The Benefits of the Post-Transplant Cyclophosphamide in Both Haploidentical and Mismatched Unrelated Donor Setting in Allogeneic Stem Cells Transplantation
Abstract
:1. Introduction
2. Results
2.1. GvHD

2.2. CMV Reactivation
2.3. Survival
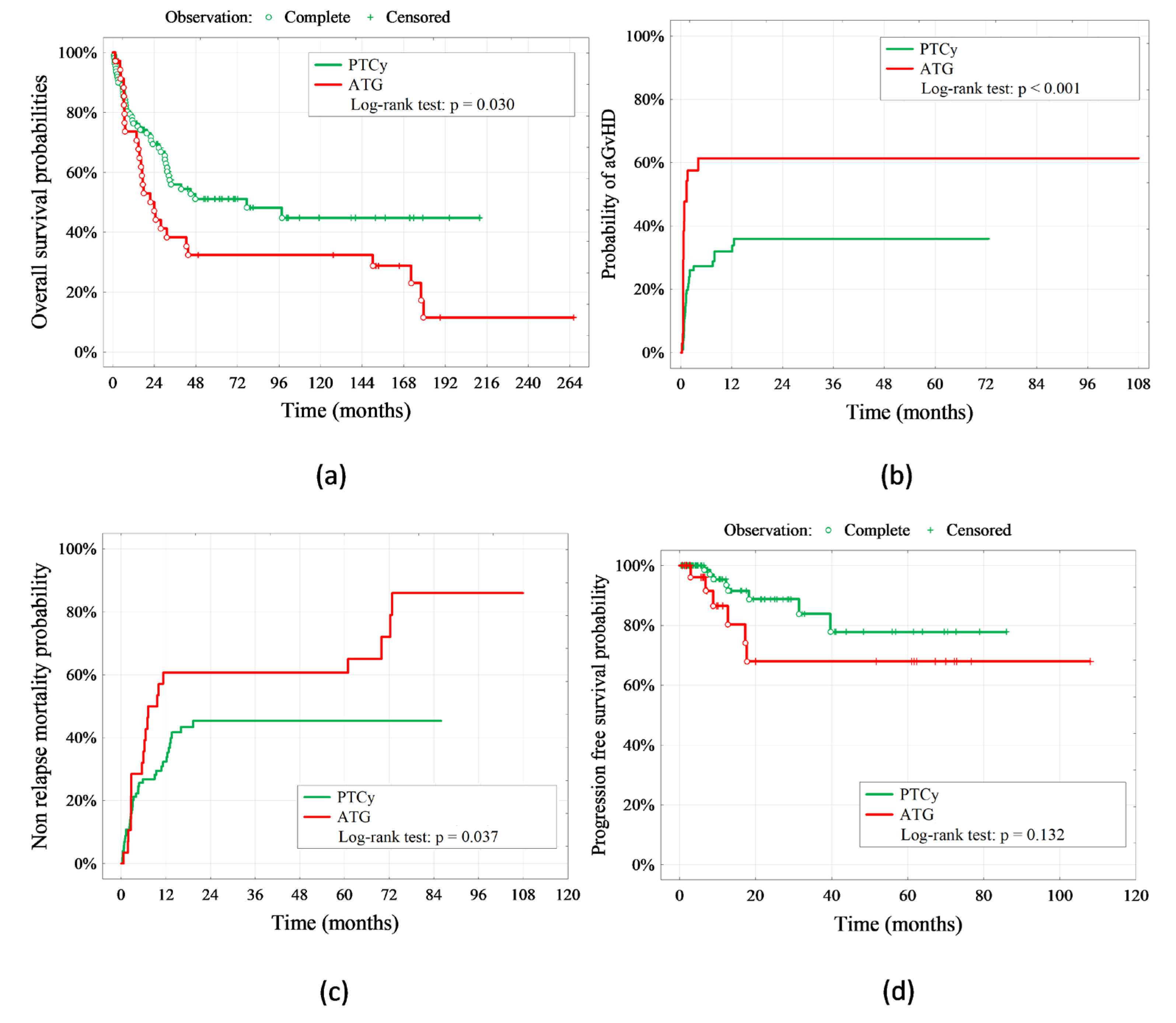
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Conditioning Regimen
4.3. GvHD Prophylaxis
4.4. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw, B.E.; Jimenez-Jimenez, A.M.; Burns, L.J.; Logan, B.R.; Khimani, F.; Shaffer, B.C.; Shah, N.N.; Mussetter, A.; Tang, X.Y.; McCarty, J.M.; et al. National Marrow Donor Program–Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J. Clin. Oncol. 2021, 39, 1971–1982. [Google Scholar] [CrossRef]
- Modi, D.; Kondrat, K.; Kim, S.; Deol, A.; Ayash, L.; Ratanatharathorn, V.; Uberti, J.P. Post-transplant Cyclophosphamide Versus Thymoglobulin in HLA-Mismatched Unrelated Donor Transplant for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Transplant. Cell. Ther. 2021, 27, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Gragert, L.; Eapen, M.; Williams, E.; Freeman, J.; Spellman, S.; Baitty, R.; Hartzman, R.; Rizzo, J.D.; Horowitz, M.; Confer, D.; et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N. Engl. J. Med. 2014, 371, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussetti, A.; Kanate, A.S.; Wang, T.; He, M.; Hamadani, M.; Finel, H.; Boumendil, A.; Glass, B.; Castagna, L.; Blaise, D.; et al. Haploidentical Vs. Matched Unrelated Donor Transplants Using Post-Transplant Cyclophosphamide for Lymphoma: A Joint CIBMTR/EBMT Study. Blood 2021, 138, 174. [Google Scholar] [CrossRef]
- Cho, B.S.; Min, G.J.; Park, S.; Park, S.S.; Shin, S.H.; Yahng, S.A.; Jeon, Y.W.; Yoon, J.H.; Lee, S.E.; Eom, K.S.; et al. Haploidentical vs matched unrelated donor transplantation for acute myeloid leukemia in remission: A prospective comparative study. Am. J. Hematol. 2021, 96, 98–109. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Bader, P.; Bonini, C.; Duarte, R.F.; Dufour, C.; Gennery, A.; Kröger, N.; Kuball, J.; Lanza, F.; et al. Use of haploidentical stem cell transplantation continues to increase: The 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017, 52, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Battipaglia, G.; Galimard, J.E.; Labopin, M.; Raiola, A.M.; Blaise, D.; Ruggeri, A.; Koc, Y.; Gülbas, Z.; Vitek, A.; Sica, S.; et al. Post-transplant cyclophosphamide in one-antigen mismatched unrelated donor transplantation versus haploidentical transplantation in acute myeloid leukemia: A study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2022, 57, 562–571. