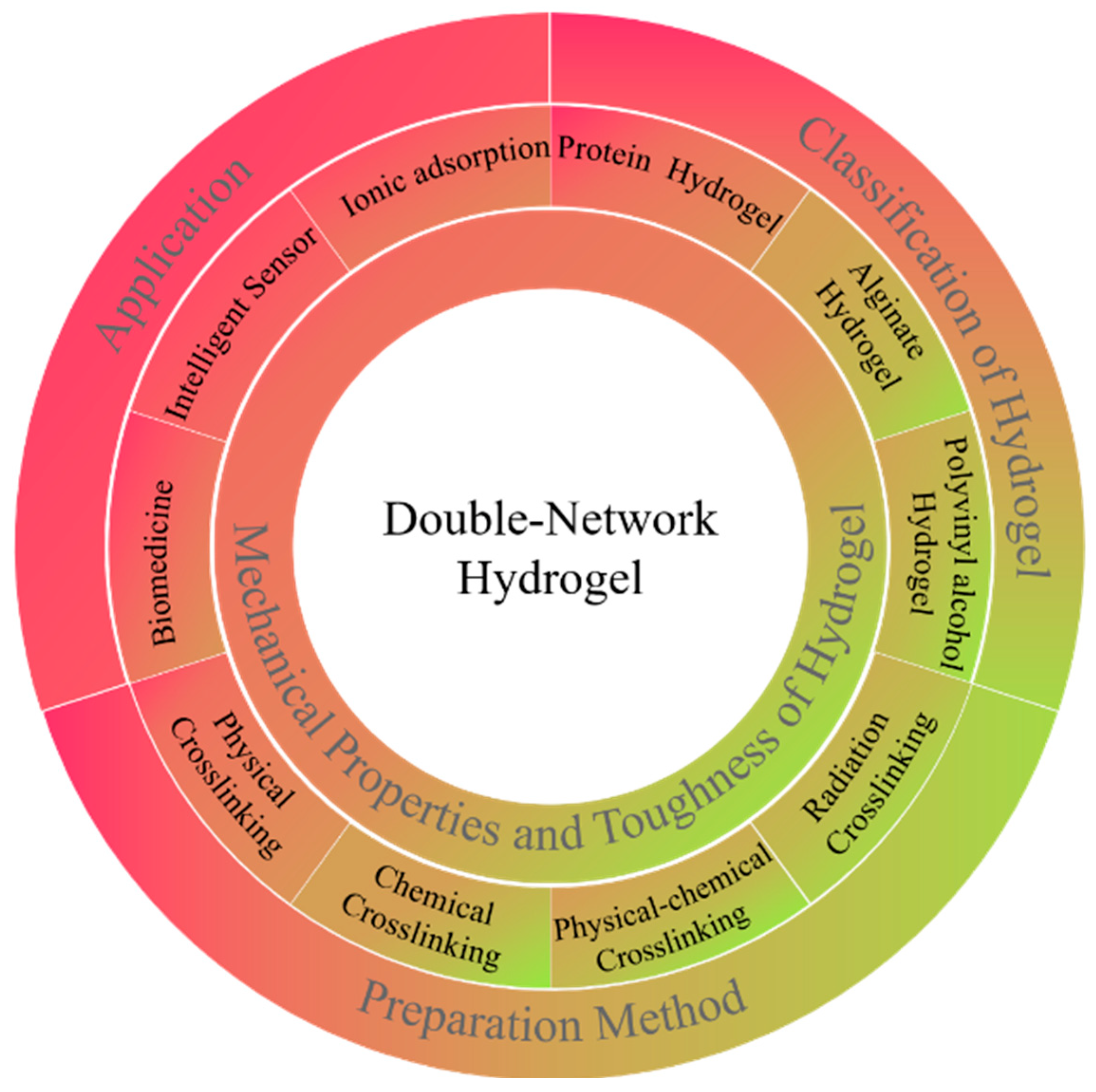
Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels
Abstract
1. Introduction
2. Preparation Method of DN Hydrogel
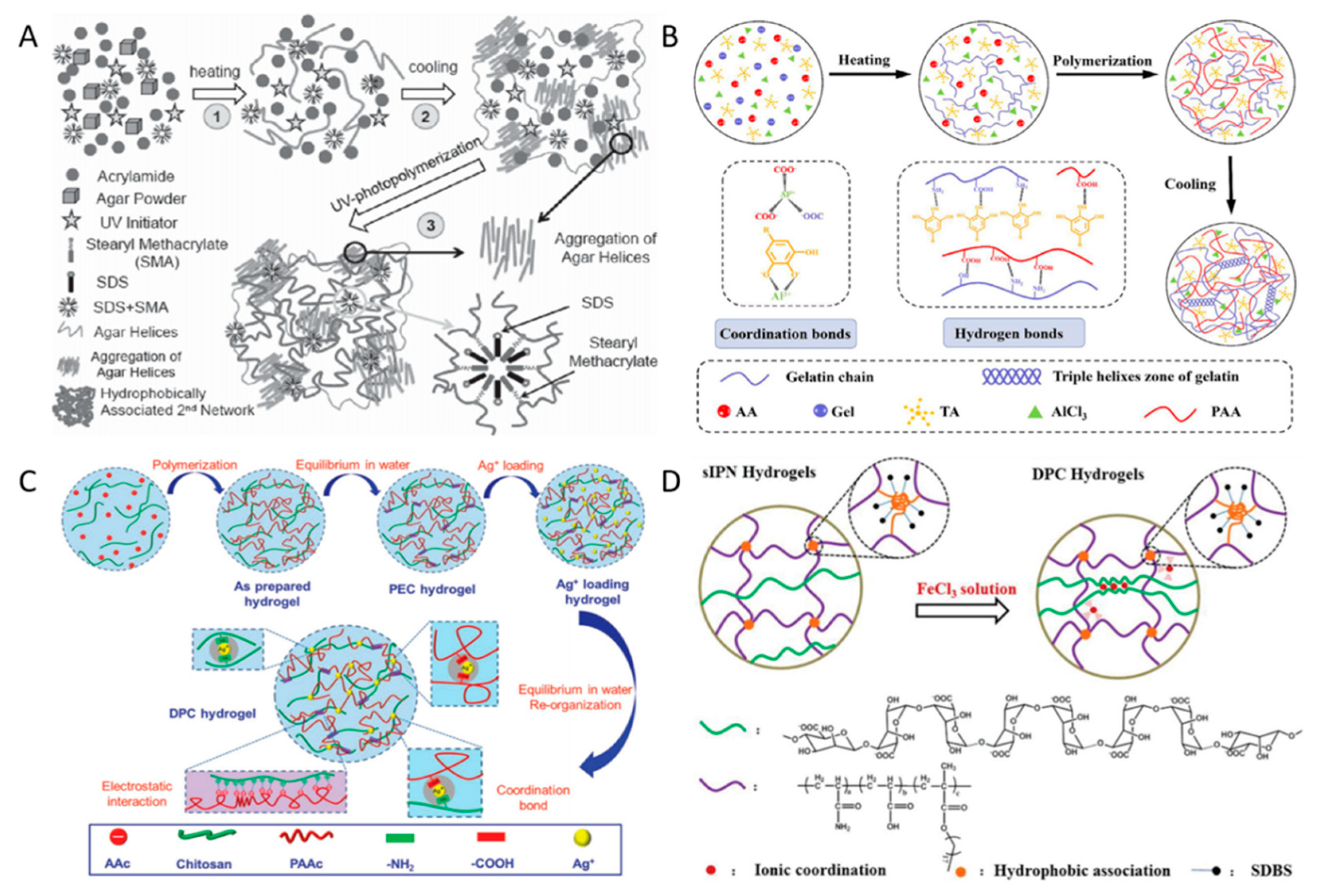
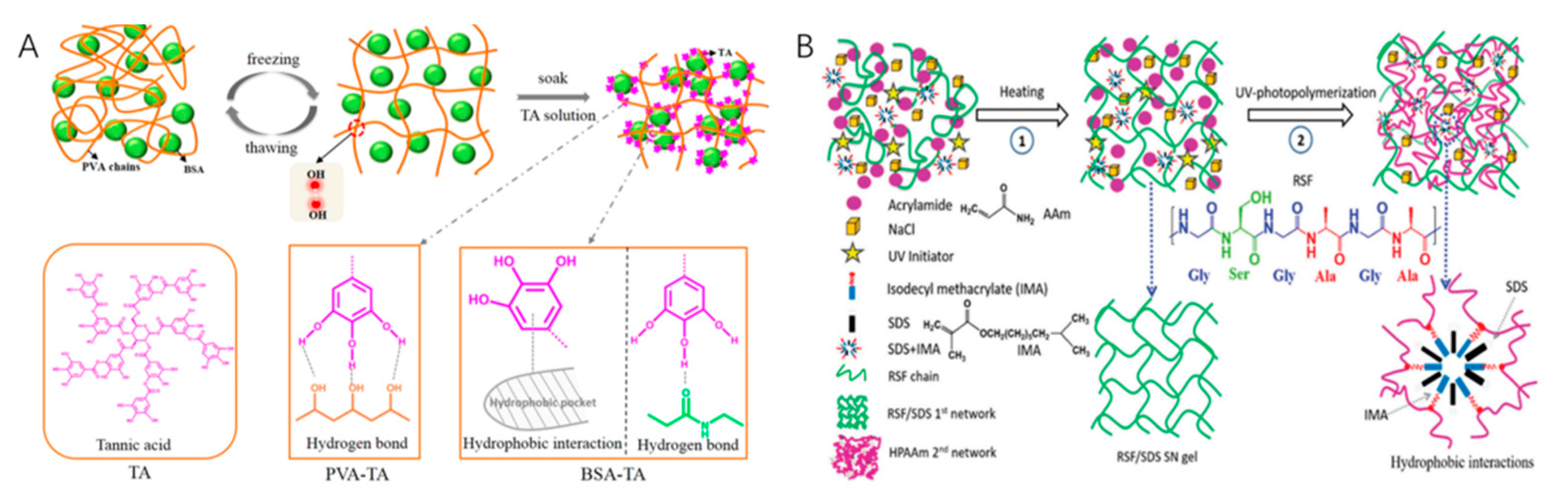
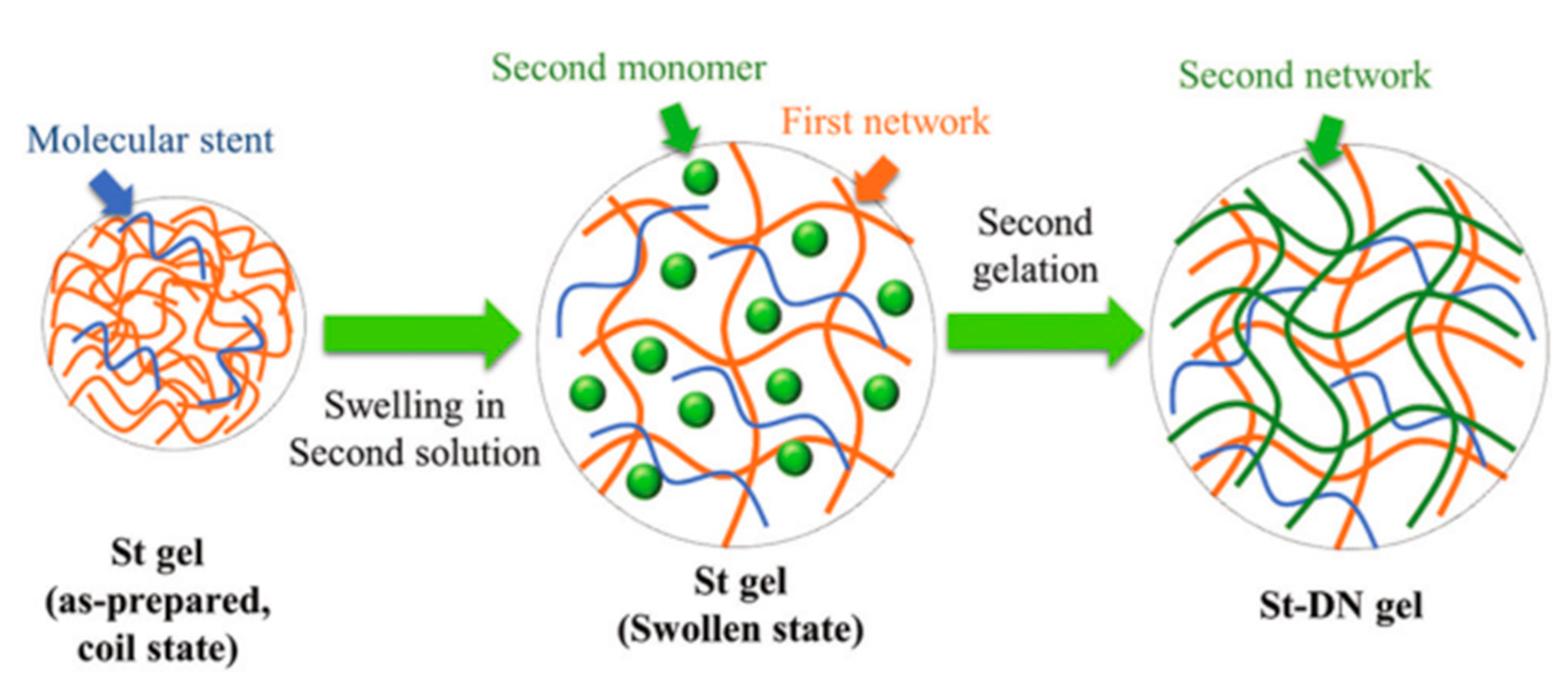
2.1. Physically Cross-Linking Hydrogels
2.2. Chemical Cross-Linking Hydrogels
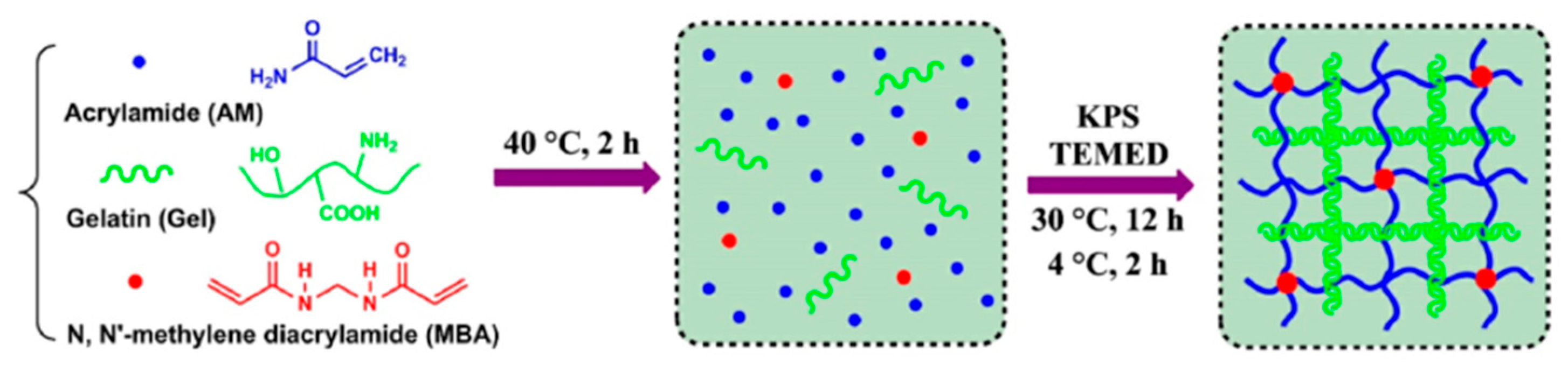
2.3. Physical–Chemical Cross-Linking Hydrogels
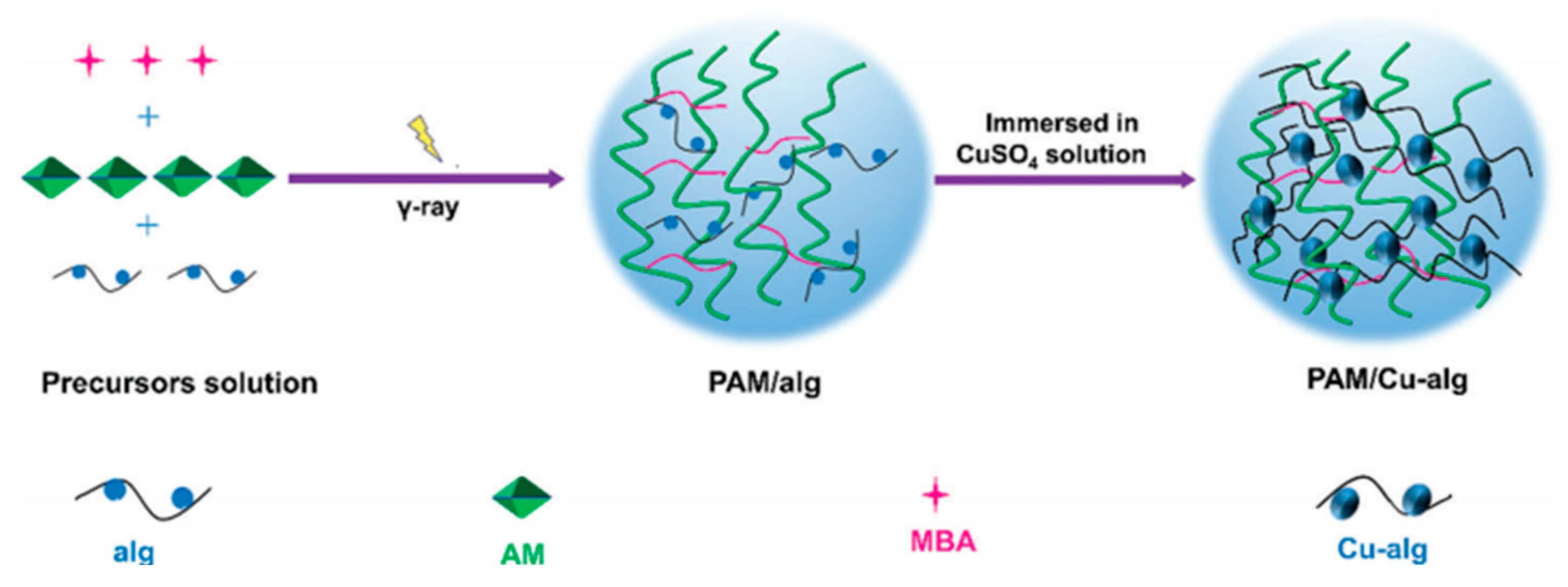
2.4. Radiation Cross-Linking Hydrogels
3. Mechanical Properties of Double Network Hydrogels
3.1. Polyvinyl Alcohol Hydrogels
3.2. Alginate Hydrogel
3.3. Protein-Based Hydrogels
3.4. Other Types of Hydrogels
4. Application
4.1. Biomedical Applications
4.2. Application of Intelligent Sensors
4.3. Application of Ion Adsorption
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhou, M.; Peng, J.; Wang, X.; Liu, Y.; Wang, W.; Wu, D. Robust, anti-freezing and conductive bonding of chitosan-based double-network hydrogels for stable-performance flexible electronic. Carbohydr. Polym. 2022, 276, 118753. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3D Bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.L.; Chen, C.; Shen, J.H.; Zhao, X.L.; Qian, S.H.; Zhu, Z.G. A Gelated Colloidal Crystal Attached Lens for Noninvasive Continuous Monitoring of Tear Glucose. Polymers 2017, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Chu, Z.; Xue, C.; Shao, K.; Xiang, L.; Zhao, X.; Chen, C.; Pan, J.; Lin, D. Photonic Crystal-Embedded Molecularly Imprinted Contact Lenses for Controlled Drug Release. ACS Appl. Bio Mater. 2022, 5, 243–251. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.W.; Wang, X.H.; Zhao, X.L.; Chen, C.; Zhu, Z.G. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Cui, Y.; Li, A.; Qiao, Y.; Qiu, D. Conjoined-network rendered stiff and tough hydrogels from biogenic molecules. Sci. Adv. 2019, 5, eaau3442. [Google Scholar] [CrossRef]
- Zhang, M.; Ren, X.; Duan, L.; Gao, G. Joint double-network hydrogels with excellent mechanical performance. Polymer 2018, 153, 607–615. [Google Scholar] [CrossRef]
