Recent Advances in the Applications of Small Molecules in the Treatment of Multiple Myeloma
Abstract
1. Introduction
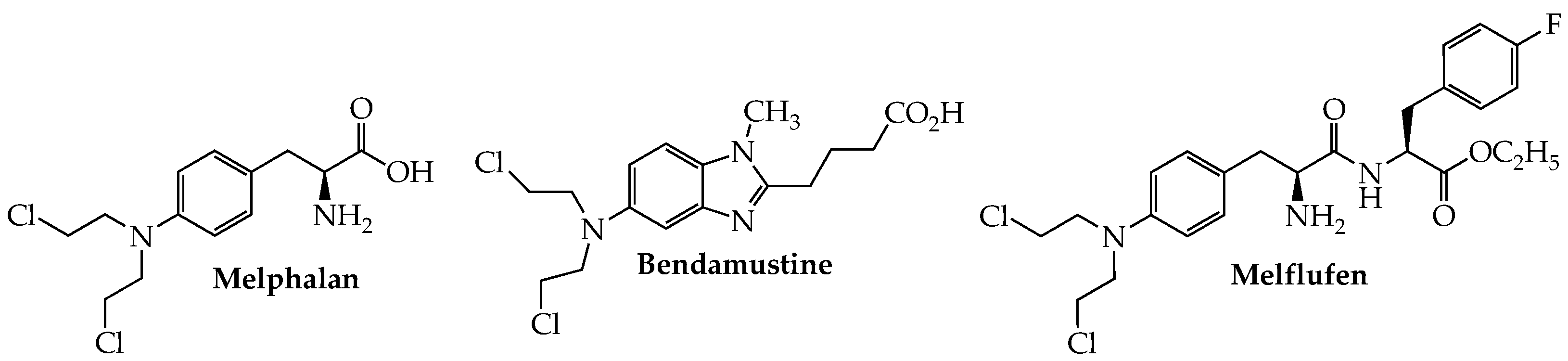
2. Alkylating Agents
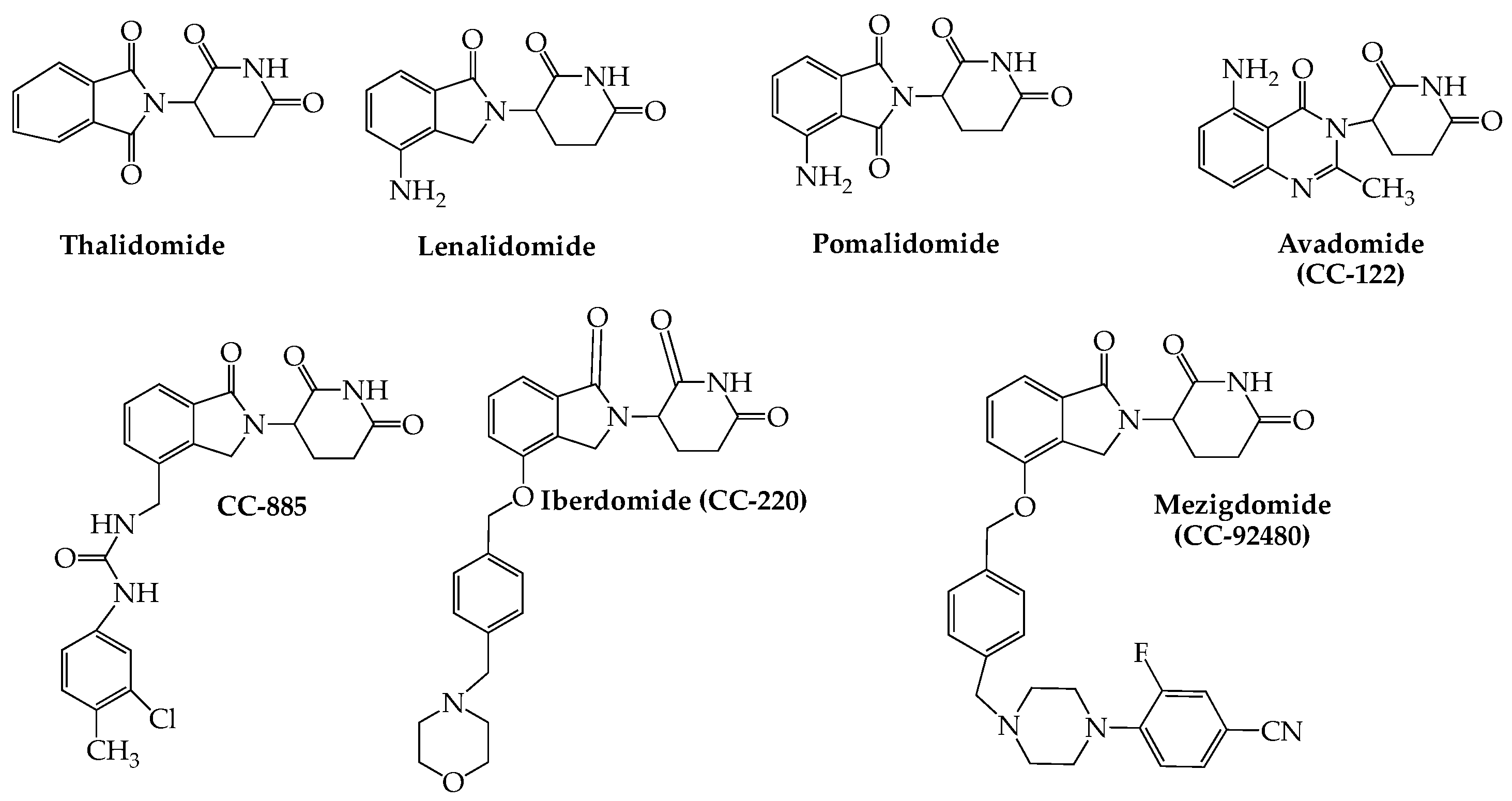
3. Cereblon E3 Ligase Modulators
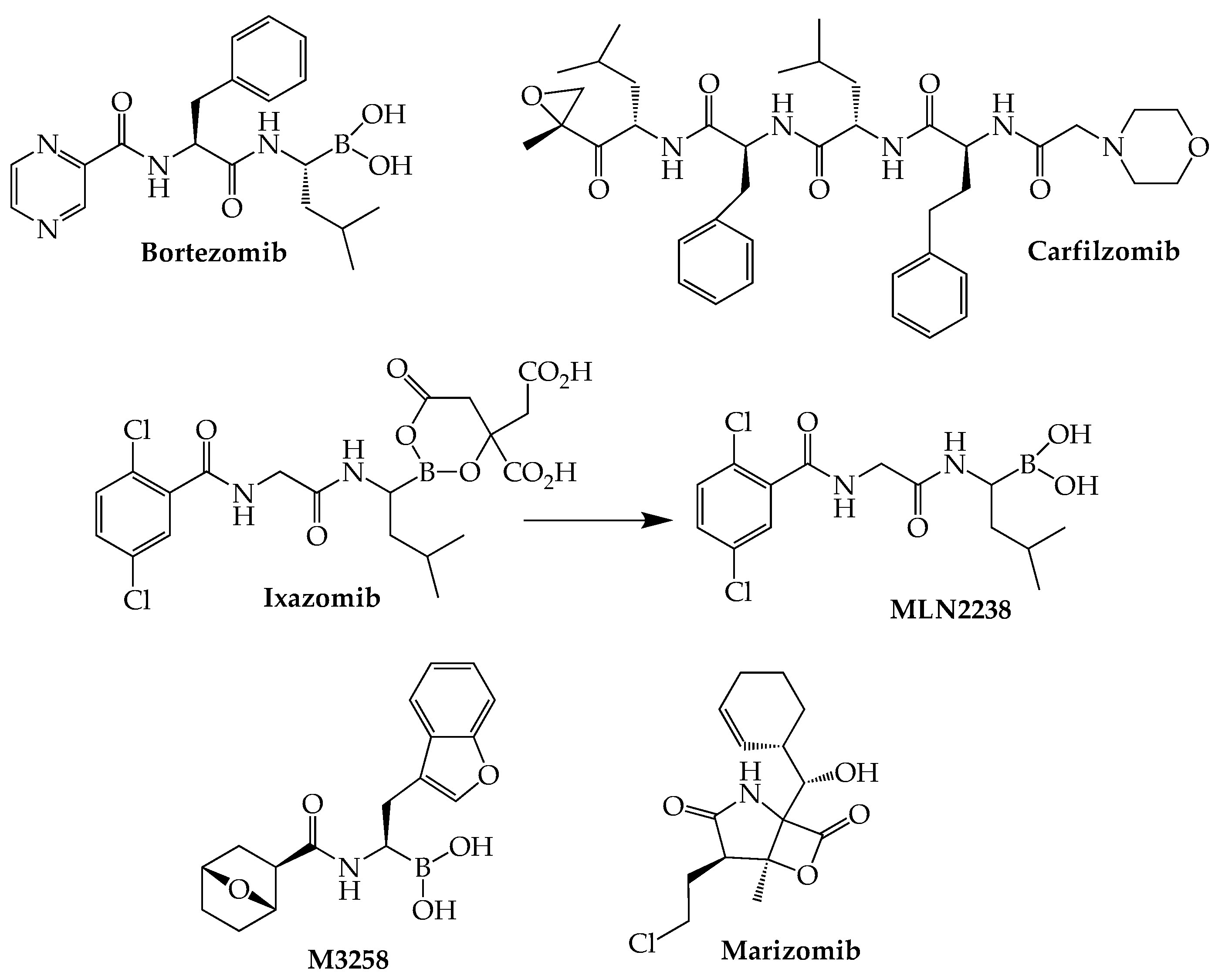
4. Proteasome Inhibitors
5. Deubiquitinase Inhibitors
6. Neddylation Inhibitors
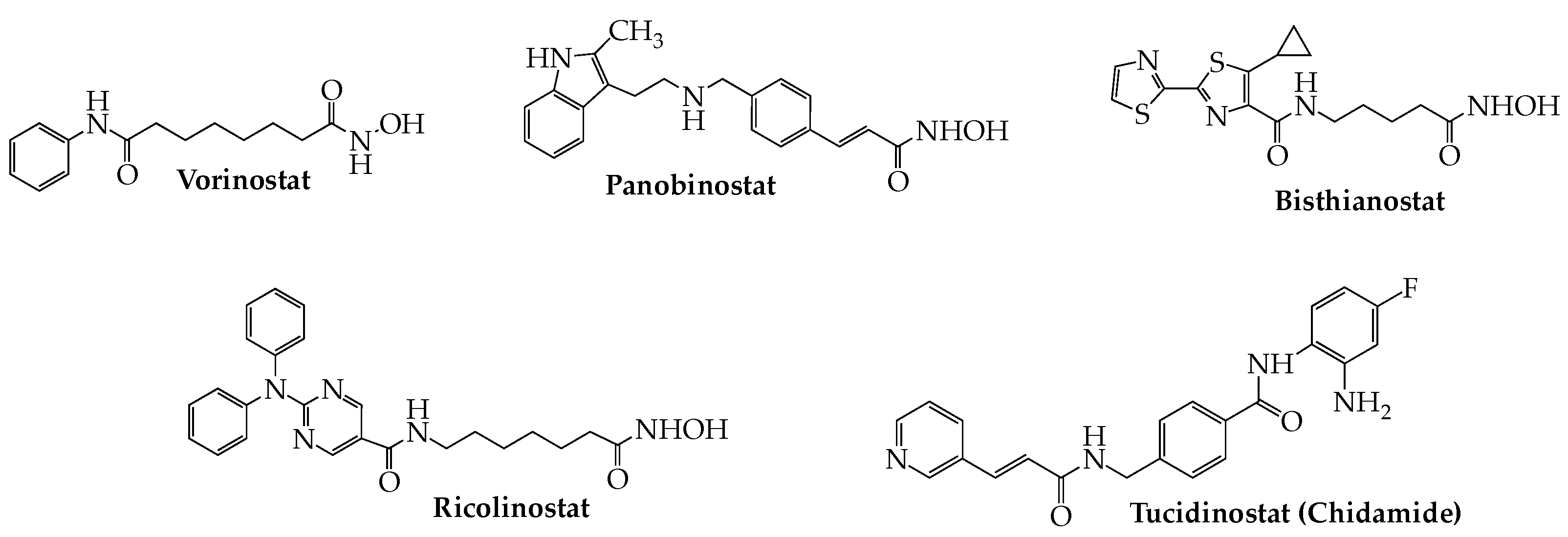
7. HDAC Inhibitors
8. Bromodomain Inhibitors
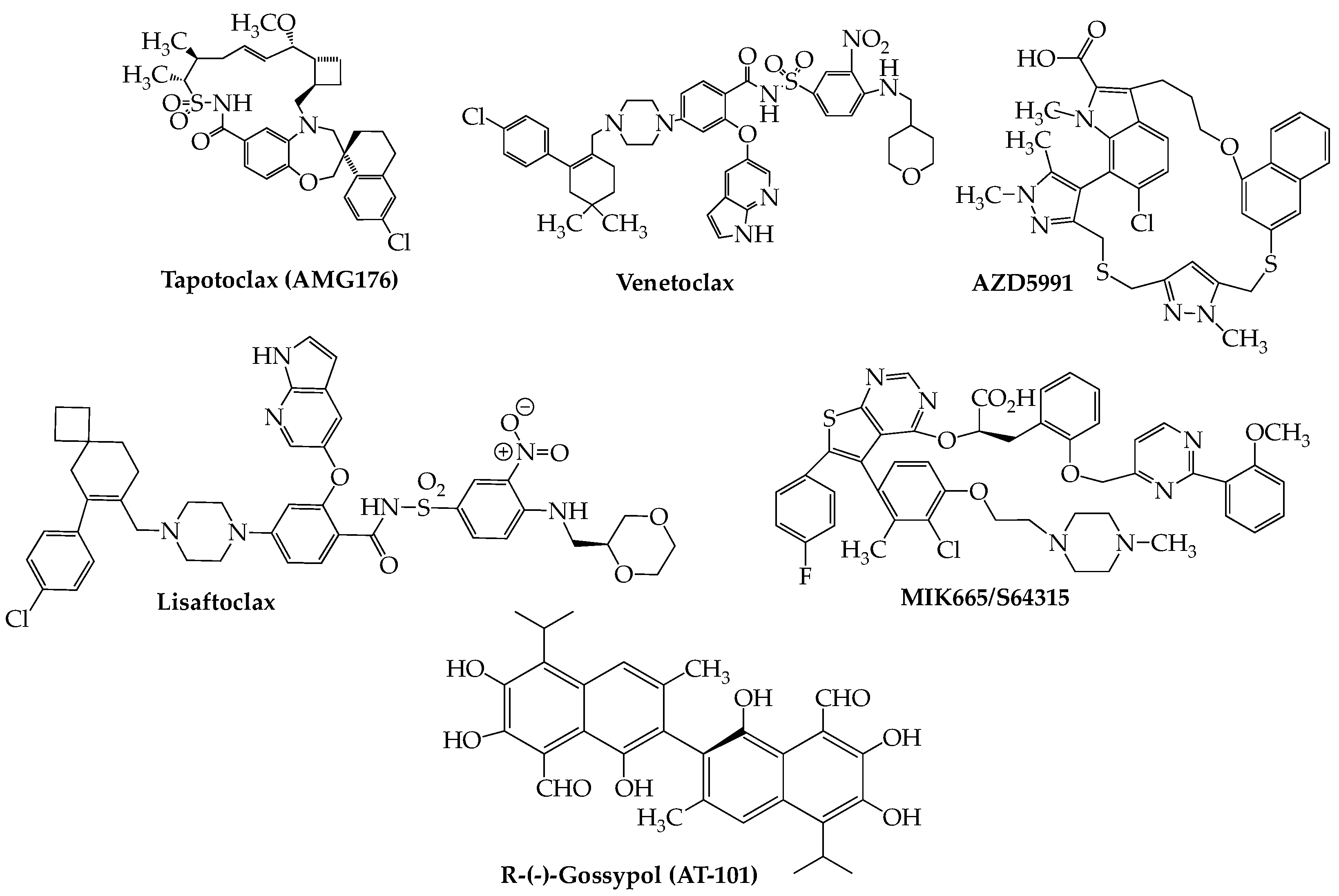
9. Apoptosis Inducers: Bcl2, IAP, and Mcl-1 Inhibitors
| Trial ID (Reference) | Phase | Drugs | Enrollment (N) | Prior Lines of Therapy (Median) | ORR (%) | PFS (Median in Months) |
|---|---|---|---|---|---|---|
| NCT03314181 [117] | I | (Ven + Dara + Dex) vs. (Ven + Dara + Dex + Bort) | Part 1: [24 with t(11;14)—Ven + Dara + Dex]; Part 2: [6 with t(11;14) + 18 other RRMM— Ven + Dara + Dex + Bort] | Part 1: 2.5; Part 2: 1 | Ven + Dara + Dex: 96; Ven + Dara + Dex + Bort: 92 | NR |
| NCT03314181 [118] | I/Ii | (Ven + Dara + Dex) vs. (Ven + Dara + Dex + Bort) | 34 all t(11;14): 11 Ven + Dara + Dex (12 at 400 mg Ven, 7 at 800 mg. Ven); 16 Ven + Dara + Dex + Bort | Ven + Dara + Dex: 1; Ven + Dara + Dex + Bort: 2 | Ven + Dara + Dex: 72.7 (at 400 mg.) and 100 (at 800 mg.); Ven + Dara + Dex + Bort: 62.5 | NR |
| NCT02899052 [119] | II | Ven + Carf + Dex | 49: 13 t(11;14); 36 non-t(11;14) | 1 | t(11;14): 92; non-t(11;14): 75 | With t(11;14): 24.8; without t(11;14): 22.8 |
| NCT01794520 [120] | I | Ven and Ven + Dex | 66: 30 t(11;14); 36 non-t(11;14) | 5 | t(11;14): 40; non-t(11;14): 6 | t(11;14): 6.6; non-t(11;14): 1.9 |