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Fuchs, E.J.; Carter, S.L.; Karanes, C.; Costa, L.J.; Wu, J.; Devine, S.M.; Wingard, J.R.; Aljitawi, O.S.; Cutler, C.S.; et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011, 118, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.S.; Saliba, R.M.; Ghanem, S.; Alousi, A.M.; Rondon, G.; Anderlini, P.; Al-Atrash, G.; Bashir, Q.; Hosing, C.M.; Im, J.S.; et al. Haploidentical versus Matched Unrelated versus Matched Sibling Donor Hematopoietic Cell Transplantation with Post-Transplantation Cyclophosphamide. Transplant. Cell. Ther. 2022, 28, e1–e395. [Google Scholar] [CrossRef]
- Gaballa, S.; Ge, I.; El Fakih, R.; Brammer, J.E.; Kongtim, P.; Tomuleasa, C.; Wang, S.A.; Lee, D.; Petropoulos, D.; Cao, K.; et al. Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer 2016, 122, 3316–3326. [Google Scholar] [CrossRef] [Green Version]
- Kröger, N.; Zabelina, T.; Binder, T.; Ayuk, F.; Bacher, U.; Amtsfeld, G.; Lellek, H.; Schrum, J.; Erttmann, R.; Eiermann, T.; et al. HLA-Mismatched Unrelated Donors as an Alternative Graft Source for Allogeneic Stem Cell Transplantation after Antithymocyte Globulin-Containing Conditioning Regimen. Biol. Blood Marrow Transplant. 2009, 15, 454–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devillier, R.; Fürst, S.; Crocchiolo, R.; El-Cheikh, J.; Castagna, L.; Harbi, S.; Granata, A.; D’Incan, E.; Coso, D.; Chabannon, C.; et al. A conditioning platform based on fludarabine, busulfan, and 2 days of rabbit antithymocyte globulin results in promising results in patients undergoing allogeneic transplantation from both matched and mismatched unrelated donor. Am. J. Hematol. 2014, 89, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, F.; Diyachenko, G.; Zabelina, T.; Panse, J.; Wolschke, C.; Eiermann, T.; Binder, T.; Fehse, B.; Erttmann, R.; Kabisch, H.; et al. Anti-thymocyte globulin overcomes the negative impact of HLA mismatching in transplantation from unrelated donors. Exp. Hematol. 2008, 36, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Finke, J.; Bethge, W.A.; Schmoor, C.; Ottinger, H.D.; Stelljes, M.; Zander, A.R.; Volin, L.; Ruutu, T.; Heim, D.A.; Schwerdtfeger, R.; et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009, 10, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, M.; Furlong, T.; O’Donnell, P.V.; Storer, B.E.; McCune, J.S.; Storb, R.; Carpenter, P.A.; Flowers, M.E.D.; Appelbaum, F.R.; Martin, P.J. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 2016, 127, 1502–1508. [Google Scholar] [CrossRef] [Green Version]