- Engkagul, V.; Sereemaspun, A.; Chirachanchai, S. One pot preparation of chitosan/hyaluronic acid-based triple network hydrogel via in situ click reaction, metal coordination and polyion complexation in water. Carbohydr. Polym. 2018, 200, 616–623. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-Inspired Multifunctional Autonomic-Intrinsic Conductive Self-Healing Hydrogels with Pressure Sensitivity, Stretchability, and 3D Printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Chen, Y.M.; Dong, K.; Liu, Z.Q.; Xu, F. Double network hydrogel with high mechanical strength: Performance, progress and future perspective. Sci. China. Tech. Sci. 2012, 55, 2241–2254. [Google Scholar] [CrossRef]
- Li, L.Q.; Wu, P.W.; Ma, J. Construction of Double Network Gel Adsorbent and Application for Pollutants Removal from Aqueous Solution. Prog. Chem. 2021, 33, 1010–1025. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Chen, H.; Yan, H.; Huang, L.; Yang, J.; Zheng, J. A Novel Design Strategy for Fully Physically Linked Double Network Hydrogels with Tough, Fatigue Resistant, and Self-Healing Properties. Adv. Funct. Mater. 2015, 25, 1598–1607. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, Z.; Huo, Y.; Shen, Y.; Qin, M.; Feng, W. Tetradic double-network physical cross-linking hydrogels with synergistic high stretchable, self-healing, adhesive, and strain-sensitive properties. J. Mater. Sci. Technol. 2022, 98, 169–176. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Chen, Y.; Zhang, L.; Zhou, J. Dual Physical Crosslinking Strategy to Construct Moldable Hydrogels with Ultrahigh Strength and Toughness. Adv. Funct. Mater. 2018, 28, 1800739. [Google Scholar] [CrossRef]
- Means, A.K.; Shrode, C.S.; Whitney, L.V.; Ehrhardt, D.A.; Grunlan, M.A. Double Network Hydrogels that Mimic the Modulus, Strength, and Lubricity of Cartilage. Biomacromolecules 2019, 20, 2034–2042. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Z.; Liu, Y.; Wang, J.; Li, Z.; Wang, M. Shape memory polyacrylamide/gelatin hydrogel with controllable mechanical and drug release properties potential for wound dressing application. Polymer 2021, 226, 123786. [Google Scholar] [CrossRef]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super flexible, fatigue resistant, self-healing PVA/Xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef]
