Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population
Abstract
1. Introduction
2. Material and Methods
3. Sleep-Disordered Breathing and Chronic Respiratory Infections: Pathophysiology and Underlying Mechanisms
- A.
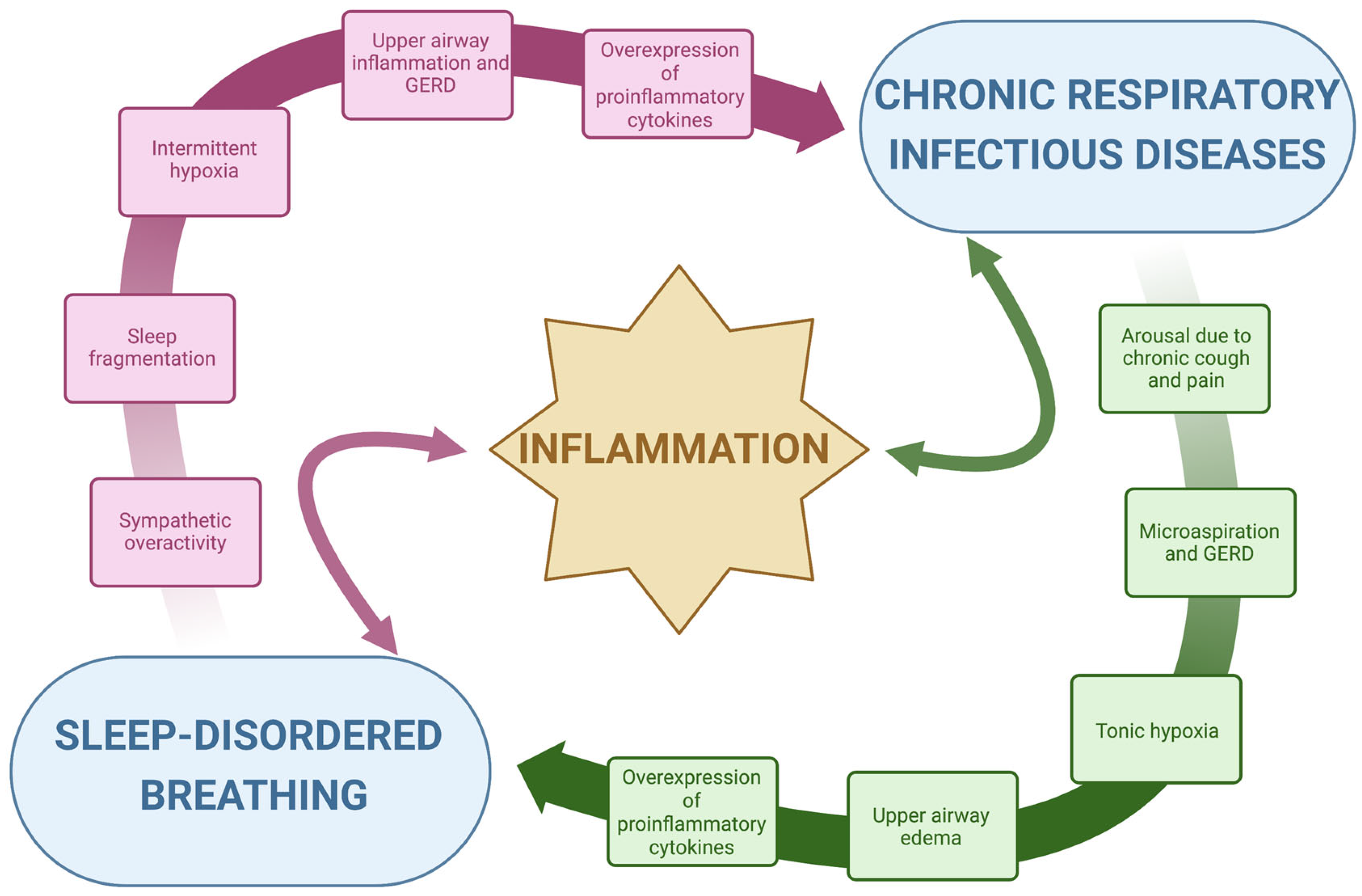
- Inflammation: The repetitive collapse of the pharyngeal airway characteristic of OSA leads to intermittent oxygen desaturation, sleep fragmentation and the consequent activation of the sympathetic nervous system, which is the major contributor to the release of systemic inflammatory mediators, Figure 1 [28,29]. Thus, intermittent hypoxia has been largely linked to major pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6), which constitute the classical prototypes of the large spectrum of systemic inflammation [29]. The subsequent cytokine-mediated inflammatory cascade, coupled with mechanical lung injury, damages the lungs and may worsen several conditions, including chronic respiratory infections [30]. Oxidative stress, such as the structural and functional alteration induced by reactive oxygen species, in response to chronic and intermittent hypoxia, is associated with airway damage [31,32]. In bronchiectasis, chronic bronchial infection and inflammation interact with each other and are responsible for progressive lung damage [33]. Oxidative stress and hypoxia in the airway are induced by the consumption of nutrients by inflammatory cells and bacteria and by a reduced supply of oxygenated blood to damaged lung segments [32]. The overexpression of pro-inflammatory cytokines due to SDB, especially in OSA, might accelerate this process precipitating the evolution of chronic respiratory diseases. On the other hand, the persistent inflammation induced by hypoxia, oxidative stress and chronic infections might predispose to SDB by increasing local phlogosis, gastroesophageal reflux disease (GERD) and, thus, upper airway edema. Upper airway inflammation and edema might increase pharyngeal collapsibility, as it will be described subsequentially. Given these premises, inflammation represents the subject of a bidirectional link between SDB and chronic respiratory infectious diseases, Figure 1. The disease-related chronic overexpression of inflammatory cytokines is enhanced in a vicious circle, with a consequent potential worsening of general clinical conditions.
- B.