- Luznik, L.; Bolaños-Meade, J.; Zahurak, M.; Chen, A.R.; Smith, B.D.; Brodsky, R.; Huff, C.A.; Borrello, I.; Matsui, W.; Powell, J.D.; et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010, 115, 3224–3230. [Google Scholar] [CrossRef] [Green Version]
- Kasamon, Y.L.; Ambinder, R.F.; Fuchs, E.J.; Zahurak, M.; Rosner, G.L.; Bolaños-Meade, J.; Levis, M.J.; Gladstone, D.E.; Huff, C.A.; Swinnen, L.J.; et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017, 1, 288–292. [Google Scholar] [CrossRef]
- Jorge, A.S.; Suárez-Lledó, M.; Pereira, A.; Gutierrez, G.; Fernández-Avilés, F.; Rosiñol, L.; Llobet, N.; Solano, T.; Urbano-Ispízua, Á.; Rovira, M.; et al. Single Antigen–Mismatched Unrelated Hematopoietic Stem Cell Transplantation Using High-Dose Post-Transplantation Cyclophosphamide Is a SuiTable Alternative for Patients Lacking HLA-Matched Donors. Biol. Blood Marrow Transplant. 2018, 24, 1196–1202. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.S.; Saliba, R.M.; Chen, J.; Rondon, G.; Hammerstrom, A.E.; Alousi, A.; Qazilbash, M.; Bashir, Q.; Ahmed, S.; Popat, U.; et al. Post-transplantation cyclophosphamide versus conventional graft-versus-host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. Br. J. Haematol. 2016, 173, 444–455. [Google Scholar] [CrossRef] [Green Version]
- Anasetti, C.; Beatty, P.G.; Storb, R.; Martin, P.J.; Mori, M.; Sanders, J.E.; Donnall Thomas, E.; Hansen, J.A. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum. Immunol. 1990, 29, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Petersdorf, E.W.; Anasetti, C.; Martin, P.J.; Gooley, T.; Radich, J.; Malkki, M.; Woolfrey, A.; Smith, A.; Mickelson, E.; Hansen, J.A. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood 2004, 104, 2976–2980. [Google Scholar] [CrossRef] [PubMed]
- Woolfrey, A.; Klein, J.P.; Haagenson, M.; Spellman, S.; Petersdorf, E.; Oudshoorn, M.; Gajewski, J.; Hale, G.A.; Horan, J.; Battiwalla, M.; et al. HLA-C Antigen Mismatch Is Associated with Worse Outcome in Unrelated Donor Peripheral Blood Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2011, 17, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Metheny, L.; de Lima, M. Hematopoietic stem cell transplant with HLA-mismatched grafts: Impact of donor, source, conditioning, and graft versus host disease prophylaxis. Expert Rev. Hematol. 