Efficacy and Safety of Plasma Exchange as an Adjunctive Therapy for Rapidly Progressive IgA Nephropathy and Henoch-Schönlein Purpura Nephritis: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Selection Criteria
2.3. Data Abstraction
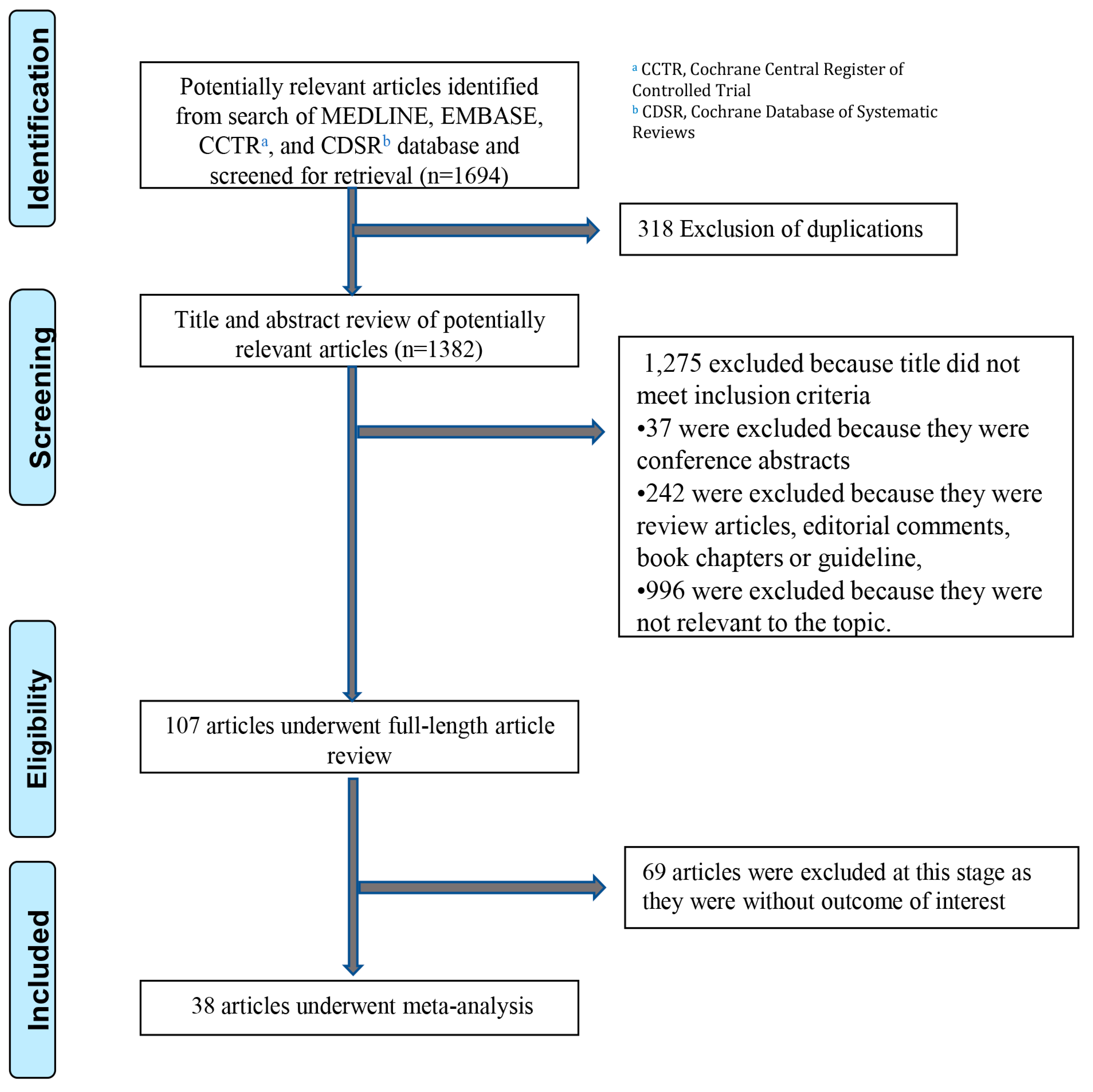
3. Results
3.1. Effect of Plasmapheresis in Native Kidneys with IgA Nephropathy
3.2. Effect of Plasmapheresis in Patients with HSP
3.3. Effect of Plasmapheresis in Patients with Transplanted Kidneys with IgA Nephropathy
3.4. Alveolar Hemorrhage with IgA Nephropathy
3.5. Adverse Events of Plasmapheresis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davin, J.C.; Ten Berge, I.J.; Weening, J.J. What is the difference between IgA nephropathy and Henoch-Schonlein purpura nephritis? Kidney Int. 2001, 59, 823–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.; Ho, K.K.; Szeto, C.C.; Yu, L.; Lai, F.M. Prognostic indicators of IgA nephropathy in the Chinese--clinical and pathological perspectives. Nephrol. Dial. Transpl. 2002, 17, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekaran, A.; Julian, B.A.; Rizk, D.V. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. Am. J. Med. Sci. 2021, 361, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Pattrapornpisut, P.; Avila-Casado, C.; Reich, H.N. IgA Nephropathy: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 78, 429–441. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Haas, M.; Reich, H.N. IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Floege, J. Primary glomerulonephritis: A review of important recent discoveries. Kidney Res. Clin. Pr. 2013, 32, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, R.J.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef] [Green Version]
- Jarrick, S.; Lundberg, S.; Welander, A.; Carrero, J.J.; Hoijer, J.; Bottai, M.; Ludvigsson, J.F. Mortality in IgA Nephropathy: A Nationwide Population-Based Cohort Study. J. Am. Soc. Nephrol. 2019, 30, 866–876. [Google Scholar] [CrossRef]
- Barbour, S.J.; Cattran, D.C.; Kim, S.J.; Levin, A.; Wald, R.; Hladunewich, M.A.; Reich, H.N. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int. 2013, 84, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Knoop, T.; Vikse, B.E.; Svarstad, E.; Leh, S.; Reisaeter, A.V.; Bjorneklett, R. Mortality in patients with IgA nephropathy. Am. J. Kidney Dis. 2013, 62, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Hetland, L.E.; Susrud, K.S.; Lindahl, K.H.; Bygum, A. Henoch-Schonlein Purpura: A Literature Review. Acta Derm. Venereol. 2017, 97, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, J.C.; Tang, S.C.; Chan, D.T.; Lui, S.L.; Lai, K.N. Increased sialylation of polymeric lambda-IgA1 in patients with IgA nephropathy. J. Clin. Lab. Anal. 2002, 16, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; Mazzucco, G.; Cagnoli, L.; Lupo, A.; Schena, F.P. Long-term prognosis of Henoch-Schönlein nephritis in adults and children. Italian Group of Renal Immunopathology Collaborative Study on Henoch-Schönlein purpura. Nephrol. Dial. Transpl. 1997, 12, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Krisanapan, P.; Tangpanithandee, S.; Thongprayoon, C.; Mao, M.A.; Cheungpasitporn, W. Efficacy of extracorporeal plasma therapy for adult native kidney patients with Primary FSGS: A Systematic review. Ren. Fail. 2023, 45, 2176694. [Google Scholar] [CrossRef]
- Sergent, S.R.; Ashurst, J.V. Plasmapheresis. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2022. [Google Scholar]
- Jayne, D.R.; Gaskin, G.; Rasmussen, N.; Abramowicz, D.; Ferrario, F.; Guillevin, L.; Mirapeix, E.; Savage, C.O.; Sinico, R.A.; Stegeman, C.A.; et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J. Am. Soc. Nephrol. 2007, 18, 2180–2188. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.B.; Turner, A.N.; Rees, A.J.; Pusey, C.D. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann. Intern. Med. 2001, 134, 1033–1042. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; Balogun, R.A.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Coppo, R.; Basolo, B.; Giachino, O.; Roccatello, D.; Lajolo, D.; Mazzucco, G.; Amore, A.; Piccoli, G. Plasmapheresis in a patient with rapidly progressive idiopathic IgA nephropathy: Removal of IgA-containing circulating immune complexes and clinical recovery. Nephron 1985, 40, 488–490. [Google Scholar] [CrossRef]
- Díaz-Tejeiro, R.; Maduell, F.; Diez, J.; Esparza, N.; Errasti, P.; Purroy, A.; Pardo, J. Loss of renal graft due to recurrent IgA nephropathy with rapidly progressive course: An unusual clinical evolution. Nephron 1990, 54, 341–343. [Google Scholar] [CrossRef]
- Streather, C.P.; Scoble, J.E. Recurrent IgA nephropathy in a renal allograft presenting as crescentic glomerulonephritis. Nephron 1994, 66, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Afessa, B.; Cowart, R.G.; Koenig, S.M. Alveolar hemorrhage in IgA nephropathy treated with plasmapheresis. South. Med. J. 1997, 90, 237–239. [Google Scholar] [CrossRef] [PubMed]
- McGregor, D.; Lynn, K.L.; Robson, R. Rapidly progressive IgA nephropathy with anti-myeloperoxidase antibodies responding to immunosuppression. Clin. Nephrol. 1998, 50, 64. [Google Scholar] [PubMed]