| NCT01794520 [121] | I/II | Ven + Dex | Phase I: 20; Phase II: 31. All t(11;14) positive | Phase I: 3; Phase II: 5 | Phase I: 60; Phase II: 48 | Phase I: 12.4; Phase II: 10.8 |
| NCT02755597 [122] | III | (Ven + Bort + Dex) vs. (Bort + Dex + Pbo) | 291: 35 with t(11;14); 194 (Ven + Bort + Dex), 97 (Bort + Dex + Pbo) | 1–3 | NR | With t(11;14): Ven + Bort + Dex: 36.8; Bort + Dex + Pbo: 9.3 |
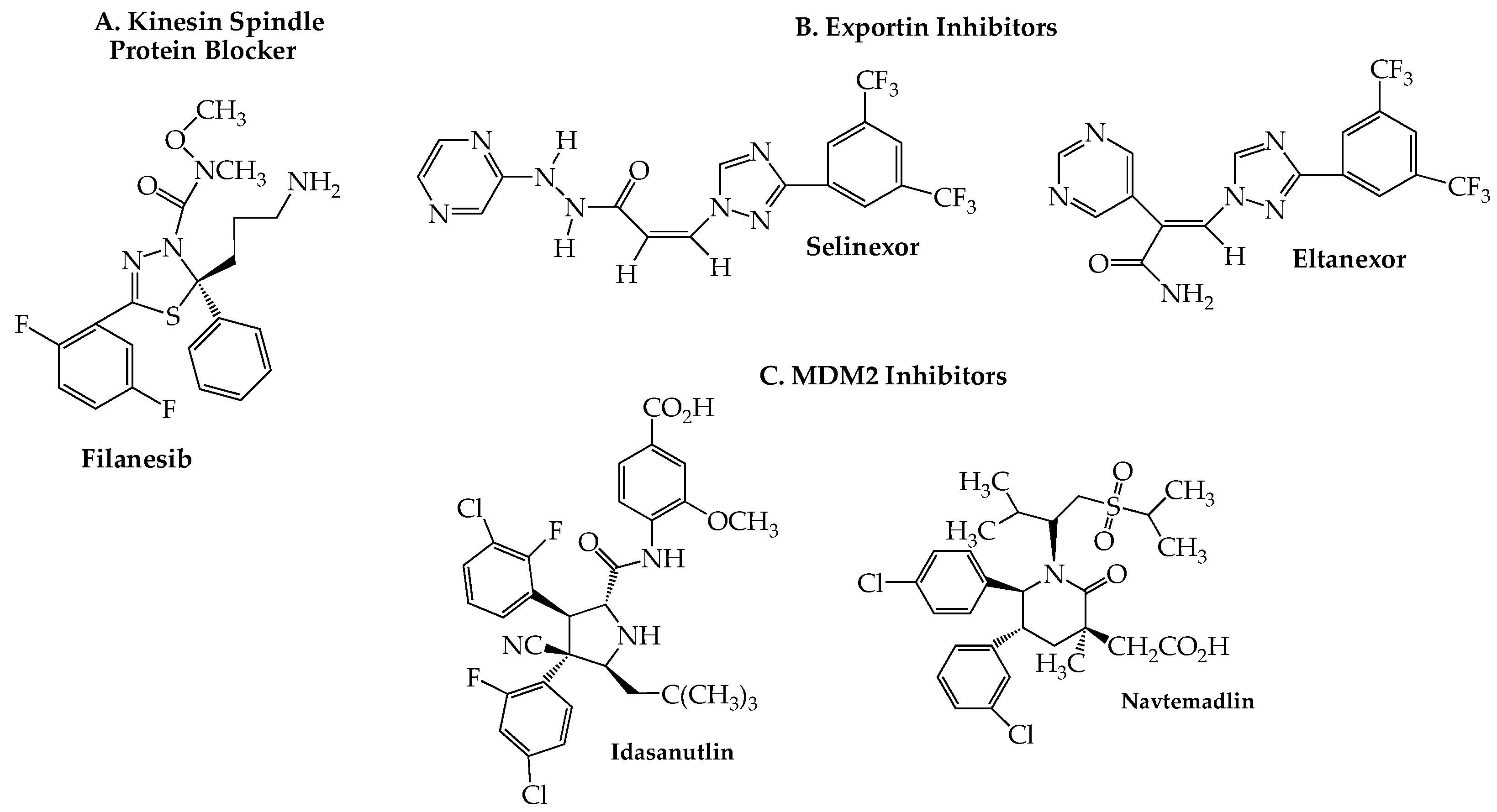
10. Kinesin Spindle Protein Inhibitors
11. Exportin Inhibitors
12. MDM2 Blockers
13. Kinase Inhibitors
13.1. Bruton’s Tyrosine Kinase Inhibitors
13.2. Transforming Growth Factor Receptor Inhibitors
13.3. Raf-Mek-Erk Pathway Inhibitors
13.4. PI3K-Akt-mTOR Pathway Inhibitors
14. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munshi, N.C.; Longo, D.L.; Anderson, K.C. Plasma cell disorders. In Harrison’s Principles of Internal Medicine, 21st ed.; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J.L., Eds.; McGraw-Hill Education: New York, NY, USA, 2022; pp. 1–22. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, staging, and management of multiple myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef]
- Callander, N.S.; Baljevic, M.; Adekola, K.; Anderson, L.D.; Campagnaro, E.; Castillo, J.J.; Costello, C.; Devarakonda, S.; Elsedawy, N.; Faiman, M.; et al. NCCN guidelines® insights: Multiple myeloma, version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Chen, C.I.; Reece, D.E. Current review on high-risk multiple myeloma. Curr. Hematol. Malig. Rep. 2017, 12, 96–108. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975–2017: Myeloma; National Cancer Institute: Bethesda, MD, USA, 2020.