2019, 12, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Carnevale-Schianca, F.; Caravelli, D.; Gallo, S. Post-transplant cyclophosphamide and tacrolimus-mycophenolate mofetil combina- tion prevents graft-versus-host disease in allo- geneic peripheral blood hematopoietic cell transplantation from HLA-matched donors. Biol Blood Marrow Transpl. 2021, 23, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggeri, A.; Labopin, M.; Bacigalupo, A.; Afanasyev, B.; Cornelissen, J.J.; Elmaagacli, A.; Itälä-Remes, M.; Blaise, D.; Meijer, E.; Koc, Y.; et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J. Hematol. Oncol. 2018, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- McCurdy, S.R.; Luznik, L. How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Blood 2019, 134, 1802–1810. [Google Scholar] [CrossRef]
- Camargo, J.F.; Ebisu, Y.; Jimenez-Jimenez, A.; Natori, Y.; Moroz, I.; Morris, M.I.; Alencar, M.; Anderson, A.D.; Lekakis, L.; Beitinjaneh, A.; et al. Lower Incidence of Cytomegalovirus Reactivation Following Post-Transplantation Cyclophosphamide HLA-Mismatched Unrelated Donor Transplantation. Transplant. Cell. Ther. 2021, 27, 1017.e1–1017.e7. [Google Scholar] [CrossRef]
- Goldsmith, S.R.; Abid, M.B.; Auletta, J.J.; Bashey, A.; Beitinjaneh, A.; Castillo, P.; Chemaly, R.F.; Chen, M.; Ciurea, S.; Dandoy, C.E.; et al. Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: A CIBMTR analysis. Blood 2021, 137, 3291–3305. [Google Scholar] [CrossRef]
- Al Malki, M.M.; Tsai, N.C.; Palmer, J.; Mokhtari, S.; Tsai, W.; Cao, T.; Ali, H.; Salhotra, A.; Arslan, S.; Aldoss, I.; et al. Posttransplant cyclophosphamide as GVHD prophylaxis for peripheral blood stem cell HLA-mismatched unrelated donor transplant. Blood Adv. 2021, 5, 2650–2659. [Google Scholar] [CrossRef]
- Hebert, C.; Watts, N.; Isaac, S.; Kukkamalla, R.; Jamy, O.; Saad, A. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation with Post-Transplant Cyclophosphamide. Biol. Blood Marrow Transplant. 2017, 23, S276–S277. [Google Scholar] [CrossRef]
- Massoud, R.; Gagelmann, N.; Fritzsche-Friedland, U.; Zeck, G.; Heidenreich, S.; Wolschke, C.; Ayuk, F.; Christopeit, M.; Kröger, N. Comparison of immune reconstitution between anti-T-lymphocyte globulin and posttransplant cyclophosphamide as acute graft-versus-host disease prophylaxis in allogeneic myeloablative peripheral blood stem cell transplantation. Haematologica 2022, 107, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Nishida, T.; Asano-Mori, Y.; Oshima, K.; Ohashi, K.; Mori, T.; Kanamori, H.; Miyamura, K.; Kato, C.; Kobayashi, N.; et al. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol. Blood Marrow Transplant. 2015, 21, 2008–2016. [Google Scholar] [CrossRef] [Green Version]