- Chen, T.C.; Chung, F.R.; Lee, C.H.; Huang, S.C.; Chen, J.B.; Hsu, K.T. Successful treatment of crescentic glomerulonephritis associated with adult-onset Henoch-Schoenlein purpura by double-filtration plasmapheresis. Clin. Nephrol. 2004, 61, 213–216. [Google Scholar] [CrossRef]
- Rech, J.; Fuchs, F.; Kallert, S.; Hueber, A.J.; Requadt, C.; Manger, B.; Kalden, J.R.; Amann, K.; Strauss, R.; Schulze-Koops, H. Plasmapheresis therapy in an elderly patient with rapidly progressive Henoch-Schonlein purpura with disseminated organ involvement. Clin. Rheumatol. 2007, 26, 112–114. [Google Scholar] [CrossRef]
- Fujinaga, S.; Ohtomo, Y.; Umino, D.; Mochizuki, H.; Murakami, H.; Shimizu, T.; Yamashiro, Y.; Kaneko, K. Plasma exchange combined with immunosuppressive treatment in a child with rapidly progressive IgA nephropathy. Pediatr. Nephrol. 2007, 22, 899–902. [Google Scholar] [CrossRef]
- Anantham, D.C.K.; Chuah, K.L.; Vathsala, A.; Eng, P. Pulmonary Capillaritis in IgA Nephropathy. South. Med. J. 2007, 100, 605–607. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Wang, G.; Zhou, Z.; Xun, Z.; Tan, X. Mesangial IgA deposits indicate pathogenesis of anti-glomerular basement membrane disease. Mol. Med. Rep. 2012, 5, 1212–1214. [Google Scholar] [CrossRef] [Green Version]
- Pipili, C.; Pantelias, K.; Papaioannou, N.; Paraskevakou, H.; Grapsa, E. Hemolytic-uremic syndrome, malignant hypertension and IgA nephropathy: Successful treatment with plasma exchange therapy. Transfus. Apher. Sci. 2012, 47, 155–158. [Google Scholar] [CrossRef]
- Herzog, A.L.; Wanner, C.; Amann, K.; Lopau, K. First Treatment of Relapsing Rapidly Progressive IgA Nephropathy With Eculizumab After Living Kidney Donation: A Case Report. Transpl. Proc. 2017, 49, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Takeda, A.; Horike, K.; Inaguma, D.; Goto, N.; Watarai, Y.; Morozumi, K. Early recurrence of active IgA nephropathy after kidney transplantation. Nephrology 2014, 19 (Suppl. 3i), 45–48. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.K.; Lee, S.T.; Cho, H. Plasmaphresis therapy for pulmonary hemorrhage in a pediatric patient with IgA nephropathy. Korean J. Pediatr. 2015, 58, 402–405. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, P.; Ogundare, O.; Raza, A.; Ponnusamy, A.; Gorton, J.; Alachkar, H.; Choudhury, J.; Barratt, J.; Kalra, P.A. Long-Term Therapeutic Plasma Exchange to Prevent End-Stage Kidney Disease in Adult Severe Resistant Henoch-Schonlein Purpura Nephritis. Case Rep. Nephrol. 2015, 2015, 269895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ring, T.; Pedersen, B.B.; Salkus, G.; Goodship, T.H. Use of eculizumab in crescentic IgA nephropathy: Proof of principle and conundrum? Clin. Kidney J. 2015, 8, 489–491. [Google Scholar] [CrossRef] [Green Version]
- Doddi, P.; Gowda, K.; Ramachandran, R.; Nada, R.; Kumar, V.; Rathi, M.; Kohli, H.S.; Gupta, K.L. Plasma exchange in Immunoglobulin A nephropathy with thrombotic microangiopathy and acute cortical necrosis. Indian J. Nephrol. 2016, 26, 42–44. [Google Scholar] [CrossRef]
- Pannu, K.M.M.; McMohan, L. Plasma exchange-resistant atypical hemolytic uremic syndrome treated with eculizumab in a patient with background IgA disease. Nephrology 2016, 21, 268. [Google Scholar]
- Nissaisorakarn, P.; D’Agati, V.; Anis, K.; Jim, B. ANCA and IgA glomerulonephritis all in one: Prognosis and complications. BMJ Case Rep. 2017, 2017, bcr2017222080. [Google Scholar] [CrossRef]
- Soltanpour, K.C.T.; Shanley, P.F.; Khanna, A. A Case of Concurrent Catastrophic Antiphospholipid Syndrome and IGA Nephropathy. In Proceedings of the ASN Kidney Week 2017, New Orleans, LA, USA, 31 October–5 November 2017; American Society of Nephrology: Washington, DC, USA; p. 1127. [Google Scholar]
- Belmar Vega, L.; Fernandez-Diaz, C.; Palmou Fontana, N.; Rodrigo Calabia, E.; Martin Penagos, L.; Arias Rodriguez, M.; Fernandez Fresnedo, G. Pulmonary hemorrhage in a patient with IgA nefropathy. Nefrologia 2017, 37, 347–349. [Google Scholar] [CrossRef]
- Surmeli-Doven, S.; Delibas, A.; Gurses, I.; Kayacan, U.R.; Coskun-Yilmaz, B.; Esen, K.; Korkmaz, E.; Ozaltin, F. Hemolytic uremic syndrome and IgA nephropathy in a child: Coincidence or not? Turk J. Pediatr. 2018, 60, 81–85. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Rajiv, A.; Kumar, S. Pleomorphic presentations of IgA nephropathy-postrenal transplantation. Indian J. Transplant. 2018, 12, 219–223. [Google Scholar] [CrossRef]
- Gani, I.; Kleven, D.; Mulloy, L. Crescentic IgA nephropathy along with simultaneous cellular and antibody-mediated rejection in a kidney transplant leading to rapid allograft failure. Clin. Case Rep. 2019, 7, 1773–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, T.; Hirose, G.; Komatsu, S.; Oshima, T.; Sugisaki, K.; Tomiyasu, T.; Yoshikawa, N.; Yamada, M.; Oda, T. Development of anti-glomerular basement membrane glomerulonephritis during the course of IgA nephropathy: A case report. BMC Nephrol. 2019, 20, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longano, A. Concurrent anti-GBM disease and IgA glomerulonephritis. Pathology 2019, 51, 336–338. [Google Scholar] [CrossRef]
- Bhuwania, P.; Veerappan, I.; Sethuraman, R. A Rare Case of Type 4 Rapidly Progressive Glomerulonephritis (Atypical) with Mesangial IgA Deposits: A Case Report. Indian J. Nephrol. 2021, 31, 488–491. [Google Scholar] [CrossRef]