- Yamamoto, C.; Minakata, D.; Koyama, S.; Sekiguchi, K.; Fukui, Y.; Murahashi, R.; Nakashima, H.; Matsuoka, S.; Ikeda, T.; Kawaguchi, S.I.; et al. Daratumumab in first-line is cost-effective in transplant-eligible newly diagnosed myeloma patients. Blood 2022, 140, 594–607. [Google Scholar] [CrossRef]
- Blommestein, H.M.; Zweegman, S. Cost-effectiveness: Maximizing impact by meticulous data. Blood 2022, 140, 525–526. [Google Scholar] [CrossRef]
- Blokhin, N.; Larionov, L.; Perevodchikova, N.; Chebotareva, L.; Merkulova, N. Clinical experiences with sarcolysin in neoplastic diseases. Ann. N. Y. Acad. Sci. 1958, 68, 1128–1132. [Google Scholar] [CrossRef]
- Ray, A.; Ravillah, D.; Das, D.S.; Song, Y.; Nordstrom, E.; Gullbo, J.; Richardson, P.G.; Chauhan, D.; Anderson, K.C. A novel alkylating agent melflufen induces irreversible DNA damage and cytotoxicity in multiple myeloma cells. Br. J. Haematol. 2016, 174, 397–409. [Google Scholar] [CrossRef]
- Holstein, S.A.; Hillengass, J.; McCarthy, P.L. Melflufen: A next-generation nitrogen mustard. J. Clin. Oncol. 2021, 39, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Schjesvold, F.H.; Bakker, N.A.; Sonneveld, P. Authors’ reply: Perspective: The approval and withdrawal of melphalan flufenamide (melflufen): Implications for the state of the FDA. Transl. Oncol. 2022, 25, 101528. [Google Scholar] [CrossRef] [PubMed]
- Schjesvold, F.H.; Dimopoulos, M.A.; Delimpasi, S.; Robak, P.; Coriu, D.; Legiec, W.; Pour, L.; Špička, I.; Masszi, T.; Doronin, V.; et al. Melflufen or pomalidomide plus dexamethasone for patients with multiple myeloma refractory to lenalidomide (OCEAN): A randomised, head-to-head, open-label, phase 3 study. Lancet Haematol. 2022, 9, e98–e110. [Google Scholar] [CrossRef] [PubMed]
- Olivier, T.; Prasad, V. The approval and withdrawal of melphalan flufenamide (melflufen): Implications for the state of the FDA. Transl. Oncol. 2022, 18, 101374. [Google Scholar] [CrossRef] [PubMed]
- Kronke, J.; Hurst, S.N.; Ebert, B.L. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology 2014, 3, e941742. [Google Scholar] [CrossRef]
- Ito, T.; Handa, H. Molecular mechanisms of thalidomide and its derivatives. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2020, 96, 189–203. [Google Scholar] [CrossRef]
- Barankiewicz, J.; Salomon-Perzyński, A.; Misiewicz-Krzemińska, I.; Lech-Marańda, E. CRL4(CRBN) E3 ligase complex as a therapeutic target in multiple myeloma. Cancers 2022, 14, 4492. [Google Scholar] [CrossRef]
- LaPlante, G.; Zhang, W. Targeting the ubiquitin-proteasome system for cancer therapeutics by small-molecule Inhibitors. Cancers 2021, 13, 3079. [Google Scholar] [CrossRef]
- Faust, T.B.; Donovan, K.A.; Yue, H.; Chamberlain, P.P.; Fischer, E.S. Small-molecule approaches to targeted protein degradation. Annu. Rev. Cancer Biol. 2021, 5, 181–201. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Siegel, D.S.; Schiller, G.J.; Samaras, C.; Sebag, M.; Berdeja, J.; Ganguly, S.; Matous, J.; Song, K.; Seet, C.S.; Talamo, G.; et al. Pomalidomide, dexamethasone, and daratumumab in relapsed refractory multiple myeloma after lenalidomide treatment. Leukemia 2020, 34, 3286–3297. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Weisel, K.; Moreau, P.; Anderson, L.D., Jr.; White, D.; San-Miguel, J.; Sonneveld, P.; Engelhardt, M.; Jenner, M.; Corso, A.; et al. Pomalidomide, bortezomib, and dexamethasone for multiple myeloma previously treated with lenalidomide (OPTIMISMM): Outcomes by prior treatment at first relapse. Leukemia 2021, 35, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Alabi, S.B.; Crews, C.M. Major advances in targeted protein degradation: PROTACs, LYTACs, and MADTACs. J. Biol. Chem. 2021, 296, 100647. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Sperling, A.S.; Ebert, B.L. Cancer therapies based on targeted protein degradation—Lessons learned with lenalidomide. Nat. Rev. Clin. Oncol. 2021, 18, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Matyskiela, M.E.; Zhang, W.; Man, H.W.; Muller, G.; Khambatta, G.; Baculi, F.; Hickman, M.; LeBrun, L.; Pagarigan, B.; Carmel, G.; et al. A cereblon modulator (CC-220) with improved degradation of Ikaros and Aiolos. J. Med. Chem. 2018, 61, 535–542. [Google Scholar] [CrossRef]
- Lonial, S.; Popat, R.; Hulin, C.; Jagannath, S.; Oriol, A.; Richardson, P.G.; Facon, T.; Weisel, K.; Larsen, J.T.; Minnema, M.C.; et al. Iberdomide plus dexamethasone in heavily pretreated late-line relapsed or refractory multiple myeloma (CC-220-MM-001): A multicentre, multicohort, open-label, phase 1/2 trial. Lancet Haematol. 2022, 9, e822–e832. [Google Scholar] [CrossRef]
- Hagner, P.R.; Man, H.W.; Fontanillo, C.; Wang, M.; Couto, S.; Breider, M.; Bjorklund, C.; Havens, C.G.; Lu, G.; Rychak, E.; et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood 2015, 126, 779–789. [Google Scholar] [CrossRef]
- Rasco, D.W.; Papadopoulos, K.P.; Pourdehnad, M.; Gandhi, A.K.; Hagner, P.R.; Li, Y.; Wei, X.; Chopra, R.; Hege, K.; DiMartino, J.; et al. A first-in-human study of novel cereblon modulator avadomide (CC-122) in advanced malignancies. Clin. Cancer Res. 2019, 25, 90–98. [Google Scholar] [CrossRef]
- Hansen, J.D.; Correa, M.; Nagy, M.A.; Alexander, M.; Plantevin, V.; Grant, V.; Whitefield, B.; Huang, D.; Kercher, T.; Harris, R.; et al. Discovery of CRBN E3 ligase modulator CC-92480 for the treatment of relapsed and refractory multiple myeloma. J. Med. Chem. 2020, 63, 6648–6676. [Google Scholar] [CrossRef]
- Wong, L.; Lamba, M.; Nunez, M.D.J.; Bauer, D.; Richardson, P.G.; Bahlis, N.J.; Vangsted, A.J.; Ramasamy, K.; Trudel, S.; Martinez-Lopez, J.; et al. Dose- and schedule-dependent immunomodulatory effects of the novel Celmod agent CC-92480 in patients with relapsed/refractory multiple myeloma. Blood 2020, 136, 47–48. [Google Scholar] [CrossRef]
- Richardson, P.G.; Vangsted, A.J.; Ramasamy, K.; Trudel, S.; Martinez, J.; Mateos, M.V.; Otero, P.R.; Lonial, S.; Popat, R.; Oriol, A.; et al. First-in-human phase I study of the novel CELMoD agent CC-92480 combined with dexamethasone (DEX) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2020, 38, 8500. [Google Scholar] [CrossRef]
- Lonial, S.; Berdeja, J.G.; Dimopoulos, M.A.; Jagannath, S.; Knop, S.; Quach, H.; Rodriguez-Otero, P.; Richardson, P.G.; Sorrell, A.; Chen, M.; et al. EXCALIBER: A phase 3 study comparing iberdomide, daratumumab, and dexamethasone (IberDd) with daratumumab, bortezomib, and dexamethasone (DVd) in patients with relapsed or refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2021, 21, S150. [Google Scholar] [CrossRef]
- Weisel, K.; Knop, S.; Lonial, S.; Richardson, P.G.; Popat, R.; Stadtmauer, E.A.; Larsen, J.T.; Oriol, A.; Jagannath, S.; Cook, G.; et al. Iberdomide (IBER) in combination with dexamethasone (DEX) and daratumumab (DARA), bortezomib (BORT), or carfilzomib (CFZ) in patients with relapsed/refractory multiple myeloma (RRMM). Oncol. Res. Treat. 2021, 44, 86–87. [Google Scholar]
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta 2004, 1695, 55–72. [Google Scholar] [CrossRef]
- Imtiaz, H.; Khan, M.; Ehsan, H.; Wahab, A.; Rafae, A.; Khan, A.Y.; Jamil, A.; Sana, M.K.; Jamal, A.; Ali, T.J.; et al. Efficacy and toxicity profile of carfilzomib-based regimens for treatment of newly diagnosed multiple myeloma: A systematic review. Onco Targets Ther. 2021, 14, 4941–4960. [Google Scholar] [CrossRef]
- Latif, A.; Kapoor, V.; Lateef, N.; Ahsan, M.J.; Usman, R.M.; Malik, S.U.; Ahmad, N.; Rosko, N.; Rudoni, J.; William, P.; et al. Incidence and management of carfilzomib-induced cardiovascular toxicity; a systematic review and meta-analysis. Cardiovasc. Hematol. Disord. Drug Targets 2021, 21, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Dasgupta, S.; Gong, Y.; Shah, U.A.; Fradley, M.G.; Cheng, R.K.; Roy, B.; Guha, A. Cardiotoxicity as an adverse effect of immunomodulatory drugs and proteasome inhibitors in multiple myeloma: A network meta-analysis of randomized clinical trials. Hematol. Oncol. 2022, 40, 233–242. [Google Scholar] [CrossRef]
- Xie, J.; Wan, N.; Liang, Z.; Zhang, T.; Jiang, J. Ixazomib—The first oral proteasome inhibitor. Leuk. Lymphoma 2019, 60, 610–618. [Google Scholar] [CrossRef]
- Richardson, P.G.; Moreau, P.; Laubach, J.P.; Gupta, N.; Hui, A.M.; Anderson, K.C.; San Miguel, J.F.; Kumar, S. The investigational proteasome inhibitor ixazomib for the treatment of multiple myeloma. Future Oncol. 2015, 11, 1153–1168. [Google Scholar] [CrossRef]
- Das, D.S.; Ray, A.; Song, Y.; Richardson, P.; Trikha, M.; Chauhan, D.; Anderson, K.C. Synergistic anti-myeloma activity of the proteasome inhibitor marizomib and the IMiD immunomodulatory drug pomalidomide. Br. J. Haematol. 2015, 171, 798–812. [Google Scholar] [CrossRef]
- Spencer, A.; Harrison, S.; Zonder, J.; Badros, A.; Laubach, J.; Bergin, K.; Khot, A.; Zimmerman, T.; Chauhan, D.; Levin, N.; et al. A phase 1 clinical trial evaluating marizomib, pomalidomide and low-dose dexamethasone in relapsed and refractory multiple myeloma (NPI-0052-107): Final study results. Br. J. Haematol. 2018, 180, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, Y.; Liang, Q.; Qu, Y.; Zhang, L.; Liu, Y.; Fang, B.; Yun, Z.; Du, X.; Xi, Y.; et al. Phase I study of a novel oral proteasome inhibitor TQB3602 in relapsed/refractory multiple myeloma. Blood 2022, 140, 4396–4397. [Google Scholar] [CrossRef]
- Tang, W.; Li, Y.; Zhong, X.; Liang, Q.; Liu, Y.; Zeng, Y.; Fang, B.; Zheng, L.; Niu, T. Phase I study of TQB3602 capsule, an oral proteasome inhibitor, in relapsed refractory multiple myeloma. Hemasphere 2022, 6, 843–844. [Google Scholar] [CrossRef]
- Lei, H.; Wang, J.; Hu, J.; Zhu, Q.; Wu, Y. Deubiquitinases in hematological malignancies. Biomark. Res. 2021, 9, 66. [Google Scholar] [CrossRef]
- Chauhan, D.; Tian, Z.; Nicholson, B.; Kumar, K.G.; Zhou, B.; Carrasco, R.; McDermott, J.L.; Leach, C.A.; Fulcinniti, M.; Kodrasov, M.P.; et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell 2012, 22, 345–358. [Google Scholar] [CrossRef]