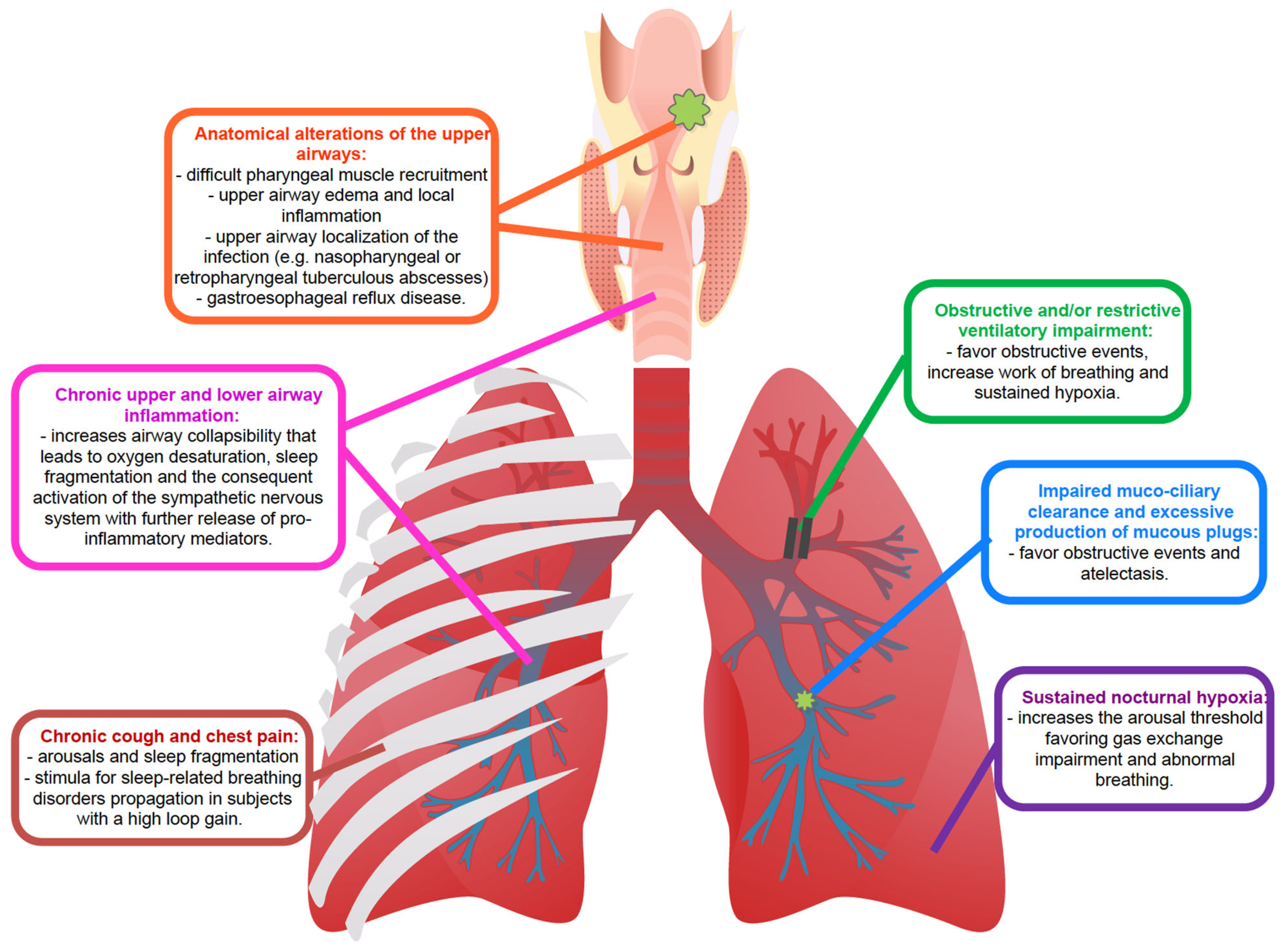
- Anatomy, upper airway edema and local inflammation: The occurrence of upper airway obstruction during sleep reflects an interplay between the removal of the wakefulness drive (which helps to maintain airway patency) and an individual anatomical predisposition with susceptibility to collapse. Pharyngeal muscle relaxation during sleep and lack of sufficient reactivation are key primary pathophysiological events leading to OSA [34]. Interstitial fluid accumulation in the upper part of the body during the night decreases the pharyngeal size and increases pharyngeal resistance and upper airway collapsibility in predisposed individuals [35,36]. A narrow upper airway importantly contributes to the development of OSA, typically worsened by a fat deposit in the parapharyngeal fat pads and pharyngeal muscles, obesity being one of the major risk factors for OSA, or by edema of the upper airway induced by local acute or chronic inflammation [28,37]. In patients with OSA, upper airway tissue is characterized by subepithelial edema and excessive inflammatory cell infiltration [38,39]. Chronic respiratory infectious diseases are characterized by chronic airway inflammation, which also involves the upper airways, GERD and microaspirations, especially at night time [40,41,42]. Acid regurgitation in the upper airway might contribute to the further narrowing of the pharyngeal region by local inflammation. In turn, OSA swings in intrathoracic pressure during apneas increase the pressure gradient between the esophagus and the stomach displacing the gastric contents into the esophagus, determining GERD. This produces a further pharyngeal spasm in patients with OSA and might decrease pharyngeal dilator muscle responsiveness by reducing specific receptors’ sensibility. GERD can also lead to bronchoconstriction or coughing in patients with lung diseases, by causing microaspiration. Additionally, OSA can also affect airway immunity leading to an increased propensity for respiratory tract infection-mediated exacerbations that can progress underlying chronic airway disease [43,44]. Indeed, the presence of upper airway symptoms was shown to increase disease duration and the exacerbation rate in patients with bronchiectasis [45]. Local upper airway inflammation related to chronic respiratory infections might therefore increase pharyngeal collapsibility, inducing pharyngeal narrowing in predisposed individuals.
- C.
- Ventilatory drive instability and loop gain: respiration regulatory disturbances are essential in SDB pathogenesis, as respiratory control plays an essential role in maintaining stable respiration during sleep in healthy humans. The respiratory control system sensitivity is modulated through a negative feedback mechanism called loop gain [46,47,48]. A high loop gain is a marker of breathing instability and determines an exaggerated increase in ventilation in response to minimal changes in blood gas tension. Changes in respiration consequent to obstructive events (reduction in ventilation) or to arousals (increase in ventilation) evoke an exaggerated respiratory response when the loop gain of the subject is high. This response becomes a disturbance itself and will propagate breathing instability, and thus apneas and hypopneas, in predisposed individuals [49]. The cardinal symptom of chronic respiratory infections is chronic productive cough [50,51,52]. Nocturnal cough arouses the subject determining a sudden increase in respiratory rate and carbon dioxide changes. Accordingly, with the loop gain of the subject, the ventilatory drive may induce an increased response that will propagate respiratory instability and respiratory events in predisposed individuals [24]. Given these premises, cough and pain might be stimuli for SDB propagation in subjects with a high loop gain.
- D.