- Apaydin, H.; Güven, S.C.; Doğan, I.; Çolak, A.; Erten, Ş. ANCA- positive IgA nephropathy presented as alveolar hemorrhage in a COVID-19 patient. Ann. Clin. Anal. Med. 2021, 12, 236–240. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, D.; Wang, W.; Zhao, F.; Zhang, X.; Li, X. Pneumocystis pneumonia secondary to intensive immunosuppression treatment for anti-GBM disease complicated with IgA nephropathy: A case report and literature review. Medicine 2021, 100, e27728. [Google Scholar] [CrossRef]
- Lai, K.N.; Lai, F.M.; Leung, A.C.; Ho, C.P.; Vallance-Owen, J. Plasma exchange in patients with rapidly progressive idiopathic IgA nephropathy: A report of two cases and review of literature. Am. J. Kidney Dis. 1987, 10, 66–70. [Google Scholar] [CrossRef]
- Nicholls, K.; Becker, G.; Walker, R.; Wright, C.; Kincaid-Smith, P. Plasma exchange in progressive IgA nephropathy. J. Clin. Apher. 1990, 5, 128–132. [Google Scholar] [CrossRef]
- Roccatello, D.F.M.; Coppo, R.; Giraudo, G.; Quattrocchio, G.; Piccoli, G. Report on intensive treatment of extracapillary glomerulonephritis with focus on crescentic IgA nephropathy. Nephrol. Dial. Transplant. 1995, 10, 2054–2059. [Google Scholar]
- Gianviti, A.T.R.; Barratt, T.M.; Lythgoe, M.F.; Dillon, M.J. Retrospective study of plasma exchange in patients with idiopathic rapidly progressive glomerulonephritis and vasculitis. Arch. Dis. Child. 1996, 75, 186–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoy, M.; Ognjanovic, M.V.; Coulthard, M.G. Treating severe Henoch-Schonlein and IgA nephritis with plasmapheresis alone. Pediatr. Nephrol. 2007, 22, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Dillon, M.J.; Tullus, K. Childhood vasculitis and plasma exchange. Eur. J. Pediatr. 2007, 166, 145–151. [Google Scholar] [CrossRef]
- Xie, X.; Lv, J.; Shi, S.; Zhu, L.; Liu, L.; Chen, M.; Wang, Y.; Cui, Z.; Wang, X.; Liu, L.; et al. Plasma Exchange as an Adjunctive Therapy for Crescentic IgA Nephropathy. Am. J. Nephrol. 2016, 44, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, M.M.B.; Hall, F.; Rabetoy, G. Plasmapheresis for Crescentic IgA Nephropathy: A Report of Two Cases and Review of the Literature. J. Clin. Apher. 1999, 14, 185–187. [Google Scholar] [CrossRef]
- Rajagopala, S.P.S.; Ajmera, J.S.; Ganesh, R.N.; Katrevula, A. Diffuse alveolar hemorrhage in IgA nephropathy: Case series and systematic review of the literature. Int. J. Rheum. Dis. 2017, 20, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.X.; Zhang, J.J.; Shi, P.P.; Fu, S.Q.; Zhang, L.G.; Wang, M.; Lu, F.X. A clinico-pathological comparison between Henoch-Schonlein purpura nephritis and IgA nephropathy in children. Zhongguo Dang Dai Er Ke Za Zhi 2012, 14, 506–509. [Google Scholar]
- Krzych, L.J.; Czok, M.; Putowski, Z. Is Antimicrobial Treatment Effective During Therapeutic Plasma Exchange? Investigating the Role of Possible Interactions. Pharmaceutics 2020, 12, 395. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Li, J.; Zhang, X.; He, J.; Wang, M.; Lv, J.; Zhang, H. Complement Activation Is Associated With Crescents in IgA Nephropathy. Front. Immunol. 2021, 12, 676919. [Google Scholar] [CrossRef]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Liu, Z.H.; Roberts, I.S.; Yuzawa, Y.; Zhang, H.; et al. Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef] [Green Version]
| Author | Year | Type of Study | n | Country | Age | Sex | HSP | Other Disease | Alveolar Hemorrhage | Crescents | Kidney Transplant | Plasma Exchange Regimen | Additional Treatment | Outcome | Adverse Event | Thrombotic Micro-angiopathy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Coppo [21] | 1985 | Case Report | 1 | Italy | 54 | M | - | - | - | 20% gloms | - | 13 cycles total: 1 session every other day for 3 weeks then weekly sessions for 4 weeks | Steroids Cytoxan | Complete remission Cr clearance improved from 30 mL/min to 120 mL/min Proteinuria 3 g/day to 0.2 g/day at 6-month follow-up | - | - |
| 2 | Tejeiro [22] | 1990 | Case Report | 1 | Spain | 54 | M | - | - | - | +60% gloms | + | 18 cycles total 22 L removed | Steroids Cytoxan | Not reported | Failed transplant and progressed to ESKD | - |
| 3 | Streather [23] | 1994 | Case Report | 1 | UK | 43 | M | - | - | - | +40% gloms | + | 3 sessions with 3 L and 4.5% albumin | Steroids | Continued improvement in Cr | - | - |
| 4 | Affessa [24] | 1997 | Case Report | 1 | USA | 66 | M | - | - | + | + | - | 3× week for 3 weeks | Steroids | Cr 6.9 to 2.8 | Catheter dislodged | - |
| 5 | McGregor [25] | 1998 | Case Report | 1 | New Zealand | 14 | M | - | P-ANCA (MPO) | + | +90% gloms | - | 10 × 2 L exchanges over 3 weeks | Steroids Cytoxan | Cr normal Proteinuria persisted No further pulmonary hemorrhage | - | - |
| 6 | Chen [26] | 2004 | Case Report | 1 | Taiwan | 33 | M | + | - | - | + | - | 9 sessions of double filtration plasmapheresis | Steroids Cytoxan | S Cr from 11.4 to 3.1 | - | - |
| 7 | Rech [27] | 2005 | Case Report | 1 | Germany | 57 | M | + | - | - | - | - | 3 days first week, 2 days second week, 40 mL/kg with FFP | Steroids Cytoxan | HD until “normal serum creatinine” and resolution of proteinuria at 1 year | - | - |
| 8 | Fujinaga [28] | 2006 | Case Report | 1 | Japan | 5 | M | - | - | - | +80% gloms | - | 5 sessions alternating days 50 mL/kg | Steroids Mizoribine | HD discontinued 3 weeks after PLEX | - | - |