- Wang, X.; Mazurkiewicz, M.; Hillert, E.K.; Olofsson, M.H.; Pierrou, S.; Hillertz, P.; Gullbo, J.; Selvaraju, K.; Paulus, A.; Akhtar, S.; et al. The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells. Sci. Rep. 2016, 6, 26979. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Paner, A.; Berdeja, J.G.; Paba-Prada, C.; Venugopal, P.; Porkka, K.; Gullbo, J.; Linder, S.; Loskog, A.; Richardson, P.G.; et al. Phase 1 study of the protein deubiquitinase inhibitor VLX1570 in patients with relapsed and/or refractory multiple myeloma. Investig. New Drugs 2020, 38, 1448–1453. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, M.; Qiu, X.; Lu, Y. Targeting the neddylation pathway in cells as a potential therapeutic approach for diseases. Cancer Chemother. Pharmacol. 2018, 81, 797–808. [Google Scholar] [CrossRef]
- Zhou, L.; Jia, L. Targeting protein neddylation for cancer therapy. Adv. Exp. Med. Biol. 2020, 1217, 297–315. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, Y.; Sun, Y. Anticancer drug discovery by targeting cullin neddylation. Acta Pharm. Sin. B 2020, 10, 746–765. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Yu, G.; Chen, P.; Li, H.; Wei, D.; Zhu, J.; Xie, L.; Jia, H.; Shi, J.; et al. Overactivated neddylation pathway as a therapeutic target in lung cancer. J. Natl. Cancer Inst. 2014, 106, dju083. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yang, J.P.; Cao, Y.; Peng, L.X.; Zheng, L.S.; Sun, R.; Meng, D.F.; Wang, M.Y.; Mei, Y.; Qiang, Y.Y.; et al. Promoting tumorigenesis in nasopharyngeal carcinoma, NEDD8 serves as a potential theranostic target. Cell Death Dis. 2017, 8, e2834. [Google Scholar] [CrossRef] [PubMed]
- McMillin, D.W.; Jacobs, H.M.; Delmore, J.E.; Buon, L.; Hunter, Z.R.; Monrose, V.; Yu, J.; Smith, P.G.; Richardson, P.G.; Anderson, K.C.; et al. Molecular and cellular effects of NEDD8-activating enzyme inhibition in myeloma. Mol. Cancer Ther. 2012, 11, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Faessel, H.M.; Mould, D.R.; Zhou, X.; Faller, D.V.; Sedarati, F.; Venkatakrishnan, K. Population pharmacokinetics of pevonedistat alone or in combination with standard of care in patients with solid tumours or haematological malignancies. Br. J. Clin. Pharmacol. 2019, 85, 2568–2579. [Google Scholar] [CrossRef]
- Yoshimura, C.; Muraoka, H.; Ochiiwa, H.; Tsuji, S.; Hashimoto, A.; Kazuno, H.; Nakagawa, F.; Komiya, Y.; Suzuki, S.; Takenaka, T.; et al. TAS4464, a highly potent and selective inhibitor of NEDD8-activating enzyme, suppresses neddylation and shows antitumor activity in diverse cancer models. Mol. Cancer Ther. 2019, 18, 1205–1216. [Google Scholar] [CrossRef]
- Shah, J.J.; Jakubowiak, A.J.; O’Connor, O.A.; Orlowski, R.Z.; Harvey, R.D.; Smith, M.R.; Lebovic, D.; Diefenbach, C.; Kelly, K.; Hua, Z.; et al. Phase I study of the novel investigational NEDD8-activating enzyme inhibitor pevonedistat (MLN4924) in patients with relapsed/refractory multiple myeloma or lymphoma. Clin. Cancer Res. 2016, 22, 34–43. [Google Scholar] [CrossRef]
- Taylor, B.C.; Young, N.L. Combinations of histone post-translational modifications. Biochem. J. 2021, 478, 511–532. [Google Scholar] [CrossRef]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef]
- Ilango, S.; Paital, B.; Jayachandran, P.; Padma, P.R.; Nirmaladevi, R. Epigenetic alterations in cancer. Front. Biosci. 2020, 25, 1058–1109. [Google Scholar] [CrossRef]
- Shvedunova, M.; Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone deacetylases (HDACs): Evolution, specificity, role in transcriptional complexes, and pharmacological actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Caprio, C.; Sacco, A.; Giustini, V.; Roccaro, A.M. Epigenetic aberrations in multiple myeloma. Cancers 2020, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Mithraprabhu, S.; Kalff, A.; Chow, A.; Khong, T.; Spencer, A. Dysregulated class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics 2014, 9, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009, 280, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ruzic, D.; Djoković, N.; Srdić-Rajić, T.; Echeverria, C.; Nikolic, K.; Santibanez, J.F. Targeting histone deacetylases: Opportunities for cancer treatment and chemoprevention. Pharmaceutics 2022, 14, 209. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Siegel, D.S.; Lonial, S.; Qi, J.; Hajek, R.; Facon, T.; Rosinol, L.; Williams, C.; Blacklock, H.; Goldschmidt, H.; et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): A multicentre, randomised, double-blind study. Lancet Oncol. 2013, 14, 1129–1140. [Google Scholar] [CrossRef]
- Richardson, P.G.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Guenther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone in previously treated multiple myeloma: Outcomes by prior treatment. Blood 2016, 127, 713–721. [Google Scholar] [CrossRef]
- Huang, H.H.; Hou, J.; Zhang, Y.M.; Zhou, Y.B.; Jia, L.; Nan, F.J. Phase 1 study of bisthianostat, an orally efficacious pan-HDAC inhibitor: Part results of safety, pharmacokinetics and efficacy in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 5591. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Zhang, Y.M.; Huang, H.H.; Shen, L.J.; Han, X.F.; Hu, X.B.; Yu, S.D.; Gao, A.H.; Sheng, L.; Su, M.B.; et al. Pharmacodynamic, pharmacokinetic, and phase 1a study of bisthianostat, a novel histone deacetylase inhibitor, for the treatment of relapsed or refractory multiple myeloma. Acta Pharmacol. Sin. 2021, 43, 1091–1099. [Google Scholar] [CrossRef]
- Vogl, D.T.; Raje, N.S.; Jagannath, S.; Richardson, P.G.; Hari, P.; Orlowski, R.Z.; Supko, J.G.; Tamang, D.; Yang, M.; Jones, S.S.; et al. Ricolinostat, the first selective histone deacetylase 6 inhibitor, in combination with bortezomib and dexamethasone for relapsed or refractory multiple myeloma. Clin. Cancer Res. 2017, 23, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.J.; Bensinger, W.I.; Supko, J.G.; Voorhees, P.M.; Berdeja, J.G.; Richardson, P.G.; Libby, E.N.; Wallace, E.E.; Birrer, N.E.; Burke, J.N.; et al. Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: A multicentre phase 1b trial. Lancet Oncol. 2016, 17, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Yan, L.; Shang, J.; Jin, S.; Shi, X.; Yan, S.; Wu, D.; Fu, C. Phase 2 Suzhou MM02 study: Chidamide with VRD versus VRD in newly diagnosed high risk transplant eligible multiple myeloma patients. Blood 2021, 138, 4765. [Google Scholar] [CrossRef]
- Sborov, D.W.; Benson, D.M.; Williams, N.; Huang, Y.; Bowers, M.A.; Humphries, K.; Efebera, Y.; Devine, S.; Hofmeister, C.C. Lenalidomide and vorinostat maintenance after autologous transplant in multiple myeloma. Br. J. Haematol. 2015, 171, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.S.; Dimopoulos, M.; Jagannath, S.; Goldschmidt, H.; Durrant, S.; Kaufman, J.L.; Leleu, X.; Nagler, A.; Offner, F.; Graef, T.; et al. VANTAGE 095: An international, multicenter, open-label study of vorinostat (MK-0683) in combination with bortezomib in patients with relapsed and refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2016, 16, 329–334.e1. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Pawlyn, C.; Tillotson, A.L.; Sherratt, D.; Flanagan, L.; Low, E.; Morgan, G.J.; Williams, C.; Kaiser, M.; Davies, F.E.; et al. Bortezomib, vorinostat, and dexamethasone combination therapy in relapsed myeloma: Results of the phase 2 MUK four trial. Clin. Lymphoma Myeloma Leuk. 2021, 21, 154–161.e3. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.L.; Mina, R.; Shah, J.J.; Laubach, J.P.; Nooka, A.K.; Lewis, C.; Gleason, C.; Sharp, C.; Harvey, R.D.; Heffner, L.T.; et al. Phase 1 trial evaluating vorinostat plus bortezomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 797–803. [Google Scholar] [CrossRef]
- Vesole, D.H.; Bilotti, E.; Richter, J.R.; McNeill, A.; McBride, L.; Raucci, L.; Anand, P.; Bednarz, U.; Ivanovski, K.; Smith, J.; et al. Phase I study of carfilzomib, lenalidomide, vorinostat, and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Br. J. Haematol. 2015, 171, 52–59. [Google Scholar] [CrossRef]
- Waldschmidt, J.M.; Keller, A.; Ihorst, G.; Grishina, O.; Muller, S.; Wider, D.; Frey, A.V.; King, K.; Simon, R.; May, A.; et al. Safety and efficacy of vorinostat, bortezomib, doxorubicin and dexamethasone in a phase I/II study for relapsed or refractory multiple myeloma (VERUMM study: Vorinostat in elderly, relapsed and unfit multiple myeloma). Haematologica 2018, 103, e473–e479. [Google Scholar] [CrossRef]
- Niesvizky, R.; Richardson, P.G.; Gabrail, N.Y.; Madan, S.; Yee, A.J.; Quayle, S.N.; Almeciga-Pinto, I.; Jones, S.S.; Houston, L.; Hayes, D.; et al. ACY-241, a novel, HDAC6 selective inhibitor: Synergy with immunomodulatory (IMiD (R)) drugs in multiple myeloma (MM) cells and early clinical results (ACE-MM-200 study). Blood 2015, 126, 3. [Google Scholar] [CrossRef]
- Niesvizky, R.; Richardson, P.G.; Yee, A.J.; Nooka, A.K.; Raab, M.S.; Shain, K.H.; Gabrail, N.Y.; Matous, J.; Agarwal, A.B.; Hoffman, J.; et al. Selective HDAC6 inhibitor ACY-241, an oral tablet, combined with pomalidomide and dexamethasone: Safety and efficacy of escalation and expansion cohorts in patients with relapsed or relapsed-and-refractory multiple myeloma (ACE-MM-200 study). Blood 2016, 128, 7. [Google Scholar] [CrossRef]
- North, B.J.; Almeciga-Pinto, I.; Tamang, D.; Yang, M.; Jones, S.S.; Quayle, S.N. Enhancement of pomalidomide anti-tumor response with ACY-241, a selective HDAC6 inhibitor. PLoS ONE 2017, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.J.; Yao, W.Q.; Yan, L.Z.; Shi, X.L.; Wang, R.J.; Yan, S.; Liu, Y.; Wu, D.P.; Cheng, F.C. Initial safety and efficacy of dose-escalating HDACs inhibitor chidamide with VRD (Chi-VRD) treatment for newly-diagnosed high-risk transplant eligible multiple myeloma patients. Blood 2019, 134, 1855. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Knapp, S. The bromodomain interaction module. FEBS Lett. 2012, 586, 2692–2704. [Google Scholar] [CrossRef]
- Gokani, S.; Bhatt, L.K. Bromodomains: A novel target for the anticancer therapy. Eur. J. Pharmacol. 2021, 911, 174523. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Petosa, C.; McKenna, C.E. Bromodomains: Structure, function and pharmacology of inhibition. Biochem. Pharmacol. 2016, 106, 1–18. [Google Scholar] [CrossRef]
- Llombart, V.; Mansour, M.R. Therapeutic targeting of “undruggable” MYC. eBioMedicine 2022, 75, 103756. [Google Scholar] [CrossRef]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.W.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 903–916. [Google Scholar] [CrossRef]
- Jung, M.; Gelato, K.A.; Fernandez-Montalvan, A.; Siegel, S.; Haendler, B. Targeting BET bromodomains for cancer treatment. Epigenomics 2015, 7, 487–501. [Google Scholar] [CrossRef]