- Arousals and arousal threshold: Arousals contribute to sleep fragmentation and poor sleep quality in subjects with SDB and chronic respiratory diseases and indirectly worsen the predisposition to develop sleep apnea. As mentioned above, recurrent abrupt arousals during sleep may contribute to the exaggerated post-event ventilatory response, reiterating respiratory instability and, thus, SDB [22,53]. The respiratory arousal threshold is the level of inspiratory mechanical effort required to wake up an individual in response to the narrowing of the upper airway during sleep. Although it has been postulated that a low arousal threshold may contribute to the development of OSA in predisposed subjects [24,54], delaying arousals in subjects with poor pharyngeal muscle responsiveness would increase the risk of severe overnight hypoxemia. Sustained isocapnic hypoxia increases the respiratory arousal threshold [55]. Increased arousal threshold together with sustained nocturnal hypoxemia may further impair the normal defense mechanisms that operate to minimize the result of abnormal breathing and gas exchange during sleep. This may have implications for disorders characterized by sustained nocturnal hypoxia, such as chronic respiratory infections, worsening the baseline hypoxic condition.
4. Sleep-Disordered Breathing and Bronchiectasis
5. Sleep-Disordered Breathing and Cystic Fibrosis
6. Sleep-Disordered Breathing and Mycobacterial Infections
7. Consequences of Coexistence of SDB and Chronic Respiratory Infectious Diseases
- A.
- Misdiagnosis: obesity is an important risk factor for OSA since OSA incidence is directly related to increased BMI [96]. Fat deposits in the upper respiratory tract narrow the airway, leading initially to snoring and, subsequently, resulting in sleep apnea with weight gain and worsening of the obstruction. Patients with chronic respiratory infections are generally normal weight or underweight due to persistent chronic infection and inflammation. The absence of snoring, as a reported symptom, and the absence of a typical OSA patient phenotype might reduce the suspicion of clinicians leading to the underestimation of SDB. Moreover, unexplained chronic cough has been reported in patients who snore and who have SDB and, as previously explained, it is also one of the peculiar symptoms of CF, NTM-PD and bronchiectasis [97]. In certain cases, chronic cough can be the sole manifestation of OSA, when specifically investigated by sleep clinicians during a visit [98]. In patients known for having respiratory diseases, such as bronchiectasis or chronic respiratory infections, cough might be explained by these underlying diseases leading to an underestimation of possible OSA symptoms and determining a misdiagnosis [15].
- B.
- Hypoxia: hypoxia has deleterious effects on the cardiovascular system, the central nervous system and all the organs of the human body [99]. Many chronic respiratory diseases, including COPD, interstitial lung diseases and chronic respiratory infectious diseases, determine normobaric hypoxia based on different pathophysiological mechanisms [99]. Susceptible subjects with chronic respiratory comorbidities show a lower SpO2 than healthy subjects, especially during night-time. Lung infections, such as those mediated by mycobacteria, and bronchiectasis are both characterized by ventilation/perfusion mismatch due to regional lack of ventilation with consequent hypoxia [31]. In the airways of patients with CF, chronic hypoxia is also driven by impaired ventilation due to airway mucus obstruction [100]. As a complication of infections, atelectasis further reduces gas exchanges. The presence of SDB, particularly OSA and CSA, can further worsen tonic hypoxia by adding intermittent episodes of oxygen reduction [11,13,14,101,102]. Thus, CF subjects with SDB had lower SpO2 and each unit increase in AHI was associated with a decline in SpO2 nadir [13]. Oxidative stress and inflammatory pathways induced by intermittent hypoxia can be compounded by inflammation due to persistent infections, airway chronic damage and gas exchange alterations due to chronic lung diseases [44,103]. The high rate of underdiagnosed – and undertreated—OSA in conditions of chronic hypoxia such as in chronic respiratory infectious disease, might act as a cofactor in worsening patients’ nocturnal hypoxia and, thus, general clinical conditions. The overlap of the two diseases represents a risk factor itself for exacerbations and an increased susceptibility to worse respiratory outcomes [14,16].
- C.
- Sleep fragmentation: cough and, secondarily, pain are hallmarks of chronic respiratory infections and when presenting at nighttime, are responsible for waking the patient and inducing sleep fragmentation. As a consequent mechanism, sleep fragmentation has effects on cognitive function, and alterations in the neuroendocrine, immune and inflammatory systems [104]. Sleep fragmentation and deterioration of sleep quality, typical of SDB, further complicate the fatigue and physical exhaustion often experienced by patients with chronic lung diseases [12]. Poor sleep quality will also impact the physiological beneficial effects on the immune system attributable to efficient sleep [105]. Moreover, poor sleep has been shown to increase the perception of pain [106]. Mori and coauthors reported worse pain experiences in subjects with poorer sleep quality [12]. OSA-related nocturnal hypoxemia, sleep fragmentation, and systemic inflammation impact pain perception by influencing the anti-nociceptive mechanism and aggravating both chronic and acute pain [107]. Worsening sleep fragmentation and the co-existence of SDB on top of a chronic respiratory infectious disease might increase systemic inflammation and pain perception aggravating the general clinical condition and patient’s quality of life [12,14,16,108].