- Ma, M.; Yang, J.; Ye, Z.; Dong, A.; Zhang, J.; Zhang, J. A Facile Strategy for Synergistic Integration of Dynamic Covalent Bonds and Hydrogen Bonds to Surmount the Tradeoff between Mechanical Property and Self-Healing Capacity of Hydrogels. Macromol. Mater. Eng. 2021, 306, 2000577. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, C.; Zhang, X.; Tsou, C.; Zhao, X.; De Guzman, M.R.; Pu, Z.; Li, X.; Lu, Y.; Zeng, C.; et al. A Dual Physical Cross-Linking Strategy to Construct Tough Hydrogels with High Strength, Excellent Fatigue Resistance, and Stretching-Induced Strengthening Effect. Macromol. Mater. Eng. 2021, 306, 2100093. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically cross-linked with glutaraldehyde. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Han, J.Q.; Lei, T.Z.; Wu, Q.L. High-water-content mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Dynamic rheological properties and hydrogel formation mechanism. Carbohydr. Polym. 2014, 102, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shao, J.; Yu, Q.; Wang, S. High-strength, anti-fatigue, stretchable self-healing polyvinyl alcohol hydrogel based on borate bonds and hydrogen bonds. J. Dispers. Sci. Technol. 2022, 43, 690–703. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Gharibi, H.; Molavian, M.R.; Norouzi, M.R.; Asefifeyzabadi, N. Physicochemical modification of hydroxylated polymers to develop thermosensitive double network hydrogels. J. Appl. Polym. Sci. 2021, 138, 50778. [Google Scholar] [CrossRef]
- Guo, C.; Zeng, Z.; Yu, S.; Zhou, X.; Liu, Q.; Pei, D.; Lu, D.; Geng, Z. Highly stretchable, compressible, adhesive hydrogels with double network. J. Polym. Res. 2021, 28, 1–9. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulanski, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- El Salmawi, K.M. Gamma radiation-induced cross-linked PVA/chitosan blends for wound dressing. J. Macromol. Sci. Part A-Pure Appl. Chem. 2007, 44, 541–545. [Google Scholar] [CrossRef]
