and encodes for myogenesis-regulating glycosidase (MYORG). It contains a short cytoplasm domain at the N-terminal, a transmembrane domain, and a long luminal fragment with a glycosidase domain at the C-terminal. MYORG is a member of the glycosyl hydrolase 31 family, and its function is to regulate protein glycosylation. In the brain, the MYORG gene is highly expressed in the cerebellum, specifically in the endoplasmic reticulum of the astrocytes [10]. Biallelic variants in the MYORG gene have been identified in 13% of genetically confirmed PFBC patients [3]. The missense change is the most common variant type in the MYORG gene, followed by in-frame indels, nonsense, and frameshift variations. There is no obvious hotspot of pathogenic variants (Figure 1e) [3,10,22,24,25,26,27,30,108,109,110,111,112,113,114,115,116,117,118]. Pathogenic variants cause the loss of the glycosidase function of MYORG, which may lead to abnormal protein glycosylation and metabolic disturbance. It is believed that MYORG variants can induce astrocyte dysfunction, which then disturbs the association between astrocytes and pericytes, resulting in neurovascular unit (NVU) dysfunction and subsequently causing the formation of brain calcification [10]. However, the exact linkage between the loss of protein glycosylation and astrocyte dysfunction remains to be elucidated.
6.6. JAM2
In 2020, another autosomal recessive PFBC-causative gene, JAM2, was identified. JAM2 is located on chromosome 21 and encodes for junctional-adhesion-molecule-2 (JAM2). The structure of JAM2 includes two immunoglobulin-like domains (V-type and C2-type). JAM2 is a member of the junctional adhesion molecule family, and it plays crucial roles in the regulation of cell polarity, endothelium permeability, leukocyte migration, and BBB function [11,12]. In the brain, JAM2 is highly expressed in the caudate nuclei. At the cellular level, JAM2 is specifically expressed in endothelial cells and astrocytes [12]. Biallelic variants in the JAM2 gene have been identified in 2% of genetically confirmed PFBC patients [3]. The nonsense change is the most common variant type in the JAM2 gene. Missense, frameshift, and structural variants have also been reported, without a mutation hotspot (Figure 1f) [3,11,12]. Variants of JAM2 gene cause the loss of cell–cell adhesion and the dysfunction of the solute passage, which may contribute to the formation of brain calcification [11,12].
6.7. CMPK2
The CMPK2 gene is the latest autosomal recessive PFBC-causative gene to be identified at the end of 2022 [13]. This gene is located on chromosome 2 and encodes for uridine monophosphate-cytidine monophosphate kinase 2 (UMP-CMPK2), which can be separated into N-terminal and C-terminal domains according to the sequence properties. CMPK2 is a member of the nucleoside monophosphate kinase family and participates in the salvage pathway for the phosphorylation of dCMP, dUMP, CMP, and UMP in the mitochondria [47]. In the brain, the CMPK2 gene is highly expressed in the hippocampus and cerebellum. At the cellular level, CMPK2 is specifically expressed in neurons and vascular endothelial cells [13]. To date, biallelic variants in the CMPK2 gene have only been reported in two PFBC families, which carry missense and start-codon loss variants (Figure 1g) [13]. The loss of UMP-CMPK2 function leads to a reduction in mitochondrial genome DNA copy numbers, the downregulation of the expression of mitochondrial protein, the decrease in ATP production, and the disturbance of mitochondrial cristae morphology. The disturbance of mitochondrial function is believed to cause impairment of energy homeostasis and the upregulation of intracellular phosphate levels, subsequently triggering the development of brain calcification [13].
6.8. Possible Pathophysiological Mechanisms of PFBC
In conclusion, three potential mechanisms underlying the pathogenesis of PFBC have been proposed. The first mechanism is the disturbance of the homeostasis of phosphate, which is supported by the transporter function of SLC20A2 and XPR1. The second mechanism is the disruption of the NVU. The NVU is composed of neurons, astrocytes, vascular SMCs, pericytes, and endothelial cells (Figure 2). Each element of the NVU interacts tightly with the others, which leads to an effective system used to regulate cerebral blood flow and maintain BBB integrity [119,120]. This is supported by the observation that PDGFRB, PDGFB, MYORG, and JAM2 are highly expressed in the composite cells of the NVU. However, the exact mechanism of how NVU dysfunction causes brain calcification remains to be elucidated [10,11,12]. The third mechanism is the impairment of mitochondrial function. This hypothesis is supported by research on CMPK2, which plays a significant role in the salvage pathway in the mitochondria [13]. Interestingly, some connections between these three mechanisms have also been proposed. First, intracellular calcification in Slc20a2−/− mice has been shown to be mainly distributed in NVU cells, such as the pericytes and astrocytes [87]. Second, the co-localization of XPR1 and PDGFRB has been reported, and they may form a complex on the cell membrane [46]. Third, CMPK2 is highly expressed in neurons and vascular endothelial cells, and the latter ones are also part of the NVU [13]. Taken together, the homeostasis of phosphate and maintaining mitochondrial function in NVU cells appear to play important roles in the pathogenesis of PFBC [12,13]. Further functional studies are needed to elucidate the detailed mechanisms.
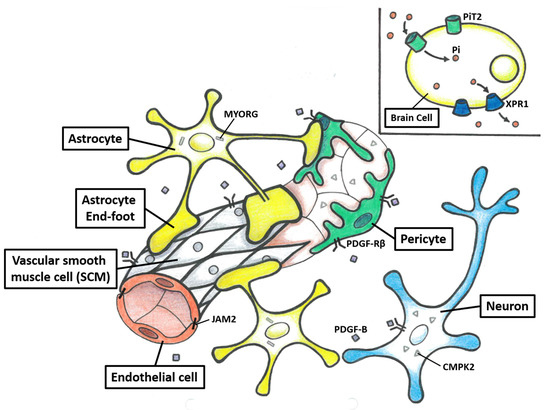
Figure 2.
The structure of the neurovascular unit (NVU) and protein model of seven PFBC-causative genes. The NVU is composed of neurons, astrocytes, vascular SMCs, pericytes, and endothelial cells. Each element of the NVU interacts tightly with the other, which leads to an effective system used to regulate cerebral blood flow and maintain BBB integrity. PDGFRB, PDGFB, MYORG, and JAM2 are highly expressed in the composite cells of the NVU. The neurons and endothelial cells secrete PDGF-B, which binds to PDGF-Rβ. The PDGF-Rβ is mainly encoded on the surface of neurons, vascular SCMs, and pericytes. MYORG is specifically encoded in the endoplasmic reticulum of the astrocytes. JAM2 is mainly encoded in endovascular cells and astrocytes. CMPK2 is highly encoded in neurons and endothelial cells. PiT2 and XPR1, which are highly encoded in the brain, mediate Pi uptake into cells and efflux from cells, respectively.