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHI | apnea-hypopnea index |
| BMI | body mass index |
| CF | cystic fibrosis |
| COPD | chronic obstructive pulmonary disease |
| CSA | central sleep apnea |
| ESS | Epworth sleepiness scale |
| GERD | gastroesophageal reflux disease |
| NIV | non-invasive ventilation |
| NTM | non-tuberculous mycobacterial |
| NTM-PD | non-tuberculous mycobacterial pulmonary disease |
| OSA | obstructive sleep |
| PCD | primary ciliary dyskinesia |
| PSQI | Pittsburgh Sleep Quality Index |
| REM | rapid eye movement |
| SDB | sleep-disordered breathing |
| SpO2 | oxygen saturation |
| TB | tuberculosis |
References
- García-Ortega, A.; Mañas, E.; López-Reyes, R.; Selma, M.J.; García-Sánchez, A.; Oscullo, G.; Jiménez, D.; Martínez-García, M.Á. Obstructive Sleep Apnoea and Venous Thromboembolism: Pathophysiological Links and Clinical Implications. Eur. Respir. J. 2019, 53, 1800893. [Google Scholar] [CrossRef]
- Lyons, O.D.; Bradley, T.D. Heart Failure and Sleep Apnea. Can. J. Cardiol. 2015, 31, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.M. Complications and Consequences of Obstructive Sleep Apnea. Curr. Opin. Pulm. Med. 2000, 6, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet. Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Peppard, P.E.; Nieto, F.J.; Hla, K.M. Burden of Sleep Apnea: Rationale, Design, and Major Findings of the Wisconsin Sleep Cohort Study. WMJ 2009, 108, 246–249. [Google Scholar] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of Sleep-Disordered Breathing in the General Population: The HypnoLaus Study. Lancet. Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Collaco, J.M. Cystic Fibrosis. Pediatr. Rev. 2021, 42, 55–67. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society Guidelines for the Management of Adult Bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Borekci, S.; Sekibag, Y.; Harbiyeli, D.O.; Musellim, B. The Frequency of Obstructive Sleep Apnea in Patients with Non-Cystic Fibrosis Bronchiectasis. Turkish Thorac. J. 2021, 22, 333–338. [Google Scholar] [CrossRef]
- Júnior, N.S.F.; Urbano, J.J.; Santos, I.R.; Silva, A.S.; Perez, E.A.; Souza, H.; Nascimento, O.A.; Jardim, J.R.; Insalaco, G.; Oliveira, L.V.F.; et al. Evaluation of Obstructive Sleep Apnea in Non-Cystic Fibrosis Bronchiectasis: A Cross-Sectional Study. PLoS ONE 2017, 12, e0185413. [Google Scholar] [CrossRef]
- Mori, K.; Tabusadani, M.; Yamane, K.; Takao, S.; Kuroyama, Y.; Matsumura, Y.; Ono, K.; Kawahara, K.; Omatsu, S.; Fujiwara, K.; et al. Effects of Pain on Depression, Sleep, Exercise Tolerance, and Quality of Life in Patients with Nontuberculous Mycobacterial Pulmonary Disease. Medicine 2021, 100, e26249. [Google Scholar] [CrossRef]
- Shakkottai, A.; Nasr, S.Z.; Hassan, F.; Irani, S.; O’Brien, L.M.; Chervin, R.D. Sleep-Disordered Breathing in Cystic Fibrosis. Sleep Med. 2020, 74, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.R.B.; Liberato, F.M.G.; de Freitas Coelho, P.; Vidal, P.D.R.; de Carvalho, R.B.C.O.; Donadio, M.V.F. Sleep-Disordered Breathing and Markers of Morbidity in Children and Adolescents with Cystic Fibrosis. Pediatr. Pulmonol. 2020, 55, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Shakkottai, A.; Irani, S.; Nasr, S.Z.; O’Brien, L.M.; Chervin, R.D. Risk Factors for Obstructive Sleep Apnea in Cystic Fibrosis. Pediatr. Pulmonol. 2022, 57, 926–934. [Google Scholar] [CrossRef]
- Lumertz, M.S.; Pinto, L.A. Sleep-Disordered Breathing in Cystic Fibrosis Pediatric Subjects. Sleep Sci. 2019, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Piper, A.J.; Grunstein, R.R. Obesity Hypoventilation Syndrome: Mechanisms and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 292–298. [Google Scholar] [CrossRef]
- Ward, S.; Chatwin, M.; Heather, S.; Simonds, A.K. Randomised Controlled Trial of Non-Invasive Ventilation (NIV) for Nocturnal Hypoventilation in Neuromuscular and Chest Wall Disease Patients with Daytime Normocapnia. Thorax 2005, 60, 1019–1024. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Hansson, D.; Schiza, S.; Grote, L. Sleep in Chronic Respiratory Disease: COPD and Hypoventilation Disorders. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2019, 28, 190064. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Xie, A.; Patz, D.S.; Wang, D. Physiology in Medicine: Obstructive Sleep Apnea Pathogenesis and Treatment—Considerations beyond Airway Anatomy. J. Appl. Physiol. 2014, 116, 3–12. [Google Scholar] [CrossRef]
- Malhotra, A.; Owens, R.L. What Is Central Sleep Apnea? Respir. Care 2010, 55, 1168–1178. [Google Scholar]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-la-Torre, M.; Campos-Rodriguez, F.; Barbé, F. Obstructive Sleep Apnoea and Cardiovascular Disease. Lancet. Respir. Med. 2013, 1, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining Phenotypic Causes of Obstructive Sleep Apnea. Identification of Novel Therapeutic Targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Wellman, A.; Eckert, D.J.; Jordan, A.S.; Edwards, B.A.; Passaglia, C.L.; Jackson, A.C.; Gautam, S.; Owens, R.L.; Malhotra, A.; White, D.P. A Method for Measuring and Modeling the Physiological Traits Causing Obstructive Sleep Apnea. J. Appl. Physiol. 2011, 110, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Wellman, A.; Edwards, B.A.; Sands, S.A.; Owens, R.L.; Nemati, S.; Butler, J.; Passaglia, C.L.; Jackson, A.C.; Malhotra, A.; White, D.P. A Simplified Method for Determining Phenotypic Traits in Patients with Obstructive Sleep Apnea. J. Appl. Physiol. 2013, 114, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Younes, M. Contributions of Upper Airway Mechanics and Control Mechanisms to Severity of Obstructive Apnea. Am. J. Respir. Crit. Care Med. 2003, 168, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Marin, J.M.; Carrizo, S.J.; Osuna, C.S.; González, R.; Marin-Oto, M.; Forner, M.; Vicente, P.; Cubero, P.; Gil, A.V.; et al. Upper Airway and Systemic Inflammation in Obstructive Sleep Apnoea. Eur. Respir. J. 2016, 48, 1108–1117. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef]