| 9 | Anantham [29] | 2007 | Case Report | 1 | Singapore | 20 | M | - | ESKD due to IgAN | + | + | - | Unclear | Cytoxan Steroids | Improvement in pulmonary hemorrhage, ESKD | - | - |
| 10 | Wang [30] | 2011 | Case Report | 1 | China | 31 | F | - | - | - | +14/17 gloms | - | 10 sessions | Steroids Cytoxan | Only mentioned Cr 3.75 after 1 mo therapy | - | - |
| 11 | Pipilli [31] | 2012 | Case Report | 1 | Greece | 35 | M | - | - | - | + | - | 17 sessions | Steroid | Cr from 7 to 2.5 | - | + |
| 12 | Herzog [32] | 2014 | Case Report | 1 | Germany | 28 | M | - | - | - | +7/12 gloms | - | 3 sessions 40 mL/kg | Steroids | ESKD | - | - |
| 13 | Otsuka [33] | 2014 | Case Report | 1 | Japan | 23 | M | - | - | - | - | + 19 days s/p | Double Filtration plasmapheresis | Steroids | Worsening Cr and proteinuria | CMV viremia | - |
| 14 | Yim [34] | 2014 | Case Report | 1 | Korea | 14 | M | - | - | + | +21/45 gloms | - | Daily plasmapheresis; weekly for 3 months | PD Steroids Cytoxan | Pulmonary symptoms resolved but progressed to ESKD | - | + |
| 15 | Hamilton [35] | 2015 | Case Report | 1 | UK | 27 | M | + | - | - | +20% gloms | - | 108 total sessions over 3 years; 2 weeks of daily sessions followed by empiric sessions every 1–2 weeks | Steroids Cytoxan Ritixumab IVIg | Gradual decline in renal function with ESKD at 3 years. Received live renal transplant at 3.5 years with stable Cr of 1.69 | - | - |
| 16 | Ring [36] | 2015 | Case Report | 1 | UK | 16 | M | + | - | - | +6/14 gloms | - | 5 Plasma exchange with 40 mL/kg | Steroids Cytoxan Eculizumab | Not mentioned | No improvement after PLAEX but after Eculizumab, then progressed to ESKD after 2 years | - |
| 17 | Doddi [37] | 2016 | Case Report | 1 | India | 25 | F | - | HUS | - | - | - | 5 sessions alternate day, 40 mL/kg | - | Cr normal in 3 months | - | + |
| 18 | Pannu [38] | 2016 | Case Report | 1 | USA | 25 | M | - | HUS | + | NR | - | PLEX >3 sessions | Eculizumab | Dialysis dependent | - | + |
| 19 | Nissaisorakarn [39] | 2017 | Case Report | 1 | USA | 75 | F | - | ANCA | - | +6/13 gloms | - | 7 sessions every other day | Steroids Cytoxan | ESKD | Influenza A, Herpes Zoster, Rothia bacteremia | - |
| 20 | Soltanpour [40] | 2017 | Case Report | 1 | USA | 42 | M | - | APLS | - | NR | - | PLEX | Steroids | Not reported | Cr improved to 1.9 from 4.5 | + |
| 21 | Vega [41] | 2017 | Case Report | 1 | Spain | 69 | M | + | - | + | - | - | 6 | Steroids IVIG 3 mo | Cr 2.1 to 1.2 (unknown time) | - | - |
| 22 | Sürmeli-Döven [42] | 2018 | Case Report | 1 | Turkey | 1.5 | M | - | HUS | - | - | - | 5 sessions with 1-day intervals | Steroids | Dialysis to Cr 0.52 | - | + |
| 23 | Rajiv [43] | 2018 | Case Report | 1 | India | 26 | M | - | - | - | + | + | 6 sessions | Steroids IVIG Cytoxan | ESKD | - | - |
| 24 | Gani [44] | 2019 | Case Report | 1 | USA | 36 | M | - | Humoral and cell-mediated rejection | - | + | + | 7 sessions | Steroids Thymoglobulin | ESKD | - | - |
| 25 | Kojima [45] | 2019 | Case Report | 1 | Japan | 66 | F | - | Anti GBM | + | +1/18 glom | - | 8 sessions | Steroids | ESKD | - | - |
| 26 | Longano [46] | 2019 | Case Report | 1 | Australia | 22 | M | - | Anti GBM | + | +2/11 gloms | - | 21 sessions | Steroids Cytoxan | Cr remained normal | - | - |
| 27 | Bhuwania [47] | 2020 | Case Report | 1 | India | 58 | F | - | ANCA Anti GBM | - | +M1S1C1 | - | 5 sessions | Steroids Cytoxan (CYCLOPS) | Cr 3.5 to 1.4 at 6 m | - | - |
| 28 | Apaydin [48] | 2021 | Case Report | 1 | Turkey | 18 | M | - | COVID PR3ANCA | + | + | - | Daily sessions for 7 days | Steroids IVIg | Cr from 0.96 to 1.15 | - | - |
| 29 | Zhang [49] | 2021 | Case Report | 1 | China | 41 | F | - | Anti GBM | - | + | - | 6 sessions | Steroids Rituximab, HD × 3 IVIG 12 mo Tacrolimus | HD discontinued, Cr 2.79–1.517 at 28 wk | PCP | - |
| Author | Year | Country /Patient no. | Study Population | Age (Yrs) | Other Disease | Initial Kidney Function | Kidney Biopsy | Treatment Regimen | Additional Treatment | Outcome | Adverse Events | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lai [50] | 1987 | UK | 2 patients; 2F | 21–24 | IgAN HTN | Each patient had a different plasma exchange regimen. | Steroids AZA | Both patients saw temporary improvement in serum creatinine following plasma exchange therapy but kidney function gradually deteriorated despite therapy. | Leukopenia | ||
| 1 | F | 24 | IgAN HTN | sCr 8.22 mg/dL (727 µmol/L) | 20 glomeruli; 13 sclerosed and 7 with fibro-cellular crescents | 4 courses consisting of 4 plasma exchanges on alternating days separated by 2–3 months. The first plasma exchange occurred 2 weeks after symptom onset. | Steroids AZA | sCr: 8.14 mg/dL (720 µmol/L) at 3 weeks 4.58 mg/dL (405 µmol/L) at 1 month 9.61 mg/dL (850 µmol/L) at 4 months 5.76 mg/dL (510 µmol/L) at 6 months 10.29 mg/dL (910 µmol/L) at 7 months 5.66 mg/dL (500 µmol/L) at 10 months 11.31 mg/dL (1000 µmol/L) at 12 months ESKD on HD at 15 month follow up | Leukopenia from AZA | |||