6.9. Composition of Calcification in PFBC
The structures of calcium deposits have been reported to be similar in Slc20a2−/− mice and PFBC patients, with the principal component being hydroxyapatite (Ca10[PO4]6[OH]2), and C, N, O, S, Fe, Zn, Al, and Mg were also observed [87,88]. The protein components in the calcification of hypomorphic mice with PDGFB (PDGFBret/ret mice) have also been identified. The most abundant proteins in calcification are known to regulate bone mineralization, including both a bone formation promotor, secreted protein acidic and rich in cysteine-like 1 (SPARCL1), and inhibitors, alpha 2-Heremans-Schmid glycoprotein (AHSG), matrix gla protein (MGP), and osteopontin (OPN) [121]. Moreover, the accumulation of reactive astrocytes and microglia around calcified area has been reported in both a PFBC patient and PDGFBret/ret mice [121,122]. Reactive astrocytes express proteins that function in oxidative damage and neurotoxic responses, and abnormal osteocyte markers, which indicate that reactive astrocytes may contribute to the formation of an osteogenic environment [14,122,123]. Reactive microglia acquire an osteoclast-like phenotype and may play a protective role in controlling brain calcification [123,124]. In summary, these findings suggest that the formation of calcification in PFBC may be a mineralization process rather than primary precipitation of calcium-phosphate products.
7. Treatment
Currently, there is no disease-modifying therapy for PFBC to reduce brain calcification or prevent its progression, and the treatment is mainly symptomatic. The discovery of PFBC-causative genes and clarifying the disease mechanisms in recent years has opened the door for therapeutic development. In one case series, biphosphonates, which modify the bone resorption and formation cycle, was shown to potentially stabilize the natural course of PFBC and showed symptomatic improvement among some younger patients. However, the study was small and included seven patients with heterogeneous PFBC, and thus, the therapeutic effect remains to be corroborated in larger clinical trials [125]. In a recent study, four SLC20A2 variants (D28N, G120R, A227V, and C496Y) were investigated functionally, and the results showed that Pi transport activity in cells was abolished, except for A227V, which was partially maintained [55]. Interestingly, the presence of the A227V variant has been reported in healthy members of the PFBC family, suggesting that the partial preservation of Pi transport function may help suppress the onset of PFBC. Therefore, a treatment to upregulate the activity of PiT2 could be a potential direction for future development [55,126]. As mentioned, the severity of brain calcification seems to be associated with increased T-cell infiltration in the brain parenchyma. Fingolimod, a sphingosine-1-phosphate receptor modulator, is used to inhibit the circulation of peripheral T-cells, and it has been shown to reduce the trafficking of T cells into the central nervous system. In a recent animal study, brain calcification was significantly alleviated in Slc20a2−/− mice after the intraperitoneal administration of fingolimod for three months. Thus, fingolimod may be a potential treatment for PFBC [89].
8. Conclusions
PFBC is a rare neurological disorder, with heterogeneous clinical presentations, neuroimaging findings, and clinical genetics. Seven genes have been linked to PFBC to date; however, around 50% of PFBC patients still have received no genetic diagnosis. Future investigations to identify novel PFBC-causative genes will likely fill the diagnostic gaps in these undiagnosed cases and shed more light on the pathogenesis of how this disease develops. The currently known PFBC genes play crucial roles in phosphate homeostasis and maintaining mitochondrial function, especially in the cells of the NVU, which may be targeted for the development of novel therapeutic interventions or gene therapy.
Author Contributions
Conceptualization, S.-Y.C. and M.-H.T.; writing—original draft preparation, S.-Y.C.; writing—review and editing, C.-J.H., Y.-T.L., C.-H.L., M.-Y.L. and M.-H.T.; supervision, M.-H.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by grants from the National Science and Technology Council (110-2314-B-182-057-MY3 and 110-2314-B-182A-076-MY3), National Health Research Institutes (NHRI-EX111-11022NI), and Chang Gung Medical Foundation Grant (CMRP8L1331, CMRPG8M1091, and CIRPG8M0091) to M.-H.T.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PFBC | primary familial brain calcification |
| TCS | total calcification score |
| CT | computerized tomography |
| MRI | magnetic resonance imaging |
| SWI | susceptibility weighted imaging |
| Pi | inorganic phosphate |
| PiT1 | inorganic phosphate transporter 1 |
| PiT2 | inorganic phosphate transporter 2 |
| Slc20a2−/− mice | Slc20a2 homozygous knockout mice |
| CSF | cerebrospinal fluid |
| PDGF | platelet-derived growth factor |
| PDGF-Rβ | platelet-derived growth factor receptor-β |
| SCMs | smooth muscle cells |
| BBB | blood–brain barrier |
| XPR1 | xenotropic and polytropic retrovirus receptor 1 |
| MYORG | myogenesis regulating glycosidase |
| JAM2 | junctional-adhesion-molecule-2 |
| UMP-CMPK2 | uridine monophosphate-cytidine monophosphate kinase 2 |
| WT | wide-type |
| NVU | neurovascular unit |
| PDGFBret/ret mice | mice hypomorphic for PFGFB |