- Guo, W.; Yang, M.; Liu, S.; Zhang, X.; Zhang, B.; Chen, Y. Chitosan/polyvinyl alcohol/tannic acid multiple network composite hydrogel: Preparation and characterization. Iran. Polym. J. 2021, 30, 1159–1168. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Yan, C.; Xiong, D. Mechanical and Tribological Study of PVA-pMPDSAH Double-Network Hydrogel Prepared by Ultraviolet Irradiation and Freeze-Thaw Methods for Bionic Arti cular Cartilage. J. Bionic Eng. 2021, 18, 1192–1201. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.H.; Bao, H.; Zhao, P.; Jiang, H.L.; Peng, L.M.; Yang, X.L.; Li, C.Z. Solvent-assisted poly(ainyl alcohol) gelated crystalline colloidal array photonic crystals. Soft Matter 2011, 7, 915–921. [Google Scholar] [CrossRef]
- Wang, X.H.; Qiu, Y.F.; Chen, G.; Chu, Z.R.; Shadike, A.; Chen, C.S.; Chen, C.; Zhu, Z.G. Self-Healable Poly(vinyl alcohol) Photonic Crystal Hydrogel. ACS Appl. Polym. Mater. 2020, 2, 2086–2092. [Google Scholar] [CrossRef]
- Elango, J.; Zamora-Ledezma, C.; Negrete-Bolagay, D.; Aza, P.N.D.; Gómez-López, V.M.; López-González, I.; Belén Hernández, A.; De Val, J.E.M.S.; Wu, W. Retinol-Loaded Poly(vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 23, 15623. [Google Scholar] [CrossRef]
- Chen, W.; Li, N.; Ma, Y.; Minus, M.L.; Benson, K.; Lu, X.; Wang, X.; Ling, X.; Zhu, H. Superstrong and Tough Hydrogel through Physical Cross-Linking and Molecular Alignment. Biomacromolecules 2019, 20, 4476–4484. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, X.; Liu, S.; Zhao, X.; Geng, C.; Wang, L.; Xia, Y. Selected Phase Separation Renders High Strength and Toughness to Polyacrylamide/Alginate Hydrogels with Large-Scale Cross-Linking Zones. ACS Appl. Mater. Interfaces 2021, 13, 25383–25391. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, T.; Li, S.; Chen, X.; Que, X.; Sheng, L.; Hu, Y.; Peng, J.; Ma, H.; Li, J.; et al. Polyacrylamide/Copper-Alginate Double Network Hydrogel Electrolyte with Excellent Mechanical Properties and Strain-Sensitivity. Macromol. Biosci. 2022, 22, 2100361. [Google Scholar] [CrossRef]
- Huang, Y.; Jayathilaka, P.B.; Islam, M.S.; Tanaka, C.B.; Silberstein, M.N.; Kilian, K.A.; Kruzic, J.J. Structural aspects controlling the mechanical and biological properties of tough, double network hydrogels. Acta Biomater. 2022, 138, 301–312. [Google Scholar] [CrossRef]