- Goldbart, A.D.; Krishna, J.; Li, R.C.; Serpero, L.D.; Gozal, D. Inflammatory Mediators in Exhaled Breath Condensate of Children with Obstructive Sleep Apnea Syndrome. Chest 2006, 130, 143–148. [Google Scholar] [CrossRef]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and Lung Damage: From Epidemiology to Pathophysiology. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2018, 27, 170077. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Chang, A.B.; Chotirmall, S.H.; Dhar, R.; McShane, P.J. Bronchiectasis. Nat. Rev. Dis. Prim. 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Fuschillo, S.; De Felice, A.; Balzano, G. Mucosal Inflammation in Idiopathic Bronchiectasis: Cellular and Molecular Mechanisms. Eur. Respir. J. 2008, 31, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Horner, R.L.; Hughes, S.W.; Malhotra, A. State-Dependent and Reflex Drives to the Upper Airway: Basic Physiology with Clinical Implications. J. Appl. Physiol. 2014, 116, 325–336. [Google Scholar] [CrossRef]
- Perger, E.; Jutant, E.-M.; Redolfi, S. Targeting Volume Overload and Overnight Rostral Fluid Shift: A New Perspective to Treat Sleep Apnea. Sleep Med. Rev. 2018, 42, 160–170. [Google Scholar] [CrossRef]
- Redolfi, S.; Yumino, D.; Ruttanaumpawan, P.; Yau, B.; Su, M.-C.; Lam, J.; Bradley, T.D. Relationship between Overnight Rostral Fluid Shift and Obstructive Sleep Apnea in Nonobese Men. Am. J. Respir. Crit. Care Med. 2009, 179, 241–246. [Google Scholar] [CrossRef]
- Kim, A.M.; Keenan, B.T.; Jackson, N.; Chan, E.L.; Staley, B.; Poptani, H.; Torigian, D.A.; Pack, A.I.; Schwab, R.J. Tongue Fat and Its Relationship to Obstructive Sleep Apnea. Sleep 2014, 37, 1639–1648. [Google Scholar] [CrossRef]
- Paulsen, F.P.; Steven, P.; Tsokos, M.; Jungmann, K.; Müller, A.; Verse, T.; Pirsig, W. Upper Airway Epithelial Structural Changes in Obstructive Sleep-Disordered Breathing. Am. J. Respir. Crit. Care Med. 2002, 166, 501–509. [Google Scholar] [CrossRef]
- Boyd, J.H.; Petrof, B.J.; Hamid, Q.; Fraser, R.; Kimoff, R.J. Upper Airway Muscle Inflammation and Denervation Changes in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2004, 170, 541–546. [Google Scholar] [CrossRef]
- McDonnell, M.J.; O’Toole, D.; Ward, C.; Pearson, J.P.; Lordan, J.L.; De Soyza, A.; Loebinger, M.; Chalmers, J.D.; Laffey, J.G.; Rutherford, R.M. A Qualitative Synthesis of Gastro-Oesophageal Reflux in Bronchiectasis: Current Understanding and Future Risk. Respir. Med. 2018, 141, 132–143. [Google Scholar] [CrossRef]
- Ledson, M.J.; Wilson, G.E.; Tran, J.; Walshaw, M.J. Tracheal Microaspiration in Adult Cystic Fibrosis. J. R. Soc. Med. 1998, 91, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Lee, J.S.; He, Z.; Ryu, J.H. Reflux-Aspiration in Chronic Lung Disease. Ann. Am. Thorac. Soc. 2020, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.D.; Walters, E.H.; Simpson, J.L.; Keely, S.; Wark, P.A.B.; O’Toole, R.F.; Hansbro, P.M. Hypoxia-Inducible Factor and Bacterial Infections in Chronic Obstructive Pulmonary Disease. Respirology 2020, 25, 53–63. [Google Scholar] [CrossRef]
- Locke, B.W.; Lee, J.J.; Sundar, K.M. OSA and Chronic Respiratory Disease: Mechanisms and Epidemiology. Int. J. Environ. Res. Public Health 2022, 19, 5473. [Google Scholar] [CrossRef]
- Shteinberg, M.; Nassrallah, N.; Jrbashyan, J.; Uri, N.; Stein, N.; Adir, Y. Upper Airway Involvement in Bronchiectasis Is Marked by Early Onset and Allergic Features. ERJ Open Res. 2018, 4, 00115–2017. [Google Scholar] [CrossRef]
- Javaheri, S.; Kazemi, H. Metabolic Alkalosis and Hypoventilation in Humans. Am. Rev. Respir. Dis. 1987, 136, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Khoo, M.C.; Kronauer, R.E.; Strohl, K.P.; Slutsky, A.S. Factors Inducing Periodic Breathing in Humans: A General Model. J. Appl. Physiol. 1982, 53, 644–659. [Google Scholar] [CrossRef]
- Ghazanshahi, S.D.; Khoo, M.C. Estimation of Chemoreflex Loop Gain Using Pseudorandom Binary CO2 Stimulation. IEEE Trans. Biomed. Eng. 1997, 44, 357–366. [Google Scholar] [CrossRef]
- White, D.P. Pathogenesis of Obstructive and Central Sleep Apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 1363–1370. [Google Scholar] [CrossRef]
- Rosen, M.J. Chronic Cough Due to Bronchiectasis: ACCP Evidence-Based Clinical Practice Guidelines. Chest 2006, 129, 122S–131S. [Google Scholar] [CrossRef]
- Penketh, A.R.; Wise, A.; Mearns, M.B.; Hodson, M.E.; Batten, J.C. Cystic Fibrosis in Adolescents and Adults. Thorax 1987, 42, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.D. Cough in Pulmonary Tuberculosis: Existing Knowledge and General Insights. Pulm. Pharmacol. Ther. 2019, 55, 89–94. [Google Scholar] [CrossRef]
- Horner, R.L.; Sanford, L.D.; Pack, A.I.; Morrison, A.R. Activation of a Distinct Arousal State Immediately after Spontaneous Awakening from Sleep. Brain Res. 1997, 778, 127–134. [Google Scholar] [CrossRef]
- Malhotra, A.; Mesarwi, O.; Pepin, J.-L.; Owens, R.L. Endotypes and Phenotypes in Obstructive Sleep Apnea. Curr. Opin. Pulm. Med. 2020, 26, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Hlavac, M.C.; Catcheside, P.G.; McDonald, R.; Eckert, D.J.; Windler, S.; McEvoy, R.D. Hypoxia Impairs the Arousal Response to External Resistive Loading and Airway Occlusion during Sleep. Sleep 2006, 29, 624–631. [Google Scholar]
- Júnior, N.S.F.; Oliveira, L.V.F.; Perez, E.A.; De Oliveira, E.F.; Apostolico, N.; Pereira, N.A.; Santos, I.D.R.D.; Urbano, J.J.; Souza, I.D.; Polonio, I.B.; et al. Observational Study of Sleep, Respiratory Mechanics and Quality of Life in Patients with Non-Cystic Fibrosis Bronchiectasis: A Protocol Study. BMJ Open 2015, 5, e008183. [Google Scholar] [CrossRef]
- Radovanovic, D.; Santus, P.; Blasi, F.; Sotgiu, G.; D’Arcangelo, F.; Simonetta, E.; Contarini, M.; Franceschi, E.; Goeminne, P.C.; Chalmers, J.D.; et al. A Comprehensive Approach to Lung Function in Bronchiectasis. Respir. Med. 2018, 145, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guan, W.; Xu, G.; Lin, Z.; Tang, Y.; Lin, Z.; Li, H.; Gao, Y.; Luo, Q.; Zhong, N.; et al. Sleep Disturbances and Health-Related Quality of Life in Adults with Steady-State Bronchiectasis. PLoS ONE 2014, 9, e102970. [Google Scholar] [CrossRef] [PubMed]