| SPARCL1 | secreted protein acidic and rich in cysteine-like 1 |
| AHSG | alpha 2-Heremans-Schmid glycoprotein |
| MGP | matrix gla protein |
| OPN | osteopontin |
References
- Tadic, V.; Westenberger, A.; Domingo, A.; Alvarez-Fischer, D.; Klein, C.; Kasten, M. Primary familial brain calcification with known gene mutations: A systematic review and challenges of phenotypic characterization. JAMA Neurol. 2015, 72, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Westenberger, A.; Balck, A.; Klein, C. Primary familial brain calcifications: Genetic and clinical update. Curr. Opin. Neurol. 2019, 32, 571–578. [Google Scholar] [CrossRef]
- Balck, A.; Schaake, S.; Kuhnke, N.S.; Domingo, A.; Madoev, H.; Margolesky, J.; Dobricic, V.; Alvarez-Fischer, D.; Laabs, B.H.; Kasten, M.; et al. Genotype-Phenotype Relations in Primary Familial Brain Calcification: Systematic MDSGene Review. Mov. Disord. 2021, 36, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Charbonnier, C.; Campion, D.; Veltman, J.A. Estimation of minimal disease prevalence from population genomic data: Application to primary familial brain calcification. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 68–74. [Google Scholar] [CrossRef]
- Chen, S.; Cen, Z.; Fu, F.; Chen, Y.; Chen, X.; Yang, D.; Wang, H.; Wu, H.; Zheng, X.; Xie, F.; et al. Underestimated disease prevalence and severe phenotypes in patients with biallelic variants: A cohort study of primary familial brain calcification from China. Park. Relat. Disord. 2019, 64, 211–219. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Shi, L.; Ren, J.; Patti, M.; Wang, T.; de Oliveira, J.R.; Sobrido, M.J.; Quintans, B.; Baquero, M.; et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat. Genet. 2012, 44, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Pottier, C.; Maltete, D.; Coutant, S.; Rovelet-Lecrux, A.; Legallic, S.; Rousseau, S.; Vaschalde, Y.; Guyant-Marechal, L.; Augustin, J.; et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology 2013, 80, 181–187. [Google Scholar] [CrossRef]
- Keller, A.; Westenberger, A.; Sobrido, M.J.; Garcia-Murias, M.; Domingo, A.; Sears, R.L.; Lemos, R.R.; Ordonez-Ugalde, A.; Nicolas, G.; da Cunha, J.E.; et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 2013, 45, 1077–1082. [Google Scholar] [CrossRef]
- Legati, A.; Giovannini, D.; Nicolas, G.; Lopez-Sanchez, U.; Quintans, B.; Oliveira, J.R.; Sears, R.L.; Ramos, E.M.; Spiteri, E.; Sobrido, M.J.; et al. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 2015, 47, 579–581. [Google Scholar] [CrossRef]
- Yao, X.P.; Cheng, X.; Wang, C.; Zhao, M.; Guo, X.X.; Su, H.Z.; Lai, L.L.; Zou, X.H.; Chen, X.J.; Zhao, Y.; et al. Biallelic Mutations in MYORG Cause Autosomal Recessive Primary Familial Brain Calcification. Neuron 2018, 98, 1116–1123 e1115. [Google Scholar] [CrossRef]
- Schottlaender, L.V.; Abeti, R.; Jaunmuktane, Z.; Macmillan, C.; Chelban, V.; O’Callaghan, B.; McKinley, J.; Maroofian, R.; Efthymiou, S.; Athanasiou-Fragkouli, A.; et al. Bi-allelic JAM2 Variants Lead to Early-Onset Recessive Primary Familial Brain Calcification. Am. J. Hum. Genet. 2020, 106, 412–421. [Google Scholar] [CrossRef]
- Cen, Z.; Chen, Y.; Chen, S.; Wang, H.; Yang, D.; Zhang, H.; Wu, H.; Wang, L.; Tang, S.; Ye, J.; et al. Biallelic loss-of-function mutations in JAM2 cause primary familial brain calcification. Brain 2020, 143, 491–502. [Google Scholar] [CrossRef]
- Zhao, M.; Su, H.Z.; Zeng, Y.H.; Sun, Y.; Guo, X.X.; Li, Y.L.; Wang, C.; Zhao, Z.Y.; Huang, X.J.; Lin, K.J.; et al. Loss of function of CMPK2 causes mitochondria deficiency and brain calcification. Cell Discov. 2022, 8, 128. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Luo, J.; Cheng, X.; Lv, W.; Luo, W.; Chen, W.J.; Xiong, Z.Q.; Liu, J.Y. The Pathology of Primary Familial Brain Calcification: Implications for Treatment. Neurosci. Bull. 2022, 39, 659–674. [Google Scholar] [CrossRef]
- Carecchio, M.; Mainardi, M.; Bonato, G. The clinical and genetic spectrum of primary familial brain calcification. J. Neurol. 2023, 270, 3270–3277. [Google Scholar] [CrossRef]
- Quintans, B.; Oliveira, J.; Sobrido, M.J. Primary familial brain calcifications. Handb. Clin. Neurol. 2018, 147, 307–317. [Google Scholar] [CrossRef]
- Zhan, F.X.; Tian, W.T.; Zhang, C.; Zhu, Z.Y.; Wang, S.G.; Huang, X.J.; Cao, L. Primary familial brain calcification presenting as paroxysmal kinesigenic dyskinesia: Genetic and functional analyses. Neurosci. Lett. 2020, 714, 134543. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, X.; Wan, H.; Hong, D. Familial IBGC caused by SLC20A2 mutation presenting as paroxysmal kinesigenic dyskinesia. Park. Relat. Disord. 2014, 20, 353–354. [Google Scholar] [CrossRef]
- Tang, L.O.; Hou, B.H.; Zhang, X.N.; Xi, Z.Y.; Li, C.X.; Xu, L. Biallelic XPR1 mutation associated with primary familial brain calcification presenting as paroxysmal kinesigenic dyskinesia with infantile convulsions. Brain Dev. 2021, 43, 331–336. [Google Scholar] [CrossRef]
- Benke, T.; Karner, E.; Seppi, K.; Delazer, M.; Marksteiner, J.; Donnemiller, E. Subacute dementia and imaging correlates in a case of Fahr’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1163–1165. [Google Scholar] [CrossRef]