| 2 | F | 21 | IgAN HTN | sCr 8.22 mg/dL (425 µmol/L) | 15 glomeruli; 5 sclerosed 10 with fibro-cellular crescents | 6 plasma exchanges on alternating days 2 months after symptom onset. | Steroids AZA | sCr: 5.09 mg/dL (450 µmol/L) at 2 months 9.61 mg/dL (850 µmol/L) at 3 months 5.77 mg/dL (510 µmol/L) at 5 months 5.66 mg/dL (500 µmol/L) at 7 months 6.78 mg/dL (600 µmol/L) at 9 months 7.35 mg/dL (650 µmol/L) at 12 months Progressive deterioration thereafter | None | |||
| 2 | Nicholls [51] | 1990 | AUS | 14 patients; 11M and 3F | 17–58 | IgAN HTN | All patients had crescents on biopsy with mean of 40% crescents in non-sclerosed glomeruli (median 34%; range 7–80%) No individualized biopsy results were provided | 4 plasma exchanges on consecutive days followed by 3 plasma exchanges weekly for 2 weeks, then weekly plasma exchange until 3 months total duration. | Dipyridamole Cytoxan | 7 patients experienced fall in sCr during treatment protocol while the renal function of the rest progressively deteriorated during the study. However, all patients ultimately experienced decline in renal function after completion of treatment with all but 4 patients requiring HD. The authors did not provide final outcomes for each individual patient. The 7 patients who had improved with plasma exchange experienced a notably slower rate of decline in renal function compared to the other patients. | Acute Tubular Necrosis in 1 patient | |
| 1 | M | 18 | IgAN HTN | sCr 1.81 mg/dL (160 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 2.14 mg/dL (190 µmol/L) at 3 months 2.04 mg/dL (180 µmol/L) at 6 months 2.26 mg/dL (200 µmol/L) at 9 months | ||||||
| 2 | M | 23 | IgAN HTN | sCr 3.73 mg/dL (330 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 4.41 mg/dL (390 µmol/L) at 3 months 4.18 mg/dL (370 µmol/L) at 6 months 4.41 mg/dL (440 µmol/L) at 9 months | ||||||
| 3 | M | 30 | IgAN HTN | sCr 3.95 mg/dL (350 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 6.11 mg/dL (540 µmol/L) at 3 months 5.66 mg/dL (500 µmol/L) at 6 months 7.58 mg/dL (670 µmol/L) at 9 months | ||||||
| 4 | M | 26 | IgAN HTN | sCr 2.26 mg/dL (200 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 2.83 mg/dL (250 µmol/L) at 3 months 1.92 mg/dL (170 µmol/L) at 6 months 2.49 mg/dL (220 µmol/L) at 9 months | ||||||
| 5 | F | 40 | IgAN HTN | sCr 2.83 mg/dL (250 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 10.63 mg/dL (940 µmol/L) at 3 months 7.58 mg/dL (670 µmol/L) at 6 months 20.36 mg/dL (1800 µmol/L) at 9 months | ||||||
| 6 | F | 50 | IgAN HTN | sCr 1.70 mg/dL (150 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 2.37 mg/dL (210 µmol/L) at 3 months 2.03 mg/dL (180 µmol/L) at 6 months 2.26 mg/dL (200 µmol/L) at 9 months | ||||||
| 7 | M | 17 | IgAN HTN | sCr 6.33 mg/dL (560 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 14.37 mg/dL (1270 µmol/L) at 3 months 8.82 mg/dL (780 µmol/L) at 6 months 15.61 mg/dL (1380 µmol/L) at 9 months | ||||||
| 8 | M | 58 | IgAN HTN | sCr 4.75 mg/dL (420 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 5.43 mg/dL (480 µmol/L) at 3 months 5.77 mg/dL (510 µmol/L) at 6 months 7.47 mg/dL (660 µmol/L) at 9 months | ||||||
| 9 | F | 20 | IgAN HTN | sCr 4.52 mg/dL (400 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 5.32 mg/dL (470 µmol/L) at 3 months 8.71 mg/dL (770 µmol/L) at 6 months 12.10 mg/dL (1070 µmol/L) at 9 months | ||||||
| 10 | M | 50 | IgAN HTN | sCr 2.83 mg/dL (250 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 3.28 mg/dL (290 µmol/L) at 3 months 3.28 mg/dL (290 µmol/L) at 6 months 3.39 mg/dL (300 µmol/L) at 9 months | ||||||
| 11 | M | 22 | IgAN HTN | sCr 4.18 mg/dL (370 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 4.18 mg/dL (370 µmol/L) at 3 months 5.43 mg/dL (480 µmol/L) at 6 months 8.03 mg/dL (710 µmol/L) at 9 months | ||||||
| 12 | M | 43 | IgAN HTN | sCr 7.35 mg/dL (650 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 8.03 mg/dL (710 µmol/L) at 3 months 10.29 mg/dL (910 µmol/L) at 6 months 22.51 mg/dL (1990 µmol/L) at 9 months | ||||||
| 13 | M | 23 | IgAN HTN | sCr 3.96 mg/dL (350 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 4.41 mg/dL (390 µmol/L) at 3 months 5.54 mg/dL (490 µmol/L) at 6 months 9.95 mg/dL (880 µmol/L) at 9 months | ||||||
| 14 | M | 44 | IgAN HTN | sCr 2.37 mg/dL (210 µmol/L) | Plasma exchange was initiated 3 months after enrollment. | sCr: 8.48 mg/dL (750 µmol/L) at 3 months 3.39 mg/dL (300 µmol/L) at 6 months 4.41 mg/dL (390 µmol/L) at 9 months | Developed ATN thought to be related to intercurrent surgery during observation period, but it was withdrawn from analysis. | |||||
| 3 | Rocatello [52] | 1995 | Italy | 6 patients; 4M and 2F | 16–61 | IgAN | All patients except controls in IgAN group received 2 month treatment of 15 mg/kg IV methylprednisolone for 3 days followed by 8 weeks of oral prednisone (1 mg/kg for first 4 weeks and 0.75 mg/kg for last 4) Oral cyclophosphamide 2.5 mg/kg/day for 8 weeks. Plasma exchange (6 treatments in 2 weeks followed by weekly PLEX for at least 2 weeks). | Steroids Cytoxan | All patients saw improvement in serum creatinine and urine abnormalities, but 3 patients eventually developed ESKD at long-term follow up. No correlation between urine abnormalities, HTN, sCr, and histological features was found. No clinical or histological parameter was significantly different between patients in the treatment group. | Pneumonia in 1 patient | ||