- Martin, M.P.; Olesen, S.H.; Georg, G.I.; Schonbrunn, E. Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains. ACS Chem. Biol. 2013, 8, 2360–2365. [Google Scholar] [CrossRef]
- Chng, W.J.; Huang, G.F.; Chung, T.H.; Ng, S.B.; Gonzalez-Paz, N.; Troska-Price, T.; Mulligan, G.; Chesi, M.; Bergsagel, P.L.; Fonseca, R. Clinical and biological implications of MYC activation: A common difference between MGUS and newly diagnosed multiple myeloma. Leukemia 2011, 25, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Aird, F.; Kandela, I.; Mantis, C. Replication study: BET bromodomain inhibition as a therapeutic strategy to target c-Myc. eLife 2017, 6, e21253. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Boi, M.; Vurchio, V.; Ercole, E.; Machiorlatti, R.; Messana, K.; Landra, I.; Urigu, S.; Aliberti, S.; Riveiro, E.; et al. OTX015, a novel BET inhibitor, is a promising anticancer agent for multiple myeloma. Cancer Res. 2014, 74, 5531. [Google Scholar] [CrossRef]
- Amorim, S.; Stathis, A.; Gleeson, M.; Iyengar, S.; Magarotto, V.; Leleu, X.; Morschhauser, F.; Karlin, L.; Broussais, F.; Rezai, K.; et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: A dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016, 3, e196–e204. [Google Scholar] [CrossRef]
- Albrecht, B.K.; Gehling, V.S.; Hewitt, M.C.; Vaswani, R.G.; Cote, A.; Leblanc, Y.; Nasveschuk, C.G.; Bellon, S.; Bergeron, L.; Campbell, R.; et al. Identification of a benzoisoxazoloazepine inhibitor (CPI-0610) of the bromodomain and extra-terminal (BET) family as a candidate for human clinical trials. J. Med. Chem. 2016, 59, 1330–1339. [Google Scholar] [CrossRef]
- Ramasamy, K.; Nooka, A.; Quach, H.; Htut, M.; Popat, R.; Liedtke, M.; Tuchman, S.A.; Laubach, J.P.; Gasparetto, C.; Chanan-Khan, A.A.; et al. Open label, multicenter, dose-escalation/ expansion phase Ib study to evaluate safety and activity of BET Inhibitor RO6870810 (RO), given as monotherapy to patients (pts) with advanced multiple myeloma. Blood 2020, 136, 12. [Google Scholar] [CrossRef]
- Siu, K.T.; Eda, H.; Santo, L.; Ramachandran, J.; Koulnis, M.; Mertz, J.; Sims, R.J.; Cooper, M.; Raje, N.S. Effect of the BET inhibitor, CPI-0610, alone and in combination with lenalidomide in multiple myeloma. Blood 2015, 126, 4255. [Google Scholar] [CrossRef]
- Klener, P.; Sovilj, D.; Renesova, N.; Andera, L. BH3 mimetics in hematologic malignancies. Int. J. Mol. Sci. 2021, 22, 10157. [Google Scholar] [CrossRef]
- Yap, J.L.; Chen, L.; Lanning, M.E.; Fletcher, S. Expanding the cancer arsenal with targeted therapies: Disarmament of the anti-apoptotic Bcl-2 proteins by small-molecules. J. Med. Chem. 2016, 60, 821–838. [Google Scholar] [CrossRef]
- Krishna, S.; Kumar, S.B.; Murthy, T.P.K.; Murahari, M. Structure-based design approach of potential BCL-2 inhibitors for cancer chemotherapy. Comput. Biol. Med. 2021, 134, 104455. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef]
- Kumar, S.; Harrison, S.J.; Cavo, M.; de La Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Updated results from BELLINI, a phase III study of venetoclax or placebo in combination with bortezomib and dexamethasone in relapsed/refractory multiple myeloma. J. Clin. Oncol. 2020, 38, 8509. [Google Scholar] [CrossRef]
- Mateos, M.V.; Moreau, P.; Dimopoulos, M.A.; Hong, W.J.; Cooper, S.; Yu, Y.; Jalaluddin, M.; Ross, J.A.; Karve, S.; Coppola, S.; et al. A phase III, randomized, multicenter, open-label study of venetoclax or pomalidomide in combination with dexamethasone in patients with t(11;14)-positive relapsed/refractory multiple myeloma. J. Clin. Oncol. 2020, 38, TPS8554. [Google Scholar] [CrossRef]
- Fu, C.C.; Chen, Z.; Li, W.S.; Men, L.C.; Wu, D.P.; Yang, D.J.; Zhai, Y.F. Trial in progress: Phase 1b/2 open-label study of lisaftoclax (APG-2575) monotherapy or in combination with lenalidomide/dexamethasone in patients with relapsed or refractory multiple myeloma (R/R MM). Blood 2021, 138, 4764. [Google Scholar] [CrossRef]
- Hu, N.; Guo, Y.H.; Xue, H.; Liu, Y.; Guo, Y.; Wang, F.; Song, X.M.; Guo, Y.; Chen, S.S.; Xu, H.P.; et al. Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. Cancer Res. 2020, 80, 3077. [Google Scholar] [CrossRef]
- Quach, H.; Rajagopal, R.; Spencer, A.; Low, M.; Kazandjian, D.; Crescenzo, R.; Du, C.; Patel, S.; Mundra, V.; Cheng, H.; et al. Preliminary safety of a Bcl-2 Inhibitor, Bgb-11417, in patients with relapsed/refractory multiple myeloma harboring t(11,14): A non-randomized, open-label, phase 1b/2 study. Blood 2022, 140, 7269–7271. [Google Scholar] [CrossRef]
- Lu, X.; Liang, H.; Orvig, C.; Chen, Z.F. Peptide and small molecule inhibitors targeting myeloid cell leukemia 1 (Mcl-1) as novel antitumor agents. Curr. Mol. Med. 2021, 21, 426–439. [Google Scholar] [CrossRef]
- Spencer, A.; Rosenberg, A.S.; Jakubowiak, A.; Raje, N.; Chatterjee, M.; Trudel, S.; Bahlis, N.J.; Siegel, D.S.; Wilop, S.; Harrison, S.J.; et al. A phase 1, first-in-human study of AMG 176, a selective MCL-1 Inhibitor, in patients with relapsed or refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, E53–E54. [Google Scholar] [CrossRef]
- Halilovic, E.; Chanrion, M.; Mistry, P.; Wartmann, M.; Qiu, S.M.; Sanghavi, S.; Chen, Y.; Lysiak, G.; Maragno, A.L.; Pfaar, U.; et al. MIK665/S64315, a novel Mcl-1 inhibitor, in combination with Bcl-2 inhibitors exhibits strong synergistic antitumor activity in a range of hematologic malignancies. Cancer Res. 2019, 79, 4477. [Google Scholar] [CrossRef]
- Bhagwat, N.; Grego, A.; Gowen-MacDonald, W.; Wang, M.; Cowart, M.; Wu, X.W.; Zhuo, J.C.; Combs, A.; Ruggeri, B.; Scherle, P.; et al. Preclinical characterization of PRT1419, a potent, selective and orally available inhibitor of MCL1. Cancer Res. 2021, 81, 983. [Google Scholar] [CrossRef]
- Matulis, S.M.; Gupta, V.A.; Brown, I.; Keats, J.J.; Secrist, P.; Cidado, J.; Tron, A.E.; Neri, P.; Bahlis, N.; Kaufman, J.L.; et al. Preclinical activity of novel MCL1 inhibitor AZD5991 in multiple myeloma. Blood 2018, 132, 952. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, S.; Wang, Y.; Mo, Y.; Xiong, F.; Zhang, S.; Zeng, Z.; Xiong, W.; Li, G.; Chen, H.; et al. Gossypol induces apoptosis of multiple myeloma cells through the JUN-JNK pathway. Am. J. Cancer Res. 2020, 10, 870–883. [Google Scholar]
- Ailawadhi, S.; Alegria, V.R.; Ahmed, S.; Laplant, B.; Manna, A.; Parrondo, R.; Roy, V.; Sher, T.; Edwards, B.; Lanier, S.; et al. Phase I Study of a novel Bcl-2 inhibitor, AT-101 in combination with lenalidomide and dexamethasone in patients with relapsed and/or refractory multiple myeloma (RRMM). Blood 2019, 134, 3137. [Google Scholar] [CrossRef]
- Parrondo, R.D.; Paulus, A.; Ailawadhi, S. Updates in the Use of BCL-2-family small molecule inhibitors for the treatment of relapsed/refractory multiple myeloma. Cancers 2022, 14, 3330. [Google Scholar] [CrossRef] [PubMed]
- Bahlis, N.J.; Baz, R.; Harrison, S.J.; Quach, H.; Ho, S.J.; Vangsted, A.J.; Plesner, T.; Moreau, P.; Gibbs, S.D.; Coppola, S.; et al. Phase I study of venetoclax plus daratumumab and dexamethasone, with or without bortezomib, in patients with relapsed or refractory multiple myeloma with and without t(11;14). J. Clin. Oncol. 2021, 39, 3602–3612. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Quach, H.; Baz, R.; Vangsted, A.J.; Ho, S.J.; Harrison, S.J.; Plesner, T.; Moreau, P.; Gibbs, S.D.; Medvedova, E.; et al. Safety and preliminary efficacy from the expansion cohort of a phase 1/2 study of venetoclax plus daratumumab and dexamethasone vs daratumumab plus bortezomib and dexamethasone in patients with t(11;14) relapsed/refractory multiple myeloma. Blood 2021, 138 (Suppl. S1), 817. [Google Scholar] [CrossRef]
- Costa, L.J.; Davies, F.E.; Monohan, G.P.; Kovacsovics, T.J.; Burwick, N.; Jakubowiak, A.J.; Kaufman, J.L.; Hong, W.J.; Dail, M.; Salem, A.H.; et al. Phase 2 study of venetoclax plus carfilzomib and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Adv. 2021, 5, 3748–3759. [Google Scholar] [CrossRef]
- Kumar, S.; Kaufman, J.L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Gasparetto, C.; Schjesvold, F.H.; Moreau, P.; Touzeau, C.; Facon, T.; Boise, L.H.; Jiang, Y.; Yang, X.; Dunbar, F.; et al. Targeting BCL-2 with venetoclax and dexamethasone in patients with relapsed/refractory t(11;14) multiple myeloma. Am. J. Hematol. 2021, 96, 418–427. [Google Scholar] [CrossRef]
- Kumar, S.; Harrison, S.J.; Cavo, M.; de la Rubia, J.; Popat, R.; Gasparetto, C.J.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Final overall survival results from BELLINI, a phase 3 study of venetoclax or placebo in combination with bortezomib and dexamethasone in relapsed/refractory multiple myeloma. Blood 2021, 138, 84. [Google Scholar] [CrossRef]
- Shahin, R.; Aljamal, S. Kinesin spindle protein inhibitors in cancer: From high throughput screening to novel therapeutic strategies. Future Sci. OA 2022, 8, Fso778. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.J.; Anderson, D.; Williams, L.; Rieger, R. ARRY-520 combined with pomalidomide displays enhanced anti-tumor activity in preclinical models of multiple myeloma. Blood 2013, 122, 3167. [Google Scholar] [CrossRef]
- Woessner, R.; Tunquist, B.; Lemieux, C.; Chlipala, E.; Jackinsky, S.; Dewolf, W., Jr.; Voegtli, W.; Cox, A.; Rana, S.; Lee, P.; et al. ARRY-520, a novel KSP inhibitor with potent activity in hematological and taxane-resistant tumor models. Anticancer Res. 2009, 29, 4373–4380. [Google Scholar] [PubMed]
- Shah, J.J.; Kaufman, J.L.; Zonder, J.A.; Cohen, A.D.; Bensinger, W.I.; Hilder, B.W.; Rush, S.A.; Walker, D.H.; Tunquist, B.J.; Litwiler, K.S.; et al. A phase 1 and 2 study of filanesib alone and in combination with low-dose dexamethasone in relapsed/refractory multiple myeloma. Cancer 2017, 123, 4617–4630. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Htut, M.; Zonder, J.A.; Fay, J.W.; Jakubowiak, A.J.; Levy, J.B.; Lau, K.; Burt, S.M.; Tunquist, B.J.; Hilder, B.W.; et al. A phase 1 dose-escalation study of filanesib plus bortezomib and dexamethasone in patients with recurrent/refractory multiple myeloma. Cancer 2016, 122, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Kaufman, J.L.; Htut, M.; Agrawal, M.; Mazumder, A.; Cornell, R.F.; Zonder, J.A.; Fay, J.W.; Modiano, M.R.; Moshier, E.L.; et al. Filanesib plus bortezomib and dexamethasone in relapsed/refractory t(11;14) and 1q21 gain multiple myeloma. Cancer Med. 2022, 11, 358–370. [Google Scholar] [CrossRef]