- Nicolas, G.; Pottier, C.; Charbonnier, C.; Guyant-Marechal, L.; Le Ber, I.; Pariente, J.; Labauge, P.; Ayrignac, X.; Defebvre, L.; Maltete, D.; et al. Phenotypic spectrum of probable and genetically-confirmed idiopathic basal ganglia calcification. Brain 2013, 136, 3395–3407. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, L.; Wallon, D.; Charbonnier, C.; Quenez, O.; Richard, A.C.; Rousseau, S.; Budowski, C.; Lebouvier, T.; Corbille, A.G.; Vidailhet, M.; et al. Biallelic MYORG mutation carriers exhibit primary brain calcification with a distinct phenotype. Brain 2019, 142, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, G.; Zhao, Z.; Zhu, M. SCL20A2 mutation presenting with acute ischemic stroke: A case report. BMC Neurol. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, J.; Jiao, B.; Weng, L. Primary familial brain calcification in a patient with a novel compound heterozygous mutation in MYORG presenting with an acute ischemic stroke: A case report. Ann. Transl. Med. 2022, 10, 423. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Dong, H.; Li, X. A novel mutation in MYORG leads to primary familial brain calcification and cerebral infarction. Int. J. Neurosci. 2022, 132, 1182–1186. [Google Scholar] [CrossRef]
- Sadok, S.H.; Borges-Medeiros, R.L.; de Oliveira, D.F.; Zatz, M.; de Oliveira, J.R.M. Report of a young patient with brain calcifications with a novel homozygous MYORG variant. Gene 2023, 859, 147213. [Google Scholar] [CrossRef]
- Song, T.; Zhao, Y.; Wen, G.; Du, J.; Xu, Q. A novel MYORG mutation causes primary familial brain calcification with migraine: Case report and literature review. Front. Neurol. 2023, 14, 1110227. [Google Scholar] [CrossRef]
- Borges-Medeiros, R.L.; de Oliveira, J.R.M. Digenic Variants as Possible Clinical Modifier of Primary Familial Brain Calcification Patients. J. Mol. Neurosci. 2020, 70, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.M.; Oliveira, J.; Sobrido, M.J.; Coppola, G. Primary Familial Brain Calcification. In GeneReviews®; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Chen, Y.; Cen, Z.; Chen, X.; Wang, H.; Chen, S.; Yang, D.; Fu, F.; Wang, L.; Liu, P.; Wu, H.; et al. MYORG Mutation Heterozygosity Is Associated With Brain Calcification. Mov. Disord. 2020, 35, 679–686. [Google Scholar] [CrossRef]
- Andretta, S.; Bonato, G.; Mainardi, M.; Salviati, L.; Antonini, A.; Carecchio, M. Symptomatic brain calcifications in two patients with JAM2 monoallelic variants. Mov. Disord. 2022, 37, S379–S380. [Google Scholar]
- Azad, R.; Mittal, P.; Malhotra, A.; Gangrade, S. Detection and Differentiation of Focal Intracranial Calcifications and Chronic Microbleeds Using MRI. J. Clin. Diagn. Res. 2017, 11, TC19–TC23. [Google Scholar] [CrossRef]
- Halefoglu, A.M.; Yousem, D.M. Susceptibility weighted imaging: Clinical applications and future directions. World J. Radiol. 2018, 10, 30–45. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Winickoff, R.N.; Heinz, E.R. Familial calcification of the basal ganglions: A metabolic and genetic study. N. Engl. J. Med. 1971, 285, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ellie, E.; Julien, J.; Ferrer, X. Familial idiopathic striopallidodentate calcifications. Neurology 1989, 39, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Manyam, B.V. What is and what is not ‘Fahr’s disease’. Park. Relat. Disord. 2005, 11, 73–80. [Google Scholar] [CrossRef]
- Ferreira, J.B.; Pimentel, L.; Keasey, M.P.; Lemos, R.R.; Santos, L.M.; Oliveira, M.F.; Santos, S.; Jensen, N.; Teixeira, K.; Pedersen, L.; et al. First report of a de novo mutation at SLC20A2 in a patient with brain calcification. J. Mol. Neurosci. 2014, 54, 748–751. [Google Scholar] [CrossRef]
- Forstl, H.; Krumm, B.; Eden, S.; Kohlmeyer, K. Neurological disorders in 166 patients with basal ganglia calcification: A statistical evaluation. J. Neurol. 1992, 239, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Eskandary, H.; Sabba, M.; Khajehpour, F.; Eskandari, M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg. Neurol. 2005, 63, 550–553; discussion 553. [Google Scholar] [CrossRef]
- Yamada, M.; Asano, T.; Okamoto, K.; Hayashi, Y.; Kanematsu, M.; Hoshi, H.; Akaiwa, Y.; Shimohata, T.; Nishizawa, M.; Inuzuka, T.; et al. High frequency of calcification in basal ganglia on brain computed tomography images in Japanese older adults. Geriatr. Gerontol. Int. 2013, 13, 706–710. [Google Scholar] [CrossRef]
- da Silva, R.J.; Pereira, I.C.; Oliveira, J.R. Analysis of gene expression pattern and neuroanatomical correlates for SLC20A2 (PiT-2) shows a molecular network with potential impact in idiopathic basal ganglia calcification (“Fahr’s disease”). J. Mol. Neurosci. 2013, 50, 280–283. [Google Scholar] [CrossRef]
- Larsen, F.T.; Jensen, N.; Autzen, J.K.; Kongsfelt, I.B.; Pedersen, L. Primary Brain Calcification Causal PiT2 Transport-Knockout Variants can Exert Dominant Negative Effects on Wild-Type PiT2 Transport Function in Mammalian Cells. J. Mol. Neurosci. 2017, 61, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Arts, F.A.; Velghe, A.I.; Stevens, M.; Renauld, J.C.; Essaghir, A.; Demoulin, J.B. Idiopathic basal ganglia calcification-associated PDGFRB mutations impair the receptor signalling. J. Cell. Mol. Med. 2015, 19, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Vanlandewijck, M.; Lebouvier, T.; Andaloussi Mae, M.; Nahar, K.; Hornemann, S.; Kenkel, D.; Cunha, S.I.; Lennartsson, J.; Boss, A.; Heldin, C.H.; et al. Functional Characterization of Germline Mutations in PDGFB and PDGFRB in Primary Familial Brain Calcification. PLoS ONE 2015, 10, e0143407. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.P.; Zhao, M.; Wang, C.; Guo, X.X.; Su, H.Z.; Dong, E.L.; Chen, H.T.; Lai, J.H.; Liu, Y.B.; Wang, N.; et al. Analysis of gene expression and functional characterization of XPR1: A pathogenic gene for primary familial brain calcification. Cell Tissue Res. 2017, 370, 267–273. [Google Scholar] [CrossRef]