| 1 | M | 16 | IgAN HTN | sCr 10.0 mg/dL (884 µmol/L) | 10 glomeruli 90% florid crescents and 10% fibrotic crescents 1+ interstitial fibrosis | 14 plasma exchanges in first month with 8 additional sessions by 2 month follow up. | Steroids Cytoxan | sCr: 2.4 mg/dL (212 µmol/L) at 2 months 2.19 mg/dL (194 µmol/L) at 6 months 5.9 mg/dL (522 µmol/L) at 16 months 7.43 mg/dL (657 µmol/L) at 24 months ESKD on HD at 36 month follow up Repeat biopsy at 16 months: 15 glomeruli 65% glomerular hyalinosis 15% florid crescents 1+ interstitial fibrosis 1+ vascular hyalinosis | - | |||
| 2 | M | 44 | IgAN HTN | sCr 1.2 mg/dL (106 µmol/L) | 12 glomeruli 15% glomerular hyalinosis 40% florid crescents 1+ interstitial infiltrates 1+ interstitial fibrosis 1+ vascular hyalinosis | 11 plasma exchanges in first month, no additional sessions. | Steroids Cytoxan | sCr: 1.1 mg/dL (97 µmol/L) at 2 months 1.49 mg/dL (132 µmol/L) at 6 months 1.49 mg/dL (132 µmol/L) at 24 months Repeat biopsy at 2 months: 26 glomeruli 30% glomerular hyalinosis 10% florid crescents 20% fibrotic crescents 1+ interstitial fibrosis 1+ vascular hyalinosis | - | |||
| 3 | F | 61 | IgAN HTN | sCr 7.19 mg/dL (636 µmol/L) | 20 glomeruli 5% glomerular hyalinosis 70% florid crescents 1+ interstitial infiltrates 1+ interstitial fibrosis 1+ vascular hyalinosis | 14 plasma exchanges in first month, no additional sessions. | Steroids Cytoxan | sCr: 3 mg/dL (265 µmol/L) at 2 months 5.1 mg/dL (451 µmol/L) at 6 months ESKD on HD at 1-year follow up Repeat biopsy at 2 months: 12 glomeruli 30% glomerular hyalinosis 50% florid crescents 1+ interstitial infiltrates 1+ interstitial fibrosis 1+ vascular hyalinosis | - | |||
| 4 | M | 39 | IgAN HTN | sCr 2.69 mg/dL (238 µmol/L) | 13 glomeruli 35% glomerular hyalinosis 50% florid crescents 1+ Interstitial fibrosis 1+ vascular hyalinosis | 10 plasma exchanges in first month with 5 additional sessions by 2 month follow up. | Steroids Cytoxan | sCr: 2.6 mg/dL (230 µmol/L) at 2 months 4.2 mg/dL (371 µmol/L) at 6 months ESKD on HD at 1-year follow up Repeat biopsy at 2 months: 14 glomeruli 30% glomerular hyalinosis 30% florid crescents 2+ interstitial fibrosis 2+ vascular hyalinosis | - | |||
| 5 | M | 55 | IgAN HTN | sCr 7.4 mg/dL (654 µmol/L) | 10 glomeruli 40% florid crescents 1+ interstitial infiltrates 2+ interstitial fibrosis | 10 plasma exchanges in first month, no additional sessions. | Steroids Cytoxan | sCr: 2.19 mg/dL (194 µmol/L) at 2 months 2.09 mg/dL (185 µmol/L) at 6 months 2.19 mg/dL (194 µmol/L) at 24 months 2.19 mg/dL (194 µmol/L) at 36 months ESKD on HD at 1-year follow up No repeat biopsy | - | |||
| 6 | F | 18 | IgAN | sCr 3.0 mg/dL (265 µmol/L) | 12 glomeruli 15% glomerular hyalinosis 80% florid crescents 1+ interstitial infiltrates 1+ interstitial fibrosis 1+ vascular hyalinosis | 18 plasma exchanges in first month with 5 additional sessions between the 2 and 6 months follow up. | Steroids Cytoxan | sCr: 1.49 mg/dL (1.32 µmol/L) at 2 months 2.3 mg/dL (2.03 µmol/L) at 6 months 1.59 mg/dL (1.41 µmol/L) at 24 months 4.2 mg/dL (371 µmol/L) at 120 months No repeat biopsy | - | |||
| 4 | Gianviti [53] | 1996 | UK | 14 patients; 10 M and 4F | 3.7–11.9 | HSP | 12/14 patients: 30–100% crescents | Children weighing below 15 kg underwent plasma filtration with a Gambro plasma filter and AK 10 blood monitor. Children above 15 kg underwent centrifugal plasma exchange with a Cobe Spectra Apheresis system. Total volume exchanged was twice the estimated plasma volume using Albumin and FFP as replacement fluids. | Cytoxan Steroids | All patients with improvement in serum Cr but 5 patients with ESKD at long-term follow up. Statistically significant improvement in kidney outcome if PLEX initiated within 1 month of disease onset. | Volume overload Cardiac arrest due to hypocalcemia Anaphylaxis | |
| 1 | F | 6.4 | HSP | sCr 1.24 mg/dL (110 µmol/L) | 60% crescents | 9 months from onset | Steroids Cytoxan | sCr 0.53 mg/dL (47 µmol/L) 2 months after PLEX ESKD at 2-year follow up | - | |||
| 2 | M | 9.0 | HSP | sCr 2.26 mg/dL (200 µmol/L) | 60% crescents | 4 months from onset | Steroids Cytoxan | sCr 1 mg/dL (88 µmol/L) 2 months after PLEX ESKD at 2-year follow up | - | |||
| 3 | M | 11.9 | HSP | sCr 0.97 mg/dL (86 µmol/L) | 80% crescents | 1 month from onset | Steroids Cytoxan | sCr 0.68 mg/dL (60 µmol/L) 2 months after PLEX sCr 0.9 mg/dL (80 µmol/L) at 2-year follow up | - | |||
| 4 | F | 9.5 | HSP | sCr 5.54 mg/dL (490 µmol/L) | 100% crescents | <1 month from onset | Steroids Cytoxan | sCr 1.36 mg/dL (120 µmol/L) 2 months after PLEX sCr 1.92 mg/dL (170 µmol/L) at 6-year follow up | - | |||
| 5 | M | 8.0 | HSP | sCr 8.03 mg/dL (710 µmol/L) | 80% crescents | 1 month from onset | Steroids Cytoxan HD | sCr 1.36 mg/dL (120 µmol/L) 2 months after PLEX sCr (76 µmol/L) at 1-year follow up | - | |||
| 6 | M | 5.1 | HSP | sCr 3.73 mg/dL (330 µmol/L) | Diffuse extra-capillary proliferation | <1 month from onset | Steroids Cytoxan HD | sCr (58 µmol/L) 2 months after PLEX sCr 0.86 mg/dL (58 µmol/L) at 2-year follow up | - | |||
| 7 | M | 10 | HSP | sCr 8.93 mg/dL (µmol/L) | Diffuse extra-capillary proliferation | 1 month from onset | Steroids Cytoxan HD | sCr (62 µmol/L) 2 months after PLEX (53 µmol/L) at 3-year follow up | - | |||