- Samp, M.A.; Iovanac, N.C.; Nolte, A.J. Sodium Alginate Toughening of Gelatin Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3176–3182. [Google Scholar] [CrossRef]
- Wen, C.; Lu, L.L.; Li, X.S. Mechanically Robust Gelatin-Alginate IPN Hydrogels by a Combination of Enzymatic and Ionic Crosslinking Approaches. Macromol. Mater. Eng. 2014, 299, 504–513. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.J.; Liu, X.; Liu, Y.; Zhu, X.; Liu, X.; You, X. A Physically Cross-Linked Sodium Alginate-Gelatin Hydrogel with High Mechanical Strength. ACS Appl. Polym. Mater. 2021, 3, 3197–3205. [Google Scholar] [CrossRef]
- Xu, R.; Ma, S.; Lin, P.; Yu, B.; Zhou, F.; Liu, W. High Strength Astringent Hydrogels Using Protein as the Building Block for Physically Cross-linked Multi-Network. ACS Appl. Mater. Interfaces 2018, 10, 7593–7601. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Yamashita, T.; Yatabe, K.; Vogel, V. Mechano-chromic protein-polymer hybrid hydrogel to visualize mechanical strain. Soft Matter 2019, 15, 9388–9393. [Google Scholar] [CrossRef]
- Chen, F.; Lu, S.; Zhu, L.; Tang, Z.; Wang, Q.; Qin, G.; Yang, J.; Sun, G.; Zhang, Q.; Chen, Q. Conductive regenerated silk-fibroin-based hydrogels with integrated high mechanical performances. J. Mater. Chem. B 2019, 7, 1708–1715. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.S.; Guan, J.; Wu, S.J. Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels. Acta Biomater. 2021, 125, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Y.; Liu, S.H.; Chen, S.F. Strong double network hydrogels reinforced by silk fibroin microfibers. J. Chem. Eng. Chin. Univ. 2021, 35, 148–154. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, J.; Qiu, C.; Xu, X.; Jin, Z. A Dual Cross-Linked Strategy to Construct Moldable Hydrogels with High Stretchability, Good Self-Recovery, and Self-Healing Capability. J. Agric. Food Chem. 2019, 67, 3966–3980. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Peng, Y.; Wang, Z.; Ran, R. Highly Stretchable, Strain-Sensitive, and Antifreezing Macromolecular Microsphere Composite Starch-Based Hydrogel. Macromol. Mater. Eng. 2021, 306, 2100198. [Google Scholar] [CrossRef]
- Nakajima, T.; Sato, H.; Zhao, Y.; Kawahara, S.; Kurokawa, T.; Sugahara, K.; Gong, J.P. A Universal Molecular Stent Method to Toughen any Hydrogels Based on Double Network Concept. Adv. Funct. Mater. 2012, 22, 4426–4432. [Google Scholar] [CrossRef]