- Xu, Y.; Johansson, M.; Karlsson, A. Human UMP-CMP kinase 2, a novel nucleoside monophosphate kinase localized in mitochondria. J. Biol. Chem. 2008, 283, 1563–1571. [Google Scholar] [CrossRef]
- Baker, M.; Strongosky, A.J.; Sanchez-Contreras, M.Y.; Yang, S.; Ferguson, W.; Calne, D.B.; Calne, S.; Stoessl, A.J.; Allanson, J.E.; Broderick, D.F.; et al. SLC20A2 and THAP1 deletion in familial basal ganglia calcification with dystonia. Neurogenetics 2014, 15, 23–30. [Google Scholar] [CrossRef]
- Batla, A.; Stamelou, M. Primary familial brain calcification in the IBGC2 kindred: All linkage roads lead to SLC20A2. Mov. Disord. 2016, 31, 1765–1766. [Google Scholar] [CrossRef]
- Giorgio, E.; Garelli, E.; Carando, A.; Bellora, S.; Rubino, E.; Quarello, P.; Sirchia, F.; Marrama, F.; Gallone, S.; Grosso, E.; et al. Design of a multiplex ligation-dependent probe amplification assay for SLC20A2: Identification of two novel deletions in primary familial brain calcification. J. Hum. Genet. 2019, 64, 1083–1090. [Google Scholar] [CrossRef]
- Guo, X.X.; Su, H.Z.; Zou, X.H.; Lai, L.L.; Lu, Y.Q.; Wang, C.; Li, Y.L.; Hong, J.M.; Zhao, M.; Lin, K.X.; et al. Identification of SLC20A2 deletions in patients with primary familial brain calcification. Clin. Genet. 2019, 96, 53–60. [Google Scholar] [CrossRef]
- Gagliardi, M.; Morelli, M.; Annesi, G.; Nicoletti, G.; Perrotta, P.; Pustorino, G.; Iannello, G.; Tarantino, P.; Gambardella, A.; Quattrone, A. A new SLC20A2 mutation identified in southern Italy family with primary familial brain calcification. Gene 2015, 568, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Zou, X.H.; Wang, C.; Yao, X.P.; Su, H.Z.; Lai, L.L.; Chen, H.T.; Lai, J.H.; Liu, Y.B.; Chen, D.P.; et al. Spectrum of SLC20A2, PDGFRB, PDGFB, and XPR1 mutations in a large cohort of patients with primary familial brain calcification. Hum. Mutat. 2019, 40, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Yao, X.P.; Zhang, Q.J.; Ni, W.; He, J.; Li, H.F.; Liu, X.Y.; Zhao, G.X.; Murong, S.X.; Wang, N.; et al. Novel SLC20A2 mutations identified in southern Chinese patients with idiopathic basal ganglia calcification. Gene 2013, 529, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Nishii, K.; Shimogawa, R.; Kurita, H.; Inden, M.; Kobayashi, M.; Toyoshima, I.; Taguchi, Y.; Ueda, A.; Tamune, H.; Hozumi, I. Partial reduced Pi transport function of PiT-2 might not be sufficient to induce brain calcification of idiopathic basal ganglia calcification. Sci. Rep. 2019, 9, 17288. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.M.; Carecchio, M.; Lemos, R.; Ferreira, J.; Legati, A.; Sears, R.L.; Hsu, S.C.; Panteghini, C.; Magistrelli, L.; Salsano, E.; et al. Primary brain calcification: An international study reporting novel variants and associated phenotypes. Eur. J. Hum. Genet. 2018, 26, 1462–1477. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tanaka, M.; Takagi, M.; Kobayashi, S.; Taguchi, Y.; Takashima, S.; Tanaka, K.; Touge, T.; Hatsuta, H.; Murayama, S.; et al. Evaluation of SLC20A2 mutations that cause idiopathic basal ganglia calcification in Japan. Neurology 2014, 82, 705–712. [Google Scholar] [CrossRef]
- Ding, Y.; Dong, H.Q. A Novel SLC20A2 Mutation Associated with Familial Idiopathic Basal Ganglia Calcification and Analysis of the Genotype-Phenotype Association in Chinese Patients. Chin. Med. J. 2018, 131, 799–803. [Google Scholar] [CrossRef]
- Koyama, S.; Sato, H.; Kobayashi, R.; Kawakatsu, S.; Kurimura, M.; Wada, M.; Kawanami, T.; Kato, T. Clinical and radiological diversity in genetically confirmed primary familial brain calcification. Sci. Rep. 2017, 7, 12046. [Google Scholar] [CrossRef]
- Magistrelli, L.; Croce, R.; De Marchi, F.; Basagni, C.; Carecchio, M.; Nasuelli, N.; Cantello, R.; Invernizzi, F.; Garavaglia, B.; Comi, C.; et al. Expanding the genetic spectrum of primary familial brain calcification due to SLC2OA2 mutations: A case series. Neurogenetics 2021, 22, 65–70. [Google Scholar] [CrossRef]
- Kasuga, K.; Konno, T.; Saito, K.; Ishihara, A.; Nishizawa, M.; Ikeuchi, T. A Japanese family with idiopathic basal ganglia calcification with novel SLC20A2 mutation presenting with late-onset hallucination and delusion. J. Neurol. 2014, 261, 242–244. [Google Scholar] [CrossRef]
- Carecchio, M.; Barzaghi, C.; Varrasi, C.; Cantello, R.; Garavaglia, B. Adult-Onset Focal Chorea in Fahr’s Disease Resulting From SLC20A2 Mutation: A Novel Phenotype. Mov. Disord. Clin. Pract. 2015, 2, 79–80. [Google Scholar] [CrossRef]
- Hsu, S.C.; Sears, R.L.; Lemos, R.R.; Quintans, B.; Huang, A.; Spiteri, E.; Nevarez, L.; Mamah, C.; Zatz, M.; Pierce, K.D.; et al. Mutations in SLC20A2 are a major cause of familial idiopathic basal ganglia calcification. Neurogenetics 2013, 14, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Blackburn, P.R.; Rozen, T.D.; van Gerpen, J.A.; Ross, O.A.; Atwal, P.S.; Wszolek, Z.K. Anticipation in a family with primary familial brain calcification caused by an SLC20A2 variant. Neurol. Neurochir. Pol. 2018, 52, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Wu, A. Association between a novel mutation in SLC20A2 and familial idiopathic basal ganglia calcification. PLoS ONE 2013, 8, e57060. [Google Scholar] [CrossRef] [PubMed]