- Erdem, E.; Ersu, R.; Karadag, B.; Karakoc, F.; Gokdemir, Y.; Ay, P.; Akpinar, I.N.; Dagli, E. Effect of Night Symptoms and Disease Severity on Subjective Sleep Quality in Children with Non-Cystic-Fibrosis Bronchiectasis. Pediatr. Pulmonol. 2011, 46, 919–926. [Google Scholar] [CrossRef]
- Yang, X.; Tang, X.; Cao, Y.; Dong, L.; Wang, Y.; Zhang, J.; Cao, J. The Bronchiectasis in COPD-OSA Overlap Syndrome Patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 605–611. [Google Scholar] [CrossRef]
- Oktem, S.; Karadag, B.; Erdem, E.; Gokdemir, Y.; Karakoc, F.; Dagli, E.; Ersu, R. Sleep Disordered Breathing in Patients with Primary Ciliary Dyskinesia. Pediatr. Pulmonol. 2013, 48, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, F.; Esposito, M.; Montella, S.; Cantone, E.; Mollica, C.; De Stefano, S.; Mirra, V.; Carotenuto, M. Sleep Disordered Breathing and Airway Disease in Primary Ciliary Dyskinesia. Respirology 2014, 19, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Gileles-Hillel, A.; Cohen-Cymberknoh, M.; Rosen, D.; Kerem, E.; Gozal, D.; Forno, E. Sleep Disorders in Cystic Fibrosis: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2020, 51, 101279. [Google Scholar] [CrossRef] [PubMed]
- Perin, C.; Fagondes, S.C.; Casarotto, F.C.; Pinotti, A.F.F.; Barreto, S.S.M.; Dalcin, P.d.T.R. Sleep Findings and Predictors of Sleep Desaturation in Adult Cystic Fibrosis Patients. Sleep Breath. 2012, 16, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Shakkottai, A.; O’Brien, L.M.; Nasr, S.Z.; Chervin, R.D. Sleep Disturbances and Their Impact in Pediatric Cystic Fibrosis. Sleep Med. Rev. 2018, 42, 100–110. [Google Scholar] [CrossRef]
- Spicuzza, L.; Sciuto, C.; Leonardi, S.; La Rosa, M. Early Occurrence of Obstructive Sleep Apnea in Infants and Children with Cystic Fibrosis. Arch. Pediatr. Adolesc. Med. 2012, 166, 1165–1169. [Google Scholar] [CrossRef]
- Villa, M.P.; Pagani, J.; Lucidi, V.; Palamides, S.; Ronchetti, R. Nocturnal Oximetry in Infants with Cystic Fibrosis. Arch. Dis. Child. 2001, 84, 50–54. [Google Scholar] [CrossRef]
- Reiter, J.; Breuer, O.; Cohen-Cymberknoh, M.; Forno, E.; Gileles-Hillel, A. Sleep in Children with Cystic Fibrosis: More under the Covers. Pediatr. Pulmonol. 2022, 57, 1944–1951. [Google Scholar] [CrossRef]
- Jagpal, S.K.; Jobanputra, A.M.; Ahmed, O.H.; Santiago, T.V.; Ramagopal, M. Sleep-Disordered Breathing in Cystic Fibrosis. Pediatr. Pulmonol. 2021, 56 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef]
- Milross, M.A.; Piper, A.J.; Dobbin, C.J.; Bye, P.T.P.; Grunstein, R.R. Sleep Disordered Breathing in Cystic Fibrosis. Sleep Med. Rev. 2004, 8, 295–308. [Google Scholar] [CrossRef]
- de Sousa, L.P.; Liberato, F.M.G.; Vendrusculo, F.M.; Donadio, M.V.F.; Barbosa, R.R.B. Obstructive Sleep Apnea in Children and Adolescents with Cystic Fibrosis and Preserved Lung Function or Mild Impairment: A Systematic Review and Meta-Analysis of Prevalence. Sleep Med. 2021, 88, 36–43. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of Obstructive Sleep Apnea in the General Population: A Systematic Review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Milross, M.A.; Piper, A.J.; Norman, M.; Willson, G.N.; Grunstein, R.R.; Sullivan, C.E.; Bye, P.T. Predicting Sleep-Disordered Breathing in Patients with Cystic Fibrosis. Chest 2001, 120, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Welsner, M.; Dietz-Terjung, S.; Stehling, F.; Schulte, T.; Niehammer, U.; Gahbiche, F.-E.; Taube, C.; Strassburg, S.; Schoebel, C.; Weinreich, G.; et al. Obstructive Sleep Apnea and Nocturnal Hypoxemia in Adult Patients with Cystic Fibrosis. BMC Pulm. Med. 2022, 22, 446. [Google Scholar] [CrossRef] [PubMed]
- McKone, E.F.; Ariti, C.; Jackson, A.; Zolin, A.; Carr, S.B.; Orenti, A.; van Rens, J.G.; Lemonnier, L.; Macek, M.J.; Keogh, R.H.; et al. Survival Estimates in European Cystic Fibrosis Patients and the Impact of Socioeconomic Factors: A Retrospective Registry Cohort Study. Eur. Respir. J. 2021, 58, 2002288. [Google Scholar] [CrossRef] [PubMed]
- Kutney, K.A.; Sandouk, Z.; Desimone, M.; Moheet, A. Obesity in Cystic Fibrosis. J. Clin. Transl. Endocrinol. 2021, 26, 100276. [Google Scholar] [CrossRef]
- Bouka, A.; Tiede, H.; Liebich, L.; Dumitrascu, R.; Hecker, C.; Reichenberger, F.; Mayer, K.; Seeger, W.; Schulz, R. Quality of Life in Clinically Stable Adult Cystic Fibrosis Out-Patients: Associations with Daytime Sleepiness and Sleep Quality. Respir. Med. 2012, 106, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Veronezi, J.; Carvalho, A.P.; Ricachinewsky, C.; Hoffmann, A.; Kobayashi, D.Y.; Piltcher, O.B.; Abreu e Silva, F.A.; Martinez, D. Sleep-Disordered Breathing in Patients with Cystic Fibrosis. J. Bras. Pneumol. Publicacao Of. Soc. Bras. Pneumol. Tisilogia 2015, 41, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.T.T.; Salles, C.; Gregório, P.B.; Barros, A.T.; Santana, A.; Araújo-Filho, J.B.; Acosta, A.X. Evaluation of the Upper Airway in Children and Adolescents with Cystic Fibrosis and Obstructive Sleep Apnea Syndrome. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1780–1785. [Google Scholar] [CrossRef]
- Katz, E.S. Cystic Fibrosis and Sleep. Clin. Chest Med. 2014, 35, 495–504. [Google Scholar] [CrossRef]