| 8 | M | 8.9 | HSP | sCr 1.27 mg/dL (112 µmol/L) | 50% crescents | 1 month from onset | Steroids Cytoxan | sCr 0.7 mg/dL (41 µmol/L) 2 months after PLEX sCr 0.68 mg/dL (60 µmol/L) at 2-year follow up | - | |||
| 9 | F | 11.5 | HSP | sCr 3.39 mg/dL (300 µmol/L) | 88% crescents | 1 month from onset | Steroids Cytoxan | sCr 0.98 mg/dL (87 µmol/L) 2 months after PLEX sCr 0.66 mg/dL (58 µmol/L) at 2-year follow up | - | |||
| 10 | M | 3.7 | HSP | sCr 1.4 mg/dL (124 µmol/L) | 30% crescents | 48 months from onset | Steroids Cytoxan | sCr 1.36 mg/dL (120 µmol/L) 2 months after PLEX ESKD at 7-year follow up | - | |||
| 11 | M | 5.6 | HSP | sCr 2.6 mg/dL (230 µmol/L) | 80% crescents | 1 month from onset | Steroids Cytoxan | sCr 0.68 mg/dL (60 µmol/L) 2 months after PLEX sCr 0.38 mg/dL (34 µmol/L) at 1.3-year follow up | - | |||
| 12 | F | 10.5 | HSP | sCr 2.26 mg/dL (200 µmol/L) | 80% crescents | 9 months from onset | Steroids Cytoxan | sCr 2.26 mg/dL (200 µmol/L) 2 months after PLEX ESKD at 1-year follow up | - | |||
| 13 | M | 8.5 | HSP | sCr 5.32 mg/dL (470 µmol/L) | 100% crescents | 2 months from onset | Steroids Cytoxan HD | sCr 2.04 mg/dL (180 µmol/L) 2 months after PLEX ESKD at 1-year follow up | - | |||
| 14 | M | 6.7 | HSP | sCr 2.6 mg/dL (230 µmol/L) | 85% crescents | 2 months from onset | Steroids Cytoxan | sCr 0.96 mg/dL (85 µmol/L) 2 months after PLEX 0.97 mg/dL (86 µmol/L) at 9-year follow up | - | |||
| 5 | Shenoy [54] | 2007 | UK | 16 (14 with HSP and 2 IgAN) pts; 6M and 10F | 3.7–13.5 | HSP IgAN | eGFR estimated using sCr and height | All patients with at least grade 3 nephritis on biopsy were treated with plasmapheresis alone. Plasmapheresis 90 mL/kg per session exchanging 80 mL/kg with 4.5% albumin and 20 mL/kg with FFP. All patients received at least 9 sessions in first 2 weeks with further increasing spaced sessions if clinical recovery was incomplete. All patients received cotrimoxazole 12 mg/kg daily for duration of treatment plus 2 months. | None | All patients had improvement in eGFR and UA/UC ratio that was stable over time, but the delayed patient ultimately required kidney transplant. Results suggest prompt treatment with plasmapheresis alone improves kidney function that remains stable over time. | Itchy rashes following FFP treated with hydrocortisone and chlorphenamine | |
| 1 | F | 11.0 | HSP | eGFR 46 | ISKDC grade 3b 20% crescents | Within 2 weeks of onset | None | eGFR 102 with negative urine dipstick for albumin at 7.5 years follow up | - | |||
| 2 | F | 6.8 | HSP | eGFR 82 | ISKDC grade 3a 40% crescents | Within 2 weeks of onset | None | eGFR 127 and UA/UC 2 at 1.1 year follow up | - | |||
| 3 | M | 5.8 | HSP | eGFR 93 | ISKDC grade 3b 24% crescents | Within 2 weeks of onset | None | eGFR 98 and UA/UC 3 at 2.1 years follow up | - | |||
| 4 | M | 15.0 | HSP | eGFR 20 | ISKDC grade 3b 20% crescents | Within 2 weeks of onset | None | eGFR 108 and UA/UC 38 at 2.5 years follow up | - | |||
| 5 | F | 3.7 | HSP | eGFR 136 | ISKDC grade 3a No crescents | Within 2 weeks of onset | None | eGFR 102 and UA/UC 2 at 6.2 years follow up | - | |||
| 6 | F | 13.5 | HSP | eGFR 28 | ISKDC grade 4b 53% crescents | Within 2 weeks of onset | None | eGFR 134 and UA/UC 42 at 2.6 years follow up | - | |||
| 7 | F | 12.5 | HSP | eGFR 61 | ISKDC grade 3b 43% crescents | Within 2 weeks of onset | None | eGFR 101 and UA/UC 10 at 3.1 years follow up | - | |||
| 8 | M | 11.8 | HSP | eGFR 33 | ISKDC grade 3b no crescents | Within 2 weeks of onset | None | eGFR 142 and UA/UC 1 at 3.8 years follow up | - | |||
| 9 | M | 12.3 | HSP | eGFR 90 | ISKDC grade 3b 10% crescents | Within 2 weeks of onset | None | eGFR 101 and UA/UC 7 at 1.1 years follow up | - | |||
| 10 | F | 10.1 | IgAN | eGFR 42 | ISKDC grade 3b 29% fibrous crescents | Within 2 weeks of onset | None | eGFR 106 and UA/UC 2 at 4.2 years follow up | - | |||
| 11 | M | 13.1 | IgAN | eGFR 17 | ISKDC grade 3b 5% crescents | Within 2 weeks of onset | None | eGFR 113 and UA/UC 16 at 3.4 years follow up | - | |||
| 12 | M | 9.9 | HSP | eGFR 43 | ISKDC grade 3b 14% fibrous crescents | Within 2 weeks of onset | None | eGFR 105 and UA/UC 9 at 5.2 years follow up | - | |||
| 13 | F | 8.4 | HSP | eGFR 64 | ISKDC grade 4b 52% crescents | Within 2 weeks of onset | None | eGFR 121 and UA/UC 14.3 at 5.5 years follow up | - | |||
| 14 | F | 8.3 | HSP | eGFR 22 | ISKDC grade 3a no crescents | Within 2 weeks of onset | None | eGFR 121 and UA/UC 2 at 4.3 years follow up | - | |||
| 15 | F | 8.9 | HSP | eGFR 67 | ISKDC grade 3b no crescents | Within 2 weeks of onset | None | eGFR 112 and UA/UC 3 at 5.4 years follow up | - | |||
| 16 | F | 7.7 | HSP | eGFR 29 | ISKDC grade 3b 26% fibrous crescents | Plasma exchange delayed until 2 months from onset due to needle phobia. | None | Kidney Transplant at 6.3 years follow up | - | |||
| 6 | Wright [55] | 2006 | UK | 32 pts; 5 with HSP, gender and specific ages not specified. | Median 9.4 (0.7–17.7 years) | 5 with HSP, Rest had collection of PAN, GPA, MPA/ICN, and NCV | eGFR obtained using Schwartz formula | All patients received at least 2 courses of plasma exchange comprised of 5 daily sessions and extra sessions based on clinical response. TPE performed using Spectra centrifugation and PF 1000 plasma filter and Gambro AK 10. Plasma volume was calculated as 50 mL/kg bodyweight with target of double volume as target with limit of 4 L. Plasma replaced with 4.5% albumin in all cases, with FFP at the end of exchange to replenish clotting factors. Median time to treatment from admission was 6 days (range 0–28 days). | Steroids Cytoxan | Hypotension Femoral vein thrombosis Sepsis | ||