- Lemos, R.R.; Ramos, E.M.; Legati, A.; Nicolas, G.; Jenkinson, E.M.; Livingston, J.H.; Crow, Y.J.; Campion, D.; Coppola, G.; Oliveira, J.R. Update and Mutational Analysis of SLC20A2: A Major Cause of Primary Familial Brain Calcification. Hum. Mutat. 2015, 36, 489–495. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Sun, H.; Zhang, S.; Zhang, J.; Wang, Y.; Fang, H.; Xu, Y. Generation of induced pluripotent stem cell line (ZZUi0012-A) from a patient with Fahr’s disease caused by a novel mutation in SLC20A2 gene. Stem Cell Res. 2019, 35, 101395. [Google Scholar] [CrossRef]
- Huang, Y.T.; Zhang, L.H.; Li, M.F.; Cheng, L.; Zou, G.Y.; Zhou, H.H. A splice site mutation causing exon 6 skipping in SLC20A2 gene in a primary familial brain calcification family. Brain Res. Bull. 2019, 150, 261–265. [Google Scholar] [CrossRef]
- Shen, Y.; Shu, S.; Ren, Y.; Xia, W.; Chen, J.; Dong, L.; Ge, H.; Fan, S.; Shi, L.; Peng, B.; et al. Case Report: Two Novel Frameshift Mutations in SLC20A2 and One Novel Splice Donor Mutation in PDGFB Associated With Primary Familial Brain Calcification. Front. Genet. 2021, 12, 643452. [Google Scholar] [CrossRef]
- Rohani, M.; Poon, Y.Y.; Naranian, T.; Fasano, A. SCL20A2 mutation mimicking fluctuating Parkinson’s disease. Park. Relat. Disord. 2017, 39, 93–94. [Google Scholar] [CrossRef]
- Knowles, J.K.; Santoro, J.D.; Porter, B.E.; Baumer, F.M. Refractory focal epilepsy in a paediatric patient with primary familial brain calcification. Seizure 2018, 56, 50–52. [Google Scholar] [CrossRef]
- Li, M.; Fu, Q.; Xiang, L.; Zheng, Y.; Ping, W.; Cao, Y. SLC20A2-Associated Idiopathic basal ganglia calcification (Fahr disease): A case family report. BMC Neurol. 2022, 22, 438. [Google Scholar] [CrossRef] [PubMed]
- Taglia, I.; Mignarri, A.; Olgiati, S.; Menci, E.; Petrocelli, P.L.; Breedveld, G.J.; Scaglione, C.; Martinelli, P.; Federico, A.; Bonifati, V.; et al. Primary familial brain calcification: Genetic analysis and clinical spectrum. Mov. Disord. 2014, 29, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Paucar, M.; Almqvist, H.; Jelic, V.; Hagman, G.; Jorneskog, G.; Holmin, S.; Bjorkhem, I.; Svenningsson, P. A SLC20A2 gene mutation carrier displaying ataxia and increased levels of cerebrospinal fluid phosphate. J. Neurol. Sci. 2017, 375, 245–247. [Google Scholar] [CrossRef]
- Lemos, R.R.; Oliveira, M.F.; Oliveira, J.R.M. Reporting a new mutation at the SLC20A2 gene in familial idiopathic basal ganglia calcification. Eur. J. Neurol. 2013, 20, e43–e44. [Google Scholar] [CrossRef]
- Uno, A.; Tamune, H.; Kurita, H.; Hozumi, I.; Yamamoto, N. SLC20A2-Associated Idiopathic Basal Ganglia Calcification-Related Recurrent Psychosis Response to Low-Dose Antipsychotics: A Case Report and Literature Review. Cureus 2020, 12, e12407. [Google Scholar] [CrossRef] [PubMed]
- Rubino, E.; Giorgio, E.; Gallone, S.; Pinessi, L.; Orsi, L.; Gentile, S.; Duca, S.; Brusco, A. Novel mutation of SLC20A2 in an Italian patient presenting with migraine. J. Neurol. 2014, 261, 2019–2021. [Google Scholar] [CrossRef]
- Coppola, A.; Hernandez-Hernandez, L.; Balestrini, S.; Krithika, S.; Moran, N.; Hale, B.; Cordivari, C.; Sisodiya, S.M. Cortical myoclonus and epilepsy in a family with a new SLC20A2 mutation. J. Neurol. 2020, 267, 2221–2227. [Google Scholar] [CrossRef]
- Brighina, L.; Saracchi, E.; Ferri, F.; Gagliardi, M.; Tarantino, P.; Morzenti, S.; Musarra, M.; Patassini, M.; Annesi, G.; Ferrarese, C. Fahr’s disease linked to a novel SLC20A2 gene mutation manifesting with dynamic aphasia. Neurodegener. Dis. 2014, 14, 133–138. [Google Scholar] [CrossRef]
- Fjaer, R.; Brodtkorb, E.; Oye, A.M.; Sheng, Y.; Vigeland, M.D.; Kvistad, K.A.; Backe, P.H.; Selmer, K.K. Generalized epilepsy in a family with basal ganglia calcifications and mutations in SLC20A2 and CHRNB2. Eur. J. Med. Genet. 2015, 58, 624–628. [Google Scholar] [CrossRef]
- Bu, W.; Hou, L.; Zhu, M.; Zhang, R.; Zhang, X.; Zhang, X.; Tang, J.; Liu, X. SLC20A2-related primary familial brain calcification with purely acute psychiatric symptoms: A case report. BMC Neurol. 2022, 22, 265. [Google Scholar] [CrossRef]
- Sun, H.; Cao, Z.; Gao, R.; Li, Y.; Chen, R.; Du, S.; Ma, T.; Wang, J.; Xu, X.; Liu, J.Y. Severe brain calcification and migraine headache caused by SLC20A2 and PDGFRB heterozygous mutations in a five-year-old Chinese girl. Mol. Genet. Genom. Med. 2021, 9, e1670. [Google Scholar] [CrossRef]
- Sekine, S.I.; Kondo, T.; Murakami, N.; Imamura, K.; Enami, T.; Shibukawa, R.; Tsukita, K.; Funayama, M.; Inden, M.; Kurita, H.; et al. Induced pluripotent stem cells derived from a patient with familial idiopathic basal ganglia calcification (IBGC) caused by a mutation in SLC20A2 gene. Stem Cell Res. 2017, 24, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Taglia, I.; Formichi, P.; Battisti, C.; Peppoloni, G.; Barghigiani, M.; Tessa, A.; Federico, A. Primary familial brain calcification with a novel SLC20A2 mutation: Analysis of PiT-2 expression and localization. J. Cell. Physiol. 2018, 233, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C.; Redmond, J.; Bradley, D.; Bede, P. Teaching NeuroImage: Primary Familial Brain Calcification in SLC20A2 Genotype. Neurology 2022, 99, 1008–1009. [Google Scholar] [CrossRef]