- Hanamura, I. Gain/amplification of chromosome arm 1q21 in multiple myeloma. Cancers 2021, 13, 256. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Barwick, B.G.; Joseph, N.; Heffner, L.T.; Hofmeister, C.C.; Bernal, L.; Dhodapkar, M.V.; Gupta, V.A.; Jaye, D.L.; Wu, J.; et al. Gain of chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J. 2019, 9, 94. [Google Scholar] [CrossRef]
- Mohan, M.; Weinhold, N.; Schinke, C.; Thanedrarajan, S.; Rasche, L.; Sawyer, J.R.; Tian, E.; van Rhee, F.; Zangari, M. Daratumumab in high-risk relapsed/refractory multiple myeloma patients: Adverse effect of chromosome 1q21 gain/amplification and GEP70 status on outcome. Br. J. Haematol. 2020, 189, 67–71. [Google Scholar] [CrossRef]
- Lee, H.C.; Shah, J.J.; Feng, L.; Manasanch, E.E.; Lu, R.; Morphey, A.; Crumpton, B.; Patel, K.K.; Wang, M.L.; Alexanian, R.; et al. A phase 1 study of filanesib, carfilzomib, and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Blood Cancer J. 2019, 9, 80. [Google Scholar] [CrossRef]
- Ocio, E.M.; Motllo, C.; Rodriguez-Otero, P.; Martinez-Lopez, J.; Cejalvo, M.J.; Martin-Sanchez, J.; Blade, J.; Garcia-Malo, M.D.; Dourdil, M.V.; Garcia-Mateo, A.; et al. Filanesib in combination with pomalidomide and dexamethasone in refractory MM patients: Safety and efficacy, and association with alpha 1-acid glycoprotein (AAG) levels. Phase Ib/II Pomdefil clinical trial conducted by the Spanish MM group. Br. J. Haematol. 2021, 192, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.G.; Passam, F.H.; Ganotakis, E.S.; Sfiridaki, K.; Xilouri, I.; Perisinakis, K.; Kyriakou, D.S. The clinical and prognostic significance of erythrocyte sedimentation rate (ESR), serum interleukin-6 (IL-6) and acute phase protein levels in multiple myeloma. Clin. Lab. Haematol. 2003, 25, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tunquist, B.; Brown, K.; Hingorani, G.; Aitchison, R.; Regensburger, J.; Lonial, S.; Kaufman, J.; Zonder, J.; Cohen, A.; Bensinger, W.; et al. Alpha 1-acid glycoprotein (AAG) is a potential patient selection biomarker for improved clinical activity of ARRY-520 in relapsed and refractory multiple myeloma (MM). Haematologica 2013, 98, 328–329. [Google Scholar]
- Algarin, E.M.; Hernandez-Garcia, S.; Garayoa, M.; Ocio, E.M. Filanesib for the treatment of multiple myeloma. Expert Opin. Investig. Drugs 2020, 29, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Zonder, J.A.; Usiinani, S.; Scott, E.C.; Harneister, C.C.; Lendvai, N.; Berdeja, J.C.; Anderson, L.D.; Hari, P.; Singhal, S.; Orloff, G.; et al. Phase 2 study of carfilzomib (CFZ) with or without filanesib (FIL) in patients with advanced multiple myeloma (MM). Blood 2015, 126, 728. [Google Scholar] [CrossRef]
- Lonial, S.; Delforge, M.; Einsele, H.; Moreau, P.; Kaiser, M.; Dimopoulos, M.A.; Oriol, A.; Gyger, M.; Hilder, B.; Ptaszynski, A.M.; et al. The AfFIRM study: A multicenter phase 2 study of single-agent filanesib (ARRY-520) in patients with advanced multiple myeloma. J. Clin. Oncol. 2015, 33, TPS8613. [Google Scholar] [CrossRef]
- Schwartz, T.U. Solving the nuclear pore puzzle. Science 2022, 376, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Raices, M.; D’Angelo, M.A. Structure, maintenance, and regulation of nuclear pore complexes: The gatekeepers of the eukaryotic genome. Cold Spring Harb. Perspect. Biol. 2022, 14, a040691. [Google Scholar] [CrossRef]
- Azmi, A.S.; Uddin, M.H.; Mohammad, R.M. The nuclear export protein XPO1—From biology to targeted therapy. Nat. Rev. Clin. Oncol. 2021, 18, 152–169. [Google Scholar] [CrossRef]
- Li, S.; Fu, J.; Lu, C.; Mapara, M.Y.; Raza, S.; Hengst, U.; Lentzsch, S. Elevated translation initiation factor eIF4E Is an attractive therapeutic target in multiple myeloma. Mol. Cancer Ther. 2016, 15, 711–719. [Google Scholar] [CrossRef]
- Tai, Y.T.; Landesman, Y.; Acharya, C.; Calle, Y.; Zhong, M.Y.; Cea, M.; Tannenbaum, D.; Cagnetta, A.; Reagan, M.; Munshi, A.A.; et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: Molecular mechanisms and therapeutic implications. Leukemia 2014, 28, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Braggio, E.; Kortuem, K.M.; Egan, J.B.; Zhu, Y.X.; Xin, C.S.; Tiedemann, R.E.; Palmer, S.E.; Garbitt, V.M.; McCauley, D.; et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia 2013, 27, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Matsumori, N.; Taoka, H.; Fujiwara, D.; Schreiner, E.P.; Wolff, B.; Yoshida, M.; Horinouchi, S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 1999, 96, 9112–9117. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Selinexor: First global approval. Drugs 2019, 79, 1485–1494. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Restrepo, P.; Bhalla, S.; Ghodke-Puranik, Y.; Aleman, A.; Leshchenko, V.; Melnekoff, D.T.; Agte, S.; Jiang, J.; Madduri, D.; Richter, J.; et al. A three-gene signature predicts response to selinexor in multiple myeloma. JCO Precis. Oncol. 2022, 6, e2200147. [Google Scholar] [CrossRef]
- Mateos, M.V.; Gavriatopoulou, M.; Facon, T.; Auner, H.W.; Leleu, X.; Hájek, R.; Dimopoulos, M.A.; Delimpasi, S.; Simonova, M.; Špička, I.; et al. Effect of prior treatments on selinexor, bortezomib, and dexamethasone in previously treated multiple myeloma. J. Hematol. Oncol. 2021, 14, 59. [Google Scholar] [CrossRef]
- Grosicki, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; Doronin, V.; et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): A randomised, open-label, phase 3 trial. Lancet 2020, 396, 1563–1573. [Google Scholar] [CrossRef]
- Cornell, R.F.; Baz, R.; Richter, J.R.; Rossi, A.; Vogl, D.T.; Chen, C.; Shustik, C.; Alvarez, M.J.; Shen, Y.; Unger, T.J.; et al. A phase 1 clinical trial of oral eltanexor in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 2022, 97, E54–E58. [Google Scholar] [CrossRef]
- Turner, J.G.; Dawson, J.L.; Grant, S.; Shain, K.H.; Dalton, W.S.; Dai, Y.; Meads, M.; Baz, R.; Kauffman, M.; Shacham, S.; et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J. Hematol. Oncol. 2016, 9, 73. [Google Scholar] [CrossRef]
- Tremblay, G.; Daniele, P.; Breeze, J.; Li, L.; Shah, J.; Shacham, S.; Kauffman, M.; Engelhardt, M.; Chari, A.; Nooka, A.; et al. Quality of life analyses in patients with multiple myeloma: Results from the selinexor (KPT-330) treatment of refractory myeloma (STORM) phase 2b study. BMC Cancer 2021, 21, 993. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Florendo, E.; Mancia, I.S.; Cho, H.; Madduri, D.; Parekh, S.; Richter, J.; Dhadwal, A.; Thomas, J.; Jiang, G.; et al. Optimal supportive care with selinexor improves outcomes in patients with relapsed/refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2021, 21, e975–e984. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, C.; Lentzsch, S.; Schiller, G.J.; Callander, N.S.; Tuchman, S.; Bahlis, N.J.; White, D.; Chen, C.; Baljevic, M.; Sutherland, H.J.; et al. Selinexor, daratumumab, and dexamethasone in patients with relapsed/refractory multiple myeloma (MM). J. Clin. Oncol. 2020, 38, 8510. [Google Scholar] [CrossRef]
- Gasparetto, C.; Schiller, G.J.; Tuchman, S.; Callander, N.S.; Baljevic, M.; Lentzsch, S.; Rossi, A.C.; Kotb, R.; White, D.; Bahlis, N.J.; et al. Once weekly selinexor, carfilzomib, and dexamethasone (SKd) in carfilzomib nonrefractory multiple myeloma (MM) patients. J. Clin. Oncol. 2021, 39, 8038. [Google Scholar] [CrossRef]
- Turner, J.G.; Cui, Y.; Bauer, A.A.; Dawson, J.L.; Gomez, J.A.; Kim, J.; Cubitt, C.L.; Nishihori, T.; Dalton, W.S.; Sullivan, D.M. Melphalan and exportin 1 inhibitors exert synergistic anti-tumor effects in preclinical models of human multiple myeloma. Cancer Res. 2020, 80, 5344–5354. [Google Scholar] [CrossRef] [PubMed]
- Jakubowiak, A.J.; Jasielec, J.K.; Rosenbaum, C.A.; Cole, C.E.; Chari, A.; Mikhael, J.; Nam, J.; McIver, A.; Severson, E.; Stephens, L.A.; et al. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br. J. Haematol. 2019, 86, 549–560. [Google Scholar] [CrossRef]
- Delimpasi, S.; Mateos, M.V.; Auner, H.W.; Gavriatopoulou, M.; Dimopoulos, M.A.; Quach, H.; Pylypenko, H.; Hájek, R.; Leleu, X.; Dolai, T.K.; et al. Efficacy and tolerability of once-weekly selinexor, bortezomib, and dexamethasone in comparison with standard twice-weekly bortezomib and dexamethasone in previously treated multiple myeloma with renal impairment: Subgroup analysis from the BOSTON study. Am. J. Hematol. 2021, 97, E83–E86. [Google Scholar] [CrossRef] [PubMed]
- Auner, H.W.; Gavriatopoulou, M.; Delimpasi, S.; Simonova, M.; Spicka, I.; Pour, L.; Dimopoulos, M.A.; Kriachok, I.; Pylypenko, H.; Leleu, X.; et al. Effect of age and frailty on the efficacy and tolerability of once-weekly selinexor, bortezomib, and dexamethasone in previously treated multiple myeloma. Am. J. Hematol. 2021, 96, 708–718. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Chen, C.; Baljevic, M.; Tuchman, S.; Bahlis, N.J.; Schiller, G.J.; Lipe, B.; Kotb, R.; Sutherland, H.J.; Madan, S.; et al. Oral selinexor, pomalidomide, and dexamethasone (XPd) at recommended phase 2 dose in relapsed refractory multiple myeloma (MM). J. Clin. Oncol. 2021, 39, 8018. [Google Scholar] [CrossRef]
- Gasparetto, C.; Lipe, B.; Tuchman, S.; Callander, N.S.; Lentzsch, S.; Baljevic, M.; Rossi, A.C.; Bahlis, N.J.; White, D.; Chen, C.; et al. Once weekly selinexor, carfilzomib, and dexamethasone (SKd) in patients with relapsed/refractory multiple myeloma (MM). J. Clin. Oncol. 2020, 38 (Suppl. S15), 8530. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; De La Calle, V.G.; Sureda, A.; De Arriba, F.; Segura, M.R.; Ribas, P.; Gonzalez, A.P.; Gonzalez-Montes, Y.; Oriol, A.; Martinez-Lopez, J.; et al. Selinexor in combination with daratumumab-bortezomib and dexamethasone for the treatment of relapse or refractory multiple myeloma: Initial results of the phase 2, open-label, multicenter GEM-Selibordara study. Blood 2021, 138, 1677. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, F.; Li, J.; Zhang, C.; Li, W.; Cheng, H.; Cai, H.; Chen, B.; Guo, J.; Mei, J.; et al. Preliminary results from the launch study-a multicenter, open-label study of selinexor, dexamethasone and chemotherapy drugs in relapsed/refractory multiple myeloma. Blood 2022, 140, 12613. [Google Scholar] [CrossRef]