- Boeckh, M.; Nichols, W.G. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 2004, 103, 2003–2008. [Google Scholar] [CrossRef] [Green Version]
- Boeckh, M.; Nichols, W.G.; Papanicolaou, G.; Rubin, R.; Wingard, J.R.; Zaia, J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol. Blood Marrow Transplant. 2003, 9, 543–558. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, A.P.; Miller Watelet, L.F.; Linder, T.; Eberly, S.; Raubertas, R.F.; Lipp, J.; Duerst, R.; Abboud, C.N.; Constine, L.; Andrews, J.; et al. Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J. Clin. Oncol. 1999, 17, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.R.; Crawford, J.M.; Cooke, K.R.; Brinson, Y.S.; Pan, L.; Ferrara, J.L.M. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood 1997, 90, 3204–3213. [Google Scholar] [CrossRef]
- Hill, G.R.; Ferrara, J.L.M. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 2000, 95, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.R.; Aubrey, M.T.; Zhang, X.; Jackson, K.C.; Morris, L.E.; Holland, H.K.; Solh, M.M.; Bashey, A. Class II HLA mismatch improves outcomes following haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv. 2020, 4, 5311–5321. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol. Blood Marrow Transplant. 2009, 15, 1628–1633. [Google Scholar] [CrossRef] [Green Version]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from hl-a-matched sibling donors1. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Immunosuppression | p Value | ||||
|---|---|---|---|---|---|
| PTC-y + TAK + MMF n = 110 | CsA + Mtx + ATG n = 35 | ||||
| Age, n (%) | 0.194 | ||||
| <60 years | 89 | 80.9% | 32 | 91.4% | |
| ≥60 years | 21 | 19.1% | 3 | 8.6% | |
| Sex, n (%) | 0.975 | ||||
| Male | 56 | 50.9% | 18 | 51.4% | |
| Female | 54 | 49.1% | 17 | 48.6% | |
| Diagnosis *, n (%) | 0.740 | ||||
| AML + MDS | 52 | 47.3% | 14 | 40.0% | |
| ALL | 15 | 13.6% | 7 | 20.0% | |
| HL + NHL + MM | 31 | 28.2% | 11 | 31.4% | |
| OMF, CML, SAA, et al. | 12 | 10.9% | 3 | 8.6% | |
| The advancement of the disease, n (%) | 0.074 | ||||
| Remission | 72 | 65.5% | 17 | 48.6% | |
| Active | 38 | 34.5% | 18 | 51.4% | |
| CMV IgG *, n (%) | 0.889 | ||||
| Negative | 20 | 18.2% | 6 | 17.1% | |
| Positive | 90 | 81.8% | 29 | 82.9% | |
| Donor age, n (%) | 0.260 | ||||
| <40 years | 67 | 60.9% | 25 | 71.4% | |
| ≥40 years | 43 | 39.1% | 10 | 28.6% | |
| Donor sex, n (%) | 0.718 | ||||
| Male | 75 | 68.2% | 25 | 71.4% | |
| Female | 35 | 31.8% | 10 | 28.6% | |