- Xu, X.W.; Jerca, V.V.; Hoogenboom, R. Bioinspired double network hydrogels: From covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels. Mater. Horiz. 2021, 8, 1173–1188. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, M.; Li, R.; Cai, Z.; Zhang, H. Fabrication of a Double-Network Hydrogel Based on Carboxymethylated Curdlan/Polyacrylamide with Highly Mechanical Performance for Cartilage Repair. ACS Appl. Polym. Mater. 2021, 3, 5857–5869. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Q.; Zong, T.; Liu, S.; Yang, X.; Luo, Y.; Zhang, D. Effect of Directional Stretching on Properties of PVA-HA-PAA Composite Hydrogel. J. Bionic Eng. 2021, 18, 1202–1214. [Google Scholar] [CrossRef]
- Luo, C.; Huang, M.; Sun, X.; Wei, N.; Shi, H.; Li, H.; Lin, M.; Sun, J. Super-Strong, Nonswellable, and Biocompatible Hydrogels Inspired by Human Tendons. ACS Appl. Mater. Interfaces 2022, 14, 2638–2649. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Lin, X.; Wang, X.; Guo, H. Muscle-inspired ion-sensitive hydrogels with highly tunable mechanical performance for versatile industrial applications. Sci. China-Mater. 2022, 65, 229–236. [Google Scholar] [CrossRef]
- Geng, L.; Hu, S.; Cui, M.; Wu, J.; Huang, A.; Shi, S.; Peng, X. Muscle-inspired double-network hydrogels with robust mechanical property, biocompatibility and ionic conductivity. Carbohydr. Polym. 2021, 262, 117936. [Google Scholar] [CrossRef] [PubMed]
- Tavakolizadeh, M.; Pourjavadi, A.; Ansari, M.; Tebyanian, H.; Tabaei, S.J.S.; Atarod, M.; Rabiee, N.; Bagherzadeh, M.; Varma, R.S. An environmentally friendly wound dressing based on a self-healing, extensible and compressible antibacterial hydrogel. Green Chem. 2021, 23, 1312–1329. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Zhu, J.; Wang, Z.; Wang, L. Preparation and characterization of double network hydrogel with high-strength and self-healing. Mater. Today Commun. 2021, 27, 102450. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Z.Q.; Shen, J.H.; Chen, H.W.; Zhu, Y.H.; Zhu, Z.G. 2D Photonic Crystal Hydrogel Sensor for Tear Glucose Monitoring. ACS Omega 2018, 3, 3211–3217. [Google Scholar] [CrossRef]