- Young, A.C.; Wilson, J.W.; Kotsimbos, T.C.; Naughton, M.T. Randomised Placebo Controlled Trial of Non-Invasive Ventilation for Hypercapnia in Cystic Fibrosis. Thorax 2008, 63, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, L.E.; Belcher, J.; Bright-Thomas, R.J. Non-Invasive Ventilation Is Associated with Long-Term Improvements in Lung Function and Gas Exchange in Cystic Fibrosis Adults with Hypercapnic Respiratory Failure. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2021, 20, e40–e45. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-Estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, E.D.D.; Gil-Santana, L.; Ramalho, D.; Tonomura, E.; Silva, E.C.; Oliveira, M.M.; Andrade, B.B.; Kritski, A. Associations between Systemic Inflammation, Mycobacterial Loads in Sputum and Radiological Improvement after Treatment Initiation in Pulmonary TB Patients from Brazil: A Prospective Cohort Study. BMC Infect. Dis. 2016, 16, 368. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Dorhoi, A. Inflammation in Tuberculosis: Interactions, Imbalances and Interventions. Curr. Opin. Immunol. 2013, 25, 441–449. [Google Scholar] [CrossRef]
- Sharma, S.K.; Upadhyay, V. Epidemiology, Diagnosis & Treatment of Non-Tuberculous Mycobacterial Diseases. Indian J. Med. Res. 2020, 152, 185–226. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Marras, T.K.; Adjemian, J.; Zhang, H.; Wang, P.; Zhang, Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large U.S. Managed Care Health Plan, 2008–2015. Ann. Am. Thorac. Soc. 2020, 17, 178–185. [Google Scholar] [CrossRef]
- Faverio, P.; Stainer, A.; Bonaiti, G.; Zucchetti, S.C.; Simonetta, E.; Lapadula, G.; Marruchella, A.; Gori, A.; Blasi, F.; Codecasa, L.; et al. Characterizing Non-Tuberculous Mycobacteria Infection in Bronchiectasis. Int. J. Mol. Sci. 2016, 17, 1913. [Google Scholar] [CrossRef]
- Vadakkan Devassy, T.; Ps, N.; Sharma, D.; Thomas, A.M. Sleep Disorders in Elderly Population Suffering from TB and Respiratory Diseases. Indian J. Tuberc. 2022, 69 (Suppl. S2), S272–S279. [Google Scholar] [CrossRef]
- Lee, T.; Tsai, M.-J.; Chung, Y.-C.; Huang, H.-L.; Chang, W.-A.; Chong, I.-W.; Huang, M.-S. Association between Sleep Apnea and Tuberculosis—A Nationwide Population-Based Study. Eur. Respir. J. 2014, 44, P2658. [Google Scholar]
- Patel, A.B.; Hinni, M.L. Tuberculous Retropharyngeal Abscess Presenting with Symptoms of Obstructive Sleep Apnea. Eur. Arch. Oto-Rhino-Laryngology 2013, 270, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.F.; Tezer, M.S.; Titiz, A.; Özlügedik, S.; Yalçin, F.; Ünal, A. Snoring and Obstructive Sleep Apnea Due to Nasopharyngeal Tuberculosis. Gazi Med. J. 2005, 16, 47–49. [Google Scholar]
- Sharma, H.S.; Kurl, D.N.; Kamal, M.Z. Tuberculoid Granulomatous Lesion of the Pharynx--Review of the Literature. Auris. Nasus. Larynx 1998, 25, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Tatsumi, K.; Kimura, H.; Honda, Y.; Kuriyama, T. Sleep Oxygen Desaturation in Late Sequelae of Pulmonary Tuberculosis. Intern. Med. 1996, 35, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Tabusadani, M.; Yamane, K.; Takao, S.; Kuroyama, Y.; Mori, K.; Ono, K.; Kawahara, K.; Omatsu, S.; Furuuchi, K.; et al. Prevalence of and Risk Factors for Depressive Symptoms in Non-Tuberculous Mycobacterial Pulmonary Disease. Int. J. Tuberc. Lung Dis. 2022, 26, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; McNicholas, W.T.; Montserrat, J.M.; Eckel, J. Adipose Tissue in Obesity and Obstructive Sleep Apnoea. Eur. Respir. J. 2012, 39, 746–767. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.Y.; Ing, A.J.; Laks, L.; Cossa, G.; Rogers, P.; Birring, S.S. Chronic Cough in Patients with Sleep-Disordered Breathing. Eur. Respir. J. 2010, 35, 368–372. [Google Scholar] [CrossRef]
- Birring, S.S.; Ing, A.J.; Chan, K.; Cossa, G.; Matos, S.; Morgan, M.D.L.; Pavord, I.D. Obstructive Sleep Apnoea: A Cause of Chronic Cough. Cough 2007, 3, 7. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Montgomery, S.T.; Mall, M.A.; Kicic, A.; Stick, S.M. Hypoxia and Sterile Inflammation in Cystic Fibrosis Airways: Mechanisms and Potential Therapies. Eur. Respir. J. 2017, 49, 1600903. [Google Scholar] [CrossRef]
- Perger, E.; Baillieul, S.; Esteve, F.; Pichon, A.; Bilo, G.; Soranna, D.; Doutreleau, S.; Savina, Y.; Ulliel-Roche, M.; Brugniaux, J.V.; et al. Nocturnal Hypoxemia, Blood Pressure, Vascular Status and Chronic Mountain Sickness in the Highest City in the World. Ann. Med. 2022, 54, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Perger, E.; Soranna, D.; Pengo, M.; Meriggi, P.; Lombardi, C.; Parati, G. Sleep-Disordered Breathing among Hospitalized Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, K.; Liu, K.; Li, Z.; Yang, J.; Dong, Y.; Nie, M.; Chen, J.; Ruan, Y.; Kang, J. Obstructive Sleep Apnea Exacerbates Airway Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Sleep Med. 2015, 16, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Faraut, B.; Boudjeltia, K.Z.; Vanhamme, L.; Kerkhofs, M. Immune, Inflammatory and Cardiovascular Consequences of Sleep Restriction and Recovery. Sleep Med. Rev. 2012, 16, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and Immune Function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Allen, J.M.; Graef, D.M.; Ehrentraut, J.H.; Tynes, B.L.; Crabtree, V.M. Sleep and Pain in Pediatric Illness: A Conceptual Review. CNS Neurosci. Ther. 2016, 22, 880–893. [Google Scholar] [CrossRef]
- Kaczmarski, P.; Karuga, F.F.; Szmyd, B.; Sochal, M.; Białasiewicz, P.; Strzelecki, D.; Gabryelska, A. The Role of Inflammation, Hypoxia, and Opioid Receptor Expression in Pain Modulation in Patients Suffering from Obstructive Sleep Apnea. Int. J. Mol. Sci. 2022, 23, 9080. [Google Scholar] [CrossRef]
- Tuleta, I.; Stöckigt, F.; Juergens, U.R.; Pizarro, C.; Schrickel, J.W.; Kristiansen, G.; Nickenig, G.; Skowasch, D. Intermittent Hypoxia Contributes to the Lung Damage by Increased Oxidative Stress, Inflammation, and Disbalance in Protease/Antiprotease System. Lung 2016, 194, 1015–1020. [Google Scholar] [CrossRef]
| Sleep-related breathing disorders (OR sleep quality OR obstructive sleep apnea OR sleep-disordered breathing) AND bronchiectasis (OR non-cystic fibrosis bronchiectasis); |
| Sleep-related breathing disorders (OR sleep quality OR obstructive sleep apnea OR sleep-disordered breathing) AND cystic fibrosis; |
| Sleep-related breathing disorders (OR sleep quality OR obstructive sleep apnea OR sleep-disordered breathing) AND tuberculosis (OR mycobacteria); |