| Conditioning *, n (%) | 0.070 | ||||
| RIC | 19 | 17.3% | 2 | 5.7% | |
| MAC | 72 | 65.5% | 30 | 85.7% | |
| NMA | 19 | 17.3% | 3 | 8.6% | |
| Unrelated donor, n (%) | <0.001 | ||||
| Haplo | 93 | 84.5% | 0 | 0.0% | |
| MMUD | 17 | 15.5% | 35 | 100.0% | |
| Acute GvHD, n (%) | <0.001 | ||||
| Yes | 40 | 36.4% | 21 | 60.0% | |
| No | 70 | 63.6% | 14 | 40.0% | |
| The degree of acute GvHD, n (%) | 0.005 | ||||
| 0 | 76 | 69.1% | 14 | 40.0% | |
| 1 | 15 | 13.6% | 10 | 28.6% | |
| 2 | 14 | 12.7% | 4 | 11.4% | |
| 3 | 4 | 3.6% | 5 | 14.3% | |
| 4 | 1 | 0.9% | 2 | 5.7% | |
| Chronic GvHD, n (%) | 0.175 | ||||
| Yes | 20 | 18.2% | 3 | 8.6% | |
| No | 90 | 81.8% | 32 | 91.4% | |
| CMV reactivation, n (%) | 0.022 | ||||
| Yes | 51 | 46.4% | 24 | 68.6% | |
| No | 59 | 53.6% | 11 | 31.4% | |
| CMV copy before treatment, n (%) | 0.008 | ||||
| <250 copies | 63 | 57.3% | 11 | 31.4% | |
| ≥250 copies | 47 | 42.7% | 24 | 68.6% | |
| CMV copy after treatment, n (%) | 0.011 | ||||
| <250 copies | 98 | 89.1% | 25 | 71.4% | |
| ≥250 copies | 12 | 10.9% | 10 | 28.6% | |
| Current patient status, n (%) | 0.006 | ||||
| Alive | 67 | 60.9% | 12 | 34.3% | |
| Died | 43 | 39.1% | 23 | 65.7% | |
| Median time of onset of aGvHD, (range) | 35 (8–375) | 18 (9–121) | 0.001 | ||
| 5-years overall survival S (t = 5) | 51.1% | 32.4% | 0.03 | ||
| Median survival function (months) | 35 | 9 | |||
| Median age, y (range) | 46 (20–70) | 45 (19–71) | 0.470 | ||
| Within the MMUD Group | PTC-y + TAK + MMF (n = 16) | CsA + Mtx + ATG (n = 35) | p-Value | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| GvHD, n (%) | 0.136 | - | ||||
| A | 4 | 25.0% | 12 | 34.3% | ||
| B | 5 | 31.1% | 3 | 8.6% | ||
| C | 4 | 25.0% | 12 | 34.3% | ||
| DQ | 1 | 6.3% | 8 | 22.9% | ||
| DQ + A | 1 | 6.3% | 0 | 0.0% | ||
| DQ allel | 1 | 6.3% | 0 | 0.0% | ||
| Patient status, n (%) | 0.003 | |||||
| Alive | 13 | 81.2% | 12 | 34.3% | 8.31 (1.97–34.9) | |
| Died | 3 | 18.8% | 23 | 65.7% | 1.00 (ref.) | |
| Acute GvHD Predictors | aGvHD | p Value | |||
|---|---|---|---|---|---|
| Yes, n = 55 | No, n = 90 | ||||
| Age < 60 years, n (%) | 48 | 87.3% | 73 | 81.1% | 0.460 |
| Female, n (%) | 29 | 52.7% | 42 | 46.7% | 0.479 |
| Diagnosis *, n (%) | 0.768 | ||||
| AML + MDS | 22 | 40.0% | 44 | 48.9% | |
| ALL | 9 | 16.4% | 13 | 14.4% | |
| HL + NHL + MM | 18 | 32.7% | 24 | 26.7% | |
| OMF, CML, SAA, et al. | 6 | 10.9% | 9 | 10.0% | |
| ELN cytogenetic risk *, n (%) | 0.209 | ||||
| Favorable | 0 | 0.0% | 2 | 2.2% | |
| Intermediate | 13 | 23.6% | 17 | 18.9% | |