- Chu, Z.; Ding, Z.; Ning, X.; Wang, M.; Shao, K.; Tang, W.; Chen, C.; Bai, J. Non-gelated polymeric photonic crystal films. Front. Chem. 2022, 10, 1009669. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, K.; Sang, P.; Hu, H.; He, M. A very mechanically strong and stretchable liquid-free double-network ionic conductor. J. Mater. Chem. A 2021, 9, 23714–23721. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, R.; Liu, J.; Zhao, L.; Yu, Y. High strength and conductive hydrogel with fully interpenetrated structure from alginate and acrylamide. e-Polymers 2021, 21, 391–397. [Google Scholar] [CrossRef]
- Qi, X.; Wu, L.; Su, T.; Zhang, J.; Dong, W. Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surf. B-Biointerfaces 2018, 170, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Chen, G.; Dong, Z.Q.; Zhu, Z.G.; Chen, C. Progress in molecular imprinted photonic crystals. J. Mater. Eng. 2020, 48, 60–72. [Google Scholar] [CrossRef]
- Ma, J.; Luo, J.; Liu, Y.; Wei, Y.; Cai, T.; Yu, X.; Liu, H.; Liu, C.; Crittenden, J.C. Pb(II), Cu(II) and Cd(II) removal using a humic substance-based double network hydrogel in individual and multicomponent systems. J. Mater. Chem. A 2018, 6, 20110–20120. [Google Scholar] [CrossRef]
- Song, L.; Liu, F.; Zhu, C.; Li, A. Facile one-step fabrication of carboxymethyl cellulose based hydrogel for highly efficient removal of Cr(VI) under mild acidic condition. Chem. Eng. J. 2019, 369, 641–651. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, Y.; Luo, K.; Wu, Y.; Gao, H.; Cheng, J.; Liu, C.; Tao, G.; Guan, Q.; Zhang, L. Preparation and Properties of Polyacrylamide/Sodium Alginate Hydrogel and the Effect of Fe3+ Adsorption on Its Mechanical Performance. J. Renew. Mater. 2021, 9, 1447–1462. [Google Scholar] [CrossRef]
- Fei, C.; Huang, D.; Feng, S. Adsorption behavior of amphoteric double-network hydrogel based on poly(acrylic acid) and silica gel. J. Polym. Res. 2012, 19, 1–7. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically cross-linked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]


| Cross-Linking Method | Hydrogel Name | First Network | Second Network | Tensile Strength | Ref. |