- Qiu, L.; Xia, Z.; Fu, C.; Chen, W.; Chang, C.; Fang, B.; An, G.; Wei, Y.; Cai, Z.; Gao, S.; et al. Selinexor plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma previously treated with an immunomodulatory agent and a proteasome inhibitor (MARCH): A phase II, single-arm study. BMC Med. 2022, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Baugh, E.H.; Ke, H.; Levine, A.J.; Bonneau, R.A.; Chan, C.S. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018, 25, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Tan, B.X.; Lane, D. How the other half lives: What p53 does when it is not being a transcription factor. Int. J. Mol. Sci. 2019, 21, 13. [Google Scholar] [CrossRef]
- Weinhold, N.; Ashby, C.; Rasche, L.; Chavan, S.S.; Stein, C.; Stephens, O.W.; Tytarenko, R.; Bauer, M.A.; Meissner, T.; Deshpande, S.; et al. Clonal selection and double hit events involving tumor suppressor genes underlie relapse from chemotherapy: Myeloma as a model. Blood 2016, 128, 1735–1744. [Google Scholar] [CrossRef]
- Melvin, A.T.; Dumberger, L.D.; Woss, G.S.; Waters, M.L.; Allbritton, N.L. Identification of a p53-based portable degron based on the MDM2-p53 binding region. Analyst 2016, 141, 570–578. [Google Scholar] [CrossRef]
- Raj, N.; Attardi, L.D. The transactivation domains of the p53 protein. Cold Spring Harb. Perspect. Med. 2017, 7, a026047. [Google Scholar] [CrossRef]
- Herrero, A.B.; Rojas, E.A.; Misiewicz-Krzeminska, I.; Krzeminski, P.; Gutierrez, N.C. Molecular mechanisms of p53 deregulation in cancer: An overview in multiple myeloma. Int. J. Mol. Sci. 2016, 17, 2003. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Liu, J.J.; Jiang, N.; Zhang, J.; Ross, T.M.; Chu, X.J.; Bartkovitz, D.; Podlaski, F.; Janson, C.; et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J. Med. Chem. 2013, 56, 5979–5983. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Al-Bahou, A. Navtemadlin. Drugs Future 2022, 47, 247–259. [Google Scholar] [CrossRef]
- Gluck, W.L.; Gounder, M.M.; Frank, R.; Eskens, F.; Blay, J.Y.; Cassier, P.A.; Soria, J.C.; Chawla, S.; de Weger, V.; Wagner, A.J.; et al. Phase 1 study of the MDM2 inhibitor AMG 232 in patients with advanced P53 wild-type solid tumors or multiple myeloma. Investig. New Drugs 2020, 38, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Good, L.; Benner, B.; Carson, W.E. Bruton’s tyrosine kinase: An emerging targeted therapy in myeloid cells within the tumor microenvironment. Cancer Immunol. Immunother. 2021, 70, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zheng, J.; Han, C.; Jin, J.; Han, H.; Liu, Y.; Lau, Y.L.; Tu, W.; Cao, X. Tyrosine kinase Btk is required for NK cell activation. J. Biol. Chem. 2012, 287, 23769–23778. [Google Scholar] [CrossRef]
- Xia, S.; Liu, X.; Cao, X.; Xu, S. T-cell expression of Bruton’s tyrosine kinase promotes autoreactive T-cell activation and exacerbates aplastic anemia. Cell. Mol. Immunol. 2020, 17, 1042–1052. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Jiang, Q.L.; Zhang, B.; Hu, A.M. Bruton’s tyrosine kinase: Potential target in human multiple myeloma. Leuk. Lymphoma 2014, 55, 177–181. [Google Scholar] [CrossRef]
- Richardson, P.G.; Bensinger, W.I.; Huff, C.A.; Costello, C.L.; Lendvai, N.; Berdeja, J.G.; Anderson, L.D., Jr.; Siegel, D.S.; Lebovic, D.; Jagannath, S.; et al. Ibrutinib alone or with dexamethasone for relapsed or relapsed and refractory multiple myeloma: Phase 2 trial results. Br. J. Haematol. 2018, 180, 821–830. [Google Scholar] [CrossRef]
- Hajek, R.; Pour, L.; Ozcan, M.; Martin Sánchez, J.; García Sanz, R.; Anagnostopoulos, A.; Oriol, A.; Cascavilla, N.; Terjung, A.; Lee, Y.; et al. A phase 2 study of ibrutinib in combination with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma. Eur. J. Haematol. 2020, 104, 435–442. [Google Scholar] [CrossRef]
- Woyach, J.A. Ibrutinib and Aspergillus: A Btk-targeted risk. Blood 2018, 132, 1869–1870. [Google Scholar] [CrossRef]
- Rogers, K.A.; Mousa, L.; Zhao, Q.; Bhat, S.A.; Byrd, J.C.; El Boghdadly, Z.; Guerrero, T.; Levine, L.B.; Lucas, F.; Shindiapina, P.; et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia 2019, 33, 2527–2530. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Cornell, R.F.; Gasparetto, C.; Karanes, C.; Matous, J.V.; Niesvizky, R.; Lunning, M.; Usmani, S.Z.; Anderson, L.D., Jr.; Chhabra, S.; et al. Final analysis of a phase 1/2b study of ibrutinib combined with carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma. Hematol. Oncol. 2020, 38, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Parrondo, R.D.; Moustafa, M.A.; LaPlant, B.R.; Alegria, V.; Chapin, D.; Roy, V.; Sher, T.; Paulus, A.; Chanan-Khan, A.A. Ibrutinib, lenalidomide and dexamethasone in patients with relapsed and/or refractory multiple myeloma: Phase I trial results. Hematol. Oncol. 2022, 40, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef]
- Kyrtsonis, M.C.; Repa, C.; Dedoussis, G.V.; Mouzaki, A.; Simeonidis, A.; Stamatelou, M.; Maniatis, A. Serum transforming growth factor-beta 1 is related to the degree of immunoparesis in patients with multiple myeloma. Med. Oncol. 1998, 15, 124–128. [Google Scholar] [CrossRef]
- Malek, E.; Hwang, S.J.; Caimi, P.F.; Metheny, L.L.; Tomlinson, B.K.; Cooper, B.W.; Boughan, K.M.; Otegbeye, F.; Gallogly, M.; Driscoll, J.J.; et al. Phase Ib trial of vactosertib in combination with pomalidomide in relapsed multiple myeloma: A corticosteroid-free approach by targeting TGF-beta signaling pathway. J. Clin. Oncol. 2021, 39, 8039. [Google Scholar] [CrossRef]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The frequency of Ras mutations in cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef]
- Xu, J.; Pfarr, N.; Endris, V.; Mai, E.K.; Md Hanafiah, N.H.; Lehners, N.; Penzel, R.; Weichert, W.; Ho, A.D.; Schirmacher, P.; et al. Molecular signaling in multiple myeloma: Association of RAS/RAF mutations and MEK/ERK pathway activation. Oncogenesis 2017, 6, e337. [Google Scholar] [CrossRef]
- Schjesvold, F.; Ribrag, V.; Rodriguez-Otero, P.; Robak, P.J.; Hansson, M.; Hajek, R.; Amor, A.A.; Martinez-Lopez, J.; Onishi, M.; Gallo, J.D.; et al. Safety and preliminary efficacy results from a phase Ib/II study of cobimetinib as a single agent and in combination with venetoclax with or without atezolizumab in patients with relapsed/refractory multiple myeloma. Blood 2020, 136, 45–46. [Google Scholar] [CrossRef]
- Sharman, J.P.; Chmielecki, J.; Morosini, D.; Palmer, G.A.; Ross, J.S.; Stephens, P.J.; Stafl, J.; Miller, V.A.; Ali, S.M. Vemurafenib response in 2 patients with posttransplant refractory BRAF V600E-mutated multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2014, 14, e161–e163. [Google Scholar] [CrossRef]
- Mey, U.J.M.; Renner, C.; von Moos, R. Vemurafenib in combination with cobimetinib in relapsed and refractory extramedullary multiple myeloma harboring the BRAF V600E mutation. Hematol. Oncol. 2017, 35, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Chau, I.; Hyman, D.M.; Ribrag, V.; Blay, J.Y.; Tabernero, J.; Elez, E.; Wolf, J.; Yee, A.J.; Kaiser, M.; et al. Vemurafenib in patients with relapsed refractory multiple myeloma harboring BRAF(V600) mutations: A cohort of the histology-independent VE-BASKET study. JCO Precis. Oncol. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Pan, D.; Richter, J. Where we stand with precision therapeutics in myeloma: Prosperity, promises, and pipedreams. Front. Oncol. 2021, 11, 819127. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Nagler, A.; Ben-Yehuda, D.; Badros, A.; Hari, P.N.; Hajek, R.; Spicka, I.; Kaya, H.; LeBlanc, R.; Yoon, S.S.; et al. Randomized, placebo-controlled, phase 3 study of perifosine combined with bortezomib and dexamethasone in patients with relapsed, refractory multiple myeloma previously treated with bortezomib. eJHaem 2020, 1, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Bahlis, N.J.; Venner, C.P.; Hay, A.E. Biomarker driven phase II clinical trial of trametinib in relapsed/refractory multiple myeloma with sequential addition of the AKT inhibitor, GSK2141795 at time of disease progression to overcome treatment failure: A trial of the Princess Margaret phase II consortium. Blood 2016, 128, 4526. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Patnaik, A.; Papadopoulos, K.P.; Rasco, D.W.; Becerra, C.R.; Allred, A.J.; Orford, K.; Aktan, G.; Ferron-Brady, G.; Ibrahim, N.; et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother. Pharmacol. 2015, 75, 183–189. [Google Scholar] [CrossRef]
- Calimeri, T.; Ferreri, A.J.M. m-TOR inhibitors and their potential role in haematological malignancies. Br. J. Haematol. 2017, 177, 684–702. [Google Scholar] [CrossRef]
- Gunther, A.; Baumann, P.; Burger, R.; Kellner, C.; Klapper, W.; Schmidmaier, R.; Gramatzki, M. Activity of everolimus (RAD001) in relapsed and/or refractory multiple myeloma: A phase I study. Haematologica 2015, 100, 541–547. [Google Scholar] [CrossRef]

| Trial ID (References) | Drugs | Enrollment (N) | Phase | Trial Title |
|---|---|---|---|---|
| NCT03374085 [32,33] | CC-92480 vs. (CC-92480 + Dex) | 201 | I/II | Multicenter, Open-label Study to Assess the Safety, Pharmacokinetics and Efficacy of CC-92480 Monotherapy and in Combination With Dexamethasone in Subjects With RRMM |
| NCT05519085 | (CC-92480 + Bort + Dex) vs. (Pom + Bort + Dex) | 760 | III | Two-Stage, Randomized, Multicenter, Open-Label Study Comparing CC-92480, Bortezomib and Dexamethasone (480Vd) Versus Pomalidomide, Bortezomib and Dexamethasone (PVd) in Subjects With RRMM (SUCCESSOR-1) |
| NCT05552976 | (CC-92480 + Carf + Dex) vs. (Carf + Dex) | 525 | III | Two-stage, Randomized, Multicenter, Open-label Study Comparing CC-92480 (BMS-986348), Carfilzomib, and Dexamethasone (480Kd) Versus Carfilzomib and Dexamethasone (Kd) in Participants With RRMM (SUCCESSOR-2) |
| NCT01421524 [30] | CC-122 | 271 | I | Multi-center, Open-label, Dose Finding Study to Assess the Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of the Pleiotropic Pathway Modifier CC-122 Administered Orally to Subjects With Advanced Solid Tumors, NHL, or MM |
| NCT05177536 | Iber | 38 | II | Iberdomide Maintenance Therapy Following ASCT in Patients With MM |
| NCT04998786 | Iber + Ixaz + Dex | 80 | II | A Multi-center Open-label Study of Ixazomib, Iberdomide and Dexamethasone in Elderly Patients With MM at First Relapse |