| Adverse | 5 | 9.1% | 18 | 20.0% | |
| Disease other than AML or MDS | 37 | 67.3% | 53 | 58.9% | |
| Negative CMV IgG *, n (%) | 12 | 21.8% | 14 | 15.6% | 0.340 |
| Donor age ≥ 40 years, n (%) | 26 | 47.3% | 27 | 30.0% | 0.036 |
| Female donor | 18 | 32.7% | 27 | 30.0% | 0.730 |
| HLA locus with a mismatch*, n (%) | 0.288 | ||||
| A | 8 | 14.5% | 8 | 8.9% | |
| B | 3 | 5.5% | 5 | 5.6% | |
| C | 10 | 18.2% | 6 | 6.7% | |
| DQ | 3 | 5.5% | 6 | 6.7% | |
| DQ + A and allel DQ, | 0 | 0.0% | 2 | 2.2% | |
| 0 | 31 | 56.4% | 63 | 70.0% | |
| Positive donor status CMV IgG, n (%) | 42 | 76.4% | 60 | 66.7% | 0.215 |
| Haplo | 31 | 56.4% | 62 | 68.9% | 0.127 |
| CMV reactivation, n (%) | 31 | 56.4% | 44 | 48.9% | 0.382 |
| ≥250 copies CMV before treatment, n (%) | 30 | 54.6% | 41 | 45.6% | 0.293 |
| ≥250 copies CMV after treatment, n (%) | 11 | 20.0% | 11 | 12.2% | 0.205 |
| Remission of the disease, n (%) | 37 | 67.3% | 52 | 57.8% | 0.255 |
| Complete remission number, n (%) | 0.217 | ||||
| 4 | 3 | 5.9% | 0 | 0.0% | |
| 3 | 2 | 3.9% | 3 | 3.5% | |
| 2 | 10 | 19.6% | 14 | 16.3% | |
| 1 | 20 | 39.2% | 36 | 41.9% | |
| GvHD prophylaxis: CsA + Mtx + ATG | 21 | 38.2% | 14 | 15.6% | 0.002 |
| Conditioning *, n (%) | 0.543 | ||||
| RIC | 10 | 18.2% | 11 | 12.2% | |
| MAC | 36 | 65.5% | 66 | 73.3% | |
| NMA | 9 | 16.4% | 13 | 14.4% | |
| Median CD34+ count, × 108/kg (range) | 7.1 [4.9–9.6] | 7.4 [5.4–9.9] | 0.442 | ||
| The first day post-transplant when a total neutrophil count > 0.5 | 17 [15–20] | 19 [15–22] | 0.206 | ||
| Median time between transplant and CMV reactivation, days (range) | 34 [31–48] | 38 [30–49] | 0.601 | ||
| <35 days between transplant and CMV, n (%) | 16 | 51.6% | 13 | 29.6% | 0.053 |
| Chronic GvHD Predictors | cGvHD | p Value | |||
|---|---|---|---|---|---|
| Yes n = 23 | No n = = 122 | ||||
| Age <60 years, n (%) | 16 | 69.6% | 105 | 86.1% | 0.051 |
| Female, n (%) | 11 | 47.8% | 60 | 49.2% | 0.905 |
| Diagnosis *, n (%) | 0.709 | ||||
| AML + MDS | 12 | 52.2% | 54 | 44.3% | |
| ALL | 4 | 17.4% | 18 | 14.8% | |
| HL + NHL + MM | 6 | 26.1% | 36 | 29.5% | |
| OMF, CML, SAA, et al. | 1 | 4.3% | 14 | 11.5% | |
| ELN cytogenetic risk *, n (%) | 0.593 | ||||
| Favorable | 0 | 0.0% | 2 | 1.6% | |
| Intermediate | 7 | 30.4% | 23 | 18.9% | |
| Adverse | 3 | 13.0% | 20 | 16.4% | |
| Disease other than AML or MDS | 13 | 56.5% | 77 | 63.1% | |
| Negative CMV * IgG, n (%) | 3 | 13.0% | 23 | 18.9% | 0.767 |
| Donor age ≥ 40 years, n (%) | 14 | 60.9% | 39 | 32.0% | 0.016 |
| Female donor | 6 | 26.1% | 39 | 32.0% | 0.754 |