|---|---|---|---|---|---|
| Physical cross-linking | Agar/Hydrophobic associated polyacrylamide hydrogels | Agar hydrogel network | Hydrophobically associated polyacrylamide gel network | 0.267 MPa | [15] |
| Gelatin/Polyacrylic acid/Tannic acid/Aluminum chloride hydrogels | Gelatin, polyacrylic acid, tannic acid hydrogen bonding network | Al3+ ligand bonding network with polyacrylic acid | 216 KPa | [16] | |
| Chitosan/Polyacrylic acid/Ag+ hydrogels | Chitosan/Ag+ cross-linking network | Polyacrylic acid network | 24 MPa | [17] | |
| Chemical cross-linking | Poly(2-acrylamido-2-methylpropanesulfonic acid)/Poly (N-isopropyl acrylamide-co-polyacrylamide) hydrogels | Poly(2-acrylamido-2-methylpropane sulfonic acid) network | poly(N-isopropyl-acrylamide-co-acrylamide) network | 25 MPa | [18] |
| Physical-chemical cross-linking | Polyacrylamide/Gelatin hydrogels | Gelatin network | Polyacrylamide network | 0.57 MPa | [19] |
| Xylan/PVA/Borax hydrogels | PVA chain with Xylan first physical network, PVA crystal domain second physical network | PVA chain and borax chemical network | 81 KPa | [20] | |
| Radiation Crosslinking | N-Acryloyl glycinamide/PVA-borax hydrogels | PVA/Dynamic boronic acid lipid bond | poly(N-acryloyl glycinamide)/PVA network | 200 KPa | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, X.; Huang, J.; A, Y.; Yuan, N.; Chen, C.; Lin, D. Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels. Int. J. Mol. Sci. 2022, 23, 15757. https://doi.org/10.3390/ijms232415757
Ning X, Huang J, A Y, Yuan N, Chen C, Lin D. Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels. International Journal of Molecular Sciences. 2022; 23(24):15757. https://doi.org/10.3390/ijms232415757
Chicago/Turabian StyleNing, Xuanjun, Jiani Huang, Yimuhan A, Ningning Yuan, Cheng Chen, and Donghai Lin. 2022. "Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels" International Journal of Molecular Sciences 23, no. 24: 15757. https://doi.org/10.3390/ijms232415757
APA StyleNing, X., Huang, J., A, Y., Yuan, N., Chen, C., & Lin, D. (2022). Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels. International Journal of Molecular Sciences, 23(24), 15757. https://doi.org/10.3390/ijms232415757