| NCT04975997 [34] | (Iber + Dara + Dex) vs. (Dara + Bort + Dex) | 864 | III | Two-Stage, Randomized, Multicenter, Open-label Study Comparing Iberdomide, Daratumumab and Dexamethasone (IberDd) Versus Daratumumab, Bortezomib, and Dexamethasone (DVd) in Subjects With RRMM (EXCALIBER) |
| NCT02773030 [28,35] | Iber vs. (Iber + Dex) vs. [Iber + Dex + (Dara or Bort or Carf)] | 449 | I/II | Multicenter, Open-label, Dose-escalation Study to Determine the Maximum Tolerated Dose, Assess the Safety, Tolerability, Pharmacokinetics and Efficacy of CC-220 as Monotherapy and in Combination With Other Treatments in Subjects With MM |
| NCT05392946 | Iber + Dara + Bort + Dex | 18 | I/II | Iberdomide in Combination With Daratumumab, Bortezomib and Dexamethasone in Patients With NDMM (IDEAL) |
| NCT05199311 | Iber + Carf + Dex | 66 | I/II | Carfilzomib, Iberdomide and Dexamethasone (KID) in Patients With Newly Diagnosed Transplant Eligible MM |
| NCT04564703 | Iber | 160 | II | Iberdomide Maintenance After ASCT in NDMM Patients |
| NCT04392037 | Iber + Ctx + Dex | 60 | II | Study of Iberdomide Combined With Low-dose Cyclophosphamide and Dexamethasone in RRMM (ICON) |
| NCT05558319 | (Bort + Isa + Dex +/− Len) vs. (Bort + Isa + Dex + Iber) | 480 | III | Trial for NDMM Patients Who Are Candidates for ASCT Comparing Extended VRD Plus Early Rescue Intervention vs. Isatuximab-VRD vs. Isatuximab-V-Iberdomide-D |
| Trial ID (References) | Drugs | Enrollment (N) | Phase | Trial Title |
|---|---|---|---|---|
| NCT00729118 [76] | Vor + Len | 19 | I | Vorinostat (SAHA) and Lenalidomide After Autologous Transplant for Patients With MM |
| NCT01502085 | Vor + Len + Dex | 25 | I/II | Vorinostat in Combination With Lenalidomide and Dexamethasone in MM Patients Refractory to Previous Lenalidomide Containing Regimens |
| NCT00642954 | Vor + Len + Dex | 31 | I | Vorinostat in Combination With Lenalidomide and Dexamethasone in Patients With RRMM |
| NCT00773747 [69] | (Vor + Bort) vs. (Bort + placebo) | 637 | III | An International, Multicenter, Randomized, Double-Blind Study of Vorinostat (MK-0683) or Placebo in Combination With Bortezomib in Patients With MM (VANTAGE 088) |
| NCT00773838 [77] | Vor + Bort + Dex | 143 | II | An International, Multicenter, Open-Label Study of Vorinostat (MK0683) in Combination With Bortezomib in Patients With RRMM |
| NCT01720875 [78] | Vor + Bort + Dex | 16 | II | Trial of Combination Treatment With Vorinostat, Bortezomib and Dexamethasone in Patients With RRMM |
| NCT01038388 [79] | Vor + Len + Bort + Dex | 30 | I | Trial Evaluating the Safety and Efficacy of Vorinostat + RVD (Lenalidomide + Bortezomib + Dexamethasone) for Patients With NDMM |
| NCT01297764 [80] | Vor + Carf + Len + Dex | 17 | I/II | Study of Carfilzomib, Lenalidomide, Vorinostat, and Dexamethasone in RRMM |
| NCT01394354 [81] | Vor + Bort + Dex + Doxo | 34 | I/II | Safety of Vorinostat in Combination With Bortezomib, Doxorubicin and Dexamethasone (VBDD) in Patients with RRMM |
| Trial Identifier (References) | Drugs | Enrollment (N) | Phase | Trial Title |
|---|---|---|---|---|
| NCT01323751 [73] | Ricol + Bort + Dex | 120 | I/II | Open-Label, Multicenter Study of ACY-1215 Administered Orally as Monotherapy and in Combination With Bortezomib and Dexamethasone for the Treatment of RRMM. |
| NCT01583283 [74] | Ricol + Len + Dex | 38 | I | Open-Label, Multicenter Study of ACY-1215 (Ricolinostat) in Combination With Lenalidomide and Dexamethasone for the Treatment of RRMM |
| NCT01997840 [73] | Ricol + Pom + Dex | 103 | I/II | Multi-Center, Open Label, Dose-Escalation Study to Determine the Maximum Tolerated Dose, Safety, and Efficacy of ACY-1215 (RICOLINOSTAT) in Combination With Pomalidomide and Low-Dose Dexamethasone in Patients With RRMM. |
| NCT02400242 [82,83,84] | Citar + Pom + Dex | 85 | I | Multicenter, Single-Arm, Open-Label, Dose-Escalation Study to Determine the Maximum Tolerated Dose, Safety, and Preliminary Activity of Oral ACY-241 Alone and in Combination With Pomalidomide and Low-Dose Dexamethasone in Patients With RRMM |
| NCT04025450 [75,85] | (Bort + Len + Dex) +/− Chid | 50 | I/II | Comparation of Chidamide Plus VRD (Bortezomib, Lenalidomide, Dexamethasone) With VRD Regimen for Primary High-Risk MM Patients, a Multiple Center, Randomized Clinical Trial. |
| NCT03605056 | Chid + Len + Dex | 25 | II | Chidamide in Combination With Lenalidomide and Dexamethasone for the Treatment of RRMM |
| Trial ID (Reference) | Drugs | Enrollment (N) | Phase | Trial Title |
|---|---|---|---|---|
| NCT00821249 [126] | Fil vs (Fil + Dex) | 55 | I/II | A Study of ARRY-520 in Patients With RRMM |
| NCT01248923 [127,128] | (Fil + Bort) vs. (Fil + Bort + Dex) | 55 | I | A Study of ARRY-520 and Bortezomib Plus Dexamethasone in Patients With RRMM |
| NCT01372540 [132] | Carf + Fil | 76 | I | Filanesib and Carfilzomib in Treating Patients With RRMM or Plasma Cell Leukemia |
| NCT02384083 [133] | Fil + Pom + Dex | 47 | I/II | Filanesib (ARRY-520) in Combination With Pomalidomide and Dexamethasone for RRMM Patients |
| NCT01989325 [137] | (Fil + Carf + Dex) vs. (Carf + Dex) | 77 | II | A Study of Filanesib (ARRY-520) and Carfilzomib in Patients With Advanced MM |
| NCT02092922 [138] | Fil | 154 | II | Trial of Filanesib in RRMM (AfFIRM) |
| Trial ID (Reference) | Drugs | Enrollment (N) | Phase | Trial Title |
|---|---|---|---|---|
| NCT02186834 [152] | Sel + Dox + Dex | 28 | I/II | Investigator-Initiated Trial of Selinexor and Liposomal Doxorubicin for RRMM |
| NCT02336815 [147,153,154] | Sel + Dex | 202 | II | Open-Label, Single-Arm Study of Selinexor Plus Low-Dose Dexamethasone (Sd) in Patients With MM Previously Treated With Lenalidomide, Pomalidomide, Bortezomib, Carfilzomib, and Daratumumab, and Refractory to Prior Treatment With Glucocorticoids, an Immunomodulatory Agent, a Proteasome Inhibitor, and Daratumumab (STORM) |
| NCT05422027 | Sel + Len + Bort + Dex | 42 | I/II | Selinexor Plus Bortezomib, Lenalidomide and Dexamethasone (XVRd) in High Risk NDMM |
| NCT04661137 [155,156] | Sel + Dex + (Pom or Carf or Dara) | 96 | II | Study of Selinexor in Combination With Carfilzomib, Daratumumab or Pomalidomide in Patients With RRMM |
| NCT04414475 | (Sel + Dex) vs. (Sel + Dex + Bort) | 134 | II | Open-label, Multi-arm Trial of Selinexor Plus Low-dose Dexamethasone (Sd) in Patients With Penta-refractory MM or Selinexor and Bortezomib Plus Low-dose Dexamethasone (SVd) in Patients With Triple-class Refractory MM |
| NCT02780609 [157] | Sel + Mel + Dex | 22 | I/II | Investigator Sponsored Study of Selinexor in Combination With High-Dose Melphalan Before ASCT for MM |
| NCT02199665 [158] | Sel + Carf + Dex | 100 | I | Study of the Combination of Selinexor With Carfilzomib and Dexamethasone in Patients With RRMM |
| NCT03110562 [149,150,158,159,160] | (Sel + Bort + Dex) vs. (Bort + Dex) | 402 | III | Randomized, Controlled, Open-label Study of Selinexor, Bortezomib, and Dexamethasone (SVd) Versus Bortezomib and Dexamethasone (Vd) in Patients With RRMM (BOSTON) |
| NCT05028348 | (Sel + Pom + Dex) vs. (Elo + Pom + Dex) | 300 | III | Randomized, Open-label Trial of Selinexor, Pomalidomide, and Dexamethasone (SPd) Versus Elotuzumab, Pomalidomide, and Dexamethasone (EloPd) in Patients With RRMM |
| NCT04764942 [161] | (Sel + Pom + Dex +/- Carf | 81 | I/II | Trial of Selinexor in Combination With Pomalidomide and Dexamethasone ± Carfilzomib for Patients With Proteasome-Inhibitor and Immunomodulatory Drug RRMM (SCOPE) |
| NCT02343042 [155,162] | Sel + Various | 518 | I/II | Study of Selinexor in Combination With Backbone Treatments for RRMM and NDMM |
| NCT03589222 [163] | Sel + Dara + Bort + Dex | 62 | II | Open-label, Multicenter Trial of Selinexor, Bortezomib and Low-dose Dexamethasone Plus Daratumumab (SELIBORDARA) for the Treatment of Patients With RRMM |
| NCT04756401 | Sel + Dara + Carf + Dex | 52 | II | Open Label Single-Arm Study of Selinexor, Daratumumab, Carfilzomib and Dexamethasone for High-Risk, RRMM Patients Who Have Received 1–3 Prior Lines of Therapy |
| NCT04877275 [164] | (Sel + Dex + Dox) vs. (Sel + Ctx + Dex) | 50 | II | Selinexor in Combination With Chemotherapy to Treat RRMM Relapsed/Refractory Multiple Myeloma Patients |
| NCT04782687 | Sel + Dex + Dara + Len | 100 | II | Trial of Daratumumab, Lenalidomide and Dexamethasone (DRd) in Combination With Selinexor for Patients With NDMM |
| NCT04941937 | (Sel + Thal + Len) vs. (Sel + Len + Dex) vs. (Sel + Pom + Dex) | 90 | II | Selinexor in Combination With Immunomodulator to Treat RRMM Patients |
| NCT04717700 | (Sel + Bort + Len + Dex) vs. (Bort + Len + Dex) | 100 | II | Selinexor With Alternating Bortezomib or Lenalidomide Plus Dexamethasone in Transplant Ineligible NDMM Patients (SABLe): An Investigator Sponsored Trial |
| NCT04891744 | Sel + Thal + Dex | 48 | I/II | Study of Selinexor in Combination With Thalidomide and Dexamethasone for RRMM |
| NCT03944057 [165] | Sel + Dex | 82 | II | Open-Label, Single-Arm Study of Selinexor Plus Dexamethasone in Patients With MM Refractory to Prior Treatment With Immunomodulatory Agents and Proteasome Inhibitor |
| NCT04939142 | (Bort + Dex) vs. (Sel + Bort + Dex) | 150 | III | Randomized, Controlled, Multicenter, Open-label Study of Selinexor, Bortezomib, and Dexamethasone (SVd) Versus Bortezomib and Dexamethasone (Vd) in Patients With RRMM |
| NCT05478993 | Sel + Pom + Dex | 21 | II | Study of Selinexor, Pomalidomide, and Dexamethasone For MM With Central Nervous System Involvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramson, H.N. Recent Advances in the Applications of Small Molecules in the Treatment of Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 2645. https://doi.org/10.3390/ijms24032645
Abramson HN. Recent Advances in the Applications of Small Molecules in the Treatment of Multiple Myeloma. International Journal of Molecular Sciences. 2023; 24(3):2645. https://doi.org/10.3390/ijms24032645
Chicago/Turabian StyleAbramson, Hanley N. 2023. "Recent Advances in the Applications of Small Molecules in the Treatment of Multiple Myeloma" International Journal of Molecular Sciences 24, no. 3: 2645. https://doi.org/10.3390/ijms24032645
APA StyleAbramson, H. N. (2023). Recent Advances in the Applications of Small Molecules in the Treatment of Multiple Myeloma. International Journal of Molecular Sciences, 24(3), 2645. https://doi.org/10.3390/ijms24032645


_Putnam.png)