| Sleep-related breathing disorders (OR sleep quality OR obstructive sleep apnea OR sleep-disordered breathing) AND non-tuberculous mycobacteria (OR non-tuberculous mycobacterial pulmonary disease OR mycobacteria other than tuberculosis OR atypical mycobacteria). |
| Author- Journal-Year | Aim | Design | Inclusion/Exclusion Criteria | Study Groups | Outcome Measure | Main Results | Strengths/Limitations/Notes |
|---|---|---|---|---|---|---|---|
| Borekci S, Turk Thorac Journal, 2021 [10] | The objective is to investigate the frequency of OSA and related parameters in patients with NCFB. | Single center, prospective, observational study. | Inclusion criteria:
Exclusion criteria:
| Including patients (n = 75), PSG performed (n = 45), and subjects enrolled (n = 43). |
| The frequency of OSA in patients with NCFB is 55.8% and increases with age. Investigating OSA using PSG is important in NCFB patients, especially at advanced ages. | Limitations:
Strengths:
|
| Faria Júnior NS, Plos One, 2017 [11] | The objective is to describe the physiological variables of sleep in patients with NCFB through PSG and to stratify these patients by the risk of OSA and excessive daytime sleepiness. | Two center, cross-sectional observational study. | Inclusion criteria:
Exclusion criteria:
| Eligible patients (n = 418), Clinical evaluation (n = 50), Subjects analyzed (n = 49). |
| Adult patients with clinically stable NCFB, especially those infected with PA, exhibit EDS and high prevalence of OSA, associated with considerable oxygen desaturation during sleep. | |
| Mori K, Medicine, 2021 [12] | The objective is to determine the prevalence and severity of body pain in patients with NTM-PD. The study also investigates the clinical indicators that contribute to pain. | Single center, retrospective cross-sectional study. | Inclusion criteria:
Exclusion criteria:
| Eligible patients (n = 180), Included and analyzed (n = 114). Divided into two groups: No pain group (n = 54), pain group (n = 60). |
| Approximately 70% of patients with NTM-PD reported experiencing pain, and of these, over 1/3 report moderate to very severe pain. Factors predicting pain included the presence of depressive symptoms, poor sleep quality, and reduced exercise tolerance. | Limitations:
|
| Shakkottai A, Sleep Medicine, 2020 [13] | Assess the frequency and severity of SDB in children and adults with and without CF (1:2), who were referred due to concerns for SDB. | A single-center, retrospective study. | Inclusion criteria:
Exclusion criteria:
| CF group included 29 children and 23 adults; The non-CF group included 58 children and 46 adults. |
| Subjects with vs. without CF had 3 times greater odds of moderate-severe SDB. Nocturnal SpO2 nadir was lower among CF vs. non-CF groups. For every 1-unit increase in AHI, the decline in minimum SpO2 was larger for subjects with vs. without CF. For every 1-unit increase in AHI, the magnitude of the decline in minimum SpO2 was larger for those with low vs. normal FEV1. | Limitations:
|
| Barbosa RRB, Pediatric Pulmonology, 2020 [14] | The objective is to evaluate the presence of SDB among children and adolescents with CF, attempting to identify associations with pulmonary function, nutritional status, days in hospital, and days taking antibiotics. | Single center, cross-sectional observational study. | Inclusion criteria:
| Assessed for eligibility (n = 93), Invited to participate (n = 57), Included and analyzed (n = 31). |
| Children and adolescents with CF show SDB, including OSA (32.3%) and nocturnal hypoxemia (29%). Individuals with nocturnal hypoxemia had lower lung function, worse clinical scores, and higher morbidity. TST with SpO2 less than 90% was associated with the length of hospitalization. | Limitations:
|
| Shakkottai A, Pediatric Pulmonology, 2022 [15] | The objective is to identify demographic and CF-specific risk factors for OSA in a cohort of sleep-laboratory referredpatients with CF. | A single center, retrospective study. | Inclusion criteria:
Exclusion criteria:
| Assessed for eligibility (n = 88), Included and analyzed (n = 74). |
| Key risk factors for OSA may differ between children and adults with CF: upper airway pathology appears important in children, overweight/obese or a crowded oropharynx in adults. Neither snoring, EDS, nor lung disease severity was associated with OSA. | Limitations:
Strengths:
|
| Lumertz MS, Sleep Science, 2019 [16] | Describe the frequency of SDB in pediatric CF patients and evaluate the associations between PSG respiratory parameters and Clinical information. | A single center, retrospective, cross-sectional study | Inclusion criteria:
Exclusion criteria:
| Assessed for eligibility (n = 91), Included and analyzed (n = 16). |
| SDB was frequently observed in children with CF. There was an association between respiratory disease progression markers and sleep breathing parameters in children. Sleep studies appear to be an important tool for the assessment of the respiratory status. | Limitations:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faverio, P.; Zanini, U.; Monzani, A.; Parati, G.; Luppi, F.; Lombardi, C.; Perger, E. Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population. Int. J. Mol. Sci. 2023, 24, 5504. https://doi.org/10.3390/ijms24065504
Faverio P, Zanini U, Monzani A, Parati G, Luppi F, Lombardi C, Perger E. Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population. International Journal of Molecular Sciences. 2023; 24(6):5504. https://doi.org/10.3390/ijms24065504
Chicago/Turabian StyleFaverio, Paola, Umberto Zanini, Anna Monzani, Gianfranco Parati, Fabrizio Luppi, Carolina Lombardi, and Elisa Perger. 2023. "Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population" International Journal of Molecular Sciences 24, no. 6: 5504. https://doi.org/10.3390/ijms24065504
APA StyleFaverio, P., Zanini, U., Monzani, A., Parati, G., Luppi, F., Lombardi, C., & Perger, E. (2023). Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population. International Journal of Molecular Sciences, 24(6), 5504. https://doi.org/10.3390/ijms24065504