- Jensen, N.; Schroder, H.D.; Hejbol, E.K.; Fuchtbauer, E.M.; de Oliveira, J.R.; Pedersen, L. Loss of function of Slc20a2 associated with familial idiopathic Basal Ganglia calcification in humans causes brain calcifications in mice. J. Mol. Neurosci. 2013, 51, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.; Schroder, H.D.; Hejbol, E.K.; Thomsen, J.S.; Bruel, A.; Larsen, F.T.; Vinding, M.C.; Orlowski, D.; Fuchtbauer, E.M.; Oliveira, J.R.M.; et al. Mice Knocked Out for the Primary Brain Calcification-Associated Gene Slc20a2 Show Unimpaired Prenatal Survival but Retarded Growth and Nodules in the Brain that Grow and Calcify Over Time. Am. J. Pathol. 2018, 188, 1865–1881. [Google Scholar] [CrossRef]
- Kimura, T.; Miura, T.; Aoki, K.; Saito, S.; Hondo, H.; Konno, T.; Uchiyama, A.; Ikeuchi, T.; Takahashi, H.; Kakita, A. Familial idiopathic basal ganglia calcification: Histopathologic features of an autopsied patient with an SLC20A2 mutation. Neuropathology 2016, 36, 365–371. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Zhang, Y.; Li, Y.; Xu, C.; Peng, Z.; Jia, Y.; Qiao, S.; Zhang, Z.; Shi, L. T-cell infiltration in the central nervous system and their association with brain calcification in Slc20a2-deficient mice. Front. Mol. Neurosci. 2023, 16, 1073723. [Google Scholar] [CrossRef]
- Guerreiro, P.M.; Bataille, A.M.; Parker, S.L.; Renfro, J.L. Active removal of inorganic phosphate from cerebrospinal fluid by the choroid plexus. Am. J. Physiol. Renal. Physiol. 2014, 306, F1275–F1284. [Google Scholar] [CrossRef]
- Jensen, N.; Autzen, J.K.; Pedersen, L. SLC20A2 is critical for maintaining a physiologic inorganic phosphate level in cerebrospinal fluid. Neurogenetics 2016, 17, 125–130. [Google Scholar] [CrossRef]
- Hozumi, I.; Kurita, H.; Ozawa, K.; Furuta, N.; Inden, M.; Sekine, S.I.; Yamada, M.; Hayashi, Y.; Kimura, A.; Inuzuka, T.; et al. Inorganic phosphorus (Pi) in CSF is a biomarker for Slc20a2-associated idiopathic basal ganglia calcification (IBGC1). J. Neurol. Sci. 2018, 388, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, X.P.; Chen, H.T.; Lai, J.H.; Guo, X.X.; Su, H.Z.; Dong, E.L.; Zhang, Q.J.; Wang, N.; Chen, W.J. Novel mutations of PDGFRB cause primary familial brain calcification in Chinese families. J. Hum. Genet. 2017, 62, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Contreras, M.; Baker, M.C.; Finch, N.A.; Nicholson, A.; Wojtas, A.; Wszolek, Z.K.; Ross, O.A.; Dickson, D.W.; Rademakers, R. Genetic screening and functional characterization of PDGFRB mutations associated with basal ganglia calcification of unknown etiology. Hum. Mutat. 2014, 35, 964–971. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Levi, M.; Sorribas, V. Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: Effects of PDGF, TNF-alpha, and Pi. Pflug. Arch. 2009, 458, 1151–1161. [Google Scholar] [CrossRef]
- Kakita, A.; Suzuki, A.; Nishiwaki, K.; Ono, Y.; Kotake, M.; Ariyoshi, Y.; Miura, Y.; Ltoh, M.; Oiso, Y. Stimulation of Na-dependent phosphate transport by platelet-derived growth factor in rat aortic smooth muscle cells. Atherosclerosis 2004, 174, 17–24. [Google Scholar] [CrossRef]
- Sekine, S.I.; Kaneko, M.; Tanaka, M.; Ninomiya, Y.; Kurita, H.; Inden, M.; Yamada, M.; Hayashi, Y.; Inuzuka, T.; Mitsui, J.; et al. Functional evaluation of PDGFB-variants in idiopathic basal ganglia calcification, using patient-derived iPS cells. Sci. Rep. 2019, 9, 5698. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Legati, A.; Nishikawa, T.; Coppola, G. First Japanese family with primary familial brain calcification due to a mutation in the PDGFB gene: An exome analysis study. Psychiatry Clin. Neurosci. 2015, 69, 77–83. [Google Scholar] [CrossRef]
- Nicolas, G.; Jacquin, A.; Thauvin-Robinet, C.; Rovelet-Lecrux, A.; Rouaud, O.; Pottier, C.; Aubriot-Lorton, M.H.; Rousseau, S.; Wallon, D.; Duvillard, C.; et al. A de novo nonsense PDGFB mutation causing idiopathic basal ganglia calcification with laryngeal dystonia. Eur. J. Hum. Genet. 2014, 22, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Keogh, M.J.; Pyle, A.; Daud, D.; Griffin, H.; Douroudis, K.; Eglon, G.; Miller, J.; Horvath, R.; Chinnery, P.F. Clinical heterogeneity of primary familial brain calcification due to a novel mutation in PDGFB. Neurology 2015, 84, 1818–1820. [Google Scholar] [CrossRef]
- Biyajima, M.; Kobayashi, Y.; Nakafuji, K.; Watanabe, R.; Tazawa, K.; Ishii, W.; Satoh, S.; Hoshi, K.; Kurita, H.; Hozumi, I.; et al. Seronegative neuromyelitis optica spectrum disorder in primary familial brain calcification with PDGFB variant. eNeurologicalSci 2022, 27, 100406. [Google Scholar] [CrossRef]
- Yao, X.P.; Wang, C.; Su, H.Z.; Guo, X.X.; Lu, Y.Q.; Zhao, M.; Liu, Y.B.; Lai, J.H.; Chen, H.T.; Wang, N.; et al. Mutation screening of PDGFB gene in Chinese population with primary familial brain calcification. Gene 2016, 597, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, X.; Xu, X.; Huang, B.; Sun, H.; Li, L.; Zhang, M.; Liu, J.Y. A PDGFB mutation causes paroxysmal nonkinesigenic dyskinesia with brain calcification. Mov. Disord. 2017, 32, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Rovelet-Lecrux, A.; Pottier, C.; Martinaud, O.; Wallon, D.; Vernier, L.; Landemore, G.; Chapon, F.; Prieto-Morin, C.; Tournier-Lasserve, E.; et al. PDGFB partial deletion: A new, rare mechanism causing brain calcification with leukoencephalopathy. J. Mol. Neurosci. 2014, 53, 171–175. [Google Scholar] [CrossRef]