| 1 | Gender not specified | -- | HSP | eGFR 64 | 48% crescents pre-TPE | Did not specify specific time/number of sessions. | Steroids Cytoxan | eGFR 106 after plasma exchange eGFR 162 at 2 months follow up | - | |||
| 2 | Gender not specified | -- | HSP | eGFR 22 | 100% crescents pre-TPE | Did not specify specific time/number of sessions. | Steroids Cytoxan | eGFR 26 after plasma exchange eGFR 66 at 2 months follow up Required HD temporarily but gradually regained kidney function | - | |||
| 3 | Gender not specified | -- | HSP | eGFR 33 | 100% crescents pre-TPE | Did not specify specific time/number of sessions. | Steroids Cytoxan | eGFR 20 after plasma exchange eGFR 10 at 2 months follow up Required HD 2 months after plasma exchange | - | |||
| 4 | Gender not specified | -- | HSP | eGFR 167 | 50% crescents pre-TPE | Did not specify specific time/number of sessions. | Steroids Cytoxan | eGFR 177 after plasma exchange eGFR 169 at 2 months follow up | - | |||
| 5 | Gender not specified | -- | HSP | eGFR 84 | 75% crescents pre-TPE | Did not specify specific time/number of sessions. | Steroids Cytoxan | eGFR 98 after plasma exchange eGFR 99 at 2 months follow up | - | |||
| 7 | Xie [56] | 2016 | China | 12 patients; 9M and 3F. No individual data available. | Mean 42.7± SD 15 | 8 patients on HD at start 2 patients with oliguria 11 patients with HTN | Mean sCr 7.98 ± 3.35 mg/dL (705.3 ± 296.4 μmol/L) | Total glomeruli 21 64.4 ± 24.4% crescents; 6 patients 50%< tubular atrophy | Mean 7 sessions (5–10) over mean of 15 days (9–30). 2.517 L exchanged per course (300) Median time of symptoms was 1.5 months (1.0–5.0). | Steroids Cytoxan Some with Mycophenolate | Compared to matched historical control group, about half of plasma exchange group were able to discontinue dialysis in 6 months. 5 patients with significant reduction in sCr to normal range that was stable in long-term follow-up (9 to 51 months). 7 patients with ESKD | Pneumonia Pulmonary Failure |
| 8 | Chambers [57] | 1999 | USA | 2 patients | ||||||||
| M | 27 | IgAN | 2.8 mg/dL (247.58 μmol/L); proteinuria 6.2 g/day | Crescentic GN | 6 × 4 L exchanges over 18 days initiated during pt’s readmission. | Steroids, Cytoxan | sCr 5.6 and proteinuria 3.5 g/day, no response to PLEX. ESKD | none | ||||
| M | 18 | IgAN | 23 mg/dL (2033.66 μmol/L); >5 g/day | Crescentic GN | 7 × 4 L exchanges over 18 days. | Steroids, Cytoxan | ESKD | Sepsis from catheter | ||||
| 9 | Rajgopala [58] | 2017 | India | 2 patients | ||||||||
| 1 | F | 38 | DAH | sCr 7.8 mg/dL (689.68 umol/L) | Crescentic GN | Did not specify regimen. | Steroid, Cytoxan | Stable on HD and DAH improved but expired from ventricular arrhythmia during HD on admission day 18 | Expired | |||
| 2 | M | 45 | DAH | sCr 5.3 mg/dL (689.68 umol/L) | Crescentic GN | Did not specify regimen. | Steroid, Cytoxan, ECMO | DAH not improved; expired from septic shock | Septic shock, Expired |
| Adverse Events | Number of Patients |
|---|---|
| Infectious complication | 8 (7.8%) |
| Mild allergic reaction | 1 (0.98%) |
| Electrolyte abnormality (hypocalcemia) | 1 (0.98%) |
| Catheter dislodgement | 1 (0.98%) |
| Volume overload | 1 (0.98%) |
| Vein thrombosis | 1 (0.98%) |
| Anaphylaxis | 1 (0.98%) |
| Leukopenia | 1 (0.98%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, B.; Acharya, C.; Tangpanithandee, S.; Miao, J.; Krisanapan, P.; Thongprayoon, C.; Amir, O.; Mao, M.A.; Cheungpasitporn, W.; Acharya, P.C. Efficacy and Safety of Plasma Exchange as an Adjunctive Therapy for Rapidly Progressive IgA Nephropathy and Henoch-Schönlein Purpura Nephritis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3977. https://doi.org/10.3390/ijms24043977
Nguyen B, Acharya C, Tangpanithandee S, Miao J, Krisanapan P, Thongprayoon C, Amir O, Mao MA, Cheungpasitporn W, Acharya PC. Efficacy and Safety of Plasma Exchange as an Adjunctive Therapy for Rapidly Progressive IgA Nephropathy and Henoch-Schönlein Purpura Nephritis: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(4):3977. https://doi.org/10.3390/ijms24043977
Chicago/Turabian StyleNguyen, Bryan, Chirag Acharya, Supawit Tangpanithandee, Jing Miao, Pajaree Krisanapan, Charat Thongprayoon, Omar Amir, Michael A. Mao, Wisit Cheungpasitporn, and Prakrati C. Acharya. 2023. "Efficacy and Safety of Plasma Exchange as an Adjunctive Therapy for Rapidly Progressive IgA Nephropathy and Henoch-Schönlein Purpura Nephritis: A Systematic Review" International Journal of Molecular Sciences 24, no. 4: 3977. https://doi.org/10.3390/ijms24043977
APA StyleNguyen, B., Acharya, C., Tangpanithandee, S., Miao, J., Krisanapan, P., Thongprayoon, C., Amir, O., Mao, M. A., Cheungpasitporn, W., & Acharya, P. C. (2023). Efficacy and Safety of Plasma Exchange as an Adjunctive Therapy for Rapidly Progressive IgA Nephropathy and Henoch-Schönlein Purpura Nephritis: A Systematic Review. International Journal of Molecular Sciences, 24(4), 3977. https://doi.org/10.3390/ijms24043977