| HLA * locus with a mismatch, n (%) | 0.289 | ||||
| A | 0 | 0.0% | 16 | 13.1% | |
| B | 1 | 4.3% | 7 | 5.7% | |
| C | 2 | 8.7% | 14 | 11.5% | |
| DQ | 0 | 0.0% | 9 | 7.4% | |
| DQ + A and allel DQ | 0 | 0.0% | 2 | 1.6% | |
| 0 | 20 | 87.0% | 74 | 60.7% | |
| Positive donor status CMV IgG, n (%) | 15 | 65.2% | 87 | 71.3% | 0.557 |
| Haplo | 20 | 87.0% | 73 | 59.8% | 0.024 |
| CMV reactivation, n (%) | 12 | 52.2% | 63 | 51.6% | 0.962 |
| ≥250 copies CMV before treatment, n (%) | 11 | 47.8% | 60 | 49.2% | 0.905 |
| ≥250 copies CMV after treatment, n (%) | 5 | 21.7% | 17 | 13.9% | 0.348 |
| Remission of the disease, n (%) | 17 | 73.9% | 72 | 59.0% | 0.178 |
| Complete remission number, n (%) | 0.065 | ||||
| 4 | 2 | 10.0% | 1 | 0.9% | |
| 3 | 1 | 5.0% | 4 | 3.4% | |
| 2 | 3 | 15.0% | 21 | 17.9% | |
| 1 | 10 | 50.0% | 46 | 39.3% | |
| GvHD prophylaxis: CsA + Mtx + ATG | 3 | 13.0% | 32 | 26.2% | 0.276 |
| Conditioning *, n (%) | 0.060 | ||||
| RIC | 7 | 30.4% | 14 | 11.5% | |
| MAC | 13 | 56.5% | 89 | 73.0% | |
| NMA | 3 | 13.0% | 19 | 15.6% | |
| Median CD34+ count, × 108/kg (range) | 5.9 [4.9–8.8] | 7.4 [5.4–9.9] | 0.189 | ||
| The first day post-transplant when a total neutrophil count > 0.5 | 17 [15–20] | 18 [15–22] | 0.959 | ||
| Median time between transplant and CMV reactivation, days (range) | 44 [30–80] | 38 [30–49] | 0.422 | ||
| <35 days between transplant and CMV, n (%) | 4 | 33.3% | 25 | 39.7% | 0.757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dybko, J.; Sobczyk-Kruszelnicka, M.; Makuch, S.; Agrawal, S.; Dudek, K.; Giebel, S.; Gil, L. The Benefits of the Post-Transplant Cyclophosphamide in Both Haploidentical and Mismatched Unrelated Donor Setting in Allogeneic Stem Cells Transplantation. Int. J. Mol. Sci. 2023, 24, 5764. https://doi.org/10.3390/ijms24065764
Dybko J, Sobczyk-Kruszelnicka M, Makuch S, Agrawal S, Dudek K, Giebel S, Gil L. The Benefits of the Post-Transplant Cyclophosphamide in Both Haploidentical and Mismatched Unrelated Donor Setting in Allogeneic Stem Cells Transplantation. International Journal of Molecular Sciences. 2023; 24(6):5764. https://doi.org/10.3390/ijms24065764
Chicago/Turabian StyleDybko, Jarosław, Małgorzata Sobczyk-Kruszelnicka, Sebastian Makuch, Siddarth Agrawal, Krzysztof Dudek, Sebatian Giebel, and Lidia Gil. 2023. "The Benefits of the Post-Transplant Cyclophosphamide in Both Haploidentical and Mismatched Unrelated Donor Setting in Allogeneic Stem Cells Transplantation" International Journal of Molecular Sciences 24, no. 6: 5764. https://doi.org/10.3390/ijms24065764