- Biancheri, R.; Severino, M.; Robbiano, A.; Iacomino, M.; Del Sette, M.; Minetti, C.; Cervasio, M.; Del Basso De Caro, M.; Striano, P.; Zara, F. White matter involvement in a family with a novel PDGFB mutation. Neurol. Genet. 2016, 2, e77. [Google Scholar] [CrossRef] [PubMed]
- Anheim, M.; Lopez-Sanchez, U.; Giovannini, D.; Richard, A.C.; Touhami, J.; N’Guyen, L.; Rudolf, G.; Thibault-Stoll, A.; Frebourg, T.; Hannequin, D.; et al. XPR1 mutations are a rare cause of primary familial brain calcification. J. Neurol. 2016, 263, 1559–1564. [Google Scholar] [CrossRef]
- Lopez-Sanchez, U.; Nicolas, G.; Richard, A.C.; Maltete, D.; Charif, M.; Ayrignac, X.; Goizet, C.; Touhami, J.; Labesse, G.; Battini, J.L.; et al. Characterization of XPR1/SLC53A1 variants located outside of the SPX domain in patients with primary familial brain calcification. Sci. Rep. 2019, 9, 6776. [Google Scholar] [CrossRef]
- Chelban, V.; Carecchio, M.; Rea, G.; Bowirrat, A.; Kirmani, S.; Magistrelli, L.; Efthymiou, S.; Schottlaender, L.; Vandrovcova, J.; Salpietro, V.; et al. MYORG-related disease is associated with central pontine calcifications and atypical parkinsonism. Neurol. Genet. 2020, 6, e399. [Google Scholar] [CrossRef]
- Zeng, Y.H.; Lin, B.W.; Su, H.Z.; Guo, X.X.; Li, Y.L.; Lai, L.L.; Chen, W.J.; Zhao, M.; Yao, X.P. Mutation Analysis of MYORG in a Chinese Cohort With Primary Familial Brain Calcification. Front. Genet. 2021, 12, 732389. [Google Scholar] [CrossRef]
- Forouhideh, Y.; Muller, K.; Ruf, W.; Assi, M.; Seker, T.; Tunca, C.; Knehr, A.; Strom, T.M.; Gorges, M.; Schradt, F.; et al. A biallelic mutation links MYORG to autosomal-recessive primary familial brain calcification. Brain 2019, 142, e4. [Google Scholar] [CrossRef]
- Ramos, E.M.; Roca, A.; Chumchim, N.; Dokuru, D.R.; Van Berlo, V.; De Michele, G.; Lieto, M.; Tedeschi, E.; De Michele, G.; Coppola, G. Primary familial brain calcification caused by a novel homozygous MYORG mutation in a consanguineous Italian family. Neurogenetics 2019, 20, 99–102. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, F.; Chen, S.; Cen, Z.; Tang, H.; Huang, J.; Xie, F.; Zheng, X.; Yang, D.; Wang, H.; et al. Evaluation of MYORG mutations as a novel cause of primary familial brain calcification. Mov. Disord. 2019, 34, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, P.; Chen, Z.; Jiang, H. A novel mutation in MYORG causes primary familial brain calcification with central neuropathic pain. Clin. Genet. 2019, 95, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Arkadir, D.; Lossos, A.; Rahat, D.; Abu Snineh, M.; Schueler-Furman, O.; Nitschke, S.; Minassian, B.A.; Sadaka, Y.; Lerer, I.; Tabach, Y.; et al. MYORG is associated with recessive primary familial brain calcification. Ann. Clin. Transl. Neurol. 2019, 6, 106–113. [Google Scholar] [CrossRef]
- Fei, B.N.; Su, H.Z.; Yao, X.P.; Ding, J.; Wang, X. Idiopathic basal ganglia calcification associated with new MYORG mutation site: A case report. World J. Clin. Cases 2021, 9, 7169–7174. [Google Scholar] [CrossRef]
- Tekin Orgun, L.; Besen, S.; Sangun, O.; Bisgin, A.; Alkan, O.; Erol, I. First pediatric case with primary familial brain calcification due to a novel variant on the MYORG gene and review of the literature. Brain Dev. 2021, 43, 789–797. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lin, W.C.; Chang, Y.Y.; Lin, T.K.; Lan, M.Y. Brain hypoperfusion and nigrostriatal dopaminergic dysfunction in primary familial brain calcification caused by novel MYORG variants: Case report. BMC Neurol. 2020, 20, 329. [Google Scholar] [CrossRef]
- Kume, K.; Takata, T.; Morino, H.; Matsuda, Y.; Ohsawa, R.; Tada, Y.; Kurashige, T.; Kawakami, H. The first Japanese case of primary familial brain calcification caused by an MYORG variant. J. Hum. Genet. 2020, 65, 917–920. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—Concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Lebouvier, T.; Andaloussi Mae, M.; Konzer, A.; Bergquist, J.; Zarb, Y.; Johansson, B.; Betsholtz, C.; Vanlandewijck, M. Astrocyte-microglial association and matrix composition are common events in the natural history of primary familial brain calcification. Brain Pathol. 2020, 30, 446–464. [Google Scholar] [CrossRef]
- Zarb, Y.; Weber-Stadlbauer, U.; Kirschenbaum, D.; Kindler, D.R.; Richetto, J.; Keller, D.; Rademakers, R.; Dickson, D.W.; Pasch, A.; Byzova, T.; et al. Ossified blood vessels in primary familial brain calcification elicit a neurotoxic astrocyte response. Brain 2019, 142, 885–902. [Google Scholar] [CrossRef]
- Maheshwari, U.; Huang, S.F.; Sridhar, S.; Keller, A. The Interplay Between Brain Vascular Calcification and Microglia. Front. Aging Neurosci. 2022, 14, 848495. [Google Scholar] [CrossRef] [PubMed]
- Zarb, Y.; Sridhar, S.; Nassiri, S.; Utz, S.G.; Schaffenrath, J.; Maheshwari, U.; Rushing, E.J.; Nilsson, K.P.R.; Delorenzi, M.; Colonna, M.; et al. Microglia control small vessel calcification via TREM2. Sci. Adv. 2021, 7, eabc4898. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.R.; Oliveira, M.F. Primary brain calcification in patients undergoing treatment with the biphosphanate alendronate. Sci. Rep. 2016, 6, 22961. [Google Scholar] [CrossRef] [PubMed]
- Inden, M.; Kurita, H.; Hozumi, I. Characteristics and therapeutic potential of sodium-dependent phosphate cotransporters in relation to idiopathic basal ganglia calcification. J. Pharmacol. Sci. 2022, 148, 152–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).